Abstract
Synthesis and characterization of four iridium(III)–octaethylporphyrins and a π-extended iridium(III)–benzoporphyrin are presented. Strong room-temperature phosphorescence was observed for all of the complexes with quantum yields of up to 30 %. Axial ligands were introduced to tune the photophysical properties and the solubility. Complexes bearing lipophilic ligands such as pyridine or N-(n-butyl)imidazole were incorporated into polystyrene to obtain optical oxygen sensors. Covalent coupling of the dye is possible by introduction of ligands with binding domains (1-imidazoleacetic acid). This enabled preparation of a water-soluble oxygen probe (by staining bovine serum albumin) and a trace oxygen sensor (by coupling to amino-modified silica gel).
Keywords: Porphyrinoids, Iridium, Phosphorescence, Optical sensors, Oxygen
Introduction
Strongly luminescent metal complexes are applied as indicators in optical sensors,1 as emitters in OLEDs2 and as labels.3 Consequently, they attract much attention from the scientific community. Among others, metalloporphyrins (especially PtII– and PdII–porphyrins), RuII–polypyridyl complexes4 and cyclometallated IrIII compounds5 areextensively studied. Phosphorescent complexes based onporphyrins and their derivatives (porphyrin–ketones, porphyrin–lactones, π-extended porphyrins) are very versatile, since different modifications of the porphyrin macrocycle are possible.6 Complexes based on cyclometallated IrIII compounds usually possess high luminescence quantum yields but often low absorption coefficients.7,8
To the best of our knowledge, nothing is known about phosphorescent IrIII–porphyrins. This is not surprising, in view of the fact that the synthesis and chemistry of such complexes are more difficult than those of the corresponding PtII– and PdII–metalloporphyrins, which are known to be strongly phosphorescent at room temperature. The question arises whether IrIII–porphyrins show any kind of luminescence at room temperature. In fact, the combination of IrIII as central metal and porphyrins as ligands is relatively rare in the literature,9–13 and mainly the catalytic properties of such complexes have been studied.14–16 Recently,barely luminescent (quantum yield from 0.03 to 1.2 %) IrIII–corrols have been reported.17
Besides possessing potentially interesting photophysical properties, complexes combining six-coordinating IrIII and porphyrins also exemplify new synthetic possibilities. In contrast to the square planar PtII– and PdII–porphyrins, two additional ligands are introduced in the IrIII–porphyrin system. The axial ligands may influence photophysical properties and affect solubility. Introducing ligands with binding domains or groups suitable for covalent linkage would also be of great interest. In this work, several IrIII–porphyrins were synthesized and characterized.
Results and Discussion
At first, the complexes were studied on the basis of an octaethylporphyrin macrocycle with varying axial ligands (Scheme 1 and Table 1). The carbonyl complex (Ir–OEP–CO–Cl9) was employed for preparation of the other complexes by using rather simple and fast ligand exchange reactions. High phosphorescence quantum yields (up to 21 %) were obtained for all the complexes, making them good candidates for oxygen sensing. Photophysical properties were affected by the axial ligands. The positively charged complexes with two identical ligands show quite similar properties in contrast to neutral Ir–OEP–CO–Cl. In fact, despite slightly lower luminescence quantum yield, Ir–OEP–CO–Cl has a significantly longer decay time (τ0 = 97 μs). Also, the absorption and emission bands are bathochromically shifted (Figure 1 and Table 1). In contrast to those of the well-known PtII–octaethylporphyrin (Pt–OEP), the absorption peaks of all IrIII–porphyrins are slightly broader (by about 5 nm at FWHM of the Soret band) and bathochromically shifted. This enables excitation with visible light (400 to 405 nm) via the strongly absorbing Soret band for all IrIII–octaethylporphyrins.
Scheme 1.
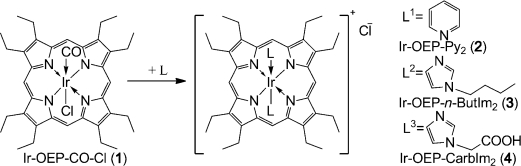
Chemical structures of the four IrIII–octaethylporphyrin complexes.
Table 1.
Photophysical properties of IrIII–octaethylporphyrins
| λmax (abs) /nm (ε /10–3cm–1m–1)[b] | λmax (em) /nm[a] | τ0 /μs | Φ /%[a] | |
|---|---|---|---|---|
| Ir–OEP–CO–Cl | 404 (165), 518 (15), 550 (31) | 672 | 97[a] (108)[e] | 14 |
| Ir–OEP–Py2 | 389 (148), 509 (11), 539 (26.6) | 655 | 40[a] (52)[e] | 19.5 |
| Ir–OEP–n-ButIm2 | 390 (150), 508 (9.7), 541 (15) | 655 | 27[a] (35)[e] | 20 |
| Ir–OEP–CarbIm2 | 388 (142), 507 (10), 538 (18)[c] | 652[c] | 37[c] 26[d] | 21[c] 8[d] |
| Pt–OEP | 382 (214), 503 (9.5), 536 (42.5) | 649 | 75[a] (86)[e] | 41.5 |
| Pd–OEP | 395 (127), 513 (9), 547 (32) | 669 | (1217)[e] | ≈ 19 |
Diluted solutions of toluene.
CHCl3.
EtOH.
Aqueous buffer (pH 7.3) at room temperature.
In polystyrene at 25 °C.
Figure 1.
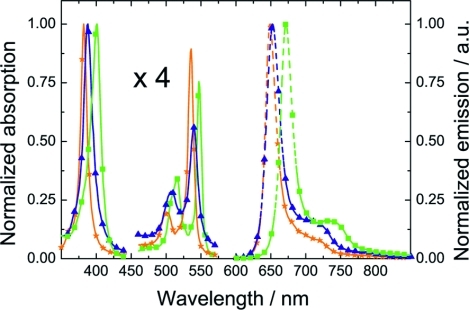
Absorption (solid line) and emission spectra (dashed line) of Ir–OEP–CO–Cl (green squares), Ir–OEP–CarbIm2 (blue triangles) and Pt–OEP (orange stars) as reference.
Axial ligands can also be used to change the solubility or to introduce binding groups. Similarly to Ir–OEP–CO–Cl, complexes bearing pyridine or N-(n-butyl)imidazole as axial ligands are well-soluble in organic solvents such as acetone, chloroform and toluene. Therefore, these complexes can be incorporated into polystyrene or other polymers to yield oxygen sensors. Stern–Volmer plots for the oxygen-sensing materials based on the above indicators in polystyrene are shown in Figure 2a. They are compared to the established Pt–OEP in the same matrix.
Figure 2.
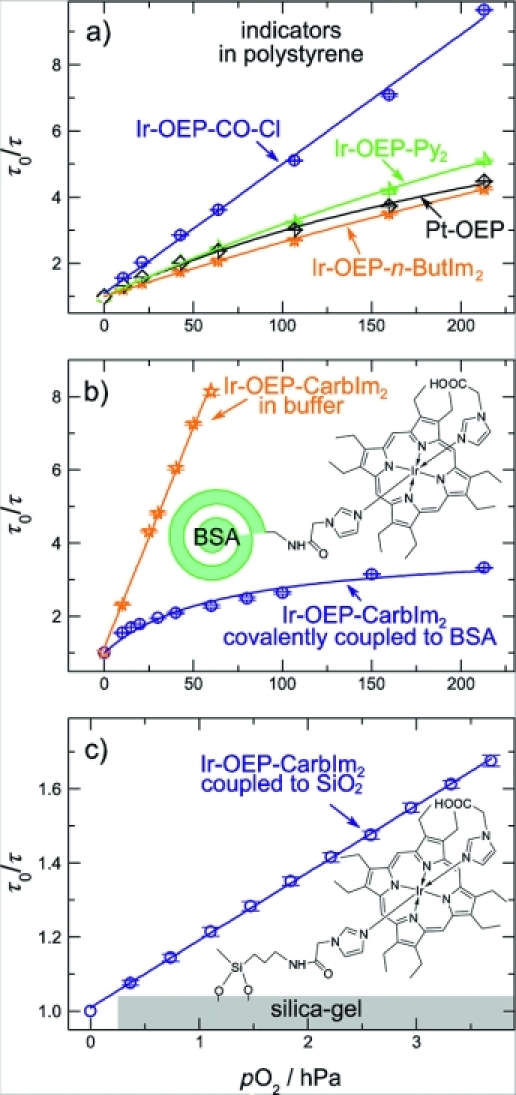
Stern–Volmer plots for the sensing materials based on IrIII–porphyrins; Pt–OEP is used as a reference.
Quenching behaviour in polymeric films is usually described by the so-called “two site model”,18 which suggests that some dye molecules are less quenchable by the analyte than others, leading to nonlinear calibration curves.
It is noticeable that the Stern–Volmer plots for the presented IrIII–porphyrins show higher linearity than the PtII analogue (Figure 2a). Surprisingly, in the case of Ir–OEP–CO–Cl, a linear Stern–Volmer plot (R2 = 0.997) from 0 to 100 % air saturation was obtained. Interestingly, the size of the axial ligands seems to affect the second-order-rate quenching constant (kq= KSV/τ0). In fact, kq increases when the size of the axial ligands increases (kq = 361, 500 and 529 [hPa–1 s–1] for Ir–OEP–CO–Cl, Ir–OEP–Py2 and Ir–OEP–n-ButIm2, respectively). This could possibly be explained by the participation of the axial ligands in the energy transfer reaction.
Axial ligands can also be used to introduce polar groups or binding moieties. For example, an imidazole ligandbearing a carboxyl group renders the porphyrin soluble in polar solvents such as ethanol, methanol and even in aqueous buffer (physiological pH).
The solubility in polar media and the presence of the carboxyl group enable coupling to biomolecules such as proteins, antibodies or lipids. To demonstrate its binding capability, Ir–OEP–CarbIm2 was coupled to bovine serum albumin, BSA, (τ0 = 24 μs). As expected, the quenching efficiency decreases upon binding to BSA, because the dye is better shielded from oxygen (Figure 2b). The highly nonlinear calibration plot can be explained by the variety of the linking positions to the protein, each having different oxygen accessibility. Protein- or peptide-bound oxygen-sensitive dyes are interesting tools to measure, for example, cell respiration,19 particularly due to their small size and good solubility in biological media.
Evidently, covalent coupling is not only attractive for biomolecules but also for polymers or functionalized surfaces. Previously, we demonstrated that trace oxygen sensors can be designed by covalently immobilizing PtII– and PdII–porphyrin complexes on the surface of amino-modified silica gel.20 For the first time Ir–OEP–CarbIm2 enables coupling to silica gel via the axial ligand (τ0= 26 μs) instead of modifying the porphyrin macrocycle. The obtained sensor is sensitive to small oxygen concentrations (Figure 2c) and features a highly linear calibration plot (R2 = 0.999).
Finally, the combination of IrIII and a π-extended benzoporphyrin was investigated. PtII– and PdII–benzoporphyrins are known to emit in the near infrared (NIR) part of the spectrum.21,22 NIR-emitting complexes are particularly interesting, as they enable subcutaneous measurements. In this work, we combined IrIII with tetraphenyltetrabenzoporphyrin and chose N-(n-butyl)imidazole as axial ligand (Ir–TPTBP–n-ButIm2). Unfortunately, bonding of the axial ligands seems to be weaker in the case of the benzoporphyrin, and they can be partly replaced, for example, by solvent molecules during the purification. Nevertheless, the results presented confirm that IrIII was complexed by the porphyrin (Figure 3). Strong NIR phosphorescence was observed (quantum yield ca. 30 %, τ0 ca. 23 μs) for Ir–TPTBP–n-ButIm2. Thus, the IrIII–porphyrin complexes represent significantly stronger emitters than the IrIII–corrols (quantum yield less than 1.2 %).17 Since the positions of the phosphorescence maxima of the IrIII–benzoporphyrin and the IrIII–corrols are similar, such a huge difference in the quantum yield cannot be explained by the lower energy gap between the triplet excited state and the ground state for the corrols (λmax ≈ 790 nm).17 The smaller cavity size of the corrols may result in nonplanarity of the IrIII corrols, which may promote nonradiative deactivation.
Figure 3.
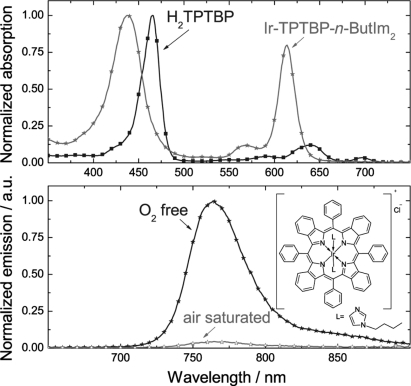
(a) Absorption spectra of the free ligand H2TPTBP and Ir–TPTBP–n-ButIm2; (b) emission spectra of Ir–TPTBP–n-ButIm2 in toluene solution.
Conclusions
It can be concluded that IrIII–porphyrin complexes are strong room-temperature emitters. In contrast to the well-known PtII– and PdII–porphyrins, the IrIII complexes bear axial ligands that have pronounced effects on the photophysical properties and solubility of the dyes. They can also be used to introduce functional groups to enable, for example, covalent coupling. The new dyes are particularly promising as indicators for oxygen sensors with tailor-made sensitivity.
Experimental Section
Ir–OEP–Cl–CO (1) was synthesized as described in the literature.9 Complexes 2, 3 and 4 were obtained by ligand exchange reactions. Ligand exchange was accelerated by using a large excess of the ligands. Ir–TPTBP–n-ButIm2 was synthesized by metallating the free porphyrin (H2TPTBP23) and subsequent replacement of the axial ligands. Ir–OEP–CarIm2 was coupled to amino-modified silica gel particles as well as to BSA through an amide bond by using 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide and N-hydroxysuccinimide as coupling reagents.
Supporting Information (see footnote on the first page of this article): Materials and instrumentation, detailed synthetic procedures, characterization of complexes, NMR and HRMS spectra, sensor preparation, measurement setup.
Acknowledgments
The authors would like to thank K. Greimel and T. Reiter from the Institute of Environmental Biotechnology at the Graz University of Technology for technical support. Financial support of the Austrian Science Fund (FWF; Research Project No. P21192-N17) is gratefully acknowledged
Supplementary material
References
- 1.Wolfbeis OS. J. Mater. Chem. 2005;15:2657–2669. [Google Scholar]
- 2.Evans R, Douglas P, Winscom C. Coord. Chem. Rev. 2006;250:2093–2126. [Google Scholar]
- 3.Papkovsky DB, O'Riordan TC. J. Fluoresc. 2005;15:569–584. doi: 10.1007/s10895-005-2830-x. [DOI] [PubMed] [Google Scholar]
- 4.Vos JG, Kelly JM. Dalton Trans. 2006:4869–4883. doi: 10.1039/b606490f. [DOI] [PubMed] [Google Scholar]
- 5.DeRosa MC, Hodgson DJ, Enright GD, Dawson B, Evans CEB, Crutchley RJ. J. Am. Chem. Soc. 2004;126:7619–7626. doi: 10.1021/ja049872h. [DOI] [PubMed] [Google Scholar]
- 6.Kadish KM, Smith KM. In: The Porphyrin Handbook. Guilard R, editor. San Diego: Academic Publishers; 2000. [Google Scholar]
- 7.Ulbricht C, Beyer B, Friebe C, Winter A, Schubert US. Adv. Mater. 2009;21:4418–4441. [Google Scholar]
- 8.Lamansky S, Djurovich P, Murphy D, Abdel-Razzaq F, Kwong R, Tsyba I, Bortz M, Mui B, Bau R, Thompson ME. Inorg. Chem. 2001;40:1704–1711. doi: 10.1021/ic0008969. [DOI] [PubMed] [Google Scholar]
- 9.Ogoshi H, Setsune J, Yoshida Z. J. Organomet. Chem. 1978;159:317–328. [Google Scholar]
- 10.Sadasivan N, Fleischer EB. J. Inorg. Nucl. Chem. 1968;30:591–601. [Google Scholar]
- 11.Kadish KM, Deng YJ, Korp JD. Inorg. Chem. 1990;29:1036–1042. [Google Scholar]
- 12.Swistak C, Cornillon JL, Anderson JE, Kadish KM. Organometallics. 1987;6:2146–2150. [Google Scholar]
- 13.Kanemitsu H, Harada R, Ogo S. Chem. Commun. 2010;46:3083. doi: 10.1039/c000263a. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CW, Chan KS. Organometallics. 2008;27:3043–3055. [Google Scholar]
- 15.Cheung C, Fung H, Lee S, Qian Y, Chan Y, Chan K. Organometallics. 2010;29:1343–1354. [Google Scholar]
- 16.Song X, Chan KS. Organometallics. 2007;26:965–970. [Google Scholar]
- 17.Palmer JH, Durrell AC, Gross Z, Winkler JR, Gray HB. J. Am. Chem. Soc. 2010;132:9230–9231. doi: 10.1021/ja101647t. [DOI] [PubMed] [Google Scholar]
- 18.Carraway ER, Demas JN, DeGraff BA, Bacon JR. Anal. Chem. 1991;63:337–42. [Google Scholar]
- 19.Papkovsky DB, Hynes J, Will Y. Expert Opin. Drug Metab. Toxicol. 2006;2:313–323. doi: 10.1517/17425255.2.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Borisov SM, Lehner P, Klimant I. Anal. Chim. Acta. 2011 doi: 10.1016/j.aca.2011.01.057. DOI: 10.1016/j.aca.2011.01.057, in press. [DOI] [PubMed] [Google Scholar]
- 21.Finikova OS, Cheprakov AV, Vinogradov SA. J. Org. Chem. 2005;70:9562–9572. doi: 10.1021/jo051580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borisov SM, Nuss G, Klimant I. Anal. Chem. 2008;80:9435–9442. doi: 10.1021/ac801521v. [DOI] [PubMed] [Google Scholar]
- 23.Borisov SM, Klimant I. Dyes Pigm. 2009;83:312–316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


