Background: The maturation process of IL-33 (IL-1F11) remains unclear.
Results: IL-33 ligand affinity column isolates neutrophil proteinase 3.
Conclusion: PR3 is an IL-33-processing enzyme.
Significance: PR3 has a dual function in IL-33 biological activity.
Keywords: Caspase, Cytokine, Inflammation, Interleukin, Signal Transduction
Abstract
IL-1 family ligand does not possess a typical hydrophobic signal peptide and needs a processing enzyme for maturation. The maturation process of IL-33 (IL-1F11), a new member of the IL-1 family ligand, remains unclear. Precursor IL-33 ligand affinity column isolates neutrophil proteinase 3 (PR3) from human urinary proteins. PR3 is a known IL-1 family ligand-processing enzyme for IL-1β (IL-1F2) and IL-18 (IL-1F4), including other inflammatory cytokines. We investigated PR3 in the maturation process of precursor IL-33 because we isolated urinary PR3 by using the precursor IL-33 ligand affinity column. PR3 converted inactive human and mouse precursor IL-33 proteins to biological active forms; however, the increase of PR3 incubation time abrogated IL-33 activities. Unlike caspase-1-cleaved precursor IL-18, PR3 cut precursor IL-33 and IL-18 at various sites and yielded multibands. The increased incubation period of PR3 abated mature IL-33 in a time-dependent manner. The result is consistent with the decreased bioactivity of IL-33 along with the increased PR3 incubation time. Six different human and mouse recombinant IL-33 proteins were expressed by the predicted consensus amino acid sequence of PR3 cleavage sites and tested for bioactivities. The human IL-33/p1 was highly active, but human IL-33/p2 and p3 proteins were inactive. Our results suggest the dual functions (activation/termination) of PR3 in IL-33 biological activity.
Introduction
IL-33 is a new member of the IL-1 family ligand and was originally discovered as a nuclear factor from high endothelial venules (1). IL-33 is considered to be critical in inducing Th2-type immune responses like immunity against nematodes and allergic diseases (2–6), mast cells (4, 7), basophils (8), and eosinophils (5, 9, 10). IL-33 also induces non-Th2 inflammatory cytokines such as TNFα, IL-1β, or IL-6 (11–13). It has also been suggested that the properties of IL-33 are cytokines or a nuclear transcription factor like IL-1β and high mobility group protein B1 (4, 5, 14–23).
ST2 (also known as IL-1RL1, DER4, Fit-1, or T1), which was originally discovered as an orphan receptor (24–28), is a ligand binding chain of IL-33 (6). IL-33 receptor complex for signaling is composed of the ligand chain ST2 and signal transducing chain IL-1 receptor accessory protein (IL-1RAcP) (2, 6, 29). This receptor complex activates downstream signaling molecules such as NF-κB and AP-1 through IL-1 receptor-associated kinase, TNF receptor-associated factor 6, and/or MAPKs (6). These cells produce inflammatory cytokines and chemokines, including IL-4, IL-5, IL-6, IL-13, and IL-8, by stimulation of IL-33 (5, 7, 9, 30). Recently, it has been reported that circulating CD34+ hematopoietic progenitors expressed ST2 and responded to IL-33 by releasing high levels of Th2-associated cytokines (31). Such results suggest potential roles of IL-33 in Th2-associated immune responses, and IL-33 seems to be closely related to allergic inflammatory diseases, including asthma and atopic dermatitis.
IL-33, similarly to IL-1β and IL-18, is produced as a precursor IL-33 molecule. This precursor IL-33 does not have a signal peptide to be secreted; it is released extracellularly as a mature protein after cleavage (6). It is known that inflammatory caspases are necessary for the cleavage of IL-1β and IL-18 (32, 33). Although it has been suggested that precursor IL-33 is processed by caspase-1 (6), the exact role of caspases in IL-33 biology still remains unclear (34, 35).
In this study, we identified and characterized a processing enzyme of precursor IL-33. PR3 converts inactive precursor IL-33 to active form, whereas a longer incubation period of PR3 abolishes IL-33 activity. This result suggests the contrasting functions (activation/termination) of PR3 in precursor IL-33 bioactivity.
MATERIALS AND METHODS
RT-PCR and Molecular Cloning for Escherichia coli Expression Vectors
Total RNA was isolated with TRI Reagent® (Sigma) from human PC-3 cells and mouse lung tissue. A pair of human IL-33 sense primers 5′-ACAGAATACTGAAAAATGAAGCC-3′ and reverse primer 5′-CTTCTCCAGTGGTAGCATTTG-3′, mouse IL-33 sense primers 5′-ATGAGACCTAGAATGAAGTATTC-3′ and reverse primer 5′-GCACAGGCGTTTTACTGCATT-3′, and β-actin sense primers 5′-ACCAACTGGGACGACATGGA-3′ and reverse primer 5′-GTGATGACCTGGCCGTCAGG-3′ was used for the RT-PCR. Moloney murine leukemia virus-RT (Beams Bio, Korea) was used for converting 2 μg of total RNA to first strand cDNA, and then PCR was performed at 94 °C for 45 s, 70 °C for 2 min, and 59 °C for 1 min for 30 cycles.
For E. coli expression vector, the PCR product of human and mouse precursor IL-33 cDNA was ligated into T&A cloning vector (RBC, Taiwan). We designed the sense primer with an EcoRI (GAATTC) site and the reverse primer with a KpnI (GGTACC) site to transfer the amplified PCR product into pProEX/HTa (Invitrogen).
The insert of human precursor IL-33, IL-33/p2, and IL-33/p3 was amplified with the same sense primer (5′-GAACGAATTCATGAAGCCTAAAATGAAG-3′). The insert of human precursor IL-33 and IL-33/p1 was amplified with the same reverse primer (5′-GAACGGTACCCTAAGTTTCAGAGAGCTT-3′). The sense primer of human IL-33/p1 (5′-TAAAGAATTCACCTATTACAGAGTATCTT-3′) and the reverse primer of IL-33/p2 (5′-TATTGGTACCTTAGACAAAGAAGGCCTG-3′) and IL-33/p3 (5′-TATTGGTACCTTATATAAACACTCCAGG-3′) were used to amplify cDNA inserts.
For mouse precursor IL-33 and IL-33/p1, the same reverse primer (5′-TATTGGTACCTTAGATTTTCGAGAGCTT-3′) and the sense primer of mouse precursor IL-33 (5′-TAAAGAATTCATGAGACCTAGAATGAAG-3′) and IL-33/p1 (5′-TAAAGAATTCTCACTTTTAACACAGTCT-3′) were used to amplify cDNA inserts. The PCR product of a single band from each construct was digested with EcoRI and KpnI and then transferred into pProEX/HTa (Invitrogen) for recombinant protein expression in E. coli.
Isolation of Urinary IL-33 Interacting Molecule from Concentrated Human Urine
Human precursor IL-33 (3 mg) was immobilized by coupling to Affi-Gel-15 beads according to the manufacturer's instructions (Bio-Rad). Batches of 300 ml of crude urinary proteins concentrated 500-fold were passed over the precursor IL-33-bound beads at 4 °C. The column was washed with 500 ml of phosphate buffer containing 0.5 m sodium chloride, pH 8. Bound proteins were eluted in a 1-ml volume of solution containing 25 mm citric acid, pH 2.2, and then the eluted fraction was immediately neutralized with 2 m glycine.
Generation of Recombinant Protein
Six recombinant (human and mouse precursor IL-33, human and mouse IL-33/p1, and human IL-33/p2, IL-33/p3) proteins were expressed in E. coli Rosetta cells (Novagen, Madison, WI) and purified by a TALON affinity column (Invitrogen). The TALON affinity-purified recombinant proteins were subjected to high performance liquid chromatography (GE Healthcare) with C4 column (Grace Vydac, Hesperia, CA). The two-step purified recombinant proteins were tested for endotoxin level (below 0.25 EU per μg of recombinant protein) by using LAL method (Cape Cod, East Falmouth, MA) according to the manufacturer's instructions and then used for experiments.
Caspase-1 and PR3 Cleavage Test
Caspase-1 (10 units from Millipore, Temecula, CA) and the recombinant proteins of precursor human IL-33, mouse IL-33, and human IL-18 (500 ng) were incubated in 20 μl of a reaction buffer (25 mm K+HEPES, 1 mm DTT, 1 mm EDTA, 0.1% CHAPS, 10% sucrose, pH 7.5) at 37 °C for 30 min. After the reaction, the sample was subjected to 10% SDS-PAGE for silver staining.
For PR3 cleavage test with different IL-1 family ligands, commercial PR3 (100 ng from Athens Research & Technology) and the recombinant proteins of precursor human IL-33, mouse IL-33, and human IL-18 (500 ng) were incubated in 20 μl of a reaction buffer (50 mm Tris, pH 8) at room temperature. The PR3-cleaved samples were subjected to 10% SDS-PAGE for silver staining.
Western Blots
For the detection of PR3-cleaved human rIL3-33, the samples were loaded on 10% SDS-PAGE. Anti-human IL-33 polyclonal antibody was developed by immunizing three BALB/c mice (6 weeks old) with mature human IL-33 (Ser112–Thr270) protein (data not shown). Human IL-33 polyclonal antibody was used for detecting a mature size of IL-33, which was cleaved by PR3. In addition, an anti-human PR3 monoclonal antibody (36) was obtained from YbdYbiotech (Seoul, Korea) and used for detecting urinary PR3. Peroxidase-conjugated goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch) was used to develop the blots by using Supex (Neuronex, Korea) and LAS-4000 imaging device (Fujifilm, Japan).
For detecting the phosphorylation of signaling molecules (IRAK1, NF-κB, p38 MAPK, p44/42 MAPK, and JNK), HMC-1 and Raw 264.7 cells were stimulated with precursor and mature IL-33 at various time points. Cells were lysed with kinase lysis buffer (Cell Signaling Technology, Beverly, MA) and then subjected to 10% SDS-PAGE. The samples were transferred to nitrocellulose membranes. The membranes were blocked in 3% BSA/TBST (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were probed first with rabbit anti-phospho-IRAK1, mouse anti-phospho-NF-κB, rabbit anti-phospho-p38 MAPK, mouse anti-phospho-p44/42 MAPK, or rabbit anti-phospho-JNK (Cell Signaling Technology). The membranes were reprobed with rabbit anti-NF-κB, p38 MAPK (Santa Cruz Biotechnology), p44/42 MAPK, JNK, or IRAK1 (Cell Signaling Technology) for normalization of each protein and then normalized finally with goat anti-actin (Santa Cruz Biotechnology).
Cell Culture and Bioassay
Mouse macrophage Raw 264.7 cell line was obtained from American Type Culture Collection (ATCC) and maintained according to the instructions. HMC-1 cells were cultured in Iscove's modified Dulbecco's medium enriched with 10% FBS. For a blockade of ST2, a fresh medium (0.2 ml) containing various concentrations of rIL-33 and 0.5 μg of anti-ST2 (R&D Systems) was used. Human HMC-1 and mouse Raw 264.7 cells were treated with different stimuli, and the cell culture supernatant was harvested the next day for cytokine assay.
Measurement of Cytokine Level
Human IL-8, mouse TNFα, mouse MIP-2, and mouse IL-6 ELISA kits were obtained from R&D Systems. Cytokine levels were measured in culture supernatants by sandwich ELISA according to manufacturer's instructions.
Statistical Analysis
The data are expressed as means ± S.E. Statistical significance of differences were analyzed by unpaired, two-tailed Student's t test. Values of p < 0.05 were considered statistically significant.
RESULTS
Isolation of an IL-33 Interacting Molecule from Urinary Proteins
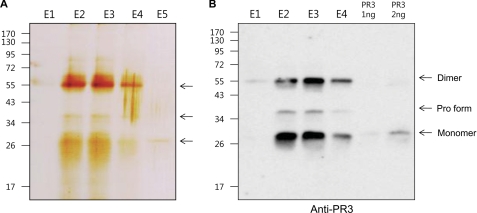
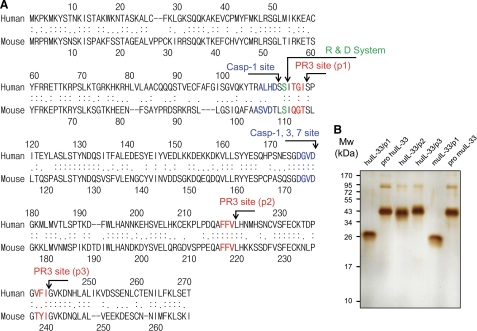
In an attempt to isolate a soluble IL-33 interacting molecule, concentrated urine was applied to a column composed of precursor IL-33 covalently immobilized to agarose beads. After extensive washing with PBS, interacting proteins were eluted at low pH citric acid solution. Aliquots of the various fractions were resolved by 10% SDS-PAGE, and the protein bands were visualized by silver staining. The broad bands corresponding to specific IL-33 interacting proteins, ∼56- and 28-kDa bands including the minor 36-kDa band, were detected mainly in fractions 2 and 3 (Fig. 1A). The 56- and 28-kDa protein bands in elution fraction 3 were excised and analyzed by mass spectrometer analysis. The result of mass spectrometer revealed that the bands were neutrophil proteinase 3 (PR3). We verified urinary PR3 in the eluted fractions with a monoclonal antibody specific to human PR3. The anti-human PR3 monoclonal antibody recognized the 56-, 36-, and 28-kDa urinary PR3 and commercial PR3 (Fig. 1B). The results suggest that 28 and 56 kDa are the monomer and dimer forms of mature PR3, respectively. The additional minor 36-kDa band could be the precursor PR3 because the anti-PR3 monoclonal antibody recognized the precursor PR3 in human monocyte cells (THP-1 and U937), and the molecular size of precursor PR3 is similar to the 36-kDa band (data not shown).
FIGURE 1.
Isolation of an IL-33 interacting molecule by using ligand affinity column. A, precursor IL-33 affinity chromatography column isolated urinary IL-33-interacting proteins and was visualized by silver-stained 10% SDS-PAGE. Four fractions were eluted from the IL-33 ligand affinity column. Molecular mass is indicated on the left. The arrows indicate the IL-33-binding proteins, 56-, 28-, and minor 36-kDa bands. B, mouse anti-human PR3 antibody was used for verifying urinary PR3 in the fractions from the IL-33 ligand affinity column. The molecular sizes of ∼56 (dimer form) and 28 kDa (monomer form), including the minor 36-kDa (precursor PR3) bands, were detected in the fractions. The data represent one of three independent experiments.
PR3 Converted Precursor IL-33 into Mature IL-33
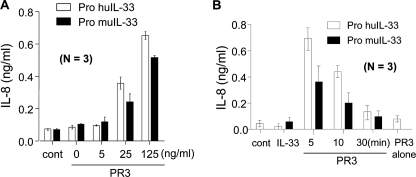
The biological activity of human and mouse precursor IL-33 was examined in the presence of PR3. The pretreatment of PR3 activated precursor IL-33 and induced IL-8 production by a dose-dependent manner, but precursor IL-33 alone did not induce IL-8 production (Fig. 2A). The time course of PR3 pretreatment revealed that PR3 induced the highest activity of IL-33 at 5 min, and its activity was decreased along with increasing incubation times (Fig. 2B). PR3 pretreatment for 30 min completely abolished IL-33 activity.
FIGURE 2.
PR3 enhancing precursor IL-33-induced cytokine production. A, PR3-preincubated precursor IL-33 induced IL-8 in a dose-dependent manner. PR3 (25 ng/ml) was preincubated for 5 min with various concentrations of precursor IL-33 as indicated on the bottom and then used for stimulating HMC-1 cells. B, precursor IL-33 (100 ng/ml) activity was gradually decreased along with increasing PR3 incubation times. The data represent one of three independent experiments. cont, control.
Examination of IL-33 Cleavage by PR3
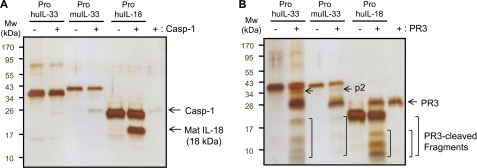
We tested the cleavage of human and mouse precursor IL-33 proteins with caspase-1 and PR3. Caspase-1 specifically cleaved precursor IL-18 resulting in the production of 18-kDa mature IL-18, which was active in the A549Rβ cell line (data not shown) (37), but precursor IL-33 proteins were not cleaved by caspase-1 (Fig. 3A). Next we examined the cleavage of precursor IL-33 proteins with PR3. As shown in Fig. 3B, PR3 cleaved the precursor IL-33 and produced multibands unlike caspase-1-cleaved IL-18 (Fig. 3A). PR3 also cleaved precursor IL-18 as multiband products in silver staining (Fig. 3B, 2nd lane from right).
FIGURE 3.
Silver staining of caspase-1 and PR3-cleaved precursor IL-33. A, process of precursor human IL-33 (250 ng/lane), mouse IL-33 (100 ng/lane), and IL-18 (500 ng/lane) was examined with caspase-1 (Casp-1) (10 units/lane). The preincubation of caspase-1 specifically cleaved precursor IL-18 and produced a single band of mature IL-18, molecular mass of 18 kDa, but both human and mouse precursor IL-33 were not affected. B, same amount of recombinant IL-1 family ligands was used for testing if these ligands were processed by PR3 (100 ng/lane). Unlike caspase-1, PR3 cleaved precursor IL-18 and produced multibands in the 2nd lane from right. The pattern of PR3-cleaved precursor IL-33 was very similar in both human and mouse. The data represent one of three independent experiments.
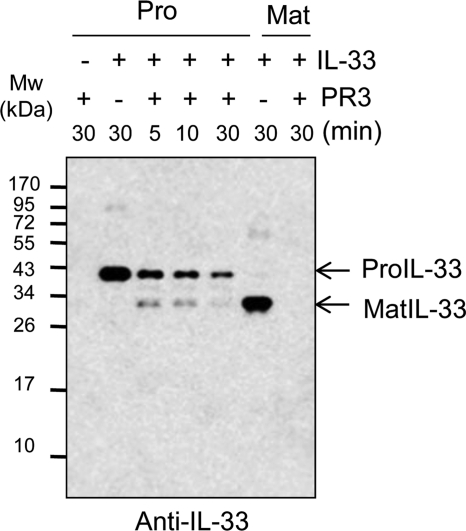
Time-dependent Reduction of a Mature Size IL-33 by PR3
IL-33 protein was incubated at different time points to examine whether the multiband products of the PR3 cleaved is a mature IL-33. We used the anti-IL-33 antibody to detect mature IL-33. The result of the Western blot exhibited that PR3-cleaved mature IL-33 was highest at 5 min and decreased gradually along with incubation time (Fig. 4). This result is consistent with bioactivity of the PR3-incubated precursor IL-33 in Fig. 2B. Mature rIL-33 (Ser112–Thr270) was completely degraded by PR3 after 30 min of incubation (Fig. 4, far right lane).
FIGURE 4.
Time-dependent reduction of a mature size of IL-33. Time course study of human precursor (Pro) IL-33 (100 ng/lane) cleavage was performed in the presence of PR3 (20 ng/lane). The precursor IL-33 was promptly reduced after 5 min of PR3 incubation. Western blot revealed that a mature (Mat) size of IL-33 was decreased by a time-dependent manner. The data represent one of three independent experiments.
Design and Expression of PR3-cleaved rIL-33 Proteins
We aligned the amino acid sequence of human and mouse IL-33 to predict PR3 cleavage sites because we failed to obtain a specific PR3 cleavage site by mass spectrometer analysis (data not shown) due to multiband productions in silver staining.
We predicted multiple PR3 cleavage sites by a previous report using a highly specific enzymatic activity testing tri-peptide library against proteinase 3 (38). A single PR3 cleavage site at the N terminus and two cleavage sites at the C terminus were predicted by the consensus sequence of the PR3 cleavage site. The predicted PR3 cleavage sites are indicated in red as p1, p2, and p3 (Fig. 5A). The previously reported caspase cleavage sites are shown in blue (Fig. 5A). We expressed six rIL-33 proteins as shown at the top of Fig. 5B. The purified rIL-33 proteins were subjected to 10% SDS-PAGE, and the result showed a single band of correct molecular size of each recombinant protein (Fig. 5B). The upper bands of precursor IL-33 and IL-33/p2–3 are dimer forms that were verified by Western blot (data not shown). Additional experiments tested the biological activity of mature human IL-33 (Ser112–Thr270) and mouse IL-33 (Ser109–Ile266) in the presence or absence of the N-terminal His tag. The N-terminal His tag did not affect the bioactivities of mature human and mouse rIL-33 (data not shown).
FIGURE 5.
Alignment of human and mouse IL-33, prediction of PR3 cleavage sites, and expression of six rIL-33 proteins. A, amino acid sequence of human (Met1–Thr270) and mouse (Met1–Ile266) was aligned to predict PR3 cleavage sites by consensus sequence. The previously reported caspase cleavage sites are indicated in blue. The predicted PR3 cleavage sites are marked in red. One cleavage site is at the N terminus, and two cleavage sites are at the C terminus. Mature IL-33 from R&D Systems is indicated in green. B, six different rIL-33 proteins were expressed in E. coli as indicated at the top. The human and mouse IL-33 proteins were purified by a Talon and HPLC and then subjected to 10% SDS-PAGE. The purity of each recombinant protein was visualized by silver staining. The data represent one of three independent experiments.
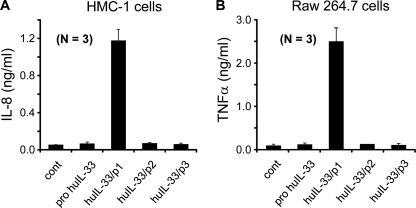
Biological Activity of rIL-33/p1 Proteins
Four human IL-33 proteins (Fig. 5B) were used to test the biological activities with human HMC-1 cells. IL-33/p1 (Ser117–Thr270) was highly active and induced IL-8 production, but precursor IL-33 (Met1–Thr270), huIL-33/p2 (Met1–Val220), and huIL-33/p3 (Met1–Ile240) were not active (Fig. 6A). We repeated cytokine assay with mouse Raw 264.7 cells, and mouse TNFα production was consistent with that of IL-8 (Fig. 6B). We did not produce the mouse form of IL-33/p2 and IL-p3 proteins because both huIL-33/p2 and huIL-33/p3 were not active (Fig. 6).
FIGURE 6.
Biological activities of precursor IL-33 and three new rIL-33 proteins from PR3-cleaved forms. A, biological activities of human IL-33 (20 ng/ml) proteins from three recombinant PR3-cleaved forms, including pro-IL-33 were examined with human HMC-1 (A) and Raw 264.7 (B) cells. Recombinant IL-33/p1 induced cytokines, but the recombinant protein of precursor IL-33, IL-33/p2, and IL-33/p3 were not active. The data represent one of three independent experiments. cont, control.
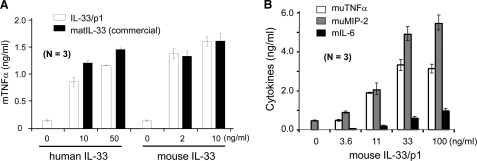
We continued to produce the large scale of human rIL-33/p1 (Ser117–Thr270) and mouse rIL-33/p1 (Ser114–Ile266) because human rIL-33/p1 was only active in the cytokine assay (Fig. 6). Human IL-33/p1 (Ser117–Thr270) has six amino acids less than the originally proposed mature human IL-33 (Ser111–Thr270) by caspase-1 cleavage (6). The biological activity of human rIL-33 (Ser117–Thr270) and mouse rIL-33/p1 (Ser114–Ile266) was compared with commercial human mature IL-33 (Ser112–Thr270) and mouse mature IL-33 (Ser109–Ile266) from R&D Systems. Our human and mouse IL-33/p1 proteins induced TNFα levels similar to that of the commercial IL-33 proteins in mouse Raw 264.7 cells (Fig. 7A). Mouse Raw 264.7 cells were treated with various concentrations of mouse IL-33/p1 (Ser114–Ile266) for additional cytokine assays. Mouse TNFα, MIP-2, and IL-6 were produced in a dose-dependent manner (Fig. 7B). We obtained a very similar result of IL-8 production in HMC-1 cells, but no additional cytokines were produced in human HMC-1 cells (data not shown).
FIGURE 7.
Comparison of IL-33/p1 with commercial mature IL-33 and a dose-dependent induction of inflammatory cytokines. A, biological activity of human and mouse IL-33/p1 was compared with commercial human and mouse IL-33 proteins (R&D Systems), respectively. Human IL-33/p1 exhibited ∼80% bioactivity of the commercial human IL-33, whereas the bioactivity of mouse IL-33/p1 was very similar to that of commercial mouse IL-33. B, several concentrations of IL-33/p1 were treated to mouse Raw 264.7 cells overnight. Inflammatory cytokines (TNFα and IL-6) and chemokine (MIP-2) were produced in mouse Raw 264.7 cells in a dose-dependent manner. The data represent one of three independent experiments.
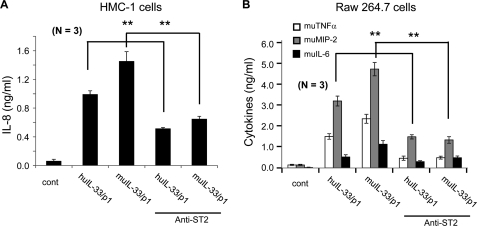
Anti-ST2 Specifically Inhibits the Cytokine Productions by IL-33/p1
We examined whether human IL-33 (Ser117–Thr270) and mouse IL-33/p1 (Ser114–Ile266) induce chemokines and inflammatory cytokines via ST2 on the cell surface of HMC-1 and Raw 264.7 cells. These cells were pretreated with anti-ST2 neutralizing antibody along with a control antibody, and the human and mouse IL-33/p1 proteins were then used to stimulate HMC-1 and Raw 264.7 cells. The anti-ST2 antibody-pretreated HMC-1 cells produced less IL-8 compared with nontreated cells (Fig. 8A), but the control antibody had no effect (data not shown). In mouse Raw 264.7 cells, TNFα, MIP-2, and IL-6 productions were specifically suppressed by the anti-ST2 antibody (Fig. 8B), and the results were statistically significant in both human and mouse cells assays (Fig. 8, A and B).
FIGURE 8.
Human and mouse IL-33/p1 produced cytokines via ST2. HMC-1 cells (A) and Raw 264.7 cells (B) were pretreated with anti-ST2 antibody, and then human and mouse IL-33/p1 proteins were treated to cells. The culture supernatant was harvested, and secreted IL-8 in HMC-1 cells (A) and TNFα, MIP-2, and IL-6 in Raw 264.7 cells (B) were measured. The anti-ST2 antibody specifically inhibited both human and mouse IL-33/p1-induced cytokine productions. Mean ± S.E. of cytokines. **, p < 0.01 (n = 3 per group). The data represent one of three independent experiments. cont, control.
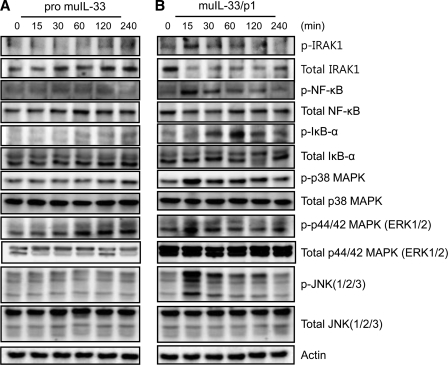
Time-dependent Activation of Signaling Molecules by IL-33/p1
Signal transduction of IL-33/p1-activated molecules was also studied. The phosphorylation of IRAK1, NF-κB, p38 MAPK, ERK, and JNK was examined with mouse IL-33/p1-treated Raw 264.7 cells because mature IL-33 proteins (Ser109–Ile266 from R&D Systems) are known to activate these inflammatory signal pathways. The recombinant precursor IL-33 phosphorylated only ERK1/2 at 60 min and remained for 2 h, but the rest of signaling molecules were not phosphorylated (Fig. 9A). Contrary to precursor IL-33, mature IL-33 stimulation promptly phosphorylated IRAK1 and NF-κB in a time-dependent manner, reached the maximal level at 15 min after exposure, and decreased dramatically at 30 min. However, the phosphorylation of I-κB was delayed and reached a maximum at 60 min. The phosphorylation of p38 MAPK, ERK1/2, and JNK was similar to that of NF-κB; however, its phosphorylation was sustained longer than that of IRAK1 and NF-κB (Fig. 9B, bottom panels). The phosphorylation pattern of NF-κB, p38 MAPK, ERK1/2, and JNK was similar to IRAK1, although there was a difference in the density of activation. This result suggested the induction of inflammatory cytokines through the activation of IRAK1, NF-κB, p38 MAPK, ERK1/2, and JNK signal pathways.
FIGURE 9.
Mouse IL-33/p1 induced the phosphorylation of IRAK1, NF-κB, I-κB, p38 MAPK, p44/42 MAPK, and JNK. Mouse precursor IL-33 (A) and IL-33/p1 (B) (50 ng/ml) were used to treat mouse macrophage Raw 264.7 cells at the indicated time points. A, p44/42 MAPK was only phosphorylated by mouse precursor IL-33 at 60 min, but the rest of the signaling molecules was not affected. B, phosphorylation of IRAK1, NF-κB, p38 MAPK, p44/42 MAPK, and JNK was significantly increased at 15 min. IL-33/p1 phosphorylated I-κB at 30 min and reached its highest at 60 min. The phosphorylation of p38 MAPK was sustained longer. The bottom of each panel exhibits the expression level of nonphosphorylated signaling molecule in cell lysates to show an equal amount of protein sample was loaded in each lane. The data represent one of three independent experiments.
DISCUSSION
In this study, we demonstrate an IL-33-processing enzyme, which is neutrophil serine proteinase 3 (PR3). PR3 processes chemokine and cytokines such as IL-8 (39), TGFβ1 (40), membrane-bound TNFα (41), IL-1β (42), IL-18 (43), and IL-32 (44) and ameliorate the biological activity of each cytokine. PR3 processes the precursor form of IL-1 family ligands such as IL-1β and IL-18 (42, 43). The biological activity of PR3-cleaved mature human IL-33 (Ser117–Thr270) and mouse IL-33 (Ser114–Ile266) proteins were studied and compared with that of mature human IL-33 (Ser112–Thr270) and mouse IL-33 (Ser109–Ile266). PR3-cleaved human and mouse mature IL-33 was highly active in producing chemokines and cytokines.
The role of caspase-1 in the activation of IL-33 was unclear in previous studies. The original study of IL-33 suggested that caspase-1 cleaves the amino acid residue (Asp110–Ser111) on human precursor IL-33. This result was generated by the incubation of in vitro-translated IL-33 rather than precursor IL-33 protein (6). Interestingly, our result in Fig. 3A showed that human and mouse precursor IL-33 proteins were not cleaved by caspase-1, whereas precursor IL-18 protein was specifically processed by caspase-1 and became an active mature IL-18 (45).
The precursor IL-33 ligand affinity column isolated urinary PR3 from concentrated human urine (Fig. 1). This result and previous reports of PR3 in the processing of IL-1β (IL-1F2) and IL-18 (IL-1F4) led us to investigate whether PR3 is an enzyme for IL-33 processing. In the presence of PR3, precursor IL-33 gained its biological function at 5 min of incubation, and a time-dependent degradation terminated its biological activity after 30 min of incubation (Fig. 2). The rIL-33 and rIL-18 proteins were cleaved resulting in productions of multibands (Fig. 3B) unlike caspase-1-cleaved precursor IL-18 (Fig. 3A).
In addition, PR3 cleaved IL-33 proteins stimulated HMC-1 cells and induced IL-8 production; however, its activity was reduced along with increasing PR3 incubation time. These results suggest that PR3 has dual functions (activation/termination) in IL-33 biology during infection or inflammation. The time-dependent reduction of IL-33 activity (Fig. 2B) was confirmed with an alternative method. We verified reduction of mature size IL-33 by Western blot (Fig. 4).
In contrast to the first report (6), it has been reported that full-length precursor IL-33 is active, and the processing by caspase-1 results in IL-33 inactivation (34). Another report on IL-33 processing by Luthi et al. (35) demonstrated that apoptotic caspases process precursor IL-33 in apoptotic cells resulting in inactivation of precursor IL-33, whereas precursor IL-33 released from necrotic cell is spontaneously active. The result suggested that the cleavage site of caspase-1 does not occur at the site initially proposed (Asp110–Ser111) but rather at amino acid residue Asp178–Gly179, which is the consensus site of cleavage by caspase-3 (34, 35, 46). Apoptotic caspase-3 and −7 abolishes the biological activity of precursor IL-33 by cleavage of the amino acid residue Asp178–Gly179. However, these studies have been performed in vitro; the biological activity of the precursor IL-33 was shown by only NF-κB luciferase assay (35) upon transfection of precursor IL-33 cDNA. Unlike the previous study, the present experiments were performed with PR3-cleaved forms of mature human IL-33 (Ser117–Thr270) and mouse IL-33 (Ser114–Ile266) proteins, inducing inflammatory cytokines and activating the phosphorylation of signaling molecules.
Another study reported calpain-dependent processing of precursor IL-33 like IL-1α, which was exhibited by treatment with calcium ionophore. Calpain processes precursor IL-33, which produces mature IL-33 in human epithelial and endothelial cells in the absence of IL-33 activity (47). Contrary to this result, Ohno et al. (48) reported that caspase-1/8 and calpain are dispensable for IL-33 release from macrophage and mast cells. Endogenous mouse IL-33 was secreted spontaneously. IL-33 secretion from cells was increased by LPS or phorbol 12-myristate 13-acetate plus ionomycin in the peritoneal macrophages of caspase-1-deficient BALB/c mice (48). The discrepancy of these results may be due to species differences. Although there is significant sequence homology between human and mouse IL-33, they share only 55% identity at amino acid level. The experiment of calpain-dependent IL-33 processing was performed with human cell lines, but the opposite report by Ohno et al. (48) was carried out using the peritoneal macrophage of caspase-1-deficient mice.
PR3-cleaved precursor IL-33 sufficiently induces inflammatory cytokines, and the biological activity of mature IL-33/p1, which was produced by PR3 cleavage site at the N terminus, was highly active. Although the predicted caspase-1 cleavage site (Asp110–Ser111) is unclear, commercial mature human IL-33 (Ser112–Thr270) and mouse IL-33 (Ser109–Ile266) are highly active in the production of cytokine. Our mature human IL-33/p1 (Ser117–Thr270) and mouse IL-33/p1 (Ser114–Ile266) exhibited potent activity, and the induction of cytokine production levels were very similar to that of commercial human and mouse mature IL-33 (Fig. 7A).
We produced human and mouse mature rIL-33 according to the consensus amino acid sequence of PR3 cleavage sites in a previous report (38). Wysocka et al. (38) described the chemical synthesis of the selective chromogenic/fluorogenic substrates and deconvolution of the tri-peptide library against proteinase 3 with the general formula ABZ-X3X2X1-ANB-NH2, which yielded the active sequence. The identification of the predicted PR3-cleaved p1, p2, and 3 fragments by enzymatic activity of PR3 was extremely difficult because PR3 cleaves pro-IL-33 transiently and eventually abolishes IL-33 activity by fragmentation (Fig. 3B). As shown in Fig. 4, PR3 digested mature IL-33 completely at 30 min. PR3-cleaved human and mouse p2 are indicated by arrows (2nd and 4th lanes of Fig. 3B), but p1 and p3 were indistinguishable because p1 migrated as PR3 (far right lane of Fig. 3B compared with Western blot in Fig. 4) and p3 migrated as pro-IL-33 (2nd lane of Fig. 5B compared with that of the 4th lane).
Although our prediction of the PR3 cleavage site Ile116–Ser117 of human IL-33 and Thr113–Ser114 of mouse IL-33 is a highly conserved consensus sequence of a potential PR3 cleavage site, as indicated by a previous report (38), we cannot exclude alternative PR3 cleavage sites of human IL-33 (Ser111–Ser112) and of mouse IL-33 (Leu108–Ser109). The alternative PR3 cleavage site was used for producing human and mouse mature rIL-33 (R&D Systems).
Collectively, we identified PR3 as a processing enzyme of IL-33. In this study, we proposed PR3 cleavage sites and expressed various forms of rIL-33 proteins to verify the contrast functions (activation/termination) of PR3 in the processing of IL-33 during infection or inflammation. Further in vivo studies on PR3-induced IL-33 processing by neutrophils or monocytes could help understand inflammatory disorders of epithelial tissues such as asthma and atopic dermatitis.
This work was supported by the National Research Foundation funded by Korean Government Grants WCU R33-2008-000-10022-0 and KRF-2008-313-C00644 and Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea Grant A100460.
- rIL
- recombinant IL.
REFERENCES
- 1. Baekkevold E. S., Roussigné M., Yamanaka T., Johansen F. E., Jahnsen F. L., Amalric F., Brandtzaeg P., Erard M., Haraldsen G., Girard J. P. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chackerian A. A., Oldham E. R., Murphy E. E., Schmitz J., Pflanz S., Kastelein R. A. (2007) IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 179, 2551–2555 [DOI] [PubMed] [Google Scholar]
- 3. Guo L., Wei G., Zhu J., Liao W., Leonard W. J., Zhao K., Paul W. (2009) IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. U.S.A. 106, 13463–13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kondo Y., Yoshimoto T., Yasuda K., Futatsugi-Yumikura S., Morimoto M., Hayashi N., Hoshino T., Fujimoto J., Nakanishi K. (2008) Administration of IL-33 induces airway hyper-responsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20, 791–800 [DOI] [PubMed] [Google Scholar]
- 5. Pecaric-Petkovic T., Didichenko S. A., Kaempfer S., Spiegl N., Dahinden C. A. (2009) Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 113, 1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 7. Allakhverdi Z., Smith D. E., Comeau M. R., Delespesse G. (2007) Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 179, 2051–2054 [DOI] [PubMed] [Google Scholar]
- 8. Sullivan B. M., Locksley R. M. (2009) Basophils. A nonredundant contributor to host immunity. Immunity 30, 12–20 [DOI] [PubMed] [Google Scholar]
- 9. Cherry W. B., Yoon J., Bartemes K. R., Iijima K., Kita H. (2008) A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzukawa M., Koketsu R., Iikura M., Nakae S., Matsumoto K., Nagase H., Saito H., Matsushima K., Ohta K., Yamamoto K., Yamaguchi M. (2008) Interleukin-33 enhances adhesion, CD11b expression, and survival in human eosinophils. Lab. Invest. 88, 1245–1253 [DOI] [PubMed] [Google Scholar]
- 11. Moulin D., Donzé O., Talabot-Ayer D., Mézin F., Palmer G., Gabay C. (2007) Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 40, 216–225 [DOI] [PubMed] [Google Scholar]
- 12. Smithgall M. D., Comeau M. R., Yoon B. R., Kaufman D., Armitage R., Smith D. E. (2008) IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT, and NK cells. Int. Immunol. 20, 1019–1030 [DOI] [PubMed] [Google Scholar]
- 13. Verri W. A., Jr., Souto F. O., Vieira S. M., Almeida S. C., Fukada S. Y., Xu D., Alves-Filho J. C., Cunha T. M., Guerrero A. T., Mattos-Guimaraes R. B., Oliveira F. R., Teixeira M. M., Silva J. S., McInnes I. B., Ferreira S. H., Louzada-Junior P., Liew F. Y., Cunha F. Q. (2010) IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 69, 1697–1703 [DOI] [PubMed] [Google Scholar]
- 14. Brint E. K., Xu D., Liu H., Dunne A., McKenzie A. N., O'Neill L. A., Liew F. Y. (2004) ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 5, 373–379 [DOI] [PubMed] [Google Scholar]
- 15. Carriere V., Roussel L., Ortega N., Lacorre D. A., Americh L., Aguilar L., Bouche G., Girard J. P. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho L. H., Ohno T., Oboki K., Kajiwara N., Suto H., Iikura M., Okayama Y., Akira S., Saito H., Galli S. J., Nakae S. (2007) IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcϵRI signals. J. Leukocyte Biol. 82, 1481–1490 [DOI] [PubMed] [Google Scholar]
- 17. Hoshino K., Kashiwamura S., Kuribayashi K., Kodama T., Tsujimura T., Nakanishi K., Matsuyama T., Takeda K., Akira S. (1999) The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurowska-Stolarska M., Stolarski B., Kewin P., Murphy G., Corrigan C. J., Ying S., Pitman N., Mirchandani A., Rana B., van Rooijen N., Shepherd M., McSharry C., McInnes I. B., Xu D., Liew F. Y. (2009) IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 [DOI] [PubMed] [Google Scholar]
- 19. Moussion C., Ortega N., Girard J. P. (2008) The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo. A novel “alarmin”? PLoS One 3, e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rank M. A., Kobayashi T., Kozaki H., Bartemes K. R., Squillace D. L., Kita H. (2009) IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 123, 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senn K. A., McCoy K. D., Maloy K. J., Stark G., Fröhli E., Rülicke T., Klemenz R. (2000) T1-deficient and T1-Fc-transgenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis. Eur. J. Immunol. 30, 1929–1938 [DOI] [PubMed] [Google Scholar]
- 22. Suzukawa M., Iikura M., Koketsu R., Nagase H., Tamura C., Komiya A., Nakae S., Matsushima K., Ohta K., Yamamoto K., Yamaguchi M. (2008) An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 181, 5981–5989 [DOI] [PubMed] [Google Scholar]
- 23. Townsend M. J., Fallon P. G., Matthews D. J., Jolin H. E., McKenzie A. N. (2000) T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bergers G., Reikerstorfer A., Braselmann S., Graninger P., Busslinger M. (1994) Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 13, 1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klemenz R., Hoffmann S., Werenskiold A. K. (1989) Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. U.S.A. 86, 5708–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tominaga S. (1989) A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 258, 301–304 [DOI] [PubMed] [Google Scholar]
- 27. Tominaga S., Jenkins N. A., Gilbert D. J., Copeland N. G., Tetsuka T. (1991) Molecular cloning of the murine ST2 gene. Characterization and chromosomal mapping. Biochim. Biophys. Acta 1090, 1–8 [DOI] [PubMed] [Google Scholar]
- 28. Tominaga S., Yokota T., Yanagisawa K., Tsukamoto T., Takagi T., Tetsuka T. (1992) Nucleotide sequence of a complementary DNA for human ST2. Biochim. Biophys. Acta 1171, 215–218 [DOI] [PubMed] [Google Scholar]
- 29. Palmer G., Lipsky B. P., Smithgall M. D., Meininger D., Siu S., Talabot-Ayer D., Gabay C., Smith D. E. (2008) The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 42, 358–364 [DOI] [PubMed] [Google Scholar]
- 30. Iikura M., Suto H., Kajiwara N., Oboki K., Ohno T., Okayama Y., Saito H., Galli S. J., Nakae S. (2007) IL-33 can promote survival, adhesion, and cytokine production in human mast cells. Lab. Invest. 87, 971–978 [DOI] [PubMed] [Google Scholar]
- 31. Allakhverdi Z., Comeau M. R., Smith D. E., Toy D., Endam L. M., Desrosiers M., Liu Y. J., Howie K. J., Denburg J. A., Gauvreau G. M., Delespesse G. (2009) CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 123, 472–478 [DOI] [PubMed] [Google Scholar]
- 32. Arend W. P., Palmer G., Gabay C. (2008) IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 [DOI] [PubMed] [Google Scholar]
- 33. Dinarello C. A. (1998) Interleukin-1β, interleukin-18, and the interleukin-1β-converting enzyme. Ann. N.Y. Acad. Sci. 856, 1–11 [DOI] [PubMed] [Google Scholar]
- 34. Cayrol C., Girard J. P. (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. U.S.A. 106, 9021–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lüthi A. U., Cullen S. P., McNeela E. A., Duriez P. J., Afonina I. S., Sheridan C., Brumatti G., Taylor R. C., Kersse K., Vandenabeele P., Lavelle E. C., Martin S. J. (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 [DOI] [PubMed] [Google Scholar]
- 36. Bae S., Choi J., Hong J., Lee S., Her E., Choi W., Kim S., Choi Y., Kim S. (2010) Generation of anti-proteinase 3 monoclonal antibodies and development of immunological methods to detect endogenous proteinase 3. Hybridoma 29, 17–26 [DOI] [PubMed] [Google Scholar]
- 37. Kim S. H., Han S. Y., Azam T., Yoon D. Y., Dinarello C. A. (2005) Interleukin-32. A cytokine and inducer of TNFα. Immunity 22, 131–142 [DOI] [PubMed] [Google Scholar]
- 38. Wysocka M., Lesner A., Guzow K., Mackiewicz L., Legowska A., Wiczk W., Rolka K. (2008) Design of selective substrates of proteinase 3 using combinatorial chemistry methods. Anal. Biochem. 378, 208–215 [DOI] [PubMed] [Google Scholar]
- 39. Padrines M., Wolf M., Walz A., Baggiolini M. (1994) Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 352, 231–235 [DOI] [PubMed] [Google Scholar]
- 40. Csernok E., Szymkowiak C. H., Mistry N., Daha M. R., Gross W. L., Kekow J. (1996) Transforming growth factor-β (TGF-β) expression and interaction with proteinase 3 (PR3) in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin. Exp. Immunol. 105, 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robache-Gallea S., Morand V., Bruneau J. M., Schoot B., Tagat E., Réalo E., Chouaib S., Roman-Roman S. (1995) In vitro processing of human tumor necrosis factor-α. J. Biol. Chem. 270, 23688–23692 [DOI] [PubMed] [Google Scholar]
- 42. Coeshott C., Ohnemus C., Pilyavskaya A., Ross S., Wieczorek M., Kroona H., Leimer A. H., Cheronis J. (1999) Converting enzyme-independent release of tumor necrosis factor α and IL-1β from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U.S.A. 96, 6261–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sugawara S., Uehara A., Nochi T., Yamaguchi T., Ueda H., Sugiyama A., Hanzawa K., Kumagai K., Okamura H., Takada H. (2001) Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 167, 6568–6575 [DOI] [PubMed] [Google Scholar]
- 44. Novick D., Rubinstein M., Azam T., Rabinkov A., Dinarello C. A., Kim S. H. (2006) Proteinase 3 is an IL-32-binding protein. Proc. Natl. Acad. Sci. U.S.A. 103, 3316–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hong J., Bae S., Jhun H., Lee S., Choi J., Kang T., Kwak A., Hong K., Kim E., Jo S., Kim S. (2011) Identification of constitutively active interleukin 33 (IL-33) splice variant. J. Biol. Chem. 286, 20078–20086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamkanfi M., Dixit V. M. (2009) IL-33 raises alarm. Immunity 31, 5–7 [DOI] [PubMed] [Google Scholar]
- 47. Hayakawa M., Hayakawa H., Matsuyama Y., Tamemoto H., Okazaki H., Tominaga S. (2009) Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem. Biophys. Res. Commun. 387, 218–222 [DOI] [PubMed] [Google Scholar]
- 48. Ohno T., Oboki K., Kajiwara N., Morii E., Aozasa K., Flavell R. A., Okumura K., Saito H., Nakae S. (2009) Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 183, 7890–7897 [DOI] [PubMed] [Google Scholar]