Background: OASIS is an ER stress transducer expressed in the large intestine.
Results: Mature goblet cells are decreased in Oasis−/− mice. Knockdown of the Oasis transcript impairs the maturation of goblet cells.
Conclusion: OASIS plays crucial roles in terminal differentiation of goblet cells.
Significance: The ER stress response mediated by OASIS signaling is involved in cell differentiation and maturation.
Keywords: Cell Differentiation, Endoplasmic Reticulum Stress, Mouse, Transcription Factors, Unfolded Protein Response, OASIS Family, bZIP Transcription Factor, Regulated Intramembrane Proteolysis
Abstract
OASIS is a basic leucine zipper transmembrane transcription factor localized in the endoplasmic reticulum (ER) that is cleaved in its transmembrane region in response to ER stress. This novel ER stress transducer has been demonstrated to express in osteoblasts and astrocytes and promote terminal maturation of these cells. Additionally, OASIS is highly expressed in goblet cells of the large intestine. In this study, we investigated the roles of OASIS in goblet cell differentiation in the large intestine. To analyze the functions of OASIS in goblet cells, we examined morphological changes and the expression of goblet cell differentiation markers in the large intestine of Oasis−/− mice. By disrupting the Oasis gene, the number of goblet cells and production of mucus were decreased in the large intestine. Oasis−/− goblet cells showed abnormal morphology of mucous vesicles and rough ER. The expression levels of mature goblet cell markers were lower, and conversely those of early goblet cell markers were higher in Oasis−/− mice, indicating that differentiation from early to mature goblet cells is impaired in Oasis−/− mice. To determine the association of OASIS with other factors involved in goblet cell differentiation, in vitro experiments using a cell culture model were performed. We found that OASIS was activated in response to mild ER stress that is induced in differentiating goblet cells. Knockdown of the Oasis transcript perturbed goblet cell terminal differentiation. Together, our data indicate that OASIS plays crucial roles in promoting the differentiation of early goblet cells to mature goblet cells in the large intestine.
Introduction
The endoplasmic reticulum (ER)2 is a central cellular organelle responsible for the synthesis, folding, and posttranslational modifications of proteins destined for the secretory pathway. A number of cellular stress conditions lead to the accumulation of unfolded or misfolded proteins in the ER lumen. These conditions, which are collectively termed ER stress, have the potential to induce cellular damage (1, 2). The ER responds to these perturbations by activating an integrated signal transduction pathway through the ER stress transducers, which is called the unfolded protein response (UPR) (3–5). The UPR involves at least three distinct components: translational attenuation to decrease the demands made on the organelle (6), transcriptional induction of genes encoding ER-resident chaperones to facilitate protein folding (7, 8), and ER-associated degradation to degrade the unfolded proteins accumulated in the ER (9, 10). If these strategies fail, cells undergo ER stress-induced apoptosis (11, 12). The UPR was originally described as a system by which cells evade damage in response to acute ER perturbation. However, recent advances have revealed that the UPR also provides important signals for regulating cell differentiation and maturation or the maintenance of basal cellular homeostasis (13–16).
Previously, we identified old astrocyte specifically induced substance (OASIS) as a novel ER stress transducer (17). OASIS is a basic leucine zipper (bZIP) transcription factor that belongs to the cAMP-responsive element (CRE)-binding protein/activating transcription factor (ATF) family. Although OASIS is localized to the ER membrane under normal conditions, it is cleaved at the membrane in response to ER stress. Consequently, its cleaved N-terminal cytoplasmic domain, which contains the bZIP domain, translocates into the nucleus, where it activates the transcription of target genes (18, 19). High expression of OASIS was observed in the osteoblasts of osseous tissues and the astrocytes of the central nervous system (20–22). From the analysis of knockout mice, OASIS has been demonstrated to be involved in terminal differentiation and osteoblasts (23–25) and astrocytes.3
The intestinal epithelium is composed of four distinct cell types, including the absorptive enterocytes and the goblet, Paneth, and enteroendocrine secretory cell lineages (26, 27). Stem cells are committed to generate these lineages by the Wnt and Notch signaling cascades. Wnt signaling is required for the generation of the secretory lineages, whereas Notch signaling is necessary for the differentiation of enterocytes (28, 29). However, the molecular mechanisms underlying the differentiation of intestinal epithelial cells are incompletely defined. Recently, the E26 transformation-specific (ETS) domain transcription factor SAM-pointed domain-containing ETS-like factor (SPDEF) (30, 31), has been reported to act downstream of ATOH1 (32), which is an essential determinant of secretory lineages downstream of β-catenin (33, 34). Maturation of Paneth and goblet cells has been shown to be impaired in Spdef-deficient mice, whereas immature secretory progenitors accumulate in the intestine (35). These observations suggest that SPDEF promotes the terminal differentiation of a secretory progenitor pool into Paneth and goblet cells. Interestingly, the expression of CREB4, an ER stress transducer that is structurally similar to OASIS, is completely abolished in Spdef-deficient Paneth and goblet cells (35). These observations suggest that the expression of CREB4 is controlled at downstream of SPDEF and that ER stress response signaling through ER stress transducers such as CREB4 mediates the differentiation and maturation of secretory cell lineages in the intestinal epithelium. In this study, we investigated the roles of the ER stress transducer OASIS, which is expressed in the crypt base of the large intestinal epithelium, in the differentiation of goblet cells. Consequently, we demonstrated that OASIS plays crucial roles in promoting the terminal differentiation of goblet cells in the large intestine.
EXPERIMENTAL PROCEDURES
Animals
3-week-old C57BL/6 mice or Oasis−/− mice were used in this study. The Oasis−/− mice were established previously in our laboratory (22–24). In all studies comparing wild-type and Oasis−/− mice, sex-matched littermates derived from the mating of Oasis+/− mice were used. The experimental procedures and housing conditions for animals were approved by the Committee of Animal Experimentation, Hiroshima University.
Cell Culture, Treatments, and Transfection
LS174T cells were cultured in Eagle's minimal essential medium supplemented with 10% FCS and 1% non-essential amino acids. LS174T cells were cultured overnight in a 9.2-cm2 dish for RNA or protein isolation and in a Lab-Tek chamber slide (Nalge Nunc) for periodic acid-Schiff (PAS) staining. Subsequently, cells were treated with 2 mm sodium butyrate, which induces LS174T cells to differentiate to mature goblet cells. For Oasis knockdown experiments, LS174T cells were transfected with 1 μg of Oasis siRNA (predesigned siRNA pool targeting Oasis: AAAAGAAGGUGGAGACAUU, GGGACCACCUGCAGCAUGA, GAAGGAGUAUGUGGAGUGU, and CAGGAGAGCCGUCGUAAGA; Thermo Scientific Dermacon, catalog no. M-008579-01-0005) or control siRNA (Silencer Cy3-labeled negative control no.1 siRNA; Invitrogen, catalog no. AM4621). Transfection was performed 12 h before treatment with sodium butyrate using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocols. 24 h after treatment with sodium butyrate, PAS staining was performed as described previously (36). The PAS-stained slide was counterstained with hematoxylin solution.
RNA Isolation and RT-PCR
Total RNA was isolated from the large intestine of 3-week-old mice or LS174T cells using ISOGEN (Wako) according to the manufacturer's protocol. First-strand cDNA was synthesized in a 20 μl of reaction volume using a random primer (Takara) and Moloney murine leukemia virus reverse transcriptase (Invitrogen). PCR was performed using each specific primer set in a total volume of 30 μl containing 0.8 μm of each primer, 0.2 mm dNTPs, 3 units of Taq polymerase, and 10× PCR buffer (Agilent). Primer sequences are summarized in supplemental Table S1. The PCR products were resolved by electrophoresis on a 4.8% acrylamide gel. The density of each band was quantified using the Adobe Photoshop Elements 2.0 program (Adobe Systems Inc.).
Western Blotting
For Western blotting, proteins were extracted from LS174T cells using cell extraction buffer containing 10% SDS, 0.5 m EDTA (pH 8.0), 100 mm methionine, and a protease inhibitor mixture (MBL International). The lysates were incubated on ice for 45 min. After centrifugation at 16,000 × g for 15 min, the protein concentrations of the supernatants were determined. Protein-equivalent samples were loaded onto sodium dodecyl sulfate-polyacrylamide gels. Anti-β-actin (Sigma, 1:3000) and anti-OASIS (purified from a hybridoma as described previously (22, 23)) antibodies were used for Western blotting. The density of each band was quantified using the Adobe Photoshop Elements 2.0 program (Adobe Systems Inc.).
Histological Analysis and in Situ Hybridization
Large intestine from 3-week-old mice was fixed overnight in 10% formalin neutral buffer solution (Wako). Samples were then dehydrated with ethanol, embedded in paraffin, and sectioned (5 μm). Hematoxylin-eosin staining and PAS staining were performed according to standard protocols. In situ hybridization was performed using digoxigenin-labeled cRNA probes (supplemental Table S2). Antisense and sense probes were made by in vitro transcription in the presence of digoxigenin-labeled dUTP using various cDNAs subcloned into the pGEM-Teasy vector (Promega) as templates. Large intestine isolated for in situ hybridization was frozen immediately and sectioned (6 μm). The frozen sections were fixed for 20 min with 4% formalin in PBS (pH 7.4). The sections were then washed with PBS and treated with 0.1% proteinase K for 5 min. After washing with PBS, the sections were refixed for 20 min with 4% formalin in PBS and treated with 0.1 m triethanolamine and 2.5% anhydrous acetic acid for 10 min, followed by washing with PBS. Sections were prehybridized for 1 h at 37 °C in hybridization buffer (0.01% dextran sulfate, 0.01 m Tris-HCl (pH 8.0), 0.05 m NaCl, 50% formamide, 0.2% sarcosyl, 1× Denhardt's solution, 0.5 mg/ml yeast tRNA, 0.2 mg/ml salmon testis DNA) and then hybridized overnight at 55 °C in hybridization solution with 100 ng/ml cRNA probe. After washing with 4× saline sodium citrate buffer for 20 min at 60 °C, the sections were further washed in 2× saline sodium citrate buffer and 50% formamide for 30 min at 60 °C. Sections were treated RNaseA in RNase buffer (10 mm Tris-HCl (pH7.4), 1 mm 0.5 m EDTA (pH 8.0), 0.5 m NaCl) for 30 min at 37 °C to remove the unhybridized probe. After RNase treatment, sections were washed with 2× saline sodium citrate buffer and 50% formamide for 30 min at 60 °C and then blocked with 1.5% blocking reagent in 100 mm Tris-HCl (pH 7.5) and 150 mm NaCl for 1 h at room temperature. For detection of digoxigenin-labeled cRNA probes, anti-digoxigenin antibody conjugated to alkaline phosphatase was used at a dilution of 1:500, and color was developed by incubation with 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate solution.
Electron Microscopy
Large intestine from 3-week-old mice was fixed in 1% glutaraldehyde in PBS for 15 min. After washing with distilled water, the tissues were post-fixed in 0.5% osmium tetroxide in 0.1 m cacodylate buffer for 30 min. Following dehydration, the tissues were embedded in EPON812, and ultra-thin sections were stained with uranyl acetate and lead citrate. Stained sections were visualized using a Hitachi 7100 electron microscope operated at 80 kV. The mean cell area was determined using ImageJ software (National Institutes of Health).
Statistical Analysis
Statistical comparisons were made using the unpaired Student's t test. Statistical significance between two samples was determined by a p value of less than 0.05. p values of less than 0.05, 0.01 or 0.001 are described as *, p < 0.05; **, p < 0.01; or ***, p < 0.001, respectively.
RESULTS
OASIS Was Highly Expressed in Immature Goblet Cells of the Large Intestine
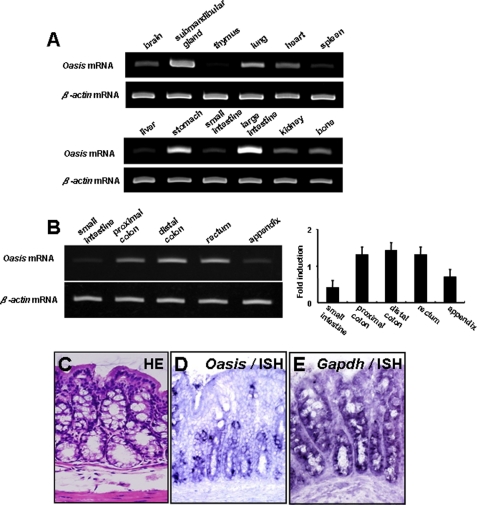
We reported previously that OASIS is expressed in osteoblasts and astrocytes (17, 20, 21, 23). To examine the tissue distribution of Oasis mRNA more precisely, we performed RT-PCR using mRNA isolated from various tissues of 3-week-old mice. We detected strong Oasis mRNA signals in the submandibular gland, lung, stomach, and large intestine, where it was most intense (Fig. 1A). Oasis mRNA was expressed highly in all portions of the large intestine, except for the appendix. In contrast, the levels of Oasis mRNA were very low in the small intestine (Fig. 1B). In the digestive tract, there are three distinct cell types, i.e. absorptive enterocytes, enteroendocrine cells, and goblet cells. To identify which of these cells expressed Oasis mRNA, we carried out in situ hybridization using Oasis cRNA probes. The Oasis signals were focally detected in the base of the crypt but not in the apical portion of the crypt (Fig. 1C). In contrast, Gapdh mRNA was observed in all cells (both basal and apical) of the crypt. The cells expressing Oasis mRNA possessed vacuoles in their cytosol, indicating that OASIS is expressed in goblet cells. Moreover, the goblet cells at the base of the crypt are immature cells that are developing from intestinal stem cells (27, 37, 38). Thus, we concluded that the cells expressing OASIS were immature goblet cells.
FIGURE 1.
The expression of Oasis mRNA in the large intestine. A, RT-PCR analysis (ISH) of Oasis mRNA in various tissues from 3-week-old mice. Oasis mRNA is highly expressed in the large intestine. B, the expression of Oasis mRNA in each region of the large intestine. Right panel, quantitative analysis of Oasis mRNA. C, hematoxylin-eosin staining (HE) in the large intestine. Many goblet cells with mucus-containing vacuoles are observed in the crypt. D and E, in situ hybridization analysis (ISH) of Oasis mRNA (D) and Gapdh mRNA (E) in the large intestine. Oasis mRNA is expressed in the lower portion of the crypt.
The Numbers of Mature Goblet Cells Decreased in Oasis−/− Mice
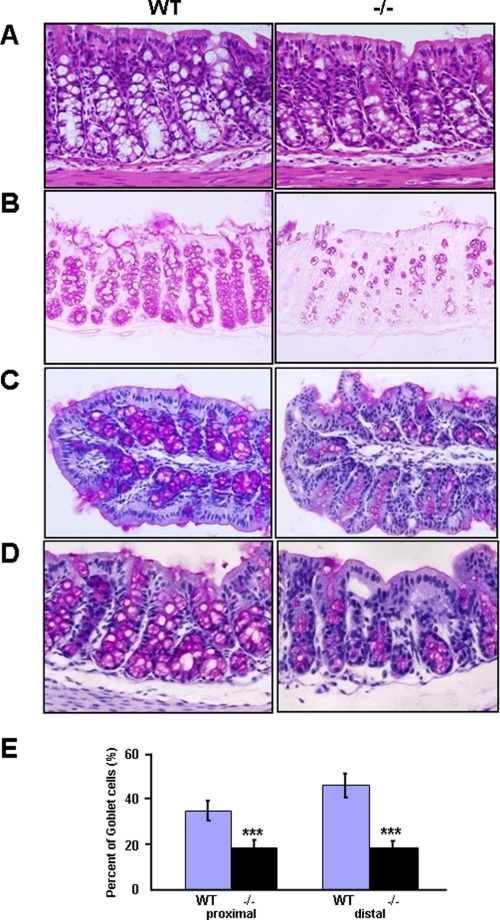
To elucidate the functions of OASIS in immature goblet cells, we first performed histological analysis in the Oasis−/− mice that we generated previously (22–24). The mice were born at the expected Mendelian ratios and were fertile but showed impaired bone formation and growth retardation. We demonstrated previously that these defects are due to impaired differentiation of osteoblasts (23) and decreased serum levels of growth hormone and insulin-like growth factor (24), respectively. Food consumption was not affected in Oasis−/− mice. Except osteopenia and growth retardation, the general morphology of all other tissues and organs was normal, and the architecture of the intestinal tract was unaffected. Additionally, formation of the crypt and differentiation of enterocytes also appeared normal. However, the apparent numbers of goblet cells that contained abundant vacuoles were decreased in large intestine of Oasis−/− mice (Fig. 2A). PAS staining showed a marked decrease in the number of cells containing mucus both in the proximal and distal large intestine of Oasis−/− mice (Fig. 2, B–E). In contrast, immature cells that contain less mucus were increased in the crypt epithelium, suggesting that the differentiation or maturation of goblet cells was inhibited in Oasis−/− mice. Goblet cells in small intestine showed no morphological changes in Oasis−/− mice. Because of the low expression level of OASIS, loss of OASIS may not affect the function of goblet cells in small intestine. Further, stomach was also morphologically intact.
FIGURE 2.
Anomalies of goblet cells in the large intestine of Oasis−/− mice. A, hematoxylin-eosin staining. B, PAS staining. The numbers of goblet cells with vacuoles are severely decreased in Oasis−/− mice. C and D, PAS-hematoxylin counterstaining of WT and Oasis−/− large intestine. C, proximal large intestine. D, distal large intestine. E, the percentages of PAS-positive goblet cells in the crypt of the large intestine in WT and Oasis−/− mice. Data are mean ± S.D. n = 5. ***, p < 0.001, unpaired Student's t test.
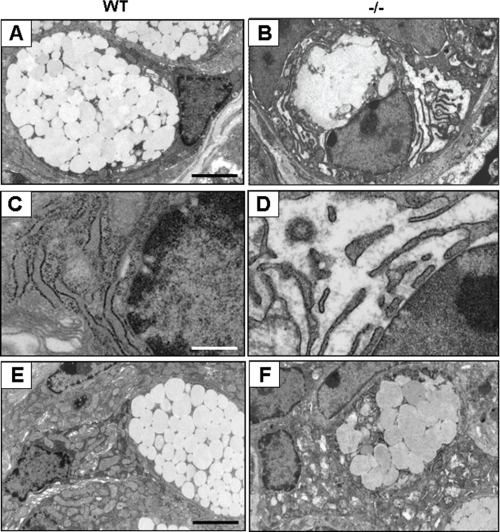
Next, we carried out electron microscopic examination of goblet cells in WT and Oasis−/− mice. Normal goblet cells have abundant cytoplasm containing large secretory vesicles (Fig. 3A). In Oasis−/− mice, almost all goblet cells at the crypt base and in the lower portion of the crypt showed an abnormal ultrastructure in which both the number and size of vesicles were decreased and the membranes of some vesicles were often fused (Fig. 3B). Moreover, the rough endoplasmic reticulum was abnormally expanded in these cells (Fig. 3D). In the upper portion of the crypt, cells with abnormal ultrastructure were also observed (Fig. 3F). The other types of cells in the crypt epithelium, such as enteroendocrine cells and enterocytes, were morphologically intact in Oasis−/− mice (data not shown). Goblet cells are known to be differentiated from intestinal stem cells in the crypt base, and the mature goblet cells move to the apical portion of the crypt. We observed abnormal morphology of goblet cells from the base to the apical portion of the crypt in Oasis−/− mice, suggesting that deletion of the Oasis gene affected the differentiation or maturation of goblet cells from the intestinal cell lineage, involving impairment of vesicle formation.
FIGURE 3.
Electron microscopy of goblet cells in the large intestine. A and B, images of goblet cells at the crypt base in the large intestine. C and D, high magnification of the rough ER in A and B. Note that rough ER in Oasis−/− goblet cells displays aberrant expansion. E and F, goblet cells in the upper portion of the crypt. The numbers of mucus vesicles are decreased, and the membrane of some vesicles are often fused in goblet cells of Oasis−/− large intestine. Scale bars = 4 μm (A, B, E, and F), 1.6 μm (C and D)
OASIS Functions in the Terminal Differentiation of Goblet Cells
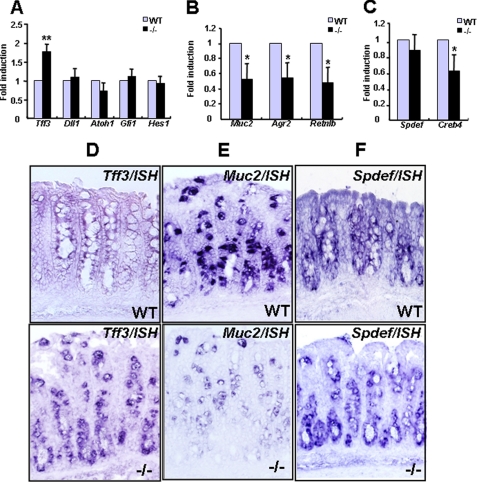
We next focused on the roles of OASIS in goblet cell differentiation. Intestinal epithelial stem cells are determined to become cells of the secretory lineage, such as goblet cells and enteroendocrine cells, by Notch ligand Delta-like 1 (Dll1) (39), whereas absorptive enterocytes are specified by Notch and the transcription factor Hairy and enhancer of split 1 (HES1) (40). Subsequently, secretory progenitor cells are specified to become goblet cells by the transcription factors atonal homolog 1 (ATOH1) (33, 34) and growth factor-independent 1 (GFI1) (41, 42). We examined the expression levels of various differentiation markers of the goblet cell lineages in the large intestine of Oasis−/− mice by RT-PCR. All genes involved in the primary differentiation from stem cells to early goblet cells or absorptive enterocytes were normally expressed in Oasis−/− mice (Fig. 4A). Therefore, intestinal epithelial stem cells are correctly specified to both the goblet cell and absorptive enterocyte lineages. Interestingly, the expression of Trefoil factor 3 (Tff3), an early goblet cell marker, was significantly increased in Oasis−/− mice (Fig. 4A). Conversely, the mature goblet cell markers Muchin 2 (Muc2), Anterior gradient 2 (Agr2), and Resistin-like β (Retnlb) were markedly decreased (Fig. 4B). In situ hybridization revealed that Tff3 mRNA signals were observed diffusely in the crypt. The intensity was very faint in WT mice but elevated in Oasis−/− mice (Fig. 4D). Muc2 mRNA signals were robustly observed throughout the crypt epithelium in WT mice but were almost abolished in Oasis−/− mice (Fig. 4E). These results suggest that differentiation from early to mature goblet cells is impaired in the large intestine of Oasis−/− mice.
FIGURE 4.
The expression of goblet cell markers in the large intestine. A, RT-PCR of genes involved in primary differentiation from intestinal stem cells and an early goblet cell marker, Tff3. Note that the expression of Tff3 is significantly elevated in Oasis−/− mice. B, RT-PCR of mature goblet cell markers. C, RT-PCR analysis of Spdef and Creb4. Data are mean ± S.D. n = 3. *, p < 0.05; **, p < 0.01, unpaired Student's t test. D–F, in situ hybridization of Tff3 (D), Muc2 (E), and Spdef (F) in large intestine.
Recently, the ETS domain transcription factor SPDEF was reported to be required for the terminal differentiation of goblet cells (35). In the large intestine, Spdef−/− mice showed a similar phenotype to that of Oasis−/− mice, i.e. goblet cells are specified correctly, but the terminal differentiation of early to mature goblet cells was impaired. CREB4 is an ER-resident CREB/ATF family transcription factor that is structurally very similar to OASIS. Interestingly, the expression of CREB4 was reported to be down-regulated in the intestine of Spdef−/− mice, indicating that CREB4 could be a direct target of SPDEF. We therefore examined whether the SPDEF-CREB4 pathway is related to the OASIS signaling pathway. The expression of Spdef mRNA was not altered in the large intestine of Oasis−/− mice (Fig. 4, C and F), implying that OASIS does not regulate the expression of the Spdef gene. In contrast, the expression of CREB4 was significantly decreased in Oasis−/− mice (Fig. 4C). These data suggest that the expression of CREB4 is regulated by OASIS. Combined with the published data, CREB4 is therefore a common downstream target of OASIS and SPDEF, but the expression of CREB4 is regulated independently. Furthermore, CREB4 may be a marker for the terminal differentiation of goblet cells and may function together with the OASIS signaling pathway in the maturation of goblet cells.
Activation of OASIS during Terminal Differentiation of Goblet Cells
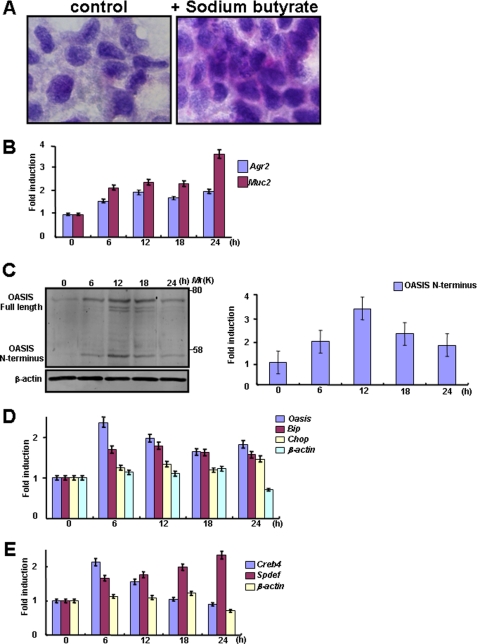
To investigate the roles of OASIS in the terminal differentiation of goblet cells, we examined the expression and activation of OASIS in the human colon cancer LS174T cell line. LS174T cells are secretory progenitors that can be induced to differentiate into goblet cells by treatment with sodium butyrate (36). In LS174T cells treated with 2 mm sodium butyrate for 24 h, PAS-positive secretory granules were produced (Fig. 5A), and the expression of mature goblet cell markers such as Muc2 and Agr2 was gradually induced from 6 h after treatment (Fig. 5B). Western blotting showed that the expression of full-length OASIS increased after 6-h treatment with sodium butyrate, peaking at 12 h, after which it decreased gradually. The cleaved fragment of OASIS, p50 OASIS (OASIS N terminus), was also increased in synchrony with the expression of full-length OASIS, indicating that OASIS is activated during the differentiation of LS174T cells to mature goblet cells (Fig. 5C). Because OASIS is activated in response to ER stress (17), it is possible that ER stress occurred during goblet cell differentiation. We examined the expression of ER stress markers after treatment with sodium butyrate. Messenger RNAs for the ER stress markers Bip and Chop were slightly but significantly up-regulated in accordance with the pattern of OASIS activation (Fig. 5D). These findings suggest that mild ER stress is induced during goblet cell differentiation and that OASIS is subjected to regulated intramembrane proteolysis in response to the mild ER stress. When differentiating into mature goblet cells, early goblet cells gradually start to produce abundant proteins such as MUC2, and these proteins are overloaded into the ER. Thus, mild ER stress during maturation of goblet cells could be derived from a high demand for synthesis and secretion of mucus materials.
FIGURE 5.
Gene expression and OASIS activation during goblet cell maturation. A, PAS staining of LS174T cells 24 h after treatment with sodium butyrate. LS174T cells are completely differentiated to mature goblet cells containing PAS-positive mucus 24 h following treatment. B, RT-PCR of mature goblet cell markers in LS174T cells treated with sodium butyrate. The expression levels of mature goblet cell markers are increased after treatment with sodium butyrate. C, Western blot analysis of OASIS in LS174T cells treated with sodium butyrate (left panel). Quantification of the amounts of OASIS N terminus (right panel). D, RT-PCR of ER stress markers during LS174T cell differentiation. E, RT-PCR of Spdef and Creb4 mRNAs in LS174T cells treated with sodium butyrate. Data are mean ± S.D. n = 5.
Our findings in Oasis−/− mice described above suggest that CREB4 could be expressed as a common downstream target of OASIS and SPDEF during differentiation of early to mature goblet cells. We therefore examined the expression of Creb4 and Spdef mRNAs after treatment of LS174T cells with sodium butyrate. The expression of Creb4 mRNA was induced 6 h after treatment, the same time point at which expression of p50 OASIS (OASIS N terminus) began (Fig. 5E). Creb4 mRNA expression then decreased gradually from 12 h after treatment. In contrast, although Spdef was also induced from 6 h, the expression level was maintained until 24 h after treatment (Fig. 5E), indicating that SPDEF is not downstream of OASIS.
OASIS Is Required for the Differentiation of Goblet Cells
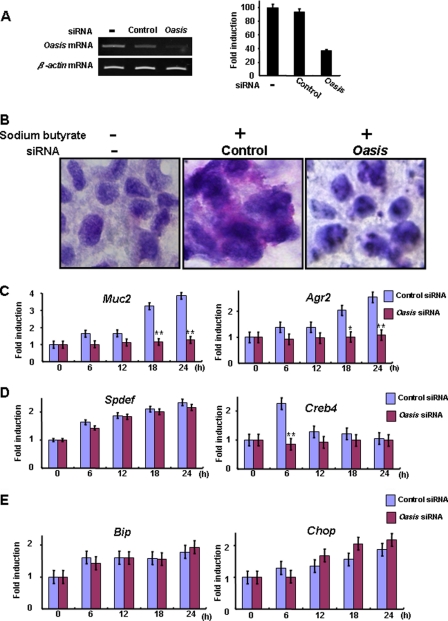
To determine whether OASIS is indispensable for the differentiation of goblet cells, we examined the effects of Oasis knockdown using Oasis siRNA on the differentiation of LS174T cells. We confirmed that Oasis siRNA successfully and significantly suppressed the expression of OASIS in LS174T cells (Fig. 6A). PAS staining showed that secretory granules were decreased significantly in LS174T cells transfected with Oasis siRNA following treatment with sodium butyrate (Fig. 6B). Furthermore, the mature goblet cell markers Muc2 and Agr2 were down-regulated dramatically (Fig. 6C). These data suggest that inhibition of OASIS expression impaired the terminal maturation of LS174T cells to goblet cells.
FIGURE 6.
OASIS is required for the terminal differentiation of goblet cells. A, RT-PCR of Oasis mRNA in LS174T cells 12 h after transfection with Oasis siRNA. The expression of Oasis mRNA is significantly suppressed by the siRNA. B, PAS staining of LS174T cells 24 h after treatment with sodium butyrate. Oasis knockdown results in inhibition of goblet cell maturation. C–E, RT-PCR of mature goblet cell markers (C), Spdef and Creb4 (D), and ER stress markers (E) in LS174T cells treated with sodium butyrate following transfection with Oasis siRNA. Data are mean ± S.D. n = 5. *, p < 0.05; **, p < 0.01; unpaired Student's t test.
Next, to analyze the relationship between the OASIS and SPDEF pathways, we checked the expression of SPDEF and CREB4 in LS174T cells treated with sodium butyrate. Even when the expression of OASIS was suppressed by siRNA, the up-regulation of Spdef mRNA following treatment with sodium butyrate was not affected. However, induction of CREB4 by sodium butyrate was inhibited significantly by transfection of LS174T cells with Oasis siRNA (Fig. 6D). These findings support our hypothesis that CREB4, but not SPDEF, is downstream of OASIS.
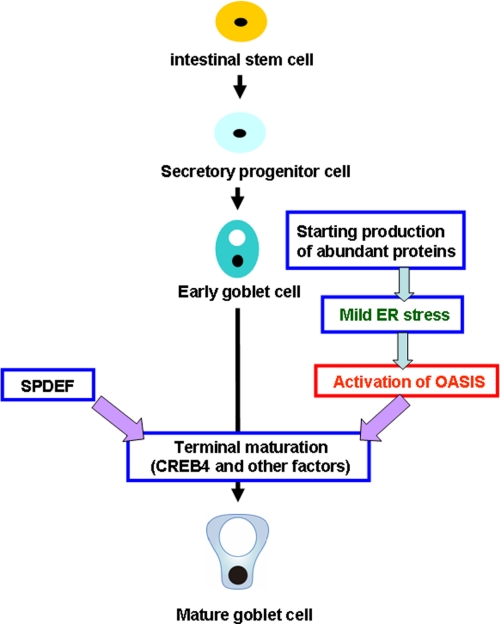
The expression of ER stress markers was slightly up-regulated during differentiation of LS174T cells to mature goblet cells (Fig. 6E). However, the slight elevation of these genes was not affected by knockdown of Oasis mRNA, indicating that mild ER stress occurs prior to OASIS activation during the maturation of goblet cells. Taken together, our data suggest that OASIS plays an essential role in goblet cell differentiation following its activation by mild ER stress (Fig. 7).
FIGURE 7.
Putative roles of OASIS in the differentiation of goblet cells in the large intestine.
DISCUSSION
We previously demonstrated that OASIS promotes the differentiation of osteoblasts and is involved in bone formation (23–25). In this study, we found that Oasis is highly expressed in goblet cells at the crypt base in the large intestine. Goblet cells synthesize and secrete large amounts of mucus proteins, including mucin, and are similar to osteoblasts in that abundant proteins are loaded into the ER. Therefore, ER stress is easily induced in secretory goblet cells (43, 44). However, immature goblet cells do not produce mucus and do not undergo ER stress. During differentiation from secretory progenitor cells to mature goblet cells, immature cells develop the machinery for dealing with abundant proteins in the ER, including the UPR system. We did, in fact, demonstrate that ER stress is induced during goblet cell differentiation and is followed by the induction of genes expressed in mature goblet cells. Thus, it is possible that ER stress is a trigger for the maturation of goblet cells. Similar phenomena are also seen in plasma cell differentiation. For the full development of antibody secretion, activation of the UPR is essential (45, 46). Taken together, previous data and our findings suggest that activation of the UPR by ER stress is required for the maturation of immature cells to “professional” secretory cells.
In the large intestine of Oasis−/− mice, the number of mature goblet cells that contain abundant secretory granules was reduced dramatically. We found that Tff3, an early goblet cell marker, was up-regulated, whereas mature goblet cell markers such as Muc2, Agr2, and Retnlb were conversely down-regulated. Genes involved in the primary differentiation of immature goblet cells were not affected, indicating that intestinal stem cells are correctly specified to both secretory progenitors and absorptive enterocyte lineages. Thus, we concluded that the abnormal architectures observed in the crypt epithelium of Oasis−/− mice are caused by impaired terminal maturation of goblet cells. The anomalies in the large intestine of Oasis−/− mice are also reminiscent of the phenotype seen in Spdef−/− mice, which showed a profound loss of mature goblet cells (35). In this study, the authors used microarray analyses to show that OASIS is not down-regulated to any extent in Spdef −/− colon. Furthermore, we observed that the expression of SPDEF was not altered in the large intestine of Oasis−/− mice. These findings suggest that both genes are involved in the terminal differentiation of goblet cells but by independent pathways. Interestingly, the expression of CREB4 was significantly down-regulated both in Oasis−/− and Spdef−/− large intestine, indicating that CREB4 is a common downstream target of OASIS and SPDEF that may be crucial for the terminal differentiation of goblet cells (Fig. 7). The promoter region of CREB4 contains several SPDEF binding sites (core sequence, GGAA/T, Ref. 30). OASIS cannot bind to the SPDEF binding site in the promoter region of CREB4 because OASIS specifically binds to the cyclic AMP responsive element (23). Therefore, it is possible that regulation of CREB4 transcription by OASIS and SPDEF is not mutually exclusive and independent. However, to understand the detailed regulation of CREB4 transcription by OASIS and SPDEF, further examination is needed. CREB4 is an ER-resident transmembrane transcription factor that is structurally similar to OASIS and contains a bZIP domain in its cytoplasmic region. Because OASIS also possesses a bZIP domain, CREB4 may form a heterodimer with OASIS that promotes the expression of target genes required for the differentiation of goblet cells. Previous characterization of Creb4 knockout mice revealed anomalies in spermatogenesis (47), but a detailed analysis of the gut has not been conducted. Because CREB4 could be a key molecule for the maturation of goblet cells, further examination of Creb4−/− mice may enable further understanding of the molecular mechanisms involved in goblet cell differentiation.
Goblet cells in the large intestine produce and secrete abundant mucus, which provides a protective barrier against bacteria and other harmful stimuli. Defects in this barrier contribute to chronic diseases, including colitis and cancer. Muc2−/− mice have been observed to develop spontaneous colitis (48). Similarly, intestinal trefoil factor-deficient mice exhibit increased susceptibility to dextran sodium sulfate-induced colitis (49). Therefore, impairment of goblet cell maturation may be linked to inflammation of the intestinal mucosa. Because mature goblet cells in Oasis−/− mice are drastically decreased, it is possible that the mice are also sensitive to colitis. Targeted deletion of the ER stress response transcription factor XBP1 and the ER stress sensor IRE1β causes sensitivity to experimental colitis (50–52). However, the mechanisms of which are sensitive to colitis are not consistent with the case of mice deleted goblet cell differentiation-related genes such as Muc2 or Intestinal trefoil factor. In both Xbp1 and Ire1β knockout mice, severe ER stress occurred followed by apoptosis and chronic inflammation in goblet cells of the large intestine. We observed abnormally expanded rough ER in the goblet cells of Oasis−/− mice, suggesting that the function of the ER in Oasis−/− goblet cells may be impaired because of sensitivity to ER stress. Indeed, overexpression of OASIS was reported to protect against ER stress-induced cell death. Conversely, knockdown of OASIS caused elevated sensitivity to ER stress-induced cell death (17). Thus, OASIS may play roles not only in the differentiation of goblet cells but also in protecting these cells from ER stress. Although further examination into the relationship between OASIS function and the onset of colitis is required, it is possible that OASIS is a novel drug target for inflammatory bowel diseases such as ulcerous colitis.
Supplementary Material
Acknowledgments
We thank S. Nakagawa for technical support.
This work was partly supported by Japan Society for the Promotion of Science KAKENHI Grants 22020030 and 22800049 and by the Sumitomo Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Astellas Foundation for Research on Metabolic Disorders, Takeda Science Foundation, the Pharmacological Research Foundation Tokyo, the Daiichi-Sankyo Foundation of Life Science, and the Naito Foundation.

This article contains supplemental Tables 1 and 2.
A. Saito, submitted for publication.
- ER
- endoplasmic reticulum
- UPR
- unfolded protein response
- OASIS
- old astrocyte specifically induced substance
- bZIP
- basic leucine zipper
- CRE
- cAMP response element
- ATF
- activating transcription factor
- ETS
- E26 transformation-specific
- SPDEF
- SAM-pointed domain-containing ETS-like factor
- PAS
- periodic acid-Schiff
- CREB
- cyclic AMP-response element-binding protein.
REFERENCES
- 1. Rutkowski D. T., Kaufman R. J. (2004) A trip to the ER. Coping with stress. Trends Cell Biol. 14, 20–28 [DOI] [PubMed] [Google Scholar]
- 2. Zhang K., Kaufman R. J. (2008) From endoplasmic reticulum stress to the inflammatory response. Nature 454, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schröder M., Kaufman R. J. (2005) ER stress and the unfolded protein response. Mutat. Res. 569, 29–63 [DOI] [PubMed] [Google Scholar]
- 4. Ron D. (2002) Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 110, 1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman R. J. (2002) Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110, 1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 7. Li M., Baumeister P., Roy B., Phan T., Foti D., Luo S., Lee A. S. (2000) ATF6 as a transcription activator of the endoplasmic reticulum stress element. Thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 20, 5096–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshida H., Haze K., Yanagi H., Yura T., Mori K. (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273, 33741–33749 [DOI] [PubMed] [Google Scholar]
- 9. Ng D. T., Spear E. D., Walter P. (2000) The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J. Cell Biol. 150, 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., Walter P. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 [DOI] [PubMed] [Google Scholar]
- 12. Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 13. Rutkowski D. T., Hegde R. S. (2010) Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 189, 783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondo S., Saito A., Asada R., Kanemoto S., Imaizumi K. (2011) Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB Life 63, 233–239 [DOI] [PubMed] [Google Scholar]
- 16. Asada R., Kanemoto S., Kondo S., Saito A., Imaizumi K. (2011) The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 149, 507–518 [DOI] [PubMed] [Google Scholar]
- 17. Kondo S., Murakami T., Tatsumi K., Ogata M., Kanemoto S., Otori K., Iseki K., Wanaka A., Imaizumi K. (2005) OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol. 7, 186–194 [DOI] [PubMed] [Google Scholar]
- 18. Murakami T., Kondo S., Ogata M., Kanemoto S., Saito A., Wanaka A., Imaizumi K. (2006) Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J. Neurochem. 96, 1090–1100 [DOI] [PubMed] [Google Scholar]
- 19. Saito A., Hino S., Murakami T., Kondo S., Imaizumi K. (2007) A novel ER stress transducer, OASIS, expressed in astrocytes. Antioxid. Redox Signal. 9, 563–571 [DOI] [PubMed] [Google Scholar]
- 20. Honma Y., Kanazawa K., Mori T., Tanno Y., Tojo M., Kiyosawa H., Takeda J., Nikaido T., Tsukamoto T., Yokoya S., Wanaka A. (1999) Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Brain Res. Mol. Brain Res. 69, 93–103 [DOI] [PubMed] [Google Scholar]
- 21. Nikaido T., Yokoya S., Mori T., Hagino S., Iseki K., Zhang Y., Takeuchi M., Takaki H., Kikuchi S., Wanaka A. (2001) Expression of the novel transcription factor OASIS, which belongs to the CREB/ATF family, in mouse embryo with special reference to bone development. Histochem. Cell Biol. 116, 141–148 [DOI] [PubMed] [Google Scholar]
- 22. Chihara K., Saito A., Murakami T., Hino S., Aoki Y., Sekiya H., Aikawa Y., Wanaka A., Imaizumi K. (2009) Increased vulnerability of hippocampal pyramidal neurons to the toxicity of kainic acid in OASIS-deficient mice. J. Neurochem. 110, 956–965 [DOI] [PubMed] [Google Scholar]
- 23. Murakami T., Saito A., Hino S., Kondo S., Kanemoto S., Chihara K., Sekiya H., Tsumagari K., Ochiai K., Yoshinaga K., Saitoh M., Nishimura R., Yoneda T., Kou I., Furuichi T., Ikegawa S., Ikawa M., Okabe M., Wanaka A., Imaizumi K. (2009) Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 11, 1205–1211 [DOI] [PubMed] [Google Scholar]
- 24. Murakami T., Hino S., Nishimura R., Yoneda T., Wanaka A., Imaizumi K. (2011) Distinct mechanisms are responsible for osteopenia and growth retardation in OASIS-deficient mice. Bone 48, 514–523 [DOI] [PubMed] [Google Scholar]
- 25. Funamoto T., Sekimoto T., Murakami T., Kurogi S., Imaizumi K., Chosa E. (2011) Roles of the endoplasmic reticulum stress transducer OASIS in fracture healing. Bone 49, 724–732 [DOI] [PubMed] [Google Scholar]
- 26. Cheng H., Leblond C. P. (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 [DOI] [PubMed] [Google Scholar]
- 27. Gordon J. I. (1989) Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J. Cell Biol. 108, 1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pinto D., Gregorieff A., Begthel H., Clevers H. (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakamura T., Tsuchiya K., Watanabe M. (2007) Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J. Gastroenterol. 42, 705–710 [DOI] [PubMed] [Google Scholar]
- 30. Oettgen P., Finger E., Sun Z., Akbarali Y., Thamrongsak U., Boltax J., Grall F., Dube A., Weiss A., Brown L., Quinn G., Kas K., Endress G., Kunsch C., Libermann T. A. (2000) PDEF, a novel prostate epithelium-specific ETS transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J. Biol. Chem. 275, 1216–1225 [DOI] [PubMed] [Google Scholar]
- 31. Ghadersohi A., Sood A. K. (2001) Prostate epithelium-derived ETS transcription factor mRNA is overexpressed in human breast tumors and is a candidate breast tumor marker and a breast tumor antigen. Clin. Cancer Res. 7, 2731–2738 [PubMed] [Google Scholar]
- 32. Noah T. K., Kazanjian A., Whitsett J., Shroyer N. F. (2010) SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 316, 452–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q., Bermingham N. A., Finegold M. J., Zoghbi H. Y. (2001) Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 [DOI] [PubMed] [Google Scholar]
- 34. Shroyer N. F., Helmrath M. A., Wang V. Y., Antalffy B., Henning S. J., Zoghbi H. Y. (2007) Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132, 2478–2488 [DOI] [PubMed] [Google Scholar]
- 35. Gregorieff A., Stange D. E., Kujala P., Begthel H., van den Born M., Korving J., Peters P. J., Clevers H. (2009) The ETS-domain transcription factor SPDEF promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137, 1333–1345.e1–3 [DOI] [PubMed] [Google Scholar]
- 36. Hatayama H., Iwashita J., Kuwajima A., Abe T. (2007) The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 356, 599–603 [DOI] [PubMed] [Google Scholar]
- 37. Mills J. C., Gordon J. I. (2001) The intestinal stem cell niche. There grows the neighborhood. Proc. Natl. Acad. Sci. U.S.A. 98, 12334–12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaker A., Rubin D. C. (2010) Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl. Res. 156, 180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crosnier C., Vargesson N., Gschmeissner S., Ariza-McNaughton L., Morrison A., Lewis J. (2005) Δ-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132, 1093–1104 [DOI] [PubMed] [Google Scholar]
- 40. Jensen J., Pedersen E. E., Galante P., Hald J., Heller R. S., Ishibashi M., Kageyama R., Guillemot F., Serup P., Madsen O. D. (2000) Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 [DOI] [PubMed] [Google Scholar]
- 41. Shroyer N. F., Wallis D., Venken K. J., Bellen H. J., Zoghbi H. Y. (2005) Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 19, 2412–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bjerknes M., Cheng H. (2010) Cell Lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev. Biol. 345, 49–63 [DOI] [PubMed] [Google Scholar]
- 43. Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E., Mori M. (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fonseca S. G., Gromada J., Urano F. (2011) Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol. Metab. 22, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reimold A. M., Iwakoshi N. N., Manis J., Vallabhajosyula P., Szomolanyi-Tsuda E., Gravallese E. M., Friend D., Grusby M. J., Alt F., Glimcher L. H. (2001) Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 [DOI] [PubMed] [Google Scholar]
- 46. Iwakoshi N. N., Lee A. H., Vallabhajosyula P., Otipoby K. L., Rajewsky K., Glimcher L. H. (2003) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 [DOI] [PubMed] [Google Scholar]
- 47. Adham I. M., Eck T. J., Mierau K., Müller N., Sallam M. A., Paprotta I., Schubert S., Hoyer-Fender S., Engel W. (2005) Reduction of spermatogenesis but not fertility in Creb3l4-deficient mice. Mol. Cell Biol. 25, 7657–7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heazlewood C. K., Cook M. C., Eri R., Price G. R., Tauro S. B., Taupin D., Thornton D. J., Png C. W., Crockford T. L., Cornall R. J., Adams R., Kato M., Nelms K. A., Hong N. A., Florin T. H., Goodnow C. C., McGuckin M. A. (2008) Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mashimo H., Wu D. C., Podolsky D. K., Fishman M. C. (1996) Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274, 262–265 [DOI] [PubMed] [Google Scholar]
- 50. Bertolotti A., Wang X., Novoa I., Jungreis R., Schlessinger K., Cho J. H., West A. B., Ron D. (2001) Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J. Clin. Invest. 107, 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaser A., Blumberg R. S. (2011) Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology 140, 1738–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.