Abstract
Combinations of KIR3DL1 and HLA-Bw4 alleles protect against HIV infection and/or disease progression. These combinations enhance NK cell responsiveness through the ontological process of education. However, educated KIR3DL1+ NK cells do not have enhanced degranulation upon direct recognition of autologous HIV-infected cells. Since antibody-dependent cellular cytotoxicity (ADCC) is associated with improved HIV infection outcomes and NK cells overcome inhibition through killer cell immunoglobulin-like receptors (KIR) to mediate ADCC, we hypothesized that KIR3DL1-educated NK cells mediate anti-HIV ADCC against autologous cells. A whole-blood flow cytometry assay was used to evaluate ADCC-induced activation of NK cells. This assay assessed activation (gamma interferon [IFN-γ] production and/or CD107a expression) of KIR3DL1+ and KIR3DL1− NK cells, from HLA-Bw4+ and HLA-Bw4− HIV-positive and HIV-negative individuals, in response to autologous HIV-specific ADCC targets. KIR3DL1+ NK cells were more functional than KIR3DL1− NK cells from HLA-Bw4+, but not HLA-Bw4−, healthy controls. In HIV-infected individuals, no differences in NK cell functionality were observed between KIR3DL1+ and KIR3DL1− NK cells in HLA-Bw4+ individuals, consistent with dysfunction of NK cells in the setting of HIV infection. Reflecting the partial normalization of NK cell responsiveness following initiation of antiretroviral therapy, a significant correlation was observed between the peripheral CD4+ T-lymphocyte counts in antiretroviral therapy-treated subjects and the functionality of NK cells. However, peripheral CD4+ T-lymphocyte counts were not correlated with an anti-HIV ADCC functional advantage in educated KIR3DL1+ NK cells. The abrogation of the functional advantage of educated NK cells may enhance HIV disease progression. Strategies to enhance the potency of NK cell-mediated ADCC may improve HIV therapies and vaccines.
INTRODUCTION
Avaccine to protect against infection with the human immunodeficiency virus (HIV) is urgently needed. However, the nature of the most effective immune responses to be induced through vaccination is not clear. Attempts to develop antibody (Ab)-based vaccines against HIV have traditionally focused on inducing broadly neutralizing antibodies (BnAbs). Although BnAbs can mediate neutralization of free virions, recent passive transfer studies suggest that their in vivo efficacy is in part dependent upon the ability of effector cells of the innate immune system, such as natural killer (NK) cells, to recognize the Ab constant region (Fc) (14). Recognition of the Fc by the NK cell Fc receptor (FcRγIII or CD16) triggers the release of cytokines, chemokines, and cytotoxic granules, through a process known as antibody-dependent cellular cytotoxicity (ADCC), which causes the lysis of HIV-infected cells bearing anti-HIV Abs. Several other investigations support a role for ADCC in protection from HIV infection and disease progression, demonstrating the activity at higher levels in HIV-exposed seronegative individuals, elite controllers, and rhesus macaques protected through vaccination (9, 19, 29). Moreover, the recent RV144 Thai vaccine trial, which provided partial protection from HIV infection, induced anti-HIV ADCC-competent Abs (35). Coinciding with the increasing evidence for ADCC as a protective anti-HIV immune response, it is also well established that NK cell responsiveness decreases with progressive HIV infection (13, 27), ultimately decreasing the ability of NK cells to mediate ADCC. While HIV infection induces altered NK cell responsiveness, experiments on NK cells from HIV-uninfected subjects have revealed remarkable variability in the ability of NK cells to mediate anti-HIV ADCC responses (11). A greater understanding of the role of NK cell ontogeny in determining the ADCC functional potential of NK cells could establish ADCC as a correlate of protection against HIV and enhance our knowledge of how to best manipulate these responses to achieve more successful vaccination strategies.
The concept that NK cells are protective against HIV infection and disease progression is supported by a large body of epidemiological evidence that links cocarriage of certain allelic combinations of killer cell immunoglobulin-like receptors (KIR) and their major histocompatibility complex (MHC) class I (or HLA-I) alleles with protection from infection and/or progression to AIDS (5, 22). In particular, alleles from the KIR3DL1 (here referred to as 3DL1) locus, which recognize HLA-B molecules carrying the HLA-Bw4 public epitope, have been associated with protection. As these protective 3DL1/HLA-Bw4 combinations have also been demonstrated to confer higher responsiveness upon NK cells expressing the 3DL1 receptor (6, 26), it has generally been assumed that these receptor/ligand combinations mediate protection through direct lysis of HIV-infected cells. Such direct lysis, however, has recently been demonstrated to be unlikely, as 3DL1+ NK cells do not degranulate upon exposure to autologous infected cells (1). As Ab-coated target cells provide additional activating signals, which have been demonstrated to overcome simultaneous inhibitory NK cell receptor signals (20), we hypothesized that an alternative mechanism of protection is that 3DL1+ NK cells demonstrate enhanced anti-HIV ADCC.
The ability of NK cells to mediate effector functions is determined by the cumulative signal generated via the ligation of activating and inhibitory NK cell receptors during the interaction of NK cells with putative target cells. NK cells obtaining similar signals during this interaction, however, will not necessarily mediate equal responses. The strength of NK cell responses is determined by the ligation of activating and inhibitory receptors during NK cell development (15). This ontological process, known as NK cell “education” (or licensing), involves the interaction of activating and inhibitory NK cell receptors with their HLA-I ligands, which can be recognized on the NK cell (cis) or on autologous cells (trans). In general, NK cell education predicts that NK cells carrying an inhibitory receptor without the HLA-I ligand will be hypofunctional, NK cells cocarrying inhibitory receptors and their HLA-I ligands will mediate higher functionality, and NK cells cocarrying activating receptors and their HLA-I ligands will demonstrate decreased functionality. This process, however, does not appear to be a simple “on-off” procedure, as the total input from all inhibitory and activating receptors contributes to the education of NK cells. Recent research demonstrates that stronger cumulative inhibitory signals during NK cell education prepare NK cells to mediate stronger effector functions against appropriate target cells (10). As such, NK cells appear to be regulated by two separate processes involving ligation of inhibitory and activating receptors. The first of these processes determines NK cell functional potential, while the second determines whether the NK cell will respond to potential target cells.
The establishment of NK cell education as the regulatory mechanism for determining the functional potential of NK cells offers a putative explanation for how certain allelic combinations of 3DL1 and HLA-Bw4 protect against HIV infection and progression to AIDS. As NK cells educated through interactions between 3DL1 and HLA-Bw4 have higher functional potential, it seemed probable that 3DL1+ NK cells could more efficiently recognize and destroy autologous HIV-infected cells, which downregulate HLA-B alleles (12). Previous research, however, has demonstrated that 3DL1+ NK cells fail to degranulate in response to autologous HIV-infected cells (1), making direct NK cell killing of HIV-infected cells an unlikely mechanism of the protective 3DL1/HLA-Bw4 effect. Activating signals through CD16 can, however, overcome inhibitory signals through KIR to facilitate NK cell activation (20). Therefore, we hypothesized that NK cells educated through 3DL1 mediate potent anti-HIV ADCC against autologous HLA-Bw4+ target cells. A prediction of this hypothesis is that 3DL1+ NK cells are a more functional ADCC-mediating NK cell subset in individuals also carrying the HLA-Bw4 ligand. We used an intracellular cytokine staining (ICS) ADCC assay to evaluate whether this was indeed the case. Whole blood from HLA-Bw4+ and HLA-Bw4− subjects was cultured with HIV envelope antigens and anti-HIV ADCC-competent Abs. NK cells from both HIV-negative and HIV-positive (HIV+) donors responding to ADCC antibodies were analyzed for 3DL1 expression, cytokine production, and degranulation.
MATERIALS AND METHODS
Study population.
Whole blood from 19 HIV-uninfected healthy control individuals and 27 HIV-infected individuals recruited through the Melbourne Sexual Health Clinic was collected into Vacutainers containing sodium heparin anticoagulant. All participants were screened for 3DL1 expression with phycoerythrin (PE)-conjugated DX9 monoclonal Ab (BD Biosciences), which detects inhibitory 3DL1 alleles that are expressed on the NK cell surface (32). Expression of 3DL1 was detected in 27/27 HIV-infected subjects and 17/19 healthy control subjects. As 3DL1-mediated education in the 2 DX9− subjects could not be evaluated, they were excluded from all subsequent analyses. HLA-B typing was performed by the Victorian Transplant and Immunogenetics Service (Parkville, Australia), using sequence-based typing. Table S1 in the supplemental material provides the HLA-B typings of the HIV-uninfected healthy controls and HIV-infected participants. All subjects provided informed consent for participating in this study, and all participating study sites approved this study.
Anti-HIV ADCC ICS assay.
A whole-blood ICS assay was used to assess NK cell activation by ADCC Abs (30). Briefly, 200 μl of sodium heparin-treated HIV-infected whole blood, or 150 μl of healthy control whole blood plus 50 μl of ADCC-competent HIV-infected plasma, was incubated at 37°C for 5 h with 1 μg/ml of HIV Env peptide pool, brefeldin A (5 μg/ml) (Sigma), and monensin (6 μg/ml) (Sigma). ADCC responses were assessed using a peptide pool containing 15-mers that overlapped by 11 amino acids. This peptide pool spanned the HIV-1 consensus subtype B Env protein (NIH AIDS Reagent Repository). Control incubations of whole blood with just peptides or just HIV-infected plasma were also set up to confirm that any observed functionality was ADCC. After the incubation, cells were surface stained with peridinin chlorophyll protein (PerCP)-conjugated anti-CD3, fluorescein isothiocyanate (FITC)-conjugated anti-CD2, PE-conjugated anti-KIR3DL1, PE-Cy7-conjugated anti-CD56, and allophycocyanin (APC)-conjugated anti-CD107a (all from BD Biosciences). Next, whole blood was treated with lysing solution (BD Biosciences) to remove red blood cells, and the remaining white blood cells were treated with permeabilization solution (BD Biosciences) and stained with Alexa 700-conjugated anti-gamma interferon (anti-IFN-γ) antibody (BD Biosciences). Flow cytometry data were collected using a FACSCanto II flow cytometer (BD Biosciences) and were analyzed using Flow Jo version 9.2 software (Tree Star).
The roles of antibody-coated target cells and immune complexes in stimulating NK cells in this assay were evaluated by assessing the activation of enriched NK cells which were exposed to HIV Env peptides and ADCC-competent plasma under the same conditions as in the whole-blood assay. NK cells were enriched by negative selection using the Easysep human NK cell enrichment kit (Stemcell Technologies). After using the kit as suggested by the manufacturer, populations of cells consisting of greater than 90% NK cells were obtained.
Statistical analysis.
Data analyses were performed using GraphPad Prism version 4.0 software. Wilcoxon matched-pair tests were used to compare within-group differences in the functionalities of 3DL1+ and 3DL1− NK cell subsets in HLA-Bw4+ and HLA-Bw4− groups. Between-group differences, i.e., differences in the functionality of 3DL1+ NK cells between HLA-Bw4+ and HLA-Bw4− groups, were assessed using Mann-Whitney tests. Spearman correlation coefficients were used to assess correlations between clinical and functional data.
RESULTS
HIV-specific ADCC activity of NK cells.
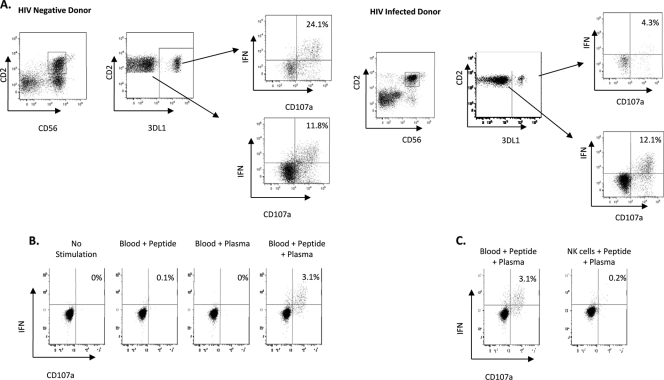
Although NK cells from healthy controls and HIV-infected individuals can mediate efficient ADCC against autologous target cells in the presence of HIV Env antigens and anti-HIV Abs, it is unknown which NK cells are most potently activated by these ADCC Abs. While educated 3DL1+ NK cells mediate enhanced natural cytotoxicity and ADCC against nonself target cells (18, 28), it is unknown if these NK cells can mediate ADCC against autologous target cells that would induce an inhibitory signal through the interaction between 3DL1 and its HLA-Bw4 ligand. To evaluate whether 3DL1-educated NK cells mediate anti-HIV ADCC, we employed a whole-blood ADCC ICS assay to examine the ADCC mediated by NK cells from healthy controls and HIV-infected individuals. This assay assesses the activation of CD56+ CD2+ NK cells as measured by IFN-γ synthesis and CD107a expression (Fig. 1). That the NK cell activation observed is ADCC and is induced by the recognition of anti-HIV antibody-coated target cells is demonstrated by the necessity of both HIV Env peptides and ADCC-competent plasma for NK cell activation (Fig. 1B). Target cells are necessary to present these peptide-antibody combinations, as enriched NK cells alone are not activated in the presence of peptides and ADCC-competent plasma (Fig. 1C). Furthermore, this activation induces immense CD16 downregulation, which is a common characteristic of stimulation through CD16 (7). Corroborating previous data (11), NK cells from both healthy controls and HIV-infected individuals mediated efficient ADCC against autologous target cells. Despite the well-known inhibitory nature of the 3DL1/HLA-Bw4 interaction (21), 3DL1+ NK cells from HLA-Bw4+ individuals were observed to mediate efficient anti-HIV ADCC against HLA-Bw4+ autologous target cells. Interestingly, when we compared the 3DL1+ and 3DL1− NK cells from an HLA-Bw4+ healthy control, we observed that the 3DL1+ NK cells were the more responsive subset.
Fig 1.
NK cell-mediated anti-HIV ADCC responses. (A) Gating was on CD3− lymphocytes. Flow cytometry plots illustrate the gating strategy used to identify the anti-HIV ADCC responses of 3DL1+ and 3DL1− NK cells. The gating strategy is illustrated for an HIV-negative donor and an HIV-infected donor. (B) Dot plots illustrate the requirement for HIV Env peptide and plasma-derived anti-HIV antibodies for NK cell activation in the whole-blood assay. (C) Dot plots depict a representative example of 3 experiments assessing the ability of NK cells within a whole-blood, HIV Env peptide, and HIV-infected plasma culture to express CD107a and produce IFN-γ, compared to enriched NK cells incubated with HIV Env peptides and HIV-infected plasma.
Impact of NK cell education on anti-HIV ADCC functionality in healthy controls.
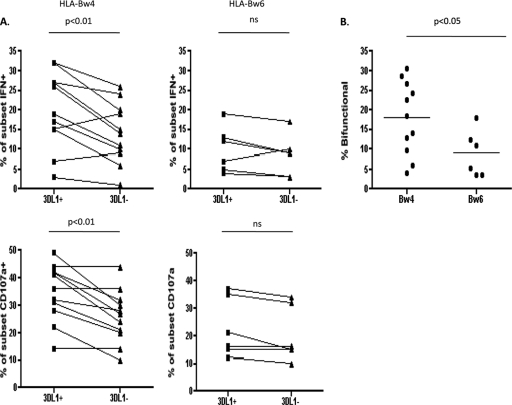
NK cells educated through 3DL1/HLA-Bw4 interactions have been previously demonstrated to exhibit higher cytokine production and degranulation against nonself target cells than 3DL1− NK cells from HLA-Bw4+ individuals (6). Our initial results (Fig. 1A) suggested that educated 3DL1+ NK cells mediate robust anti-HIV ADCC against HLA-Bw4+ autologous target cells. We therefore recruited a total of 11 HLA-Bw4+ healthy control subjects to investigate more thoroughly whether 3DL1+ NK cells were more functional than 3DL1− NK cells from within HLA-Bw4+ individuals. As a control, we also recruited 6 HLA-Bw6 homozygous subjects, who do not carry HLA-Bw4 alleles and would not be expected to demonstrate NK cell education through 3DL1. The whole-blood anti-HIV ADCC ICS assay demonstrated 3DL1+ NK cells to be a more functional NK cell subset than 3DL1− NK cells in HLA-Bw4 carriers. In the HLA-Bw4 carriers, significantly higher mean percentages of the 3DL1+ than of 3DL1− NK cell subset produced IFN-γ (20.2 ± 2.9 versus 14.2 ± 2.3; P < 0.01) and/or expressed the CD107a degranulation marker (34.8 ± 3.2 versus 25.9 ± 2.9; P < 0.01) (Fig. 2A). In HLA-Bw6 homozygous individuals, no difference was observed in IFN-γ production (10.1 ± 2.3 versus 8.5 ± 2.1; not significant [NS]) or CD107a expression (22.7 ± 4.4 versus 20.3 ± 4.1; NS) between the 3DL1+ and 3DL1− NK cells (Fig. 2A). These results demonstrate that NK cell education through 3DL1/HLA-Bw4 interactions enhances the anti-HIV ADCC functionality of 3DL1+ NK cells against HLA-Bw4+ autologous target cells.
Fig 2.
Anti-HIV ADCC responsiveness of 3DL1+ and 3DL1− NK cells from HLA-Bw4+ and HLA-Bw6 homozygous HIV-uninfected healthy controls. (A) The graphs on the left illustrate the relative abilities of 3DL1+ and 3DL1− NK cell subsets from HLA-Bw4+ individuals to produce IFN-γ and express CD107a. The graphs on the right illustrate the relative abilities of 3DL1+ and 3DL1− NK cell subsets from HLA-Bw6 homozygous individuals to produce IFN-γ and express CD107a. The significance of within-group differences of 3DL1+ and 3DL1− NK cell subset responses was assessed with Wilcoxon matched-pair tests. (B) The scatter plot depicts the relative anti-HIV ADCC bifunctional (i.e., CD107a+ IFN-γ+) activities of the 3DL1+ NK cell subsets in HLA-Bw4+ and HLA-Bw6 homozygous individuals. The significance of the between-group difference of responses from 3DL1+ NK cells originating from HLA-Bw4+ and HLA-Bw6 homozygous individuals was assessed with a Mann-Whitney t test.
Previous research has demonstrated that polyfunctional cell-based immune responses are associated with better control of viral infections. Indeed, this has been demonstrated in the context of HIV infection for both cytotoxic T lymphocytes and NK cells from individuals with protective KIR/HLA allelic combinations (6, 26, 31). We investigated whether the functional advantage of 3DL1+ NK cells, compared to 3DL1− NK cells, from HLA-Bw4 carriers was observed for IFN-γ and CD107a monofunctionality and bifunctionality. The functional advantage of the 3DL1+ NK cell subset was largely restricted to bifunctional responses (CD107a+ IFN-γ+). While 3DL1+ and 3DL1− NK cells demonstrated similar monofunctional CD107a (CD107a+ IFN-γ−) and IFN-γ (CD107a− IFN-γ+) responses, a significantly higher percentage of 3DL1+ NK cells than of 3DL1− NK cells mediated bifunctional responses (Table 1). It should be noted that this difference was still significant after the Bonferroni correction was applied. A similar comparison of monofunctional and bifunctional 3DL1+ and 3DL1− NK cell responses in HLA-Bw6 homozygotes revealed no significant differences in any of the functional permutations (Table 1). These data demonstrate that NK cell education enhances the anti-HIV ADCC function of 3DL1+ NK cells and that this enhanced function is driven primarily by an increased ability of 3DL1-educated NK cells to mediate multiple functions simultaneously.
Table 1.
Impact of NK cell education on anti-HIV ADCC functional profile
| Healthy control group and functional profile | % of NK cell subset expressing effector molecule (mean ± SE) |
Pa | |
|---|---|---|---|
| 3DL1+ | 3DL1− | ||
| HLA-Bw4 | |||
| IFN-γ+ CD107a− | 2.2 ± 0.5 | 1.7 ± 0.3 | 0.23 |
| IFN-γ− CD107a+ | 16.8 ± 2.7 | 13.4 ± 1.8 | 0.07 |
| IFN-γ+ CD107a+ | 18.0 ± 2.8 | 12.5 ± 2.3 | 0.01 |
| HLA-Bw6 | |||
| IFN-γ+ CD107a− | 1.2 ± 0.3 | 1.3 ± 0.3 | 0.84 |
| IFN-γ− CD107a+ | 13.8 ± 2.8 | 13.1 ± 2.8 | 0.31 |
| IFN-γ+ CD107a+ | 8.9 ± 2.3 | 7.2 ± 1.9 | 0.22 |
P values obtained from Wilcoxon matched-pair tests.
NK cell education through 3DL1 and HLA-Bw4 interactions has been demonstrated to induce higher responsiveness against nonself target cells in 3DL1+ NK cells than in 3DL1− NK cells from HLA-Bw4 subjects, and it drives higher responsiveness in 3DL1+ NK cells from HLA-Bw4+ subjects than in those from HLA-Bw6 homozygotes (6, 18, 28). Given that our results demonstrated higher bifunctional responses in 3DL1+ NK cells than in 3DL1− NK cells in HLA-Bw4+ individuals, but not in HLA-Bw6 homozygotes, we hypothesized that 3DL1+ NK cells would be more responsive to autologous ADCC target cells in HLA-Bw4+ individuals than in HLA-Bw6 homozygotes. Indeed, HLA-Bw4+ individuals did have overall higher frequencies of 3DL1+ NK cells capable of mediating bifunctional anti-HIV ADCC than did HLA-Bw6 homozygotes (18.0 ± 2.8 versus 8.9 ± 2.3; P < 0.05) (Fig. 2B). Cumulatively, these results demonstrate that NK cell education enhances the ability of NK cells to mediate anti-HIV ADCC against autologous target cells.
Effect of HIV infection on the impact of NK cell education on the relative anti-HIV ADCC functionalities of 3DL1+ and 3DL1− NK cells.
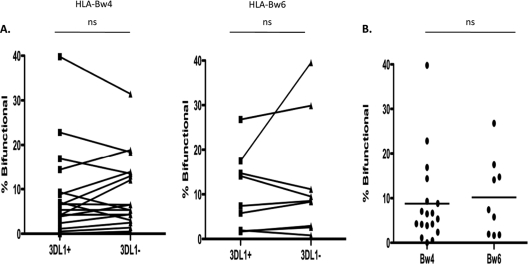
Numerous studies demonstrate that HIV infection has a detrimental effect on NK cell functional potential (13, 23, 33). Given that NK cell education determines the functional potential of NK cells, it might be expected that HIV-infected individuals would experience a decrease in the functionality of NK cells educated through 3DL1 and HLA-Bw4 interactions. Therefore, we recruited 27 HIV-infected subjects to evaluate ADCC activity in NK cell subsets. This unselected heterogenous cohort included consecutive subjects attending an outpatient clinic and included viremic and aviremic patients at different stages of disease progression and treatment status (see Table S1 in the supplemental material). We used a whole-blood anti-HIV ADCC ICS assay to evaluate whether HIV+ HLA-Bw4+ individuals maintain a functional advantage in their 3DL1+ NK cells. HIV+ HLA-Bw6 homozygous individuals were also evaluated as a control group. No difference in bifunctional anti-HIV ADCC responses was observed between the 3DL1+ and 3DL1− NK cell subsets from the either HLA-Bw4+ (8.8 ± 2.3 versus 9.2 ± 1.9; NS) or the HLA-Bw6 homozygous (10.2 ± 2.9 versus 12.6 ± 4.4; NS) group (Fig. 3A). It should also be known that no differences were observed in monofunctional IFN-γ or monofunctional CD107a responses between the 3DL1+ and 3DL1− NK cell subsets from the HLA-Bw4+ (1.03 ± 0.33 versus 1.16 ± 0.39 [NS] and 6.42 ± 1.29 versus 7.23 ± 1.15 [NS]) or HLA-Bw6 homozygous (0.05 ± 0.05 versus 0.21 ± 0.11 [NS] and 8.54 ± 1.95 versus 8.54 ± 1.63 [NS]) group (data not shown). Although some HLA-Bw4+ individuals were found to have higher bifunctionality in their 3DL1+ NK cell subset than in their 3DL1− NK cells, the population as a whole demonstrates no functional advantage in their 3DL1+ NK cells. Coinciding with this observation, no difference was observed in the frequencies of bifunctional 3DL1+ NK cells in HLA-Bw4 carriers and HLA-Bw6 homozygous individuals (8.8 ± 2.3 versus 10.2 ± 2.9; NS) (Fig. 3B). These results suggest that HIV infection has a negative impact on the ADCC functional advantage of NK cells educated through the interaction of 3DL1 and HLA-Bw4.
Fig 3.
Anti-HIV ADCC responsiveness of 3DL1+ and 3DL1− NK cells from HLA-Bw4+ and HLA-Bw6 homozygous HIV-infected individuals. (A) The graph on the left illustrates the relative abilities of 3DL1+ and 3DL1− NK cell subsets from HLA-Bw4+ individuals to mediate bifunctional anti-HIV ADCC responses (IFN-γ+ CD107a+). The graph on the right illustrates the relative abilities of 3DL1+ and 3DL1− NK cell subsets from HLA-Bw6 homozygous individuals to mediate bifunctional anti-HIV ADCC responses (IFN-γ+ CD107a+). The significance of within-group differences in responses of 3DL1+ and 3DL1− NK cell subsets was assessed with Wilcoxon matched-pair tests. (B) The scatter plot depicts the relative anti-HIV ADCC bifunctional (i.e., CD107a+ IFN-γ+) activities of the 3DL1+ NK cell subsets from HLA-Bw4+ and HLA-Bw6 homozygous individuals. The significance of the between-group difference in responses of 3DL1+ NK cell subsets from HLA-Bw4+ and HLA-Bw6 homozygous individuals was assessed with a Mann-Whitney t test.
Clinical correlates of post-HAART NK cell functionality.
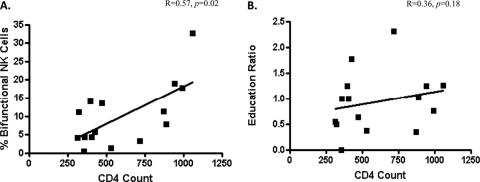
NK cell responsiveness decreases with progressive HIV infection and subsequent immunodeficiency (13, 23, 33). We studied the relationship between 3DL1 expression and ADCC activity of NK cells from HIV-infected HLA-Bw4 carriers. Our results demonstrate a loss of the functional advantage of educated 3DL1+ NK cells in both viremic and aviremic HIV+ individuals (Fig. 3 and data not shown). As the majority of the HLA-Bw4+ HIV-infected subjects recruited were on effective highly active antiretroviral therapy (HAART) and had undetectable viral loads (see Table S1 in the supplemental material), subsequent analyses of the impact of disease progression on the function of NK cells focused on the latest peripheral CD4+ T-lymphocyte count. We hypothesized that the relative normalization of NK cell functionality observed after immune reconstitution following the initiation of HAART would result in a reestablishment of the functional advantage within the educated NK cell subset. We therefore expected to see a correlation between treatment efficacy, as measured by counts of peripheral CD4+ T lymphocytes, and the bifunctionality of total NK cells. We also expected to observe a correlation between the counts of peripheral CD4+ T lymphocytes and the NK cell education ratio in HLA-Bw4+ subjects (i.e., ratio of bifunctionality observed in 3DL1+ NK cells to bifunctionality observed in 3DL1− NK cells). Thus, we calculated the percentage of bifunctional total NK cells and the NK cell education ratio for the HLA-Bw4+ treated and aviremic HIV+ individuals and correlated these values with the counts of peripheral CD4+ T lymphocytes. A significant correlation was observed between the percentage of bifunctional total NK cells and the count of peripheral CD4+ T lymphocytes (r = 0.57; P < 0.05) (Fig. 4A). However, no significant correlation was observed between the NK cell education ratio and the CD4+ T-lymphocyte counts within these patients (r = 0.36; P > 0.05) (Fig. 4B). It should be noted that a similar pattern was observed when we correlated the increase in CD4+ T-lymphocyte counts with the percentage of bifunctional total NK cells (r = 0.52; P < 0.05) and the NK cell education ratio (r = 0.26; P > 0.05) (data not shown). These data demonstrate that the post-HAART counts of peripheral CD4+ T lymphocytes are associated with a partial normalization of NK cell functionality. However, the partial reconstitution of immune competence on HAART is not associated with a full restoration of an anti-HIV ADCC functional advantage for educated 3DL1+ NK cells over 3DL1− NK cells.
Fig 4.
Relationship between HAART efficacy and the functionalities of total and 3DL1-educated NK cells of HIV-infected HLA-Bw4+ individuals. (A) The graph shows the relationship between the post-HAART counts of peripheral CD4+ T lymphocytes and the percentage of bifunctional total NK cells in post-HAART aviremic HLA-Bw4+ individuals. (B) The graph shows the relationship between the post-HAART counts of peripheral CD4+ T lymphocytes and the ratio of bifunctionality between 3DL1+ and 3DL1− NK cells in post-HAART aviremic HLA-Bw4+ individuals. The strengths of the relationships were evaluated with Spearman correlation coefficients.
DISCUSSION
Several epidemiological studies demonstrate an association between cocarriage of several 3DL1 and HLA-Bw4 alleles and protection from HIV infection and/or disease progression (5, 22). Although these 3DL1/HLA-Bw4 combinations increase the responsiveness of 3DL1+ NK cells against target cells devoid of HLA (6, 26), 3DL1+ NK cells do not degranulate upon direct exposure to autologous HIV-infected cells (1). As such, the mechanism of 3DL1/HLA-Bw4-conferred protection remains undefined. In this study, we evaluated the role of 3DL1-driven NK cell education in anti-HIV ADCC activity to autologous target cells. A simple and robust whole-blood ICS assay was employed to assess the activation of NK cell subsets from both HIV-uninfected healthy controls and a cohort of HIV-infected patients. This experimental system involves the combination of whole blood with HIV Env peptides and plasma from an HIV-infected source. This results in presentation of HIV peptides, in an as-yet-nonelucidated mechanism, by target cells, which become coated with anti-HIV Abs and activate NK cells for ADCC (I. Stratov et al., unpublished data). As these targets are nucleated cells that express surface HLA-I, this assay serves to evaluate the ability of NK cells to mediate anti-HIV ADCC in a self-HLA-I environment. Our results demonstrate that educated NK cells from healthy controls mediate responses against autologous target cells which express HLA-Bw4 ligands that have a known inhibitory effect upon binding inhibitory KIR (21). Not only were educated NK cells capable of responding to autologous ADCC targets; they also represented a more responsive subset. This study also linked functional alterations in NK cells from HIV-infected individuals to the abrogation of the functional benefit of educated NK cells. Further, we demonstrated that although successful HAART partially restores the function of total NK cells, treated aviremic individuals, regardless of peripheral CD4+ T-lymphocyte counts, still exhibit a loss of the functional advantage of educated 3DL1+ NK cells for mediating anti-HIV ADCC.
This study provides the first evidence that educated human NK cells are a more functional NK cell subset than noneducated NK cells against autologous ADCC target cells. Although this observation coincides with previous research demonstrating that educated NK cells are more responsive to Ab-coated murine P815 cells and Ab-coated heterologous Epstein-Barr virus (EBV)-transformed human B lymphocytes (2, 28), the results contrast with previous evidence of nonresponsive educated NK cells in the context of infections. For example, a recent investigation of the role of educated NK cells in the context of murine cytomegalovirus demonstrated that noneducated NK cells were the major effector cells in infected mice (25). Corroborating this observation is the demonstration, in humans, that 3DL1+ NK cells do not mediate Ab-independent degranulation against autologous HIV-infected target cells (1). Since previous research has demonstrated that Ab-dependent NK cell responses can overcome inhibitory signals through inhibitory KIR to mediate functionality (20), we investigated whether educated NK cells represented a more functional subset in the context of Ab-dependent responses against autologous target cells. Indeed, our results demonstrated that Ab-dependent responses can overcome inhibitory signals and that educated NK cells preferentially mediate Ab-dependent responses against autologous target cells. This observation is particularly intriguing, as the ADCC measured in our assay was mediated against target cells that did not have altered HLA-B expression. Although previous work has suggested that autologous HIV-infected target cells need to downregulate HLA-B to be susceptible to NK cell-mediated functions (3), our results suggest that with optimal stimulation, NK cells can be triggered to mediate effector functions against autologous target cells with normal HLA-B expression.
Our results also provide the first evidence linking the NK cell dysfunction observed in HIV infection with an abrogation of the functional advantage of educated NK cells mediating ADCC effector functions. Although higher bifunctional responses were observed in the educated 3DL1+ NK cell subset of HIV-uninfected HLA-Bw4+ healthy controls than in their 3DL1− subset, no significant difference in bifunctional responses was observed between these NK cell subsets in HLA-Bw4+ HIV-infected individuals. Coinciding with this observation, it was also noted that while HIV-uninfected HLA-Bw4+ healthy controls demonstrate a higher proportion of bifunctional 3DL1+ NK cells than HIV-uninfected HLA-Bw6 homozygous healthy controls, no significant difference was observed between bifunctional 3DL1+ responses in HIV-infected HLA-Bw4+ and HLA-Bw6 homozygous individuals. Several NK cell abnormalities have been noted in HIV infection, including a decrease in total functionality and NK cell-mediated destruction of uninfected CD4+ T-lymphocytes (13, 23, 33, 34). The abrogated function of educated NK cells observed in this study may reflect an overall reduction in NK cell functionality. Although it is unknown how HIV infection decreases the function of educated 3DL1+ NK cells, it is possible that recently described escape peptides that abrogate the 3DL1/HLA-Bw4 interaction could interfere with NK cell education (8). Alternatively, the alteration of educated 3DL1+ NK cell functionality may reflect an infection-induced increase in the function of noneducated NK cells that would be more likely to mediate the destruction of uninfected autologous CD4+ T lymphocytes. Lastly, alterations to other NK cell receptors that influence NK cell education could contribute the dysfunction observed in educated 3DL1+ NK cells. However, this situation is unlikely, as the dysfunction in educated 3DL1+ NK cells was observed in both treated and untreated HIV-infected subjects, and successful treatment of HIV infection has been reported to normalize the expression levels of most receptors that exhibit altered expression due to viremia (13). Indeed, we investigated the expression of one such receptor, NKG2A, on total NK cells and 3DL1+ NK cells from HIV-uninfected subjects and HAART-treated HIV-infected subjects, and we observed no differences between the groups (data not shown). Future studies using serial samples from HIV-infected individuals, prior to and following initiation of HAART, will help further clarify these observations.
HIV infection abrogated the functional advantage of educated NK cells. A correlation between the effectiveness of immune reconstitution following antiretroviral treatment, measured as CD4+ T-lymphocyte counts, and the bifunctionality of total NK cells demonstrated that more effective control of HIV disease was associated with higher NK cell functionality. However, the lack of correlation between treatment efficacy and the NK cell education ratio is interesting, as it may provide a partial explanation for the higher incidence of non-AIDS-defining malignancies (NADMs) in HAART-treated HIV-infected individuals than in the general population (24). Future studies using larger sample sizes and following subjects over time will help elucidate the factors determining why some HIV-infected individuals exhibit normal NK cell education ratios and the potential role of these ratios in susceptibility to NADMs.
Although the present study demonstrates that NK cells educated through 3DL1/HLA-Bw4 interactions can mediate potentially beneficial anti-HIV ADCC responses against autologous target cells, it is conceivable that the similar activity of 3DL1-educated NK cells in the presence of autoantibodies or uninfected cells carrying viral proteins could mediate immunopathology. As HIV-infected individuals often carry antilymphocyte Abs (4) and potentially demonstrate soluble gp120 binding to uninfected CD4+ T lymphocytes (16), future research needs to evaluate whether 3DL1-educated NK cells are functional in these potentially pathological situations. It is essential to establish which educated NK cell-mediated effector functions are truly beneficial and which are potentially pathogenic before such effector functions can be targeted as therapies and/or vaccines to HIV-infected individuals or individuals at risk.
Demonstrating that educated NK cells mediate anti-HIV ADCC against autologous target cells is essential to understanding the NK cell education process and its potential implications for vaccines and therapies. While this study evaluated the anti-HIV ADCC activity of NK cells educated through the epidemiologically interesting 3DL1/HLA-Bw4 interactions (22), future research should investigate the impact of other HLA/KIR combinations and the cumulative HLA/KIR phenotype of NK cells on the ability of NK cells to mediate autologous anti-HIV ADCC. Interestingly, Kamya et al. recently showed that while HLA-Bw4/KIR3DL1 combinations contribute to the ability of NK cells to be activated by K562 cells devoid of HLA, HLA-C/KIR2DL1/2/3 combinations did not contribute to the ability of NK cells to be activated by this stimulation (17). In contrast, both HLA-Bw4/KIR3DL1 and HLA-C/KIR2DL1/2/3 combinations contributed to the ability of NK cells from healthy controls to be activated by K562 stimulation. These results illustrate the importance of future research investigating other HLA/KIR interactions that may be important in the context of NK cell-mediated ADCC. Furthermore, it will be necessary to elucidate the effector functions mediated by educated NK cells that are important for viral control. Such a detailed understanding of the impact of NK cell education on anti-HIV ADCC is essential for understanding NK cell antiviral responses and for the design of more effective ADCC-inducing HIV vaccines.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge J. Silvers, H. Kent, M. Chen, R. Moore, J. Sasadeusz, C. Fairley, T. Schmidt, C. Bradshaw, and T. Read, from the Melbourne Sexual Health Centre, and Christos M. Tsoukas, from the McGill University Health Center, for their assistance in collecting clinical samples. We are also grateful to all of the study's participants.
None of the authors has a commercial or other association that might pose a potential conflict of interest related to this paper.
This work was supported by National Health and Medical Research Council grant 510448, Australian Research Council grant LP0991498, and National Institutes of Health grant R21AI081541 and by the Australian Centre for HIV and Hepatitis Virology Research, the Royal Australasian College of Physicians, and the Ramaciotti Foundation. M.S.P. is supported by a Vanier Scholarship from the Canadian Institutes for Health Research (CIHR) and is a visiting scholar at the University of Melbourne through a Michael Smith Foreign Study Supplement from the CIHR.
Footnotes
Published ahead of print 15 February 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Alter G, et al. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204:3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anfossi N, et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25:331–342 [DOI] [PubMed] [Google Scholar]
- 3. Bonaparte MI, Barker E. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087–2094 [DOI] [PubMed] [Google Scholar]
- 4. Bonner BC, Poulton TA. 1991. Cytofluorometric analysis of anti-lymphocyte antibodies in AIDS. FEMS Microbiol. Immunol. 4:33–40 [DOI] [PubMed] [Google Scholar]
- 5. Boulet S, et al. 2008. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS 22:1487–1491 [DOI] [PubMed] [Google Scholar]
- 6. Boulet S, et al. 2010. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J. Immunol. 184:2057–2064 [DOI] [PubMed] [Google Scholar]
- 7. Bowles JA, Weiner GJ. 2005. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J. Immunol. Methods 304:88–99 [DOI] [PubMed] [Google Scholar]
- 8. Brackenridge S, et al. 2011. An early HIV mutation within an HLA-B*57-restricted T cell epitope abrogates binding to the killer inhibitory receptor 3DL1. J. Virol. 85:5415–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brocca-Cofano E, et al. 2011. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine 29:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. 2009. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113:2434–2441 [DOI] [PubMed] [Google Scholar]
- 11. Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. 2009. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J. Immunol. 182:1202–1210 [DOI] [PubMed] [Google Scholar]
- 12. Cohen GB, et al. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671 [DOI] [PubMed] [Google Scholar]
- 13. Fauci AS, Mavilio D, Kottilil S. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835–843 [DOI] [PubMed] [Google Scholar]
- 14. Hessell AJ, et al. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104 [DOI] [PubMed] [Google Scholar]
- 15. Hoglund P, Brodin P. 2010. Current perspectives of natural killer cell education by MHC class I molecules. Nat. Rev. Immunol. 10:724–734 [DOI] [PubMed] [Google Scholar]
- 16. Iannello A, Debbeche O, Samarani S, Ahmad A. 2008. Antiviral NK cell responses in HIV infection. II. Viral strategies for evasion and lessons for immunotherapy and vaccination. J. Leukoc. Biol. 84:27–49 [DOI] [PubMed] [Google Scholar]
- 17. Kamya P, et al. Inhibitory killer immunoglobulin-like receptors to self HLA-B and HLA-C ligands contribute differentially to natural killer cell functional potential in HIV infected slow progressors. Clin. Immunol., in press [DOI] [PubMed] [Google Scholar]
- 18. Kim S, et al. 2008. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl. Acad. Sci. U. S. A. 105:3053–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambotte O, et al. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang P, et al. 2002. Clinical scale isolation of T cell-depleted CD56+ donor lymphocytes in children. Bone Marrow Transplant. 29:497–502 [DOI] [PubMed] [Google Scholar]
- 21. Lanier LL, et al. 1995. The NKB1 and HP-3E4 NK cells receptors are structurally distinct glycoproteins and independently recognize polymorphic HLA-B and HLA-C molecules. J. Immunol. 154:3320–3327 [PubMed] [Google Scholar]
- 22. Martin MP, et al. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mavilio D, et al. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. U. S. A. 100:15011–15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen ML, Farrell KJ, Gunthel CJ. 2010. Non-AIDS-defining malignancies in patients with HIV in the HAART era. Curr. Infect. Dis. Rep. 12:46–55 [DOI] [PubMed] [Google Scholar]
- 25. Orr MT, Murphy WJ, Lanier LL. 2010. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parsons MS, et al. 2010. Mind the gap: lack of association between KIR3DL1*004/HLA-Bw4-induced natural killer cell function and protection from HIV infection. J. Infect. Dis. 202(Suppl. 3):S356–S360 [DOI] [PubMed] [Google Scholar]
- 27. Parsons MS, Grant M. 2009. Natural killer cell receptors in human immunodeficiency virus infection: pathways to protection or doors to disappointment? Curr. HIV Res. 7:487–496 [DOI] [PubMed] [Google Scholar]
- 28. Parsons MS, Zipperlen K, Gallant M, Grant M. 2010. Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J. Leukoc. Biol. 88:905–912 [DOI] [PubMed] [Google Scholar]
- 29. Scott-Algara D, et al. 2003. Increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 171:5663–5667 [DOI] [PubMed] [Google Scholar]
- 30. Stratov I, Chung A, Kent SJ. 2008. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J. Virol. 82:5450–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thobakgale CF, et al. 2011. CD8(+) T cell polyfunctionality profiles in progressive and nonprogressive pediatric HIV type 1 infection. AIDS Res. Hum. Retroviruses 27:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trundley A, Frebel H, Jones D, Chang C, Trowsdale J. 2007. Allelic expression patterns of KIR3DS1 and 3DL1 using the Z27 and DX9 antibodies. Eur. J. Immunol. 37:780–787 [DOI] [PubMed] [Google Scholar]
- 33. Ullum H, et al. 1995. Defective natural immunity: an early manifestation of human immunodeficiency virus infection. J. Exp. Med. 182:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vieillard V, Strominger JL, Debre P. 2005. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl. Acad. Sci. U. S. A. 102:10981–10986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wren L, Kent SJ. 2011. HIV vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum. Vaccin. 7:466–473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.