Background: Angiopoietin-like 4 (Angptl4) is a secreted protein involved in triacylglycerol homeostasis.
Results: Angptl4 was required for fasting, glucocorticoids, and catecholamines to stimulate cAMP-dependent signaling and triacylglycerol hydrolysis in murine fat, a response reproduced by treating adipocytes with purified ANGPTL4.
Conclusion: Angptl4 is a physiological mediator of lipolysis.
Significance: This finding may impact aberrant lipolytic states like insulin resistance.
Keywords: Adipocyte, Catecholamines, Cyclic AMP (cAMP), Glucocorticoids, Lipolysis, Fasting, Angiopoietin-like 4
Abstract
Intracellular triacylglycerol (TG) hydrolysis and fatty acid release by the white adipose tissue (WAT) during a fast is stimulated by counter-regulatory factors acting in concert, although how adipocytes integrate these lipolytic inputs is unknown. We tested the role of angiopoietin-like 4 (Angptl4), a secreted protein induced by fasting or glucocorticoid treatment, in modulating intracellular adipocyte lipolysis. Glucocorticoid receptor blockade prevented fasting-induced tissue Angptl4 expression and WAT TG hydrolysis in mice, and TG hydrolysis induced by fasts of 6 or 24 h was greatly reduced in mice lacking Angptl4 (Angptl4−/−). Glucocorticoid treatment mimicked the lipolytic effects of fasting, although with slower kinetics, and this too required Angptl4. Thus, fasting-induced WAT TG hydrolysis requires glucocorticoid action and Angptl4. Both fasting and glucocorticoid treatment also increased WAT cAMP levels and downstream phosphorylation of lipolytic enzymes. Angptl4 deficiency markedly reduced these effects, suggesting that Angptl4 may stimulate lipolysis by modulating cAMP-dependent signaling. In support of this, cAMP levels and TG hydrolysis were reduced in primary Angptl4−/− murine adipocytes treated with catecholamines, which stimulate cAMP-dependent signaling to promote lipolysis, and was restored by treatment with purified human ANGPTL4. Remarkably, human ANGPTL4 treatment alone increased cAMP levels and induced lipolysis in these cells. Pharmacologic agents revealed that Angptl4 modulation of cAMP-dependent signaling occurs upstream of adenylate cyclase and downstream of receptor activation. We show that Angptl4 is a glucocorticoid-responsive mediator of fasting-induced intracellular lipolysis and stimulates cAMP signaling in adipocytes. Such a role is relevant to diseases of aberrant lipolysis, such as insulin resistance.
Introduction
An essential step in intermediary metabolism that occurs during a physiological fast or prolonged exercise involves the organized flux of energy in the form of free fatty acids (FFAs)3 from the white adipose tissue (WAT) to the liver and skeletal muscle for utilization. Several counter-regulatory factors, including neurotransmitters, such as catecholamines, and hormones, such as thyroid hormone, growth hormone, glucagon, and glucocorticoids, play critical roles in regulating the flux of FFAs during fasting. However, the way in which adipocytes integrate these inputs is incompletely understood.
A net flux of FFAs out of the WAT can result when the rate at which adipocytes hydrolyze intracellular triacylglycerols (TGs) and release FFAs is greater than the rate at which they take up and esterify dietary fats. The uptake of dietary fats stored within circulating lipoproteins by adipocytes requires the action of lipoprotein lipase (Lpl) enzymes (extracellular lipolysis), whereas the mechanisms governing TG hydrolysis (intracellular lipolysis) by adipocytes are more complex.
One factor that is known to regulate extracellular lipolysis and that is induced by fasting is angiopoietin-like 4 (Angptl4; also known as fasting-induced adipose factor, FIAF), a glycoprotein that is secreted by the WAT and liver in response to fasting and inhibits the action of Lpl (1, 2). Studies using genetically altered mice confirm that Angptl4 plays a key role in fat metabolism (2, 3). For example, transgenic mice overexpressing Angptl4 in the WAT have increased levels of plasma TG and FFAs (4, 5), whereas the opposite is true of mice lacking Angptl4 (Angptl4−/−) (6).
In considering how Angptl4 functions, it is intriguing to note that, in addition to inhibiting Lpl, Angptl4 also promotes the expression of WAT genes involved in TG hydrolysis and the lipolytic release of intracellular FFAs by adipocytes (5). Therefore, it is possible that Angptl4 may modulate both extracellular and intracellular lipolysis.
Much effort has been put into determining the mechanisms governing the induction of Angptl4 transcription during fasting. We previously focused on exploring Angptl4 gene regulation by glucocorticoids. Glucocorticoids act by binding to the glucocorticoid receptor (GR) and promoting its recruitment to the nucleus, where it binds to genomic response elements in order to modulate the transcription of nearby genes. Treating cultured primary hepatocytes and adipocytes with the synthetic glucocorticoid dexamethasone (DEX), we identified Angptl4 as a direct transcriptional target of GR (6).
Glucocorticoids act to modulate intermediary metabolism in a nutritionally dependent manner, stimulating lipogenesis and TG formation in the fed state (7, 8) and WAT lipolysis in the fasted state (9–11). Glucocorticoid levels fluctuate throughout the day in accordance with this, displaying their largest peak in the morning following an overnight fast (12). Although the involvement of glucocorticoid action in fasting-induced WAT lipolysis has been described (13, 14), the extent to which glucocorticoids regulate intracellular adipocyte lipolysis and the mechanisms by which this occurs remain to be determined.
This contrasts with the knowledge gathered from studying pathological states of chronic glucocorticoid excess, such as Cushing syndrome, where normal dietary and diurnal regulation of systemic lipid flux gives way to a tonically enhanced flux of FFAs away from peripheral WAT depots into visceral ones and leading to the development of central obesity, hepatic steatosis, and dyslipidemia (14–17).
The relationship between Angptl4 and fat metabolism is seen in humans as well as in mice. For example, a large population-based study showed that sequence variations in human ANGPTL4 are associated with reduced plasma TG levels (18), and another showed that ANGPTL4 levels in the WAT correlate with body weight in monozygotic twins (19). Angptl4 is therefore an attractive target for studying how fasting and glucocorticoids induce WAT lipolysis.
Here we examine the role of Angptl4 in mediating intracellular lipolysis and FFA release by adipocytes in response to stimulation by fasting, glucocorticoids, and catecholamines. Using WT and Angptl4−/− mice, we demonstrate the importance of glucocorticoid action in WAT lipolysis stimulated by fasting within the physiologic range and identify Angptl4 as a key downstream effector in this process. Using primary murine adipocytes and purified human ANGPTL4, we go on to explore how Angptl4 exerts its prolipolytic effects, showing that Angptl4 participates in cAMP-dependent signaling and the phosphorylation of key lipolytic enzymes and represents a common regulatory point in the lipolytic cascade induced by multiple stimuli.
EXPERIMENTAL PROCEDURES
Animals
Angptl4−/− mice were provided by the laboratories of Andras Nagy (Samuel Lunenfeld Research Institute, Mount Sinai Hospital) and Jeff Gordon (Washington University) (20). Angptl4−/− mice were generated on a mixed B6:129 Sv background. WT mice were the littermates of Angptl4−/− mice. The PCR protocols and strategy for mouse genotyping were as described (20). C57BL/6J mice were from Charles River Laboratories (Wilmington, MA). Glucocorticoid treatment of mice (2–4 months old) involved the intraperitoneal injection of water-soluble dexamethasone (5 mg/kg body weight; Sigma) in PBS once between 9 and 10 a.m. and the collection of tissues 6 or 24 h after the injection. GR antagonism was achieved in vivo by providing 2–4-month-old C57BL/6J mice with water containing 0.12 mg/ml mifepristone (RU486; Sigma) dissolved in DMSO for 3 days. Control mice were given water containing only DMSO. The Office of Laboratory Animal Care at the University of California, Berkeley approved all of the animal experiments (approval number R306-0111).
Immunoblots
The protocol for Western blot was as described (6). Proteins were detected by fluorescence imaging (LI-COR Odyssey imager) using the following antibodies: β-actin (C4) mouse monoclonal IgG1 (sc-47778, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)), Hsl (rabbit polyclonal, 4107S, Cell Signaling), phospho-Hsl (serine 660 rabbit polyclonal, 4126S, Cell Signaling), Pnpla2 (rabbit polyclonal, 2138S, Cell Signaling), Plin1 (rabbit polyclonal, ab3526, AbCam), serine 492-phospho-Plin1 (serine 492 mouse monoclonal, 4855, Vala Sciences), Abhd5 (goat polyclonal, ab111984, AbCam), β1AR (rabbit polyclonal, sc-568, Santa Cruz Biotechnology, Inc.), β2AR (rabbit polyclonal, sc-570, Santa Cruz Biotechnology, Inc.), β3AR (rabbit polyclonal, sc-50436, Santa Cruz Biotechnology, Inc.), GR (provided by Keith Yamamoto, University of California, San Francisco), goat anti-rabbit IRDye 800CW (LI-COR), and goat anti-mouse IRDye 800CW (LI-COR). The optical density of the bands was quantified using ImageJ software (National Institutes of Health) and normalized to β-actin.
Lipolysis Assays
Lipolysis was measured as described (21). Explants from freshly removed epididymal and inguinal WAT depots (∼100 mg) were incubated at 37 °C in 500 μl of Krebs-Ringer buffer (12 mm HEPES, 121 mm NaCl, 4.9 mm KCl, 1.2 mm MgSO4, and 0.33 mm CaCl2) with 3% BSA and 3 mm glucose. Glycerol release was determined over time using a free glycerol reagent (Sigma). Measurements were normalized to the total protein content of the explants using Bradford protein dye (Bio-Rad).
Measurement of Corticosterone Levels
An ELISA (Enzo Life Sciences) was used to measure corticosterone levels from plasma immediately after isolation from the whole blood of mice.
WAT cAMP Measurement
Epididymal WAT was isolated, weighed, and homogenized in Krebs-Ringer buffer. Tissues homogenates were centrifuged (13,000 × g for 10 min at 4 °C), and the cAMP content of the tissue supernatants was measured by ELISA (Enzo Life Sciences) (6).
Quantitative Real-time PCR (qPCR)
Total RNA was isolated from the livers and epididymal WAT of mice using Tri-reagent (Molecular Research Center Inc.). Reverse transcription was performed as described (6). The resulting cDNA was diluted to 170 μl, and 3.5 μl was used to perform qPCR in a 25-μl reaction using the EVA qPCR SuperMix kit (Biochain) per the manufacturer's protocol. qPCR was performed on a StepOne PCR system (Applied Biosystems) and analyzed using the ΔΔCT method as supplied by the manufacturer. Primer sequences were as follows: Rpl19 (forward, AGCCTGTGACTGTCCATTCC; reverse, GGCAGTACCCTTCCTCTTCC; used for internal normalization). Pnpla2 (forward, CCAACGCCACTCACATCTAC; reverse, CCTCAATAATGTTGGCACCTG), Angptl4 (forward, GCCATTCCAATCTCAATGG; reverse, ATCAACAGGGTGGTAGCCTG), Pepck (forward, GTCCGATCCCCGTTTATTCT; reverse, ACCTTGGTTTTGGGGGTAAC), Adcy3 (forward, CCACGATGATAGCACACAGG; reverse, CTTGTGTTGGGGGTCACTGT), Adcy6 (forward, CAGCAGGGTAGTGTGTGCAG; reverse, TCTGCATTTGATTTTGGCCT), Adcy8 (forward, AGGTGCTCATCCTCCACATT; reverse, CTCCGCTTGGAGACAGAGAA), PDE3B (forward, TCCTGAACATCTTGCCACTG; reverse, AGTACCGCGGAGGAAAAAGT), Gnas (forward, GCAGGATCCTCATCTGCTTC; reverse, CTAATGGGTGACTCCGTCCA), Gnb1 (forward, TTTCTGGTCTGGTTTCCCAC; reverse, GCTGGGACTGGAACAGCC). Primers were from Elim Biopharmaceuticals.
FFA Assays
Plasma was isolated from whole blood immediately after collection, and a colorimetric kit (Wako) was used to measure plasma FFA levels.
Measurements of Liver TG
Liver samples were weighed and homogenized in a buffer consisting of 50 mm Tris-HCl (pH 7.4), 250 mm sucrose, and protease inhibitors. Lipids were extracted in chloroform/methanol (2:1) and separated by TLC on silica gel G-60 plates with the solvent hexane/ethyl ether/acetic acid (v/v/v, 80:20:1). The TG bands were visualized by exposure to iodine and then scraped and analyzed as described (22), with triolein (Sigma) as a standard, and expressed per tissue weight.
Body Fat Measurements
Mice that were either fasted or fed ad libitum for 24 h were anesthetized with isofluorane and scanned by dual energy x-ray absorptiometry (DEXA) with a PixiMus2 scanner (GE Healthcare Lunar) in order to measure body fat content. Body composition was also measured prior to fasting experiments to ensure that there were no basal differences between treatment groups.
Isolation of Adipocytes
WT and Angptl4−/− mice were euthanized, and epididymal WAT was harvested and minced thoroughly in Krebs-Ringer buffer containing collagenase. The mixture was transferred to a conical tube and shaken at 220 rpm and 37 °C for 1 h. After digestion, the mixture was filtered through a 250-mm gauze mesh and spun for 5 min at 200 rpm. The buffer was carefully removed using a needle and syringe, and the floating layer of adipocytes was washed three times with 10 ml of Krebs-Ringer buffer and then resuspended in medium containing 10% stripped FBS. Aliquots of cells were placed into microcentrifuge tubes containing medium at a final volume of 500 μl for further treatments. Cells were incubated at 37 °C with shaking at 220 rpm for all treatments. Agents used to treat adipocytes included isoproterenol, norepinephrine, forskolin, and 3-isobutyl-1-methylxanthine, from Sigma or 8-bromo-cAMP from Santa Cruz Biotechnology, Inc.
Purification of Human ANGPTL4
HEK293 cells cultured in medium with 5% FBS were infected with adenovirus expressing a FLAG-tagged version of human ANGPTL4 (provided by Sara Vienberg and Ronald Kahn, Joslin Diabetes Center) for 1 h, at which point the medium was replaced. After an additional 72 h, the medium was collected, and Angptl4 protein was purified using an anti-FLAG M2 affinity gel (Sigma). The purified protein was then dialyzed and concentrated 10× using Slide-A-Lyzer dialyzing cassettes and concentrating solution (Thermo Scientific). Western blot and Coomassie staining confirmed protein purity. The affinity gel elution buffer (Tris-buffered saline) was also dialyzed and concentrated to serve as a control.
Determination of Tissue and Plasma Angptl4 Levels
Plasma and epididymal WAT were isolated from C57B/6J mice that had been fed ad libitum or fasted for 24 h. Approximately 200 mg of the WAT was homogenized in 500 μl of radioimmune precipitation buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA, 150 mm NaCl, 5% glycerol, 0.1% sodium deoxycholate, 0.1% SDS, and 1% Triton X-100) supplemented with protease inhibitors. The homogenates were centrifuged (13,000 × g for 10 min at 4 °C), and the Angptl4 content of the tissue supernatants and plasma samples were analyzed by ELISA (USCN Life Sciences).
Statistics
Data are expressed as S.E. for each group, and comparisons were analyzed by Student's t test.
RESULTS
Glucocorticoid Action Is Required for Fasting-induced Angptl4 Expression
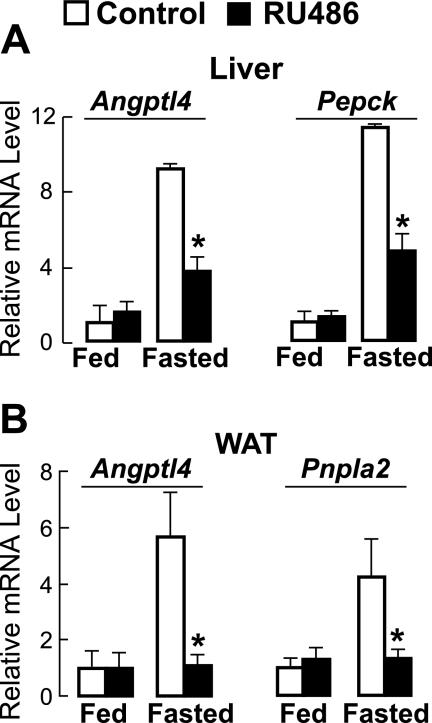
Fasting, a potent inducer of Angptl4 expression (23), also increases circulating glucocorticoid levels. We therefore investigated whether glucocorticoid action is required for the induction of Angptl4 by physiological fasting in mice. To do this, we provided mice with drinking water containing either the GR antagonist RU486 or DMSO (control). The mice were treated for 3 days and either fasted or allowed to feed ad libitum for the final 17 h, after which liver and WAT samples were collected for measurement of Angptl4 mRNA levels. There was no difference in hepatic Angptl4 mRNA levels when mice fed ad libitum were treated with either RU486 or DMSO (Fig. 1A). However, fasting induced a 9.1-fold increase in Angptl4 mRNA levels, and this was reduced by ∼60% in mice treated with RU486 (Fig. 1A). A similar reduction by RU486 treatment was observed for mRNA levels of the phosphoenolpyruvate carboxykinase gene (Pepck), a hepatic GR target known to play an important role during fasting (Fig. 1A) (24, 25).
FIGURE 1.
A, relative mRNA levels (versus fed controls) of hepatic Angptl4 and Pepck measured by qPCR from the livers of mice treated with either RU486 (0.12 mg/ml in the drinking water) or control (DMSO) for 3 days and either allowed to feed ad libitum or fasted for 17 h, showing reduced levels in fasted Angptl4−/− mice (n = 8; *, p < 0.05 versus fasted WT mice). B, as in A, except that mRNA levels were for Angptl4 and Pnpla2 and were measured from epididymal WAT. Error bars, S.E.
As in the livers, Angptl4 mRNA levels in the WAT were not altered by RU486 treatment in mice fed ad libitum, but the increase induced by fasting in control mice (5.7-fold over fed) was abolished by RU486 treatment (Fig. 1B). This reduction matched that seen for the patatin-like phospholipase domain containing 2 (Pnpla2) gene (Pnpla2; also known as adipose triglyceride lipase/ATGL), which is known to be a direct GR target gene in the WAT (Fig. 1B) (26). Together these results indicate that glucocorticoids are required for the induction of Angptl4 by physiological fasting in the liver and WAT.
Glucocorticoid Action Is Important for Fasting-induced Lipolysis
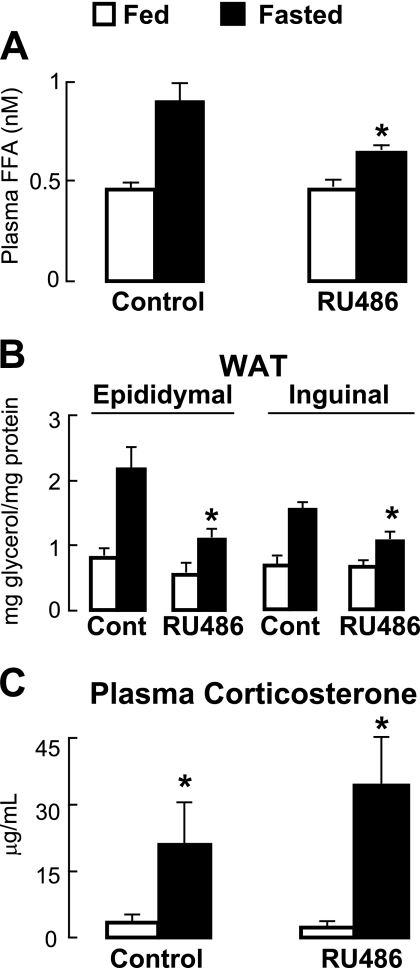
Physiological fasting also stimulates the hydrolysis and mobilization of intracellular TG by adipocytes in the WAT. To determine the importance of glucocorticoid action on WAT lipolysis during such a fast, we analyzed the blood and WAT samples collected from the control and RU486-treated mice described above. Fasting increased plasma FFA levels in control mice by 2-fold, an effect that was reduced ∼30% by RU486 treatment (Fig. 2A). There was also an increase in the amount of glycerol released from WAT samples explanted from mice fasted for 24 h (2.7-fold for epididymal WAT and 2-fold for inguinal WAT), and this was similarly reduced (1.8-fold increase for epididymal WAT and 1.6-fold increase for inguinal WAT) by RU486 treatment (Fig. 2B). These reductions were not a function of lowered circulating levels of endogenous corticosteroids because fasting increased the levels of plasma corticosterone to a similar extent in both control and RU486-treated mice (Fig. 2C). Together, these findings establish the physiological importance of glucocorticoid action in fasting-induced WAT TG hydrolysis and FFA release.
FIGURE 2.
A, plasma FFA levels from mice treated with RU486 or control (DMSO) for 3 days and either fasted or allowed to feed ad libitum for the final 17 h of treatment, showing that RU486 treatment lowers fasting-induced increases in FFA levels (n = 5–6; *, p < 0. 05 versus fasted controls). B, glycerol concentration measured from epididymal and inguinal WAT explants taken from the mice in A after 2 h in medium, showing an RU486-dependent reduction in glycerol release (n = 6; *, p < 0.05 versus fed; **, p < 0.05 versus WT fasted). Data are normalized to total protein. C, plasma corticosterone levels from treated mice (n = 5–6; *, p < 0.05 versus fed). Error bars, S.E.
Angptl4 Is Needed for Fasting-induced WAT Lipolysis
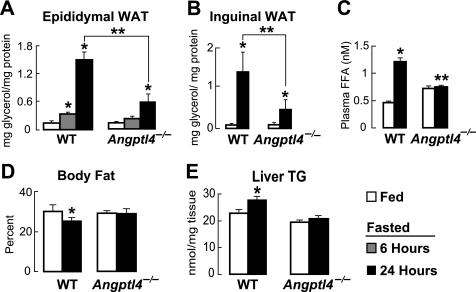
Given that fasting in mice both induces WAT Angptl4 expression and stimulates WAT lipolysis, we wondered whether Angptl4 could be a mediator of the fasting-induced lipolytic response in the WAT. We tested this hypothesis by fasting WT and Angptl4−/− mice. Measuring the amount of glycerol released into medium over 2 h by WAT samples explanted from WT mice that had either been fed ad libitum or fasted revealed that 6 h of fasting induced a 3-fold increase in the concentration of glycerol released from epididymal WAT (Fig. 3A). However, this increase was greatly reduced when the explants were from Angptl4−/− mice (Fig. 3A). The increase in glycerol release from WT explants was more pronounced when the duration of fasting was prolonged to 24 h (10.7-fold) but remained reduced (4.9-fold) when from Angptl4−/− WAT (Fig. 3A). A similar relationship (∼17-fold increase for WT versus ∼7.4-fold for Angptl4−/−) was observed from inguinal WAT samples after a 24-h fast (Fig. 3B) and from both depots when glycerol release was measured over 1 or 4 h (data not shown). Thus, fasting for as little as 6 h, a duration well within the daily physiological range, stimulates WAT lipolysis by a mechanism that is compromised in Angptl4−/− mice.
FIGURE 3.
A, the concentration of glycerol released by epididymal WAT explants taken from mice that were fed ad libitum or fasted for 6 or 24 h, showing decreased release from Angptl4−/− WAT (n = 6; *, p < 0.05 versus fed; **, p < 0.0001 versus WT 24-h fasted). Glycerol concentrations were normalized to protein. B, as in A, using inguinal WAT explants (n = 6; *, p < 0.05 versus WT fasted). C, FFA levels measured from the plasma of WT and Angptl4−/− mice that were either fed ad libitum or fasted for 24 h, showing a reduction in the fasting-induced plasma FFA levels in Angptl4−/− mice (n = 6; *, p < 0.01 versus WT fed; **, p < 0.001 versus WT fasted). D, body composition as measured by DEX, showing a lack of body fat loss induced by fasting in Angptl4−/− mice (n = 9; *, p < 0.05 versus WT fed). E, liver triglyceride content measured by TLC in response to a 24-h fast, showing a loss of fasting-induced hepatic steatosis in Angptl4−/− mice (n = 9; *, p < 0.05 versus WT fed). Error bars, S.E.
To confirm this conclusion, we also measured changes in the circulating FFA levels and body composition of WT and Angptl4−/− mice fasted for 24 h. Fasting greatly increased circulating FFA levels in WT mice, but not in Angptl4−/− mice (Fig. 3C and as seen previously (15)). Fasting also markedly reduced the percentage of body fat and increased hepatic TG content in WT mice, both of which remained relatively constant in fasted Angptl4−/− mice (Fig. 3, D and E). Together, these results support the concept that Angptl4−/− mice have a reduction in fasting-induced WAT lipolysis and that Angptl4 is therefore important in this process.
Angptl4 Is Required for Normal Glucocorticoid-induced WAT Lipolysis
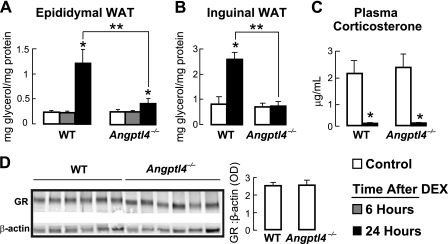
Given our findings that fasting-induced WAT lipolysis is dependent on glucocorticoid action and that glucocorticoid administration, like fasting, induces Angptl4 expression in tissues (7), we wanted to know whether Angptl4 functions to mediate WAT lipolysis when directly stimulated by glucocorticoids. To determine this, we measured the release of glycerol into medium over 2 h from WAT samples explanted from WT and Angptl4−/− mice that were first administered a single dose of either PBS (control) or a synthetic glucocorticoid (DEX). Unlike with fasting, we could not measure a change in the release of glycerol from epididymal WAT explants taken within 6 h of DEX treatment, regardless of genotype (Fig. 4A). However, when the WAT explants were taken 24 h after DEX treatment, we could clearly observe an increase in glycerol release from WT epididymal (Fig. 4A) and inguinal (Fig. 4B) WAT explants, demonstrating that WAT lipolysis stimulated solely by glucocorticoids mirrors that stimulated by fasting in mice although with a slower kinetic onset.
FIGURE 4.
A, the concentration of glycerol released into the medium over 2 h from epididymal WAT explants taken from control (PBS-treated) mice and from mice either 6 or 24 h following a single 5 mg/kg intraperitoneal dose of DEX, showing a reduction in the concentration of glycerol released by Angptl4−/− WAT 24 h after DEX treatment (n = 7–8; *, p < 0.05 versus control; **, p < 0.05 versus WT 24-h DEX). B, as for A, using inguinal WAT explants (n = 7–8; *, p < 0.0001 versus WT control; **, p < 0.001 versus WT 24-h DEX). C, plasma corticosterone levels, showing DEX-induced suppression across genotypes (n = 5–6; *, p < 0.05 versus control). D, WAT GR protein abundance by immunoblot (n = 6). The image is a grouping of representative images from different areas of the same gel. Error bars, S.E.
In contrast, glycerol release by WAT explants taken from Angptl4−/− mice 24 h following DEX treatment was greatly reduced (epididymal WAT; Fig. 4A) to essentially absent (inguinal WAT; Fig. 4B). The differences in glycerol release between WT and Angptl4−/− WAT were also present when measured over 1 or 4 h (data not shown) and demonstrate that Angptl4 is necessary for WAT lipolysis following a single administration of glucocorticoids in mice.
We also monitored the levels of plasma corticosterone, the primary endogenous glucocorticoid in mice, and WAT GR expression in response to DEX treatment. Plasma corticosterone levels were similar at base line and were suppressed to a comparable degree by DEX treatment (95 and 94%, respectively) in WT and Angptl4−/− mice (Fig. 4C). GR expression levels in the WAT were also similar in WT and Angptl4−/− mice (Fig. 4D). Together, these data indicate that impaired DEX-induced WAT lipolysis in Angptl4−/− mice is not due to differences in circulating levels of endogenous glucocorticoids or WAT GR expression.
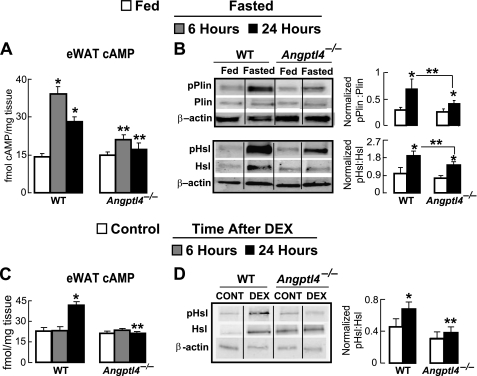
Fasting and DEX Treatment Stimulate Angptl4-dependent cAMP Signaling in WAT
In response to acute lipolytic stimuli, such as physiological fasting, cAMP levels in adipocytes rise and stimulate the activation of protein kinase A (PKA), which then phosphorylates Hsl and perilipin-1 (Plin1), two important lipolytic enzymes (27, 28). We therefore investigated the role of Angptl4 in regulating cAMP levels and the activation of components of the lipolytic machinery in the WAT of fasted mice. Fasting for 6 and 24 h increased cAMP levels by 2.8- and 1.9-fold, respectively, in the WAT of WT mice (Fig. 5A). However, this was greatly reduced in the WAT of Angptl4−/− mice, where a 6-h fast produced a relatively modest (1.5-fold) increase in WAT cAMP levels that was absent altogether when fasting was extended to 24 h (Fig. 5A).
FIGURE 5.
A, cytosolic cAMP levels (normalized to sample weight) measured from epididymal WAT, showing decreased values in WAT from Angptl4−/− mice fasted for 6 and 24 h (n = 6; *, p < 0.01 versus WT fed; **, p < 0.05 versus WT similarly fasted). B, immunoblots probing for the levels of total Plin and pPlin as well as Hsl and pHsl in epididymal WAT explants taken from mice fed ad libitum or fasted for 24 h, showing that Angptl4−/− mice have a decrease in the relative induction of pPlin and pHsl by fasting. The intensity of bands quantified from five separate blots per treatment condition were expressed as the ratio of pPlin to total Plin and pHsl to total Hsl and normalized to actin (*, p < 0.05 versus fed; **, p < 0.05 versus WT fasted). C, cAMP levels measured as in A from epididymal WAT taken from control (PBS-treated) mice or either 6 or 24 h following a single intraperitoneal dose of DEX, showing that the increase in WAT cAMP levels 24 h after DEX treatment is absent in Angptl4−/− mice (n = 4–5; *, p < 0.001 versus WT control; **, p < 0.05 versus WT 24 h after DEX). D, total Hsl and pHsl measured as in B from epididymal WAT 24 h following DEX treatment, showing a reduction in the DEX-induced increase in the ratio of pHsl to total Hsl (*, p < 0.05 versus WT control; **, p < 0.05 versus WT DEX). Data are the mean band intensities measured from eight separate blots per treatment condition. Each immunoblot image is a grouping of representative images from different parts of the same gel. Error bars, S.E.
Fasting also produced a marked increase in the absolute abundance of the phosphorylated forms of both Hsl (serine 660; pHsl) (29, 30) and Plin1 (serine 492; pPlin1) (31, 32) as well as the ratio of phosphorylated to total enzyme for each (Fig. 5B). In contrast, both the absolute levels and relative abundances of pHsl and pPlin1 were lower in the WAT of fasted Angptl4−/− mice (Fig. 5B), indicating that Angptl4 is needed for cAMP-dependent protein phosphorylation events that are important in the fasting-induced lipolytic response.
To determine whether DEX treatment could mirror the increase in WAT cAMP levels stimulated by fasting, we analyzed cAMP levels in the WAT of mice following a single dose of DEX. As for glycerol release from WAT explants, cAMP levels within the WAT were not altered 6 h after an intraperitoneal dose of DEX but were markedly increased (1.8-fold) by 24 h. Remarkably, DEX treatment was unable to increase cAMP levels in the WAT of Angptl4−/− mice, as was the case when fasting was used as the stimulus (Fig. 5C). Consistent with these data, DEX treatment increased the ratio of pHsl to total Hsl by ∼35% in the WAT of WT mice but did not alter this ratio in the WAT of Angptl4−/− mice (Fig. 5D). Together these findings indicate that a single DEX treatment can mimic physiologic fasting to increase cAMP levels in murine WAT, albeit with a slower time course. Moreover, as for glycerol release, the ability of DEX to increase cAMP levels in the WAT and stimulate downstream phosphorylation events is dependent on Angptl4.
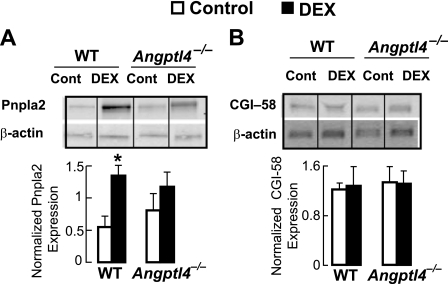
Other enzymes are involved in mobilizing TG from its storage depot within adipocyte lipid droplets during WAT lipolysis, including Pnpla2 and CGI-58 (Abhd5), both of which are implicated in diseases associated with aberrant lipolysis (33, 34). We found that DEX treatment stimulated an increase in the overall abundance of Pnpla2 within the WAT of WT mice despite some variability in expression levels between individual mice. However, we did not see this relative increase in the epididymal WAT of DEX-treated Angptl4−/− mice (Fig. 6A). On the other hand, CGI-58 protein levels were not altered by DEX treatment within the WAT of either WT or Angptl4−/− mice (Fig. 6B). These data suggest that DEX may act to transcriptionally regulate the expression of some lipolytic genes, such as Pnpla2, but not others, such as Abhd5, and that this transcriptional control may be at least partially determined by the presence or absence of Angptl4. In any case, the impairment of glucocorticoid-induced WAT lipolysis in Angptl4−/− mice was not associated with a reduction in the basal expression of either Hsl, Plin1, Pnpla2, or CGI-58 in the WAT.
FIGURE 6.
A, immunoblots probing for the levels of Pnpla2 in epididymal WAT explants taken 24 h after DEX treatment, showing a significant increase in expression after DEX treatment in WT mice but not in Angptl4−/− mice. The intensity of bands quantified from five separate blots per treatment condition was expressed as the ratio of Pnpla2 normalized to actin (*, p < 0.05 versus fed; **, p < 0.05 versus WT control). B, expression of CGI-58 was also measured by immunoblot in WAT of the same animals. No differences in expression were observed in response to DEX treatment in either genotype. Data are the mean band intensities measured from four separate blots per treatment condition. Error bars, S.E.
Angptl4 Is a Mediator of Catecholamine-induced Lipolysis in Adipocytes
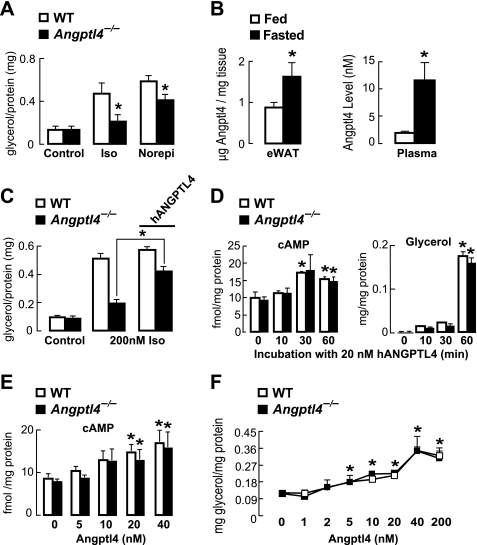
Beyond glucocorticoids, catecholamines are regarded as dominant regulators of intracellular adipocyte lipolysis during fasting and do so by stimulating cAMP-dependent signaling (28, 35). Because Angptl4−/− WAT had less lipolysis and less cAMP-dependent signaling in response to both physiological fasting and glucocorticoid treatment, we explored whether Angptl4 plays a role in catecholamine-induced lipolysis. We measured the concentration of glycerol released into the medium by primary adipocytes isolated from WT and Angptl4−/− mice during a 1-h treatment with either 200 nm isoproterenol or 1 μm norepinephrine. Glycerol release by Angptl4−/− adipocytes in response to either isoproterenol or norepinephrine treatment was significantly lower than for WT adipocytes (Fig. 7A), indicating that the requirement of Angptl4 for normal lipolysis in response to fasting or glucocorticoid treatment in vivo is also present in cultured murine adipocytes treated with catecholamines.
FIGURE 7.
A, glycerol concentration measured in the medium bathing primary murine adipocytes treated for 1 h with isoproterenol (200 nm) or norepinephrine (1 μm), showing a decrease in catecholamine-stimulated glycerol release by Angptl4−/− adipocytes (n = 6; *, p < 0.05 versus similarly treated WT cells). B, Angptl4 concentrations measured by ELISA in the epididymal WAT and plasma of mice fed ad libitum or fasted for 24 h (n = 5–6; *, p < 0.05 versus fed). C, glycerol concentration measured in the medium bathing primary murine adipocytes treated for 1 h with isoproterenol alone or plus 20 nm hANGPTL4, showing that the addition of hANGPTL4 largely rescues the impaired catecholamine-stimulated glycerol release seen in Angptl4 deficiency (n = 6; *, p < 0.05). D, cytosolic cAMP levels and glycerol release measured from WT and Angptl4−/− adipocytes treated with 20 nm hANGPTL4, showing the kinetics for each response over 60 min (n = 4–5; *, p < 0.05 versus time 0). E, dose-response relationship for the increase in cytosolic cAMP in WT and Angptl4−/− adipocytes stimulated by treatment with hANGPTL4 for 1 h (n = 8; *, p < 0.05 versus buffer alone). F, dose-response relationship for the stimulation of glycerol release into the medium by WT and Angptl4−/− adipocytes treated with hANGPTL4 for 1 h (n = 4; *, p < 0.05 versus buffer alone). Error bars, S.E.
We affinity-purified human angiopoietin-like 4 protein (hANGPTL4) from HEK293 cells to further explore how Angptl4 regulates the lipolytic response of adipocytes. In order to determine the concentration range over which treatments with hANGPTL4 should be performed, we first measured the extent to which Angptl4 levels in the WAT and plasma are elevated by physiological fasting. In both compartments, fasting for 24 h resulted in a marked increase in Angptl4 protein concentration (Fig. 7B). Although the concentration reached in the epididymal WAT (1.6 μg/mg tissue; ∼32.5 nm) was notably higher than that in the plasma (11.6 nm), these values determined the range of hANGPTL4 concentrations (20 nm) used to treat primary adipocytes in subsequent studies.
We tested whether purified hANGPTL4 could rescue the impaired lipolytic response of Angptl4−/− adipocytes to catecholamine treatment. Indeed, the concentration of glycerol released into the medium in response to isoproterenol treatment by Angptl4−/− adipocytes was restored to near WT levels by the addition of 20 nm hANGPTL4 into the medium (Fig. 7C), indicating that extracellular Angptl4 is a mediator of catecholamine-stimulated TG hydrolysis in adipocytes. hANGPTL4 treatment did not further enhance isoproterenol-stimulated glycerol release by WT adipocytes; however, the response of these cells to isoproterenol treatment was already quite robust even without added hANGPTL4.
Extracellular hANGPTL4 Increases cAMP Levels and TG Hydrolysis in Adipocytes
We treated primary WT and Angptl4−/− adipocytes with 20 nm hANGPTL4 to determine if this alone was sufficient to increase cAMP levels and stimulate lipolysis. hANGPTL4 treatment increased cytosolic cAMP levels similarly in WT and Angptl4−/− adipocytes and did so within 30 min (Fig. 7D). hANGPTL4 treatment also stimulated glycerol release, again to a similar degree, from WT and Angptl4−/− adipocytes (Fig. 7D). However, the kinetics of hANGPTL4-stimulated glycerol release had a slower onset than that for elevation of cytosolic cAMP, increasing markedly after 60 min. Together these findings indicate that the addition of extracellular Angptl4 alone is sufficient to increase the level of cytosolic cAMP and stimulate lipolysis in adipocytes and that, as seen in response to lipolytic stimuli in vivo, the time course for glycerol release by adipocytes is slower than that needed to elevate cytosolic cAMP.
We also tested the concentration dependence of the effect of hANGPTL4 treatment on increasing cytosolic cAMP levels and stimulating lipolysis in adipocytes. WT and Angptl4−/− adipocytes were treated with hANGPTL4 for 60 min at concentrations ranging up to 200 nm. Over this period, 20 nm hANPTL4 was the minimum concentration sufficient to increase cAMP levels, and it did so similarly in WT and Angptl4−/− adipocytes (Fig. 7E). On the other hand, glycerol release over 60 min was stimulated by as little as 5 nm hANGPTL4, although the concentration of glycerol released did not increase further until 40 nm hANGPTL4 was used (Fig. 7F).
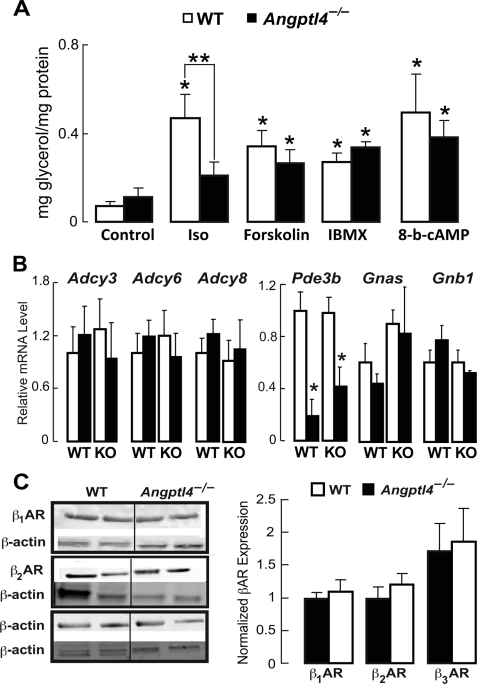
Angptl4 Regulates Lipolysis at Point Upstream of Adenylate Cyclase
The cascade leading from β-adrenergic receptor (βAR) stimulation to TG hydrolysis is well characterized, and we took advantage of established pharmacologic approaches to dissect the point within this cascade at which hANGPTL4 exerts its lipolytic effects. WT and Angptl4−/− adipocytes were treated with either isoproterenol or one of several other agents that also stimulate lipolysis but do so by engaging signaling steps distal to βAR activation. Interestingly, Angptl4 deficiency in adipocytes impaired glycerol release in response to treatment with isoproterenol but not forskolin, which directly activates adenylate cyclase (Fig. 8A). Angptl4 deficiency likewise did not affect lipolysis stimulated by treatment of adipocytes with 3-isobutyl-1-methylxanthine, which inhibits the hydrolysis of cAMP by phosphodiesterases (PDEs), or with 8-bromo-cAMP, which is resistant to such hydrolysis (Fig. 8A). Taken together, these data indicate that Angptl4 modulates cellular signaling at a point downstream of βAR but upstream of adenylate cyclase and more distal modulators.
FIGURE 8.
A, glycerol concentration in the medium bathing WT or Angptl4−/− adipocytes measured after a 1-h treatment with PBS (control), 200 nm isoproterenol (Iso), 10 μm forskolin, 100 μm 3-isobutyl-1-methylxanthine, or 10 mm 8-bromo-cAMP, showing that only isoproterenol-stimulated glycerol release was decreased by Angptl4 deficiency (n = 6; *, p < 0.05 versus WT control; **, p < 0.05 versus WT isoproterenol). B, mRNA levels of several genes encoding proteins involved in the cAMP-dependent signaling cascade in epididymal WAT taken from control (PBS-treated) mice and from mice 24 h after a single intraperitoneal treatment with DEX, showing a DEX-induced reduction only in the levels of Pde3b but no differences between genotypes (n = 12; *, p < 0.05 versus control). C, immunoblots from epididymal WAT, showing no differences in the protein levels of β1-, β2-, or β3AR between WT and Angptl4−/− mice (n = 6 separate blots/group). Data are the mean band intensities measured from six separate blots per treatment condition. Error bars, S.E.
We wanted to make sure that Angptl4 deficiency was not associated with differences in the expression of genes and proteins critical to the cAMP-dependent lipolytic cascade that would have confounded the interpretation of data obtained using pharmacologic inhibitors. The mRNA levels of genes encoding several adenylate cyclase isoforms (Adcy3, Adcy6, and Adcy8), PDE3B (Pde3b), the stimulatory G protein α-subunit (Gnas), and the G protein β subunit (Gnb1) at base line or following DEX treatment were all similar in WT and Angptl4−/− adipocytes, as was the abundance of β1-, β2-, and β3AR proteins (Fig. 8, B and C). Therefore, the effect of Angptl4 deficiency on cAMP levels and glycerol release in adipocytes is not due to a reduction in the expression of elements critical to cAMP-dependent signaling.
DISCUSSION
Lipolysis is an essential component of the response to fasting, in which TG stores in the WAT are hydrolyzed in order to mobilize substrates for hepatic gluconeogenesis and β-oxidation in the liver and skeletal muscle. Our studies in mice reveal that, beyond inhibiting extracellular Lpl, the secreted protein Angptl4 also stimulates intracellular TG hydrolysis and FFA release by adipocytes during fasting in response to classical physiologic cues. Mice lacking Angptl4 failed to appropriately release glycerol, a marker of TG hydrolysis, in response to a physiological fast. We built on these findings by exploring the role of glucocorticoid action, which directly induces Angptl4 transcription during fasting (6), in WAT TG hydrolysis. We show that glucocorticoid action is a determinant of the lipolytic potential of WAT during fasting in mice and that these effects require Angptl4. In addition to stimulating TG hydrolysis, short term fasting and glucocorticoid treatment in vivo and catecholamine treatment in vitro each also increased cAMP levels in adipocytes, and Angptl4 was necessary for the ability of each of these stimuli to do so. In exploring this role further, we found that purified human ANGPTL4, when added on its own to cultured murine adipocytes, can remarkably increase intracellular cAMP levels and rescue the lipolytic impairment produced by Angptl4 deficiency. Our studies combine to implicate Angptl4 as a common downstream mediator that integrates the acute lipolytic actions of glucocorticoids and catecholamines during fasting in adipocytes by increasing WAT cAMP levels and the phosphorylation of cAMP-dependent components of the lipolytic machinery.
Although administration of RU486, a GR antagonist, greatly reduced fasting-induced WAT TG hydrolysis and FFA release, the role of Angptl4 in this reduction may be tissue-specific. Although RU486 treatment completely abolished the fasting-induced increase in Angptl4 expression in the WAT, it only suppressed it by ∼60% in the liver. These findings together suggest that Angptl4 expressed locally within the WAT may be more closely linked to TG hydrolysis than that secreted into the circulation by the liver and that other, glucocorticoid-independent, signaling pathways probably contribute to the induction of hepatic Angptl4 expression during fasting. Indeed, the array of signals that modulate Angptl4 expression is likely to be diverse and tissue-specific. In the hypothalamus, for example, it was recently shown that Angptl4 levels in mice are increased by the CNS administration of leptin or insulin or the intake of dietary macronutrients (36). On the other hand, both leptin and insulin may signal to repress Angptl4 expression in peripheral tissues (37).
We next explored the temporal order in which catecholamines and glucocorticoids induce WAT TG hydrolysis during fasting. An increase in glycerol release from the WAT was initially detectable after 6 h of fasting and was markedly ramped up by prolonging the fast to 24 h. By contrast, 6 h of fasting could maximally increase WAT cAMP levels, suggesting that activation of cAMP-dependent signaling probably precedes TG hydrolysis and FFA release by adipocytes. Interestingly, Angptl4 deficiency severely blunted both glycerol release from the WAT and the increase in WAT cAMP levels following fasts of either 6 or 24 h, strongly suggesting that Angptl4 can modulate events early in the cascade leading to WAT TG hydrolysis. Indeed, we were able to validate this concept in cultured adipocytes treated with catecholamines, which stimulate cAMP-dependent lipolysis within 60 min. Here too, Angptl4 deficiency was associated with reduced cAMP levels and rates of glycerol release.
On the other hand, lipolysis stimulated solely by a single dose of DEX was much slower; whereas fasting could elevate WAT cAMP levels within 6 h, a single dose of DEX alone required 24 h to do so. Despite these temporal differences, both fasting and DEX treatment still required Angptl4 to elevate WAT cAMP levels and stimulate glycerol release. Taken together, these findings suggest that early on during fasting, the Angptl4 required for TG hydrolysis is either already present in the WAT or induced by a mechanism independent of glucocorticoid action, which displays slower transcriptional kinetics. As fasting is prolonged, or in response to DEX treatment, the role of Angptl4 induced directly by GR activation becomes more prominent, playing an important role in maintaining WAT lipolysis.
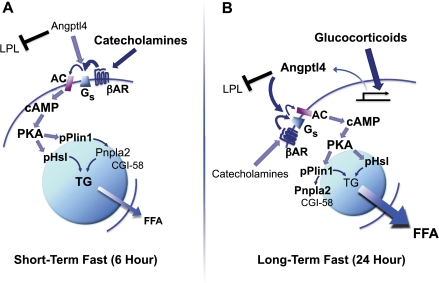
Our in vivo and in vitro findings together allow for the construction of a model piecing together the temporal contribution of several components of fasting-induced lipolysis and the integrative role of Angptl4 in this process (Fig. 9). During a physiological fast, catecholamines and other counter-regulatory defenses act on the WAT early on to increase cAMP levels, leading to activation of PKA and phosphorylation Hsl and Plin1, two proteins that localize to lipid droplets and undergo specific PKA-dependent phosphorylation in order to participate in lipolysis. During this early phase of fasting (as modeled here by fasting mice for 6 h), Angptl4 serves two roles; it inhibits Lpl to limit extracellular lipolysis and fat uptake by adipocytes and also potentiates the actions of catecholamines by enhancing their effect on cAMP-dependent TG hydrolysis. When fasting is carried out longer (as modeled by fasting mice for 24 h), the contribution of glucocorticoid action to WAT TG hydrolysis increases and is characterized by transcriptional effects on many genes, one of which is Angptl4. For both catecholamines and glucocorticoids, the ability to stimulate the release of stored TG by the WAT is linked to their capacity to increase adipocyte cAMP levels. For this, they require Angptl4.
FIGURE 9.
Model depicting the proposed role of Angptl4 in integrating hormonal inputs to modulate intracellular adipocyte lipolysis during short and long term fasting. A, hormonal inputs, notably catecholamines, act early on (6-h fast) to stimulate WAT lipolysis. Angptl4 is required for this, inhibiting extracellular Lpl activity and facilitating intracellular cAMP formation and the downstream activation of hydrolytic enzymes (pHsl) and lipid droplet proteins (pPlin1). B, as fasting is extended (24 h), glucocorticoid action becomes relatively more important, regulating the transcription of several genes, including Angptl4. Angptl4 potentiates the lipolytic effects of catecholamines and glucocorticoids, acting upstream of adenylate cyclase.
Our next goal was to more deeply explore the role of cAMP-dependent signaling in the mechanism by which Angptl4 stimulates TG hydrolysis during fasting. We first measured the phosphorylation of Hsl at serine 660 and of Plin at serine 492, two sites of action for cAMP-dependent PKA. The relative induction of the phosphorylated forms of these two enzymes by fasting (pHsl) or DEX treatment (pHsl and pPlin1) was greatly reduced in the WAT of Angptl4−/− mice, indicating that Angptl4 modulates the PKA-dependent functional status of TG hydrolytic enzymes.
We went on to treat primary adipocytes isolated from WT or Angptl4−/− mice with norepinephrine or isoproterenol, catecholamines that rapidly raise adipocyte cAMP levels and stimulate TG hydrolysis during fasting and other stressful states (28, 35). These studies yielded several important findings. First, the capacity of catecholamine treatment to increase glycerol release from Angptl4−/− adipocytes was greatly reduced. Remarkably, this reduction could largely be rescued by co-treatment of Angptl4−/− adipocytes with hANGPTL4. Moreover, hANGPTL4 was able to increase cAMP levels within 30 min when added to adipocytes, regardless of whether these were from WT or Angptl4−/− mice. These findings, when combined with those from our in vivo studies using WAT explants, support the concept that Angptl4 deficiency impairs intracellular WAT lipolysis by limiting its ability to increase cAMP and initiate PKA-dependent phosphorylation events in response to multiple physiologic stimuli. Providing cultured adipocytes with hANGPTL4 can bypass this limitation, raising intracellular cAMP to similar levels in WT and Angptl4−/− adipocytes. Furthermore, stimulation with forskolin, PDE inhibitors, and cAMP analogs could also bypass the limitation on lipolysis afforded by Angptl4 deficiency, suggesting that this limitation is due to an impairment at or upstream of adenylate cyclase and downstream of receptor activation.
Several possible mechanisms emerge when considering how Angptl4 could modulate this point in the cAMP-dependent signaling cascade. One is that it may influence the rate at which Gαs cycles between the GTP- and GDP-bound states. Enhanced cycling could affect the availability Gα to activate adenylate cyclase, invoking potential roles for GTPase-activating proteins and guanine exchange factors other than Gα itself. Angptl4 could also modulate the turnover rate of cAMP upon activation of adenylate cyclase, invoking the action of PDEs. We compared the WAT mRNA levels of genes encoding adenylate cyclase isoforms (Adcy3, Adcy6, and Adcy8), PDEs (Pde3b), and G protein subunits (Gnas and Gnb1) and did not find differences between WT and Angptl4−/− mice at base line or following DEX treatment. We further did not find differences in the abundance of βAR1, -2, or -3 proteins in the WAT between either genotype. However, these measurements do not exclude the possibility that WT and Angptl4−/− adipocytes have functional or regulatory differences in G proteins, adenylate cyclase, or PDEs or in receptor-G protein interaction. Determining the role of such functional differences in Angptl4 action is the subject of future studies and is of particular interest, given that catecholamines raise cAMP levels in adipocytes by activating adenylate cyclase (38, 39), whereas glucocorticoids may do so by inhibiting PDE3b (31). Because Angptl4 is required for the normal lipolytic response to both of these stimuli, it may act on adipocytes by altering cAMP formation, hydrolysis, or both of these.
Our findings do not preclude the possibility that pathways other than cAMP-dependent signaling may also mediate, at least in part, the effect of glucocorticoids on fasting-induced lipolysis. Beyond regulating the expression of Angptl4, GR activation probably modulates a network of genes to affect lipolysis. For example, glucocorticoids increase mRNA levels of Hsl, an effect that may be directly dependent on transcriptional regulation by GR (40). We used chromatin immunoprecipitation along with massively parallel sequencing to identify an intronic GR binding region in murine Hsl that, when inserted into a reporter plasmid, could stimulate reporter activity in response to glucocorticoid treatment (40). Glucocorticoid action also increases the expression of Pnpla2 (26), although the mechanism(s) governing this process are unclear.
Similarly, we do not suggest that all catecholamine-stimulated lipolysis is Angptl4-dependent. Indeed, as opposed to its effects on fasting or glucocorticoid treatment in vivo, Angptl4 deficiency was less able to limit increases in cAMP or glycerol release in adipocytes stimulated by treatment with norepinephrine in vitro, pointing to an Angptl4-independent component of catecholamine-stimulated lipolysis. Therefore, the need for Angptl4 to couple external inputs to cAMP-dependent signaling in order to stimulate TG hydrolysis in the WAT during fasting may be bypassed, at least when lipolysis is stimulated solely by pharmacologic doses of catecholamines.
Of note, Angptl4 is secreted in both full-length and truncated isoforms, and the full-length isoform of hANGPTL4 was used in our cell-based experiments. In measuring levels of Angptl4 in the plasma and the WAT of mice by ELISA, however, we are unable to distinguish between the two. Furthermore, although the C terminus of Angptl4 alone can inhibit Lpl, it is unclear whether truncated isoforms can modulate TG hydrolysis. Studies are currently under way to tackle this important question.
Also, levels of lipases and lipid droplet-associated proteins involved in TG hydrolysis were measured by immunoblot from lysates of whole WAT taken from mice subjected to fasting or DEX treatment and often displayed a high degree of intersample variability within genotypes. For Pnpla2, this was associated with a small increase in protein levels in Angptl4−/− WAT under control (basal) conditions. The physiological importance of this increase with respect to the lack of further increase seen in response to fasting is unclear. Additionally, certain proteins interact with each other at the surface of lipid droplets during the course of TG hydrolysis; for example, Pnpla2 interacts with CGI-58. Although we measured protein levels of both Pnpla2 and CGI-58 (total CGI-58 measured in this way was not altered by Angptl4 deficiency), further experiments are necessary to determine how Angptl4 affects the interaction between these and other components of the lipolytic machinery at the surface of lipid droplets.
In summary, we have uncovered a physiologically important role for glucocorticoids as inducers of Angptl4 expression during a fast, both in the WAT and in the liver. Angptl4, in turn, is a key determinant of the lipolytic response of adipocytes, acting directly to increase the levels of cAMP and the phosphorylation of downstream TG hydrolytic enzymes. These findings enhance the framework for understanding intermediary metabolism and identify hormonal and WAT-specific regulators of lipolysis with relevance to conditions of insulin resistance, where lipolysis is aberrant. Given that polymorphisms in human ANGPTL4 result in dyslipidemia, the impact of these on WAT lipolysis is also worthy of investigation.
Acknowledgments
We thank Drs. Charlie Harris (University of California, San Francisco), Carlos Pantoja (UC Berkeley), Hei Sook Sul (UC Berkeley), and Maryam Ahmadian (UC Berkeley) for helpful comments on the manuscript as well as Joyce Lee (UC Berkeley) and Simply Flor Cruz (UC Berkeley) for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant DK83591.
- FFA
- free fatty acid
- WAT
- white adipose tissue
- TG
- triacylglycerol
- Lpl
- lipoprotein lipase
- GR
- glucocorticoid receptor
- DEX
- dexamethasone
- qPCR
- quantitative real-time PCR
- pHsl and pPlin1
- phosphorylated form of Hsl and Plin1, respectively
- βAR
- β-adrenergic receptor
- PDE
- phosphodiesterase
- hANGPTL4
- human ANGPTL4.
REFERENCES
- 1. Kersten S. (2005) Regulation of lipid metabolism via angiopoietin-like proteins. Biochem. Soc. Trans. 33, 1059–1062 [DOI] [PubMed] [Google Scholar]
- 2. Miida T., Hirayama S. (2010) Impacts of angiopoietin-like proteins on lipoprotein metabolism and cardiovascular events. Curr. Opin. Lipidol. 21, 70–75 [DOI] [PubMed] [Google Scholar]
- 3. Lichtenstein L., Kersten S. (2010) Modulation of plasma TG lipolysis by angiopoietin-like proteins and GPIHBP1. Biochim. Biophys. Acta 1801, 415–420 [DOI] [PubMed] [Google Scholar]
- 4. Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., Heuer J. G., Jaskunas S. R., Eacho P. (2005) Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3. Regulation of triglyceride metabolism. Endocrinology 146, 4943–4950 [DOI] [PubMed] [Google Scholar]
- 5. Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Müller M., Kersten S. (2006) The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 934–944 [DOI] [PubMed] [Google Scholar]
- 6. Koliwad S. K., Kuo T., Shipp L. E., Gray N. E., Backhed F., So A. Y., Farese R. V., Jr., Wang J. C. (2009) Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J. Biol. Chem. 284, 25593–25601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berdanier C. D. (1989) Role of glucocorticoids in the regulation of lipogenesis. FASEB J. 3, 2179–2183 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y., Jones Voy B., Urs S., Kim S., Soltani-Bejnood M., Quigley N., Heo Y. R., Standridge M., Andersen B., Dhar M., Joshi R., Wortman P., Taylor J. W., Chun J., Leuze M., Claycombe K., Saxton A. M., Moustaid-Moussa N. (2004) The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr. 134, 1032–1038 [DOI] [PubMed] [Google Scholar]
- 9. Gravholt C. H., Dall R., Christiansen J. S., Møller N., Schmitz O. (2002) Preferential stimulation of abdominal subcutaneous lipolysis after prednisolone exposure in humans. Obes. Res. 10, 774–781 [DOI] [PubMed] [Google Scholar]
- 10. Tomlinson J. W., Sherlock M., Hughes B., Hughes S. V., Kilvington F., Bartlett W., Courtney R., Rejto P., Carley W., Stewart P. M. (2007) Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 activity in vivo limits glucocorticoid exposure to human adipose tissue and decreases lipolysis. J. Clin. Endocrinol. Metab. 92, 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samra J. S., Clark M. L., Humphreys S. M., MacDonald I. A., Bannister P. A., Frayn K. N. (1998) Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 83, 626–631 [DOI] [PubMed] [Google Scholar]
- 12. Krieger D. T., Allen W., Rizzo F., Krieger H. P. (1971) Characterization of the normal temporal pattern of plasma corticosteroid levels. J. Clin. Endocrinol. Metab. 32, 266–284 [DOI] [PubMed] [Google Scholar]
- 13. Campbell J. E., Peckett A. J., D'souza A. M., Hawke T. J., Riddell M. C. (2011) Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am. J. Physiol. Cell Physiol. 300, C198–C209 [DOI] [PubMed] [Google Scholar]
- 14. Macfarlane D. P., Forbes S., Walker B. R. (2008) Glucocorticoids and fatty acid metabolism in humans. Fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 197, 189–204 [DOI] [PubMed] [Google Scholar]
- 15. Walker B. R. (2006) Cortisol. Cause and cure for metabolic syndrome? Diabet. Med. 23, 1281–1288 [DOI] [PubMed] [Google Scholar]
- 16. Arnaldi G., Scandali V. M., Trementino L., Cardinaletti M., Appolloni G., Boscaro M. (2010) Pathophysiology of dyslipidemia in Cushing's syndrome. Neuroendocrinology 92, Suppl. 1, 86–90 [DOI] [PubMed] [Google Scholar]
- 17. Pivonello R., De Leo M., Vitale P., Cozzolino A., Simeoli C., De Martino M. C., Lombardi G., Colao A. (2010) Pathophysiology of diabetes mellitus in Cushing's syndrome. Neuroendocrinology 92, Suppl. 1, 77–81 [DOI] [PubMed] [Google Scholar]
- 18. Romeo S., Pennacchio L. A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H. H., Cohen J. C. (2007) Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39, 513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robciuc M. R., Naukkarinen J., Ortega-Alonso A., Tyynismaa H., Raivio T., Rissanen A., Kaprio J., Ehnholm C., Jauhiainen M., Pietiläinen K. H. (2011) Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 52, 1575–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., Gordon J. I. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaworski K., Ahmadian M., Duncan R. E., Sarkadi-Nagy E., Varady K. A., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., Kim K. H., de Val S., Kang C., Sul H. S. (2009) AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 15, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snyder F., Stephens N. (1959) A simplified spectrophotometric determination of ester groups in lipids. Biochim. Biophys. Acta 34, 244–245 [DOI] [PubMed] [Google Scholar]
- 23. Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., Wahli W. (2000) Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28488–28493 [DOI] [PubMed] [Google Scholar]
- 24. Seitz H. J., Kaiser M., Krone W., Tarnowski W. (1976) Physiologic significance of glucocorticoids and insulin in the regulation of hepatic gluconeogenesis during starvation in rats. Metabolism 25, 1545–1555 [DOI] [PubMed] [Google Scholar]
- 25. Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. (1984) Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J. Biol. Chem. 259, 15242–15251 [PubMed] [Google Scholar]
- 26. Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids. Ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 27. Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaworski K., Sarkadi-Nagy E., Duncan R. E., Ahmadian M., Sul H. S. (2007) Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest Liver Physiol. 293, G1–G4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anthonsen M. W., Rönnstrand L., Wernstedt C., Degerman E., Holm C. (1998) Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 273, 215–221 [DOI] [PubMed] [Google Scholar]
- 30. Su C. L., Sztalryd C., Contreras J. A., Holm C., Kimmel A. R., Londos C. (2003) Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 278, 43615–43619 [DOI] [PubMed] [Google Scholar]
- 31. Zhang H. H., Souza S. C., Muliro K. V., Kraemer F. B., Obin M. S., Greenberg A. S. (2003) Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J. Biol. Chem. 278, 51535–51542 [DOI] [PubMed] [Google Scholar]
- 32. Brasaemle D. L., Subramanian V., Garcia A., Marcinkiewicz A., Rothenberg A. (2009) Perilipin A and the control of triacylglycerol metabolism. Mol. Cell Biochem. 326, 15–21 [DOI] [PubMed] [Google Scholar]
- 33. Lass A., Zimmermann R., Oberer M., Zechner R. (2011) Lipolysis. A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamaguchi T. (2010) Crucial role of CGI-58/α/β hydrolase domain-containing protein 5 in lipid metabolism. Biol. Pharm. Bull. 33, 342–345 [DOI] [PubMed] [Google Scholar]
- 35. Ahmadian M., Duncan R. E., Sul H. S. (2009) The skinny on fat. Lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol. Metab. 20, 424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim H. K., Youn B. S., Shin M. S., Namkoong C., Park K. H., Baik J. H., Kim J. B., Park J. Y., Lee K. U., Kim Y. B., Kim M. S. (2010) Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes 59, 2772–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon J. C., Chickering T. W., Rosen E. D., Dussault B., Qin Y., Soukas A., Friedman J. M., Holmes W. E., Spiegelman B. M. (2000) Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20, 5343–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins S., Surwit R. S. (2001) The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog. Horm. Res. 56, 309–328 [DOI] [PubMed] [Google Scholar]
- 39. Evans B. A., Sato M., Sarwar M., Hutchinson D. S., Summers R. J. (2010) Ligand-directed signaling at β-adrenoceptors. Br. J. Pharmacol. 159, 1022–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu C. Y., Mayba O., Lee J. V., Tran J., Harris C., Speed T. P., Wang J. C. (2010) Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One 5, e15188. [DOI] [PMC free article] [PubMed] [Google Scholar]