Background: RbgA is a GTPase involved in a late assembly step of the 50 S subunit.
Results: RbgA is maximally stimulated by 50 S subunits and associates with the ribosome in a nucleotide-dependent manner.
Conclusion: Biochemical characterization of RbgA supports that GTPase activity promotes a late step in ribosome assembly and RbgA dissociation.
Significance: Learning how RbgA promotes ribosome assembly is critical for understanding how GTPases regulate ribosome biogenesis.
Keywords: Bacillus, Enzyme Kinetics, GTPase, Ribosome Assembly, Ribosomes
Abstract
The ribosome biogenesis GTPase A protein RbgA is involved in the assembly of the large ribosomal subunit in Bacillus subtilis, and homologs of RbgA are implicated in the biogenesis of mitochondrial, chloroplast, and cytoplasmic ribosomes in archaea and eukaryotes. The precise function of how RbgA contributes to ribosome assembly is not understood. Defects in RbgA give rise to a large ribosomal subunit that is immature and migrates at 45 S in sucrose density gradients. Here, we report a detailed biochemical analysis of RbgA and its interaction with the ribosome. We found that RbgA, like most other GTPases, exhibits a very slow kcat (14 h−1) and has a high Km (90 μm). Homology modeling of the RbgA switch I region using the K-loop GTPase MnmE as a template suggested that RbgA requires K+ ions for GTPase activity, which was confirmed experimentally. Interaction with 50 S subunits, but not 45 S intermediates, increased GTPase activity by ∼55-fold. Stable association with 50 S subunits and 45 S intermediates was nucleotide-dependent, and GDP did not support strong interaction with either of the subunits. GTP and guanosine 5′-(β,γ-imido)triphosphate (GMPPNP) were sufficient to promote association with the 45 S intermediate, whereas only GMPPNP was able to support binding to the 50 S subunit, presumably due to the stimulation of GTP hydrolysis. These results support a model in which RbgA promotes a late step in ribosome biogenesis and that one role of GTP hydrolysis is to stimulate dissociation of RbgA from the ribosome.
Introduction
Ribosome assembly is a complex tightly regulated process that involves the coordinated assembly of three RNA molecules and >50 proteins (1, 2). Although >150 accessory proteins required for the assembly of ribosomes in eukaryotes have been identified (3), relatively few proteins dedicated to the assembly of ribosomes have been discovered in bacteria (4–6). Several ribosome-associated GTPases, such as RbgA (YlqF), Era, YqeH, YphC (EngA), CgtEA, YloQ (YjeQ and RsgA), and YsxC, have been implicated in the assembly of either the 50 S or 30 S ribosomal subunits in bacteria (6–13), however, their precise functions in ribosome assembly have remained elusive. The ribosome-associated GTPase RbgA is required for a late maturation step of the 50 S subunit in Bacillus subtilis (8, 10), and its homologs have been shown to be required for the assembly of the large ribosomal subunit in eukaryotes (14–17).
Depletion of RbgA in B. subtilis stalls 50 S subunit assembly, resulting in the accumulation of a large ribosomal intermediate that migrates more slowly (45 S) through a sucrose gradient and lacks three ribosomal proteins, L16, L27, and L36 (8, 10, 11). RbgA can interact with the 45 S intermediate and 50 S subunits, but the latter interaction was observed only in the presence of a non-hydrolyzable analog of GTP (8, 10, 11). It was also observed that the intact 50 S subunit stimulates the GTPase activity of RbgA (8). These observations led to the proposal of a model in which RbgA promotes a late step in ribosome assembly in which GTPase activation of RbgA occurs upon correct assembly of the 50 S subunit, followed by dissociation of RbgA from the subunit (8, 10). In this way, RbgA would serve as a checkpoint to ensure proper formation of the 50 S subunit. However, it was later reported that the GTPase activity of RbgA is maximally stimulated by the 45 S intermediate (referred to as pre-50 S in this model) and “free” 50 S subunits, but not by mature 50 S subunits (those isolated by dissociation of subunits from 70 S ribosomes) (18). These additional results led to a revised model in which RbgA promotes a GTPase-dependent structural rearrangement of the 45 S complex, which then allows L16 and L27 to subsequently bind. GDP-bound RbgA remains associated with the ribosome until some undetermined signal triggers the release of RbgA (18). The later model posits that a structural difference exists between free 50 S and mature 50 S subunits to explain the differential activation of RbgA by free 50 S subunits.
To address these two conflicting models, we undertook a more thorough biochemical analysis of RbgA and explored its interaction with the ribosome in details. Our results demonstrate that the GTPase activity of RbgA was maximally stimulated by both mature and free 50 S subunits, by >10 times over the stimulation observed with the 45 S intermediate. Interaction assays of RbgA with the different ribosomal subunits showed that GDP-bound RbgA did not stably associate with the ribosome and suggested that the GTPase activity of RbgA promoted dissociation from the ribosome. We discuss our findings in the context of the two conflicting models and further clarify the role of GTPase activity in RbgA function during ribosome assembly.
EXPERIMENTAL PROCEDURES
Growth Conditions and Strain Construction
All experiments were performed at 37 °C in LB medium. When necessary, antibiotics were added at the following concentrations: 5 μg/ml chloramphenicol (Sigma) and 100 μg/ml ampicillin. All B. subtilis strains used in this study were derived from the wild-type strain JH642 (RB247). B. subtilis RB301 (Pspank-rbgA) and RB418 (Pspank-infB) strains were constructed as described previously (10). RB301 and RB418 accumulate 45 S and 50 S subunits, respectively, when grown in the absence of isopropyl β-d-thiogalactopyranoside (IPTG)2 and were used to purify 45 S and free 50 S ribosomal subunits. Escherichia coli BL21(DE3) cells transformed with plasmids containing full-length rbgA placed under the control of the T7 promoter were used to overexpress RbgA proteins (10).
Purification of RbgA Proteins
RbgA protein with a His6 tag at the C terminus was isolated as described previously (10). Briefly, E. coli BL21(DE3) cells transformed with plasmid containing full-length rbgA under the control of the IPTG-inducible T7 promoter (10) were grown to A600 = 0.5 at 37 °C in LB medium containing 100 μg/ml ampicillin and then induced by the addition of 1 mm IPTG. The cells were harvested by centrifugation after 3 h and resuspended in binding buffer (20 mm sodium phosphate (pH 7.5), 0.5 m NaCl, and 20 mm imidazole). The cells were lysed by three consecutive passes through a French press between 1400 and 1600 p.s.i. and then clarified by centrifugation at 16,000 × g for 20 min. RbgA-His6 was isolated from the cell lysate by affinity chromatography using a HisTrap HP column (nickel-nitrilotriacetic acid resin; GE Healthcare) in a Bio-Rad BioLogic LP chromatography system. The cell lysate was injected into the column, which was pre-equilibrated with binding buffer and then allowed to equilibrate for 5 min and washed with 5 column volumes of wash buffer (20 mm sodium phosphate (pH 7.5), 0.5 m NaCl, and 60 mm imidazole), followed by step elution with binding buffer containing 250 mm imidazole. Elution was monitored by UV absorption at 280 nm, and RbgA-containing fractions were verified by SDS-PAGE, concentrated, and desalted by exchanging the buffer to desalting buffer (50 mm Tris-HCl (pH 7.5), 750 mm KCl, 5 mm MgCl2, 20 mm imidazole, 2 mm DTT, and 10% glycerol) and then to storage buffer (50 mm Tris-HCl (pH 7.5), 750 mm KCl, 5 mm MgCl2, 2 mm DTT, and 10% glycerol). The purity of isolated RbgA was verified by SDS-PAGE before storage at −20 °C. K59A, P129R, S134A, and F180A mutations were introduced into rbgA using a QuikChange IIXL kit (Stratagene).
Preparation of Ribosomal Subunits
Mature 50 S, free 50 S, and 45 S large ribosomal subunits were prepared by sucrose density centrifugation (19, 20). Free 50 S and 45 S complexes were isolated from lysates of RB418 and RB301 cells, respectively. Mature 50 S subunits were isolated by subjecting the RB247 lysate to low Mg2+ buffer (10 mm Tris-HCl (pH 7.6), 1 mm MgCl2, and 50 mm NH4Cl) thereby dissociating the 70 S ribosome into 50 S and 30 S subunits. The cells were grown to A600 = 0.5 at 37 °C in LB medium with or without IPTG. RB301 and RB418 cells were grown without IPTG for several generations to deplete the cells of RbgA and initiation factor 2, respectively, until a doubling time of ∼150 min was reached. Chloramphenicol was added to a final concentration of 100 μg/ml 5 min prior to harvesting. Cells were harvested by centrifugation at 5000 × g for 10 min and resuspended in lysis buffer (10 mm Tris-HCl (pH 7.5), 60 mm KCl, 10 mm MgCl2, 0.5% Tween 20, 1 mm DTT, 1× Complete EDTA-free protease inhibitors (Roche Applied Science), and 10 units/ml RNase-free DNase (Roche Applied Science)). Cells were lysed by three consecutive passes through a French press set at 1400–1600 p.s.i. and then clarified by centrifugation at 16,000 × g for 20 min. Clarified cell lysates were loaded on top of 10–25% sucrose density gradients equilibrated in buffer A and centrifuged using a SureSpin 630 rotor (Sorvall) for 4.5 h at 30,000 rpm. Gradients were then fractionated in a Bio-Rad BioLogic LP chromatography system by monitoring UV absorbance at 254 nm. Fractions corresponding to the ribosomal subunits of interest were pooled, concentrated using 100-kDa cutoff column (Millipore), and stored in buffer B (10 mm Tris-HCl (pH 7.6), 10 mm MgCl2, 60 mm KCl, and 1 mm DTT) at −80 °C. To purify mature 50 S subunits, RB247 cells were grown in LB medium at 37 °C to A600 = 0.5, harvested, and resuspended in lysis buffer containing only 1 mm Mg2+. The lysate was incubated on ice for 30 min and then centrifuged for 14 h at 20,600 rpm through a 25–40% sucrose gradient prepared in buffer A containing 1 mm Mg2+ (10 mm Tris-HCl (pH 7.6), 1 mm MgCl2, and 50 mm NH4Cl). 50 S subunit peak fractions were pooled, concentrated, and stored in buffer B.
Characterization of GTPase Activity of RbgA
The GTP hydrolysis activity of RbgA was determined by incubating RbgA with GTP for 30 min and then measuring the released free phosphate by the malachite green/ammonium molybdate colorimetric assay (BioAssays Systems) (21). Intrinsic GTPase activity was assayed in triplicate at a constant RbgA protein concentration of 2 μm and a range of GTP concentrations (8–650 μm) (USB Corp.). GTPase reactions was started by the addition of protein to GTP solutions at 37 °C in reaction buffer (50 mm Tris-HCl (pH 7.5), 200 mm KCl, 5 mm MgCl2, and 1 mm DTT) for 30 min and terminated by the addition of malachite reagent. We determined that, under these conditions, GTP hydrolysis was in the linear range of the assay and that <10% of the substrate was consumed. Released phosphate was detected by monitoring color formation at 620 nm using a 96-well plate reader (Tecan SunriseTM). Experiments were repeated a minimum of three times, and values for Km and kcat were calculated by fitting the data to the Michaelis-Menten equation with nonlinear regression curve fitting using GraphPad Prism (version 5.0, GraphPad Software Inc.).
Stimulation of GTPase Activity of RbgA by Ribosomal Subunits
To probe the effect of ribosomal subunits on the GTPase activity of RbgA, purified ribosomal subunits (mature 50 S subunits from dissociated 70 S subunits, free 50 S subunits from initiation factor 2-depleted cells, and 45 S subunits from RbgA-depleted cells) at 100 nm were individually incubated with 100 nm RbgA for 15 min at 37 °C in reaction buffer and then added to 8–650 μm GTP to start the reaction. We predetermined that, under these conditions, the values were in the linear range of the assay. The activity of the RbgA-subunit complex was determined by standard malachite green assay as described above for RbgA proteins alone. Three biological replicates (independent RbgA and ribosome preparations) were performed, each with three technical replicates. 50 S subunits isolated from dissociated 70 S subunits, free 50 S subunits isolated from wild-type profiles and 50 S subunits isolated from initiation factor 2-depleted cells behaved similarly in GTPase assays.
Importance of K+ for GTPase Activity of RbgA
To test the GTPase activity in the presence of K+, the protein was purified as described above and stored in storage buffer. To test the effects of Na+, the protein was exchanged and stored in NaCl buffer (50 mm Tris-HCl (pH 7.5), 750 mm NaCl, 5 mm MgCl2, 2 mm DTT, and 10% glycerol). The intrinsic activity of RbgA was assayed by incubation of 2 μm RbgA with 200 μm GTP for 15 min at 37 °C. Stimulation of GTPase activity was tested by incubation of 100 nm free 50 S subunits with 100 nm RbgA and 200 μm GTP for 15 min at 37 °C. The activity was determined by standard malachite green assay as described above.
Homology Modeling of K-loop of RbgA
Homology modeling of the K-loop of RbgA was carried out with MODELLER (22) using MnmE as a template. For the remainder of RbgA, the existing crystal structure was used (PDB code 1puj). A total of 20 initial models were constructed and the best model was chosen based on the lowest energy (molpdf) and the lowest discrete objective protein energy (DOPE) function. A ligand-based superimposition of RbgA (Protein Data Bank code 1puj) and MnmE (code 2gj8) was carried out using the LigAlign script (23) in PyMOL (24). The figure was generated using Chimera (25).
Interaction between RbgA and Ribosomal Particles
RbgA protein and ribosomal particles were purified as described above. The RbgA subunit binding assay was performed as described previously (8). The protocol was modified, however, to include a 15-min incubation with high salt buffer C (10 mm Tris-HCl (pH 7.5), 60 mm KCl, 500 mm NH4Cl, 10 mm MgCl2, and 1 mm DTT) to further test the specificity of RbgA-subunit interaction. Briefly, 60 pmol of RbgA was preincubated with different guanine nucleotides at 1.5 mm for 15 min, followed by the addition of 10 pmol of purified subunits. The binding was allowed to proceed for 15 min at 37 °C, followed by centrifugation once through Microcon 100 columns (Millipore) with a cutoff of 100 kDa. The RbgA-subunit complexes were washed once with buffer B and twice with buffer C before elution. Ribosome-bound RbgA was detected by separating the complexes on NuPAGE 12% BisTris/SDS gels (Invitrogen), followed by Western blot analysis using rabbit anti-RbgA and HRP-conjugated goat anti-rabbit polyclonal antibodies. RbgA-specific bands were visualized with a Western Lightning chemiluminescent detection system (PerkinElmer Life Sciences).
RESULTS
RbgA Has Low Intrinsic GTPase Activity
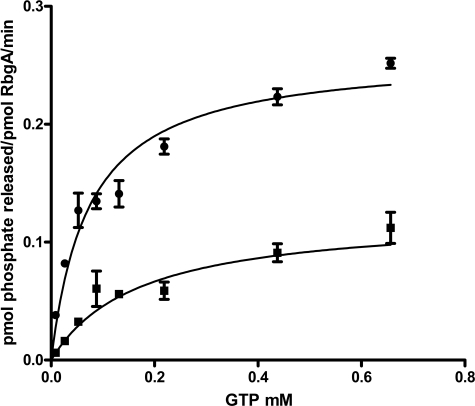
Most ribosome-associated GTPases have low intrinsic GTPase activity in the absence of an effector protein or molecule. To characterize the intrinsic GTPase activity of RbgA, we purified RbgA-His6 and measured GTP hydrolysis using a malachite green assay. We have shown previously that the C-terminal fusion of six histidine residues to RbgA yields a functional protein that can support wild-type growth of B. subtilis as well as interaction with the ribosome (10). We performed a steady-state kinetic analysis of RbgA GTPase activity using the RbgA-His6 protein (Fig. 1). Wild-type RbgA had a kcat of 14 h−1, a Km of 90 μm, and a kcat/Km of 43 m−1 s−1 (Table 1). To confirm that the low rate of hydrolysis observed was due to RbgA and not a contaminant, we created three mutants with important residues altered in the P-loop (P129R and S134A) or in the G4 region that specifies binding for guanine nucleotides (K59A) and tested their ability to hydrolyze GTP (Fig. 1 and supplemental Table 1). All three mutants displayed lower GTPase activity and a reduced kcat/Km, indicating that the GTPase activity observed for wild-type RbgA was not due to a contaminant.
FIGURE 1.
Kinetic analysis of GTP hydrolysis rates of RbgA proteins. GTP hydrolysis rates of RbgA proteins were determined by monitoring the release of free phosphate using the malachite green-ammonium molybdate colorimetric assay as described “Experimental Procedures.” Reactions were carried out under initial rate conditions (<10% of substrate consumed). Values of kcat and Km were determined by fitting the Michaelis-Menten equation using nonlinear regression algorithms provided by the GraphPad Prism software. Representative GTP hydrolysis curves for wild-type RbgA (●) and the P-loop variant S134A (■) are shown. Error bars represent S.D. of three technical replicates.
TABLE 1.
Kinetic parameters of RbgA in presence and absence of ribosomal subunits
The intrinsic activity of RbgA was assayed by incubation of 2 μm RbgA with various concentrations of GTP for 30 min at 37 °C. Stimulation of RbgA GTPase activity by ribosomes was assessed by determining the GTP hydrolysis rates of RbgA in the presence of various purified ribosomal particles. 100 nm purified ribosome was incubated with 100 nm RbgA for 15 min at 37 °C in the presence of various concentrations of GTP and assayed as described under “Experimental Procedures.” S.D. values of three biological replicates are presented.
| Ribosome | kcat | Km | kcat/Km |
|---|---|---|---|
| h−1 | μm | m−1s−1 | |
| None | 14 ± 2 | 90 ± 13 | 43 |
| Free 50 S subunit | 755 ± 207 | 32 ± 6 | 6533 |
| Mature 50 S subunit | 807 ± 158 | 62 ± 16 | 3616 |
| 45 S intermediate | 69 ± 16 | 4 ± 3 | 4791 |
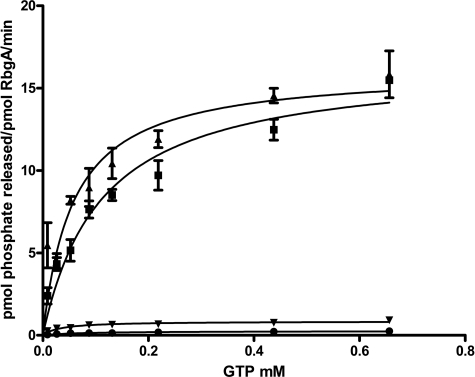
GTPase Activity of RbgA Is Maximally Stimulated by Mature or Free 50 S Ribosomal Subunits
To better understand the role of GTPase activity in the function of RbgA, we characterized GTP hydrolysis of RbgA in the presence of 50 S subunits and 45 S intermediates. We tested three types of large ribosomal subunits: mature 50 S subunits isolated by dissociating subunits from 70 S ribosomes, free 50 S subunits isolated by depleting initiation factor 2, and 45 S intermediates purified from RbgA-depleted cells. Previous work had indicated that 45 S intermediates and free 50 S subunits had higher stimulatory activity than mature 50 S subunits (18). Ribosomal subunits were mixed with RbgA in a ratio of 1:1, and the GTPase activity of RbgA was monitored using a malachite green assay. Both mature and free 50 S subunits were capable of stimulating the GTPase activity of RbgA by ∼55-fold (Fig. 2 and Table 1). In contrast, the 45 S intermediate isolated from RbgA-depleted cells showed a markedly reduced ability to stimulate the GTPase activity of RbgA (5-fold) over intrinsic GTPase activity. Michaelis-Menten analysis of the GTPase activity of RbgA indicated that the increase in GTPase activity with 50 S subunits was governed primarily by an increase in kcat (Table 1). Conversely, interaction with the 45 S intermediate decreased the Km of RbgA for GTP to 4 μm, indicating a tighter affinity for GTP in the presence of the 45 S complex, whereas the kcat was largely unaffected. Our results show that the GTPase activity of RbgA is maximally stimulated by the 50 S subunits (free and mature), in contrast to previously published results (18).
FIGURE 2.
Stimulation of GTPase activity of RbgA by ribosomal particles. Stimulation of RbgA GTPase activity by ribosomes was assessed by determining the GTP hydrolysis rates of RbgA in the presence of various purified ribosomal particles. 100 nm purified ribosome was incubated with 100 nm RbgA for 15 min at 37 °C in the presence of various concentrations of GTP and assayed as described under “Experimental Procedures.” The representative curves are of GTP hydrolysis rates determined based on the free phosphate produced after the reactions had proceeded for 15 min at 37 °C with reaction mixtures containing RbgA only (●), RbgA and the mature 50 S subunit (■), RbgA and the free 50 S subunit (▴), and RbgA and the 45 S intermediate (▾). Error bars represent S.D. of three technical replicates.
Potassium Is Required for Optimal GTPase Activity
Recent work has indicated that three translation-associated GTPases (MnmE, FeoB, and YqeH) utilize a unique mechanism for GTP hydrolysis in which a K+ ion participates in the activation of GTP hydrolysis (26–28). The structures of these GTPases demonstrate that a K+ ion functions as a GTPase-activating element, analogous to GTPase-activating proteins in eukaryotic GTPases.
This K+ ion occupies a position, usually occupied by an arginine residue, known as the “arginine finger” in GTPase-activating proteins of Ras-related GTPases (29). The positive charge provided by arginine or K+ at this position stabilizes the transition state by neutralizing negative charges that build up during the transition state. In GTPases that require K+, the K+ ion is held in position by a region of the GTP-binding domain that has been designated the “K-loop” (28). The K-loop is located upstream of switch I, interacts with K+ through the peptide backbone of amino acids, and shields the K+ ion from the bulk solvent. An additional side chain interaction is provided by a conserved asparagine residue situated within the P-loop. Inspection of the primary sequence of RbgA indicated a possible K-loop-type sequence; however, this part of the protein is not resolved in any of the crystal structures of RbgA to date. Therefore, we modeled this K-loop in RbgA using the transition state structure of MnmE (Fig. 3A) (28). Of 20 homology models generated, the most energetically favorable model shows a high degree of similarity to the K-loop of MnmE. Moreover, the asparagine residue from the P-loop, which makes a direct contact with the K+ ion in other K+-activated GTPases, is oriented similarly to the P-loop asparagine (GIPNVGKS) in the RbgA crystal structure (Fig. 3B). This suggests that RbgA may also utilize K+ in the activation of GTPase activity similarly to MnmE, FeoB, and YqeH.
FIGURE 3.
Superimposition of MnmE and homology model of RbgA. A, an MnmE and RbgA homology model was superimposed using the LigAlign script in PyMOL, which aligned the two structures based on their ligand positions, which is GDP in this case. The K-loops of MnmE (green) and RbgA (brown) occupy a similar position (boxed) around the bound potassium in the crystal structure. B, an enlarged view of the catalytic pocket with GDP and bound potassium is shown in which Asn-130 from the P-loop of RbgA (brown) coordinates the bound K+ ion (indicated by a purple sphere) in a similar fashion as Asn-226 from the P-loop of MnmE (green) (indicated by arrows).
Next, we investigated whether K+ is required for maximal stimulation of RbgA GTPase activity. The intrinsic GTPase activity and stimulation of RbgA GTPase were monitored in the presence of 250 mm NaCl or KCl. The intrinsic GTPase activity of RbgA was reduced by 133-fold when NaCl was substituted for KCl, demonstrating that K+ is required for optimal RbgA GTPase activity (Table 2). The stimulation of RbgA GTPase activity by the ribosome was also affected by Na+ but to a lesser degree, showing a 22-fold reduction in GTPase activity when co-incubated with 50 S subunits compared with K+ conditions. These results show that K+ is required for maximal GTPase activity.
TABLE 2.
GTPase activity of RbgA in presence of Na+ and K+ ions
The intrinsic activity of RbgA was assayed by incubation of 2 μm RbgA with 200 μm GTP for 15 min at 37 °C in the presence of 250 mm NaCl or KCl. Stimulation of GTPase activity by the ribosome was tested by incubation of 100 nm free 50 S subunits with 100 nm RbgA and 200 μm GTP for 15 min at 37 °C in the presence of 250 mm NaCl or KCl. The activity was determined by standard malachite green assay as described under “Experimental Procedures.” S.D. values for three biological replicates are presented.
| Intrinsic GTPase activity | Stimulation of GTPase activity in presence of 50 S subunit | |
|---|---|---|
| pmol phosphate/pmol RbgA/min | ||
| RbgA + 250 mm KCl | 0.26 ± 0.02 | 13.8 ± 0.85 |
| RbgA + 250 mm NaCl | <0.003 | 0.78 ± 0.1 |
RbgA Interacts with 50 S Ribosomes and 45 S Intermediates in Nucleotide-specific Manner
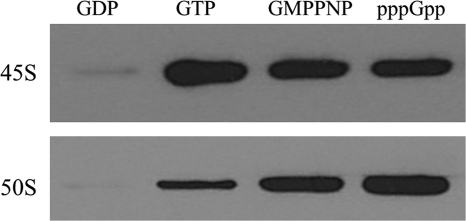
Previous work showed that RbgA interacts with the large ribosomal subunit when incubated with a non-hydrolyzable analog of GTP (8). To explore the interaction of RbgA with the subunits in detail, the interaction of RbgA with both purified 50 S subunits and the 45 S intermediate was characterized in the presence of GTP, GDP, and GMPPNP. Saturating levels of guanine nucleotides were used to ensure that all RbgA molecules were bound to nucleotide. 45 S intermediates or 50 S subunits were incubated with RbgA for 15 min at 37 °C and spun through a 100-kDa cutoff Microcon filter to remove unbound RbgA. RbgA bound to the subunits was retained on top of the filter and was detected using RbgA-specific polyclonal antibodies. We found that RbgA associated most stably with the 45 S subunit in the GTP- and GMPPNP-bound forms and that binding was greatly reduced in the presence of GDP (Fig. 4). In the presence of the 50 S subunit, RbgA binding was maximally enhanced in the presence of GMPPNP but was greatly reduced in the presence of GTP and GDP. The difference between GTP and GMPPNP is likely due to GTP hydrolysis in the presence of the 50 S ribosome, resulting in dissociation from the ribosome. We also tested if the alarmone pppGpp affects RbgA interaction with the ribosome. We found that the addition of pppGpp enhanced the interaction of RbgA with both the 45 S intermediate and 50 S subunits.
FIGURE 4.
Interaction between RbgA and ribosome in presence of different guanine nucleotides. Binding of RbgA to purified ribosomal subunits was tested by an in vitro binding assay in which 60 pmol of RbgA was preincubated with 1.5 mm GDP, GTP, GMPPNP, or pppGpp before the addition of 10 pmol of purified 45 S or 50 S ribosomal subunits. The mixtures were incubated for an additional 15 min at 37 °C, and free RbgA was filtered off by centrifugation through a 100-kDa cutoff Microcon column. The columns were washed three times, first with buffer B and then twice with buffer C (high salt buffer). RbgA-ribosome complexes were eluted, and bound RbgA was detected by immunoblotting using anti-RbgA antibody.
DISCUSSION
Previous genetic and biochemical studies aimed at elucidating the role of RbgA in large ribosomal subunit assembly have yielded two conflicting models. One model posits that RbgA couples a final maturation step of the 50 S subunit with the activation of GTP hydrolysis, which signals RbgA to leave the ribosome (8, 10). This maturation step involves the incorporation of ribosomal proteins L16, L27, and L36. However, a revised model was proposed suggesting that RbgA uses GTP hydrolysis to induce a conformational change in the 45 S subunit that is independent of the incorporation of L16, L27, and L36 (18). The revised model is based upon the finding that the 45 S subunit can maximally stimulate RbgA GTPase activity in vitro, although one major complication of the model is that free 50 S subunits, but not mature 50 S subunits, can also stimulate GTP hydrolysis to the same level as the 45 S intermediate. Because both of these models rely on limited biochemical evidence, we undertook a detailed analysis of RbgA interaction with the ribosome to clarify the role of GTP hydrolysis in RbgA function.
Several lines of evidence presented here support the model in which RbgA utilizes GTPase activity to dissociate from the ribosome. First, GTPase stimulation by 50 S subunits (free or mature) was 10 times more than that observed with 45 S intermediates. Second, RbgA interaction with 50 S subunits was nucleotide-dependent, with reduced levels of binding observed in the presence of GTP and GDP compared with the non-hydrolyzable analog GMPPNP. In contrast, the 45 S intermediate displayed similar binding in the presence of GTP and GMPPNP, with greatly reduced binding in the presence of GDP. Decreased binding of RbgA to the 50 S subunit in the presence of GTP, but not GMPPNP, is consistent with GTP hydrolysis governing dissociation of RbgA from the ribosome. Third, steady-state kinetic analysis of RbgA in the presence of the different ribosomal subunits showed that the kcat of RbgA was dramatically increased only in the presence of the 50 S subunit. This indicates that the 50 S subunit, but not the 45 S intermediate, serves a GTPase-activating protein-like function for RbgA. Additionally, Km was decreased only in the presence of the 45 S intermediate, but not the 50 S subunit, which suggests a cooperative binding of the 45 S subunit and GTP to RbgA. These data support a model in which the GTPase activity of RbgA is required to dissociate RbgA from the 50 S subunit. It is possible either that RbgA serves to monitor correct ribosome formation with its GTPase activity or that it couples the hydrolysis of GTP to a conformational change in the subunit while subsequently dissociating from the subunit.
An important question to address is why we observed maximal stimulation of RbgA GTPase activity in the presence of 50 S subunits (free or mature), whereas previous work showed maximal stimulation with the 45 S intermediate and free 50 S subunits, but not mature subunits (18). Our steady-state kinetic analysis of RbgA indicates a potential answer to this question. The Km of RbgA in the absence of the ribosome is ∼90 μm, and given the slow forward reaction in the absence or presence of the ribosome, the Km is a good estimate for the KD of RbgA for GTP. In the presence of 50 S subunits, the Km is only marginally reduced (62 μm for mature 50 S subunits and 32 μm for free 50 S subunits). However, in the presence of the 45 S intermediate, the Km of RbgA is reduced to 4 μm, indicating that interaction with the 45 S intermediate fosters tighter association with GTP. In previous work, GTPase assays were performed using GTP at a concentration of 10 μm, well below the Km of RbgA for GTP in the presence of the 50 S subunit (18). These assay conditions would result in most of the RbgA protein being unbound to GTP and should underestimate the activity of RbgA in the presence of 50 S subunits. Because the GTP concentration in the cell is in the range of 0.5–5 mm (30, 31), our assay likely reflects a more physiologically relevant condition. Finally, our results demonstrate that RbgA is a member of a growing family of GTPases that utilize K+ as part of the mechanism of GTP hydrolysis. Previous work on the biochemistry of RbgA was performed with NH4Cl (18). Although NH4+ supports hydrolysis of GTP in YqeH, it is reduced by 2-fold in its activity, indicating that NH4+ is not sufficient to replace K+ for full activity (26). Future experiments on the biochemistry of ribosome-associated GTPases thus warrant investigation of K+ as a possible GTPase-activating element.
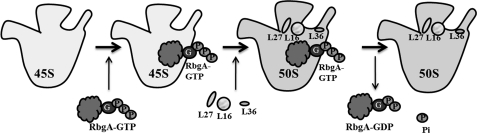
The results presented here support a model in which the guanine nucleotide state of RbgA regulates the association of RbgA with the ribosome (Fig. 5). GDP significantly inhibits RbgA interaction with either the 45 S intermediate or the 50 S subunit, and the interaction with the 50 S subunit is stabilized only in the presence of non-hydrolyzable analogs of GTP. These results indicate that GTP hydrolysis promotes RbgA dissociation from the 50 S subunit, similar to other translation factor GTPases such as elongation factor Tu. Elongation factor Tu couples proper accommodation of the aminoacyl-tRNA to GTPase activity, resulting in release of elongation factor Tu from the ribosome (32). We propose that RbgA promotes a late step in large subunit assembly that allows the stable incorporation of ribosomal proteins L16, L27, and L36. Although the nature of this rearrangement is still unknown, once properly achieved, we propose that RbgA “senses” the correct assembly of the ribosome, which in turn promotes GTP hydrolysis and RbgA dissociation. Alternatively, RbgA could act directly on the 45 S intermediate independent of incorporation of L16, L27, or L36. Finally, we previously proposed that regulating a late assembly process would allow RbgA to act as a checkpoint governing the release of active 50 S subunits into the translation active pool of ribosomes in the cell. Previous work with BipA has also shown that pppGpp can regulate GTPase association with ribosomal subunits (33). It is intriguing that the alarmone pppGpp enhances the association of RbgA with both 45 S and 50 S subunits, given the fact that RbgA likely binds to the subunit interface and would block association with the 30 S subunit. Under times of translational stress, pppGpp could block the additional maturation of 45 S intermediates and prevent newly formed 50 S subunits from participating in translation.
FIGURE 5.
Proposed model for role of RbgA in 50 S subunit maturation. RbgA interacts with the 45 S intermediate in the GTP-bound form and introduces conformational changes that further facilitate the binding of late ribosomal proteins such as L16, L27, and L36 (depicted by ovals). Binding of these proteins promotes maturation of the 45 S intermediate (white) to a mature 50 S subunit (gray). Along with the complete maturation of the 50 S subunit, GTP at RbgA is hydrolyzed to GDP, and this GDP-bound RbgA and inorganic phosphate leave the mature 50 S subunit, which is now ready to take part in translation. It is also possible that RbgA completes maturation of a late step that does not end in the final maturation of the 50 S subunit.
Supplementary Material
Acknowledgments
We thank Marci Baranski and Loren Palmeri for technical assistance and Jade Wang (Baylor College of Medicine) for providing pppGpp.
This work was supported by National Science Foundation CAREER Award 0643565 (to R. A. B.).

This article contains supplemental Table 1.
- IPTG
- isopropyl β-d-thiogalactopyranoside
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- GMPPNP
- guanosine 5′-(β,γ-imido)triphosphate
- pppGpp
- guanosine pentaphosphate.
REFERENCES
- 1. Nierhaus K. H. (1991) The assembly of prokaryotic ribosomes. Biochimie 73, 739–755 [DOI] [PubMed] [Google Scholar]
- 2. Nomura M., Erdmann V. A. (1970) Reconstitution of 50 S ribosomal subunits from dissociated molecular components. Nature 228, 744–748 [DOI] [PubMed] [Google Scholar]
- 3. Dez C., Tollervey D. (2004) Ribosome synthesis meets the cell cycle. Curr. Opin. Microbiol. 7, 631–637 [DOI] [PubMed] [Google Scholar]
- 4. Culver G. M. (2003) Assembly of the 30 S ribosomal subunit. Biopolymers 68, 234–249 [DOI] [PubMed] [Google Scholar]
- 5. Wilson D. N., Nierhaus K. H. (2007) The weird and wonderful world of bacterial ribosome regulation. Crit. Rev. Biochem. Mol. Biol. 42, 187–219 [DOI] [PubMed] [Google Scholar]
- 6. Britton R. A. (2009) Role of GTPases in bacterial ribosome assembly. Annu. Rev. Microbiol. 63, 155–176 [DOI] [PubMed] [Google Scholar]
- 7. Gollop N., March P. E. (1991) A GTP-binding protein (Era) has an essential role in growth rate and cell cycle control in Escherichia coli. J. Bacteriol. 173, 2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsuo Y., Morimoto T., Kuwano M., Loh P. C., Oshima T., Ogasawara N. (2006) The GTP-binding protein YlqF participates in the late step of 50 S ribosomal subunit assembly in Bacillus subtilis. J. Biol. Chem. 281, 8110–8117 [DOI] [PubMed] [Google Scholar]
- 9. Schaefer L., Uicker W. C., Wicker-Planquart C., Foucher A. E., Jault J. M., Britton R. A. (2006) Multiple GTPases participate in the assembly of the large subunit in Bacillus subtilis. J. Bacteriol. 188, 8252–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uicker W. C., Schaefer L., Britton R. A. (2006) The essential GTPase RbgA (YlqF) is required for 50 S ribosome assembly in Bacillus subtilis. Mol. Microbiol. 59, 528–540 [DOI] [PubMed] [Google Scholar]
- 11. Uicker W. C., Schaefer L., Koenigsknecht M., Britton R. A. (2007) The essential GTPase YqeH is required for proper ribosome assembly in Bacillus subtilis. J. Bacteriol. 189, 2926–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang M., Datta K., Walker A., Strahler J., Bagamasbad P., Andrews P. C., Maddock J. R. (2006) The Escherichia coli GTPase CgtAE is involved in late steps of large ribosome assembly. J. Bacteriol. 188, 6757–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell T. L., Daigle D. M., Brown E. D. (2005) Characterization of the Bacillus subtilis GTPase YloQ and its role in ribosome function. Biochem. J. 389, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bassler J., Grandi P., Gadal O., Lessmann T., Petfalski E., Tollervey D., Lechner J., Hurt E. (2001) Identification of a 60 S preribosomal particle that is closely linked to nuclear export. Mol. Cell 8, 517–529 [DOI] [PubMed] [Google Scholar]
- 15. Jensen B. C., Wang Q., Kifer C. T., Parsons M. (2003) The NOG1 GTP-binding protein is required for biogenesis of the 60 S ribosomal subunit. J. Biol. Chem. 278, 32204–32211 [DOI] [PubMed] [Google Scholar]
- 16. Kallstrom G., Hedges J., Johnson A. (2003) The putative GTPases Nog1p and Lsg1p are required for 60 S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively. Mol. Cell. Biol. 23, 4344–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saveanu C., Bienvenu D., Namane A., Gleizes P. E., Gas N., Jacquier A., Fromont-Racine M. (2001) Nog2p, a putative GTPase associated with pre-60 S subunits and required for late 60 S maturation steps. EMBO J. 20, 6475–6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuo Y., Oshima T., Loh P. C., Morimoto T., Ogasawara N. (2007) Isolation and characterization of a dominant-negative mutant of Bacillus subtilis GTP-binding protein, YlqF, essential for biogenesis and maintenance of the 50 S ribosomal subunit. J. Biol. Chem. 282, 25270–25277 [DOI] [PubMed] [Google Scholar]
- 19. Charollais J., Pflieger D., Vinh J., Dreyfus M., Iost I. (2003) The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48, 1253–1265 [DOI] [PubMed] [Google Scholar]
- 20. Lin B., Thayer D. A., Maddock J. R. (2004) The Caulobacter crescentus CgtAC protein cosediments with the free 50 S ribosomal subunit. J. Bacteriol. 186, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100, 95–97 [DOI] [PubMed] [Google Scholar]
- 22. Sali A., Potterton L., Yuan F., van Vlijmen H., Karplus M. (1995) Evaluation of comparative protein modeling by MODELLER. Proteins 23, 318–326 [DOI] [PubMed] [Google Scholar]
- 23. Heifets A., Lilien R. H. (2010) LigAlign: flexible ligand-based active site alignment and analysis. J. Mol. Graph. Model. 29, 93–101 [DOI] [PubMed] [Google Scholar]
- 24. DeLano W. L. (2002) The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, San Diego, CA [Google Scholar]
- 25. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 26. Anand B., Surana P., Prakash B. (2010) Deciphering the catalytic machinery in 30 S ribosome assembly GTPase YqeH. PLoS ONE 5, e9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ash M. R., Maher M. J., Guss J. M., Jormakka M. (2011) The initiation of GTP hydrolysis by the G-domain of FeoB: insights from a transition-state complex structure. PLoS ONE 6, e23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scrima A., Wittinghofer A. (2006) Dimerization-dependent GTPase reaction of MnmE: how potassium acts as GTPase-activating element. EMBO J. 25, 2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheffzek K., Ahmadian M. R., Kabsch W., Wiesmüller L., Lautwein A., Schmitz F., Wittinghofer A. (1997) The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 [DOI] [PubMed] [Google Scholar]
- 30. Neidhardt F. C., Ingraham J. L., Schaechter M. (1990) Physiology of the Bacterial Cell: A Molecular Approach, Sinauer Associates Inc., Sunderland, MA [Google Scholar]
- 31. Lopez J. M., Marks C. L., Freese E. (1979) The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim. Biophys. Acta 587, 238–252 [DOI] [PubMed] [Google Scholar]
- 32. Schmeing T. M., Voorhees R. M., Kelley A. C., Gao Y. G., Murphy F. V., 4th, Weir J. R., Ramakrishnan V. (2009) The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326, 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. deLivron M. A., Robinson V. L. (2008) Salmonella enterica serovar Typhimurium BipA exhibits two distinct ribosome-binding modes. J. Bacteriol. 190, 5944–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.