Background: Leptin promotes breast tumor progression and is overexpressed in breast tumors.
Results: Leptin induces EMT in breast cancer cells via concurrent modulation of Akt/GSK3β and MTA1/Wnt1 axes to mediate β-catenin activation.
Conclusion: β-Catenin is required for leptin-induced EMT in breast cancer cells.
Significance: Learning how leptin regulates EMT is important for understanding leptin-mediated breast cancer growth and metastasis.
Keywords: Adipokines, Breast Cancer, Epithelial-Mesenchymal Transition, Leptin, Signal Transduction, MTA1
Abstract
Perturbations in the adipocytokine profile, especially higher levels of leptin, are a major cause of breast tumor progression and metastasis; the underlying mechanisms, however, are not well understood. In particular, it remains elusive whether leptin is involved in epithelial-mesenchymal transition (EMT). Here, we provide molecular evidence that leptin induces breast cancer cells to undergo a transition from epithelial to spindle-like mesenchymal morphology. Investigating the downstream mediator(s) that may direct leptin-induced EMT, we found functional interactions between leptin, metastasis-associated protein 1 (MTA1), and Wnt1 signaling components. Leptin increases accumulation and nuclear translocation of β-catenin leading to increased promoter recruitment. Silencing of β-catenin or treatment with the small molecule inhibitor, ICG-001, inhibits leptin-induced EMT, invasion, and tumorsphere formation. Mechanistically, leptin stimulates phosphorylation of glycogen synthase kinase 3β (GSK3β) via Akt activation resulting in a substantial decrease in the formation of the GSK3β-LKB1-Axin complex that leads to increased accumulation of β-catenin. Leptin treatment also increases Wnt1 expression that contributes to GSK3β phosphorylation. Inhibition of Wnt1 abrogates leptin-stimulated GSK3β phosphorylation. We also discovered that leptin increases the expression of an important modifier of Wnt1 signaling, MTA1, which is integral to leptin-mediated regulation of the Wnt/β-catenin pathway as silencing of MTA1 inhibits leptin-induced Wnt1 expression, GSK3β phosphorylation, and β-catenin activation. Furthermore, analysis of leptin-treated breast tumors shows increased expression of Wnt1, pGSK3β, and vimentin along with higher nuclear accumulation of β-catenin and reduced E-cadherin expression providing in vivo evidence for a previously unrecognized cross-talk between leptin and MTA1/Wnt signaling in epithelial-mesenchymal transition of breast cancer cells.
Introduction
A growing body of evidence suggests a pivotal role of leptin in breast tumor progression and metastasis (1–7). Leptin exerts its biological functions through its specific transmembrane receptors (LEPR)2 expressed widely in a variety of tissues transducing leptin-mediated downstream signaling events (8). Leptin and LEPR are highly abundant in human breast tumor tissues compared with benign or normal tissues (9, 10). The degree of leptin mRNA expression in the peritumoral adipose tissue is significantly higher in the breast cancer patients than the control women. Also, a positive relationship exists between blood leptin levels and breast cancer risk (11). In vivo studies evaluating the effect of leptin on breast cancer progression utilizing genetic loss-of-function mutants for leptin or the LEPR show that leptin or LEPR-deficient MMTV-transforming growth factor-α (TGF-α) mice do not develop oncogene-induced mammary tumors (12, 13). Recently, hypothalamic LEPR-B (long-form LEPR)-reconstituted db/db (LEPR-null) mice (db/dbNse+/+) (14) crossed with MMTV-PyMT mice show that an LEPR-B-mediated signaling promotes mammary tumor growth and metastasis (15). Diet-induced obese mouse models have been used for manipulating leptin levels in vivo. Obese MMTV-TGFα mice indeed show elevated levels of leptin as well as faster growth of mammary tumors (16). Also, xenografts of MMTV-Wnt1 tumors transplanted in diet-induced obese mice grow faster as compared with lean counterparts (17). Consistent with the important role of leptin in breast tumorigenesis, xenografts of MMTV-Wnt1 cancer cells transplanted into leptin-deficient obese (Ob/Ob) mice display stunted growth (18). Cellular studies from our laboratory and others have shown that leptin signaling regulates various molecules involved in proliferation, adhesion, invasion, migration, inflammation, and angiogenesis, such as cyclin D1, survivin, β3 integrin, interleukin-1 (IL-1), IL-1 receptor, vascular endothelial growth factor, and its receptor type 2 in breast carcinogenesis (6, 19–25). Leptin regulates the cell cycle and increases breast cancer cell growth by inducing cyclin D1 expression modulating its local chromatin structure recruiting specific coactivator complexes, including Med1 and SRC1 (19). It has also been shown that leptin cross-talks with other contributors of mammary tumorigenesis and progression, including IGF1, IL6, Notch, and sex hormones (3, 6, 21, 26), and enhances invasion potential. Our recent work shows that leptin increases survivin expression, via activation of EGFR-Notch1 axis, leading to enhanced breast cancer cell migration (6). Hence, highly active leptin-induced signaling could contribute to various aspects of tumorigenesis and metastasis by modulating various key regulatory steps. However, the involvement of leptin in epithelial-mesenchymal transition (EMT: an early step during tumor metastasis) remains elusive. Given the association between leptin and high metastatic potential, we speculate that leptin promotes EMT in breast cancer cells.
EMT is a crucial step in the induction of cell motility and enhanced survival during physiological processes such as embryonic development and wound healing as well as in pathological situations like malignant cells undergoing invasion and metastasis (27). A hallmark of EMT is down-regulation of epithelial markers such as E-cadherin that can be achieved by transcriptional suppression mediated by transcription factors Twist, Snail, and Slug (28–31). Disruption of cell-cell adhesion and increase in nuclear β-catenin that also functions as a transcription factor for TCF/LEF-regulatory genes has been observed in EMT (32–34). Because leptin modulates the expression of various gene products that are regulated by β-catenin and potentiates tumor cell migration and invasion, which are also in part regulated by β-catenin, we postulated that leptin might mediate its effects on EMT through modulation of the Wnt/β-catenin pathway. To test this hypothesis, we investigated the effect of leptin on β-catenin activation. In this report, we show that leptin increases activation of β-catenin that is integral to leptin-induced EMT and tumorsphere formation. We found that leptin treatment induced concomitant activation of Akt/GSK3 and MTA1/Wnt1 signaling leading to destruction of the LKB1-GSK3β-Axin complex and stabilization of β-catenin. In addition, we provide molecular evidence supporting the regulatory role of MTA1 in leptin-induced Wnt1 up-regulation, β-catenin stabilization, and nuclear translocation. This study provides in vitro as well as in vivo evidence that leptin promotes EMT, which is implicated in breast cancer progression to invasive and metastatic state.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
The human breast cancer cell lines MCF7, MDA-MB-231, MDA-MB-468, and MCF-10A were obtained from the American Type Culture Collection (ATCC) and cultured according to supplier's instructions. Cell line authentication was done by analysis of known genetic markers or response (e.g. expression of estrogen receptor and p53 and estrogen responsiveness). MDA-MB-231 cell line is a highly invasive “basal B” type and estrogen-independent fibroblastic human breast cancer cell line with stellate morphology. MCF7 cell line is a well accepted representative of estrogen receptor-positive “luminal” type breast cancer and exhibits epithelial phenotype. MDA-MB-468 is a moderately aggressive, estrogen receptor-negative human breast cancer cell line with “basal A” type breast cancer. For treatment, cells were seeded at a density of 1 × 106/100-mm tissue culture dish. After 16 h of serum starvation, the culture media were changed to serum-free media containing treatments as indicated. Cultures were treated with human recombinant leptin (Sigma) at 100 ng/ml. In other sets of experiments, cells were treated with phosphatidylinositol 3-kinase inhibitor LY294002 (Cell Signaling tumorsphere) at 10 μm. Antibodies for β-catenin, cyclin D1, p-GSK3β (phospho-GSK3β), GSK3β, p-Akt (phospho-Akt), Akt, Wnt1, MTA1, tubulin, histone, vimentin, E-cadherin, N-cadherin, Occludin, Snail, and Slug were purchased from Cell Signaling Technology and Santa Cruz Biotechnology. ICG-001 was obtained from Enzo Life Sciences (Farmingdale, NY).
Tumorsphere Assay
Cells were plated at an initial density of 1 × 104 cells/well as a single cell suspension into 6-well plates coated with 1.2% poly-(2-hydroxyethyl methacrylate). Cells were grown as suspension cultures for 1–2 weeks for tumorsphere formation. Colonies were counted in 10 randomly selected fields at 10× magnification using Olympus IX50 inverted microscope.
Western Blotting
Whole cell lysate was prepared by scraping MCF7 and MDA-MB-231 cells in 250 μl of ice-cold modified RIPA buffer (21). Equal amount of lysate protein was resolved on SDS-polyacrylamide gel and transferred to nitrocellulose membrane, and Western blot analysis was performed. Immunodetection was performed using enhanced chemiluminescence (ECL system, Amersham Biosciences) according to the manufacturer's instructions.
Scratch Migration Assay
Migration assay was performed according to our published protocol (6). Cells were treated with leptin as indicated. Plates were photographed after 24 and 48 h at the identical location of the initial image.
Invasion Assay
For an in vitro model system for metastasis, a Matrigel invasion assay was performed by using a Matrigel invasion chamber from BD Biocoat Cellware (San Jose, CA) (35). The slides were coded to prevent counting bias, and the number of invaded cells on representative sections of each membrane were counted under a light microscope. The number of invaded cells for each experimental sample represents the average of triplicate wells.
Preparation of Subcellular Fractions
Cellular cytosolic and nuclear fractions were prepared by incubating cells in 100 μl of ice-cold lysis buffer (10 mm Tris-HCl (pH 7.4), 10 mm NaCl, 3 mm MgCl2, 0.5% Nonidet P-40, 2 mm DTT, and 0.1 mm PMSF). The lysates were incubated for 5 min on ice followed by centrifugation at 4,000 × g for 10 min at 4 °C to precipitate nuclei. Supernatant was stored as a cytoplasmic fraction. Nuclear pellet was incubated with 100 μl of ice-cold extraction buffer (20 mm Tris-HCl (pH 7.9), 0.42 m KCl, 0.2 mm EDTA, 10% glycerol, 2 mm DTT, and 0.1 mm PMSF) for 10 min followed by centrifugation at 12,000 × g for 10 min at 4 °C to clear the nuclear debris. Total protein was quantified using the Bradford protein assay kit (Bio-Rad). Equal amount of protein was subjected to Western blot analysis.
β-Catenin Stable Knockdown Using Lentiviral Short Hairpin RNA
Pre-made lentiviral β-catenin short hairpin RNA (shRNA) constructs and a negative control construct created in the same vector system (pLKO.1) were purchased from Open Biosystems (Huntsville, AL). Paired β-catenin stable knockdown cells were generated following our previously established protocol (36).
Chromatin Immunoprecipitation (ChIP)
ChIP analyses were performed using our published procedure with the following modifications (37). Chromatin samples were sonicated on ice three times for 10 s each (i.e. until the average length of sheared genomic DNA was 1–1.5 kb) followed by centrifugation for 10 min. The immunoprecipitated DNA was ethanol-precipitated and resuspended in 25 μl of water. Total input samples were resuspended in 100 μl of water and diluted 1:100 before PCR analysis. Initially, PCR was performed with different numbers of cycles and/or dilutions of input DNA to determine the linear range of amplification; all results shown fall within this range. Following 28–30 cycles of amplification, PCR products were run on 1% agarose gel and analyzed by ethidium bromide staining.
RNA Interference
For RNA interference, cells were seeded in 6-well plates and transfected at 50% confluency with 100 nm of control siRNA or targeted siRNA (as indicated) (Invitrogen) using Oligofectamine according to the manufacturer's instructions. Twenty four hours after transfection, the cells were treated with leptin or left untreated as indicated. Forty eight hours post-transfection, the cells were harvested, and luciferase and Renilla activities were measured using a dual-luciferase kit (Promega, Madison, WI). The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to Renilla luciferase activities.
Immunoprecipitation
For immunoprecipitation, whole cell lysates were incubated with specific antibodies for LKB1 and Axin, and the mixture was rotated slowly at 4 °C for 16 h. A total of 20 μl of packed protein A/G-agarose beads was added, and the mixture was incubated at 4 °C for 1 h with rotation. The beads were collected by gentle centrifugation and washed twice with 1.5 ml of ice-cold buffer (50 mm Tris-Cl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin). After the final wash, the precipitated protein-beads complexes were resuspended in SDS sample loading buffer, fractionated by SDS-PAGE, and transferred to nitrocellulose membrane. Immunodetection was performed by blocking the membranes for 1 h in TBS buffer (20 mm Tris-Cl (pH 7.5), 137 mm NaCl, 0.05% Tween 20) containing 5% powdered nonfat milk followed by addition of the primary antibody (as indicated) in TBS for 2 h at room temperature. Specifically bound primary antibodies were detected with peroxidase-coupled secondary antibodies and developed by enhanced chemiluminescence (ECL system, Amersham Biosciences) according to the manufacturer's instructions.
Immunofluorescence and Confocal Imaging
Breast cancer cells (5 × 105 cells/well) were plated in 4-well chamber slides (Nunc, Rochester, NY) followed by treatment with leptin and subjected to immunofluorescence analysis as described. Fixed and immunofluorescently stained cells were imaged using a Zeiss LSM510 Meta (Zeiss) laser scanning confocal system configured to a Zeiss Axioplan 2 upright microscope with a 63× (NA 1.4) plan-apochromat objective. All experiments were performed multiple times using independent biological replicates.
Immunohistochemical Analysis
We have shown previously that leptin administration increases growth of MDA-MB-231 cells implanted in female athymic nude mice (6). Briefly, MDA-MB-231 (5 × 106) cells in 0.1 ml of Hanks' balanced saline solution were subcutaneously injected into the right gluteal region of 4–6-week-old female athymic nude mice. After 2 weeks of initial implantation, animals were placed into two experimental groups. Mice were treated with intraperitoneal injections of saline or recombinant leptin (dosage of 5 mg/kg), 5 days a week for the duration of the experiment. Tumors were measured using vernier calipers, with tumor volume calculated using the formula (V = a/2 × b2), where V is the tumor volume in mm3, and a and b are the largest and smallest diameters in millimeters, respectively. All animals were killed after 6 weeks of treatment. Tumors were collected, weighed, fixed in 10% neutral-buffered formalin and subjected to further analysis by immunohistochemistry. All animal studies were conducted in accordance with the guidelines of IACUC. We used tumor sections from the same study to determine the effect of leptin on expression of β-catenin, Wnt1, pGSK3β, vimentin and Ki-67 by immunohistochemistry. Immunohistochemistry was performed essentially as described by us previously for other proteins (6). At least four nonoverlapping representative images from each tumor section from five mice of each group were captured using ImagePro software for quantitation of β-catenin, Wnt1, pGSK3β, vimentin, and Ki-67 expression.
RNA Isolation, RT-PCR
Total cellular RNA was extracted using the TRIzol reagent kit (Invitrogen). RT-PCR was performed using specific sense and antisense PCR primers.
Statistical Analysis
All experiments were performed three times in triplicate. Statistical analysis was performed using Microsoft Excel software. Significant differences were analyzed using Student's t test and two-tailed distribution. Results were considered to be statistically significant if p < 0.05. Results were expressed as mean ± S.E. between triplicate experiments performed three times.
RESULTS
Leptin Exposure Induces Epithelial-Mesenchymal Transition in Breast Cancer Cells
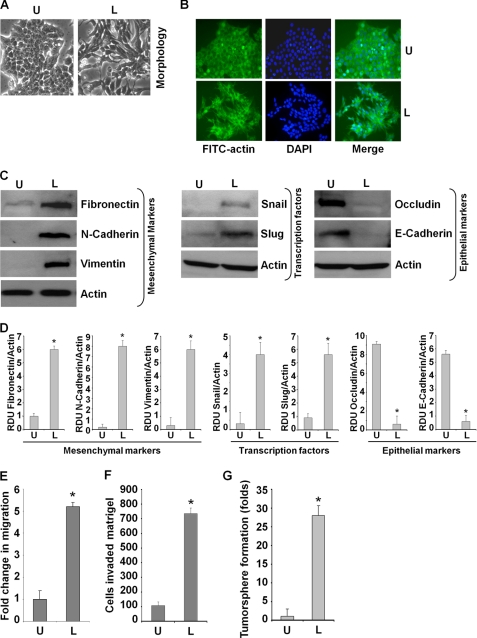
Epithelial to mesenchymal transition of cancer cells is an early event leading to local invasion and distant metastasis. Despite accumulating evidence showing a strong link between high leptin levels and cancer invasion and metastasis, the specific involvement of leptin in EMT remains elusive. Therefore, we aimed to examine whether leptin stimulation induced EMT in breast cancer cells. Following treatment with leptin for 3 and 5 days, blinded investigators observed morphological differences in leptin-treated MCF7 cells as compared with untreated cells. The phenotypic changes observed included acquisition of fibroblast-like appearance and increased formation of pseudopodia observed emanating from the cell membrane (Fig. 1A). In addition, leptin stimulation led to reorganization of actin from a predominantly membrane-bound location to newly formed pseudopodia. Actin was also observed to form stress fibers throughout the cytoplasm following leptin treatment (Fig. 1B). These changes represent typical mesenchymal phenotype rather than the usual epithelial phenotype of MCF7 cells, indicating that MCF7 cells have undergone EMT upon leptin treatment. To unequivocally establish that leptin-induced EMT changes were not restricted to MCF7 cells, we next examined the effect of leptin treatment in MDA-MB-468 and MDA-MB-231 cells. As evident in supplemental Fig. 2, MDA-MB-468 cells undergo distinct morphological changes upon leptin treatment acquiring spindle cell-like appearance and increased intracellular separation signifying loss of intercellular adhesion as opposed to cobblestone-like shape, a typical feature of epithelial cells, displayed by untreated cells (supplemental Fig. S2A). MDA-MB-231 cells are highly invasive “basal” type breast cancer cells that exhibit fibroblast-like morphology. Upon exposure to leptin, MDA-MB-231 cells retained their fibroblast-like morphology and acquired elongated pseudopodia (supplemental Fig. S2A).
FIGURE 1.
Leptin induces epithelial-mesenchymal transition, tumorsphere formation, migration, and invasion of breast cancer cells. A, MCF7 cells were serum-starved for 16 h followed by treatment with 100 ng/ml leptin (L) or were untreated (U) for 3 days. Morphological changes associated with EMT are shown in phase-contrast images. The presence of spindle-shaped cells, increased intercellular separation, and pseudopodia were noted in treated cells but not in untreated cells. B, MCF7 cells were treated as in A. Fluorescent confocal microscopy showed the alterations in the cytoskeleton by leptin treatment. Reorganization of actin into pseudopodia and stress fibers were noted in leptin-treated cells. Nuclei were visualized with DAPI staining. C, MCF7 cells were treated with 100 ng/ml leptin (L) or were untreated (U). Total lysates were immunoblotted for fibronectin, N-cadherin, vimentin, Snail, Slug, Occludin, and E-cadherin expression levels. Leptin treatment increased fibronectin, N-cadherin, vimentin, Snail, and Slug expression, whereas E-cadherin and occludin expression was decreased. Actin was used as control. D, densitometric analysis was performed on multiple blots for each target protein. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for target proteins normalized to actin. *, p < 0.005 compared with untreated controls. E, MCF7 cells were subjected to the scratch-migration assay in the absence (U) or presence of 100 ng/ml leptin (L) for 24 h. Fold-change in migration is shown in bar graphs. *, p < 0.005, compared with untreated controls. F, MCF7 cells were cultured in Matrigel invasion chambers followed by treatment with leptin for 24 h. The number of cells that invaded through the Matrigel was counted in five different regions. The slides were blinded to remove counting bias. The results show mean of three independent experiments performed in triplicate. *, p < 0.005 compared with untreated controls (U). G, MCF7 cells were subjected to tumorsphere formation in the absence (U) or presence of 100 ng/ml leptin (L) as indicated. Number of tumorsphere was counted in five different views under a microscope. The results are presented as fold-change with untreated. *, p < 0.005, compared with untreated controls.
A biochemical hallmark of EMT is loss of expression of epithelial marker proteins such as E-cadherin and keratin-18 with a concurrent increase in mesenchymal marker expression (e.g. vimentin and fibronectin) (38, 39). Exposure of MCF7 cells to leptin resulted in up-regulation of mesenchymal marker (vimentin, N-cadherin, and fibronectin) expressions that was accompanied by a marked decrease in E-cadherin and occludin expression (Fig. 1, C and D). MDA-MB-468 cells exhibited induction of fibronectin expression along with a decrease in keratin-18 expression levels. Basal expression of fibronectin was higher in MDA-MB-231 cells, which further increased upon leptin stimulation (supplemental Fig. S2B). We next examined whether E-cadherin repressors, Snail and Slug, may be involved in leptin-induced EMT. Western blot analysis showed that leptin treatment increased the expression of Snail and Slug (Fig. 1, C and D). Twist, a basic helix-loop-helix transcription factor, and Zeb1 function as transcriptional repressors for epithelial marker proteins, are frequently detected in metastatic cancer cells, and are known to be involved in EMT (28, 29, 38). Treatment of MCF7 cells with leptin increased mRNA levels of Snail, Slug, Zeb1, and Twist (supplemental Fig. S1A). Alteration in cytoplasmic-nuclear translocation of Snail and Slug has been observed in EMT (34, 38). Indeed, leptin treatment not only increased the expression of Snail and Slug but also increased nuclear translocation of these transcription factors (supplemental Fig. S1B). Similarly, increased expression of Snail and Slug was observed in MDA-MB-468 and MDA-MB-231 cells upon leptin treatment (supplemental Fig. S2B). Consistent with these molecular changes, cell motility and invasion potential was significantly enhanced in leptin-treated MCF7 (Fig. 1, E and F), MDA-MB-468, and MDA-MB-231 (supplemental Fig. S2, C and D).
According to the stem cell hypothesis, tumors can arise from a small set of self-renewing cells termed cancer stem cell or progenitor or cancer-initiating cells, and interestingly, many laboratories have shown that EMT can endow cells with stem-like characteristics (40–42). Induction of EMT in immortalized human mammary epithelial cells, treatment with transforming growth factor-β, or overexpression of key EMT inducers such as Snail and Twist result in an increased ability to form tumorspheres and increased expression of CD44 (40, 41, 43). Tumorsphere formation assays rely on the unique property of cancer cells with stem cell-like potential to form large, round, unattached floating spheroid colonies (termed tumorsphere), and it has been successfully used to establish long term cultures enriched in stem cells from invasive tumor samples. Leptin treatment induces EMT changes as well as an increase in migration and invasion potential; therefore, we hypothesize that leptin treatment may induce tumorsphere formation in breast cancer cells. Indeed, leptin treatment induced about a 25–31-fold enhancement in tumorsphere formation (Fig. 1G).
Leptin Induces Activation of β-Catenin in Breast Cancer Cells
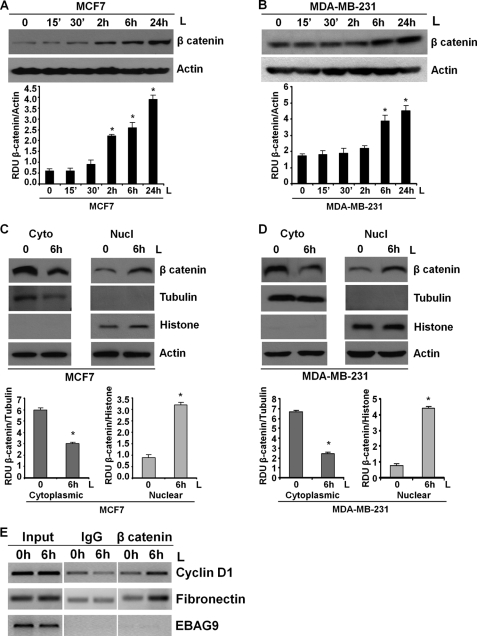
Aberrant activation of β-catenin has been reported in many malignancies, including breast cancer (44, 45). Although up-regulation of nuclear/cytoplasmic β-catenin is found in 40–60% of human breast tumors and is correlated with poor prognosis (46, 47), genetic mutations of APC, Axin, or β-catenin as reported in other tumors are rarely observed in breast cancer (48). These observations suggest that alternate/additional mechanisms may lead to β-catenin activation. Also, β-catenin has been shown as an important regulator of EMT and tumorsphere formation (27, 32, 33). We questioned whether leptin modulates β-catenin expression and activity. Leptin exposure increased the expression of β-catenin in a temporal manner with a significant increase observed at 2–6 h post-leptin treatment intervals, although a subtle increase was observed as early as 15-min post-leptin treatment in MCF7 cells (Fig. 2A). MDA-MB-231 cells also showed a significant increase in β-catenin expression upon leptin treatment (Fig. 2B). Once stabilized, β-catenin translocates to the nucleus and participates in transcriptional activation of responsive genes critical for tumor cell EMT via interacting with TCF/LEF (49, 50). Western blot analysis of nuclear and cytoplasmic fractions from leptin-treated cells showed that leptin treatment increased nuclear translocation of β-catenin as evidenced by elevated accumulation of β-catenin in the nuclear fraction of leptin-treated cells as compared with untreated cells (Fig. 2, C and D). The purity of nuclear and cytoplasmic fractions was examined by tubulin and histone expression. Next, we used chromatin immunoprecipitation analyses to examine the recruitment of β-catenin to specific TCF/LEF-responsive promoters upon leptin treatment. Cyclin D1 and fibronectin represent two important TCF/LEF-responsive genes that play key roles in breast cancer growth and metastatic progression. Leptin increased the expression of cyclin D1 and fibronectin in breast cancer cells in a temporal manner as evident by RT-PCR and Western blot analysis (supplemental Figs. S3, A and B, and S4). ChIP analysis clearly showed that leptin treatment induced binding of β-catenin to cyclin D1 and fibronectin gene promoters at TCF/LEF-binding sites. EBAG9 was used as a negative control (Fig. 2E). We previously showed that leptin induces recruitment of Stat3 to cyclin D1 promoter in breast cancer cells (19). Recruitment of Stat3 to cyclin D1 promoter in a ChIP assay was used to standardize the conditions for leptin stimulation (supplemental Fig. S3C).
FIGURE 2.
Leptin increases expression, nuclear accumulation, and recruitment of β-catenin to responsive gene promoters. A, MCF7; B, MDA-MB-231 cells were treated with 100 ng/ml leptin for indicated time intervals. Total lysates were immunoblotted for β-catenin expression. The membranes were re-blotted using actin antibody as control. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for β-catenin normalized to actin. *, p < 0.005 compared with untreated controls. Leptin (L) increases the expression of β-catenin. C and D, breast cancer cells were treated with 100 ng/ml leptin for the indicated time intervals followed by nuclear (Nucl) and cytoplasmic (Cyto) extract isolation. Equal amounts of proteins were immunoblotted using antibodies against β-catenin. The membranes were re-blotted using tubulin, histone, and actin antibodies as controls. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for β-catenin normalized to either tubulin or histone. *, p < 0.005 compared with untreated controls. Leptin treatment induces nuclear accumulation of β-catenin. E, soluble chromatin was prepared from breast cancer cells treated and untreated with leptin as indicated and immunoprecipitated with 5 μg of specific antibodies against β-catenin overnight at 4 °C. The immune complexes were pulled down with protein A-agarose/salmon sperm DNA beads and washed extensively as described under “Experimental Procedures,” and cross-linking was reversed. The purified DNA was analyzed by PCR using primers spanning the TCF/LEF site at cyclin D1 and fibronectin promoter. EBAG9 was used as a control gene. Leptin increases recruitment of β-catenin to responsive gene promoters.
Leptin Induces Concomitant Activation of Akt-GSK3β and Wnt-GSK3β Axes to Mediate β-Catenin Activation
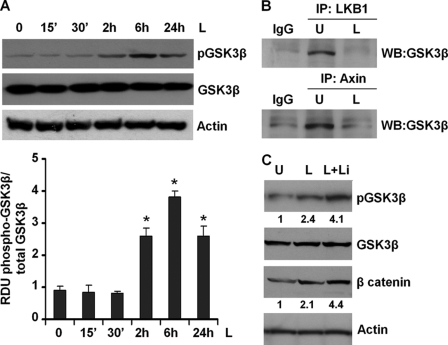
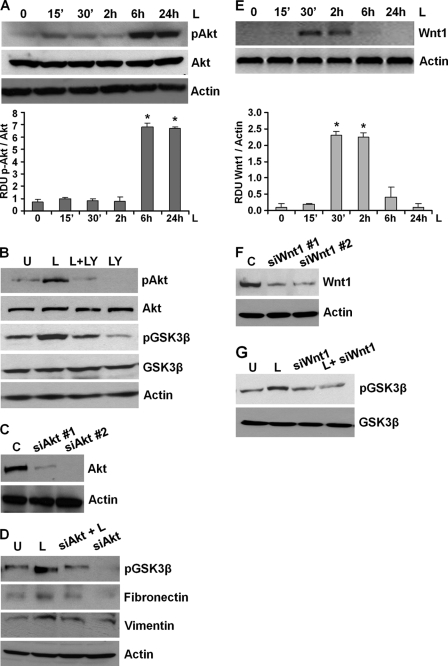
Glycogen synthase kinase-3β (GSK3β), a widely expressed serine/threonine kinase, regulates numerous cellular functions, including cytoskeletal rearrangement, apoptosis, gene expression, and proliferation in response to growth factor or hormonal stimulation (51). Unlike most kinases, GSK-3β is catalytically active in resting cells. GSK3β enzymatic activity is inhibited by phosphorylation leading to modulation of downstream targets (51). We investigated whether GSK3β inactivation is involved in mediating the ability of leptin to stabilize β-catenin. As shown in Fig. 3A, induction with leptin resulted in increased phosphorylation of GSK3β. In the absence of an upstream stimulus, β-catenin is sequestered in an inactive state by a multimeric “destruction complex” composed of GSK3β, LKB1, Axin, and APC (50, 52). APC and Axin function as scaffold proteins, allowing GSK3β-mediated phosphorylation of critical residues of β-catenin hence marking β-catenin for ubiquitination and subsequent proteasomal degradation (52). We questioned whether leptin-mediated GSK3β phosphorylation led to disintegration of the “destruction complex.” To address this question, breast cancer cells were treated with leptin for 6 h followed by immunoprecipitation with LKB1 or Axin antibodies, representing two important components of the complex. Immunoprecipitated protein complexes were analyzed for the presence of GSK3β using Western blot analysis. GSK3β immunoprecipitated with LKB1 and Axin in the absence of leptin treatment indicated the presence of an intact destruction complex, and leptin treatment led to dissociation of LKB1-GSK3β and Axin-GSK3β protein complexes (Fig. 3B). Cotreatment of breast cancer cells with leptin and LiCl, a known inhibitor of GSK3β, further increased GSK3β phosphorylation as compared with leptin treatment alone and resulted in increased accumulation of β-catenin (Fig. 3C). Studies in different cells have suggested that Akt/protein kinase B phosphorylates GSK3β rendering it inactive (51). We previously showed that leptin concomitantly activates multiple signaling pathways, including Akt in multiple cancers (20, 21). We asked whether leptin-mediated GSK3β inactivation and involvement in metastatic properties involved Akt. As expected, we observed Akt activation upon leptin treatment (Fig. 4A). Pretreatment of breast cancer cells with the Akt inhibitor LY294002 not only inhibited leptin-induced Akt phosphorylation but also reduced GSK3β phosphorylation (Fig. 4B). Next, we silenced Akt in breast cancer cells using Akt-siRNA (Fig. 4C). Akt depletion reduced leptin-mediated increase in GSK3β phosphorylation (Fig. 4D). Akt silencing also reduced two important mesenchymal markers, resulting in decreased fibronectin and vimentin expression even in the presence of leptin treatment (Fig. 4D).
FIGURE 3.
Leptin elevates phosphorylation of GSK3β and decreases the formation of GSK3β-LKB1-Axin complex. A, MCF7 breast cancer cells were treated with 100 ng/ml leptin for indicated time intervals. Total lysates were immunoblotted using specific antibodies for phospho-GSK3β. The membranes were re-blotted using total GSK3β and actin antibodies as control. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for phosphorylated GSK3β normalized to total GSK3β. *, p < 0.005 compared with untreated controls. Leptin treatment increases phosphorylation of GSK3β. B, breast cancer cells were untreated (U) or treated with 100 ng/ml leptin (L) and subjected to immunoprecipitation (IP) analysis using specific antibodies against LKB1 and Axin. The immunoprecipitates were analyzed by immunoblot analysis using anti-GSK3β antibodies. Immunoprecipitation with IgG was used as control. Leptin decreases the formation of the GSK3β-LKB1-Axin complex. C, breast cancer cells were treated with 100 ng/ml leptin alone (L) and in combination with LiCl (L+Li). Untreated cells are denoted with U. Total lysates were immunoblotted using antibodies against phospho-GSK3β and β-catenin. The membranes were re-blotted using total GSK3β and actin antibodies as control. The blots are representative of multiple independent experiments. Combined treatment with leptin and LiCl further increases β-catenin expression. WB, Western blot.
FIGURE 4.
Leptin-mediated increase in GSK3β phosphorylation involves activation of Akt as well as Wnt1. A, breast cancer cells were treated with 100 ng/ml leptin for indicated time intervals. Total lysates were immunoblotted for phospho-Akt expression. The membranes were re-blotted using total Akt and actin antibodies as control. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for phosphorylated Akt normalized to total Akt. *, p < 0.001 compared with untreated controls. Leptin treatment increases phosphorylation of Akt. B, breast cancer cells were untreated (U) or treated with 100 ng/ml leptin alone (L), 10 μm Akt inhibitor LY294002 alone (LY), and in combination (L+LY). Total lysates were immunoblotted for phospho-GSK3β and phospho-Akt expression levels. The membranes were re-blotted using total GSK3β, total Akt, and actin antibodies as control. LY294002 reduces leptin-induced phosphorylation of GSK3β. C, breast cancer cells were transiently transfected with siAkt#1 or siAkt#2 siRNAs (Invitrogen) using FuGENE (Invitrogen). Immunoblot analysis was performed using Akt antibodies, and actin antibodies were used as control. D, breast cancer cells were transfected with siAkt#2 for 24 h followed by leptin treatment for 6 h. Total lysates were subjected to immunoblot analysis using specific antibodies for phospho-GSK3β, fibronectin, and vimentin. The membranes were re-blotted using actin. The blots are representative of multiple independent experiments. Akt silencing inhibits leptin-induced GSK3β phosphorylation, fibronectin, and vimentin expression. E, breast cancer cells were treated with 100 ng/ml leptin for indicated time intervals. Total lysates were immunoblotted using anti-Wnt1 antibodies. Immunoblots for actin levels were used as control. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for Wnt1 normalized to actin. *, p < 0.005 compared with untreated controls. Leptin increases Wnt1 expression. F, breast cancer cells were transiently transfected with siWnt1#1 or siWnt1#2 siRNAs (Invitrogen) using FuGENE (Invitrogen). Immunoblot analysis was performed using Wnt1 antibodies, and actin antibodies were used as control. G, breast cancer cells were transfected with siWnt1#1 for 24 h followed by leptin treatment for 6 h. Total lysates were subjected to immunoblot analysis using specific antibodies for phospho-GSK3β. The membranes were re-blotted using total GSK3β. The blots are representative of multiple independent experiments. Wnt1 inhibition inhibits leptin-induced GSK3β phosphorylation.
It is interesting to note that completely suppressing phosphorylated Akt levels in leptin-treated cells reduced leptin-induced GSK3β phosphorylation but did not totally inhibit it. As evident, leptin was able to stimulate GSK3β phosphorylation in the absence of phosphorylated Akt, although to a lesser extent (Fig. 4, B and D), indicating the presence of an Akt-independent mechanism for leptin-induced GSK3β phosphorylation. Also, significant leptin-induced GSK3β phosphorylation was observed at 2 h post-leptin treatment prior to leptin-induced Akt phosphorylation (Figs. 3A and 4A). Phosphorylation of GSK3β and inhibition of its ability to phosphorylate β-catenin are also regulated by cytoplasmic signaling events initiated by Wnt1 acting as a paracrine and/or autocrine factor (53). Leptin has been known to cross-talk with multiple signaling pathways to impact a wide variety of cellular systems and biological processes (3, 6, 20, 21). Hence, we investigated whether Wnt1 played a role in mediating leptin-stimulated GSK3β phosphorylation in breast cancer cells. Exposure of breast cancer cells with leptin resulted in increased expression of Wnt1 within 30 min of leptin treatment, which was diminished after 6 h returning to basal levels at 24 h post-treatment (Fig. 4E). To evaluate the contribution of the increased Wnt1 expression in breast cancer cells toward leptin-induced GSK3β phosphorylation, we next performed the Western blot analysis for phosphorylated GSK3β levels after Wnt1 knockdown by Wnt1-siRNA. Results indicated that indeed Wnt1 knockdown (as shown in Fig. 4F) reduced leptin-induced GSK3β phosphorylation (Fig. 4G), suggesting the involvement of Wnt1 in the stimulatory effect of leptin.
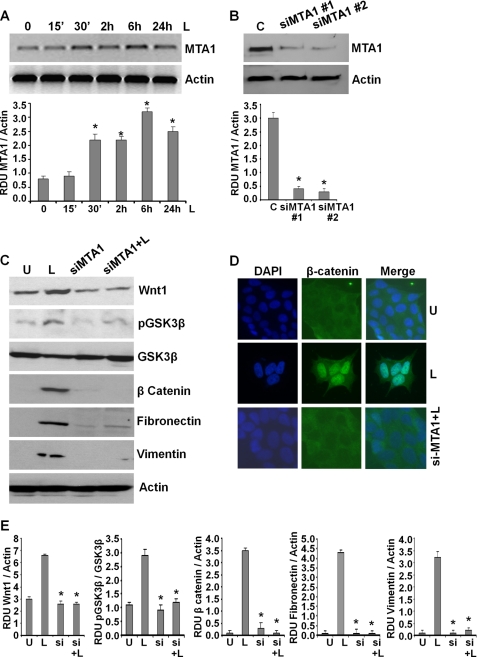
Leptin Induces an Important Modifier of Wnt1 Signaling, MTA1
Wnt1 expression is up-regulated in various cancers, including breast cancer (53). Recent studies show that metastatic tumor antigen (MTA1) stimulates the expression of bioactive Wnt1 in breast cancer cells (54, 55). MTA1 expression correlates with tumor aggressiveness and is known to contribute to the invasiveness and anchorage-dependent potential of breast cancer cells (56). Interestingly, we found that leptin-treated MCF7 (Fig. 5A) and MDA-MB-231 cells (supplemental Fig. S5) exhibited increased expression of MTA1. We questioned whether MTA1 had a role in leptin-induced Wnt1 up-regulation in breast cancer cells. To address this question, we silenced MTA1 in breast cancer cells using MTA1-siRNA (Fig. 5B). MTA1 depletion compromised the ability of leptin to induce Wnt1 expression (Fig. 5, C and E). In parallel, MTA1 inhibition also inhibited two important downstream effectors of Wnt1 signaling, resulting in decreased GSK3β phosphorylation and β-catenin expression. Also, exposure of si-MTA1 cells to leptin did not induce GSK3β phosphorylation and β-catenin expression as compared with control cells (Fig. 5, C and E). In addition, siMTA1 cells showed reduced levels of fibronectin and vimentin that remained lower even upon leptin stimulation (Fig. 5, C and E). Functional MTA1-Wnt1 signaling results in nuclear translocation of β-catenin. Corroborating the results from Western blot analysis, si-MTA1-MCF7 cells showed inhibition of nuclear β-catenin expression even in the presence of leptin (Fig. 5D). These findings suggest that MTA1 is an important effector of leptin signaling modulating the leptin-induced Wnt1-GSK3β-β-catenin axis, and its net levels affect biological functions of leptin.
FIGURE 5.
Leptin induces the expression of MTA1 that plays an integral role in leptin-induced GSK3β phosphorylation, β-catenin accumulation, and nuclear translocation. A, MCF7 breast cancer cells were treated with 100 ng/ml leptin for indicated time intervals. Total protein lysates were immunoblotted using specific antibodies for MTA1. Immunoblot for actin levels was used as control. The blots are representative of multiple independent experiments. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for MTA1 normalized to actin. *, p < 0.001 compared with untreated controls. Leptin increases MTA1 expression in breast cancer cells. B, MCF7 cells were transiently transfected with siMTA1#1 or siMTA1#2 siRNAs (Invitrogen) using FuGENE (Invitrogen). Immunoblot analysis was performed using MTA1 antibodies, and actin antibodies were used as control. C, breast cancer cells were transfected with siMTA1#1 for 24 h followed by leptin treatment for 6 h. Total lysates were immunoblotted using antibodies against Wnt1, phospho-GSK3β, β-catenin, fibronectin, and vimentin along with total GSK3β and actin. The blots are representative of multiple independent experiments. Inhibition of MTA1 inhibits leptin-induced GSK3β phosphorylation, β-catenin, fibronectin, and vimentin expression. D, breast cancer cells were transfected with siMTA1#1 for 24 h followed by leptin treatment for 6 h (siMTA1+L), untreated (U) or treated with leptin alone (L) for 6 h and subjected to immunofluorescence analysis of β-catenin. MTA1 silencing inhibits leptin-induced nuclear localization of β-catenin. E, densitometric analysis was performed on multiple blots for each target protein. The histogram is the mean of densitometric analysis showing relative density units (RDU) of the Western blot signal for target proteins (Wnt1, p-GSK3β, β-catenin, fibronectin, and vimentin) normalized to actin or unphosphorylated total protein. *, p < 0.001 compared with untreated controls. Inhibition of MTA1 inhibits leptin-induced GSK3β phosphorylation, β-catenin, fibronectin, and vimentin expression.
β-Catenin Activation Is Integral to Leptin-induced Epithelial-Mesenchymal Transition
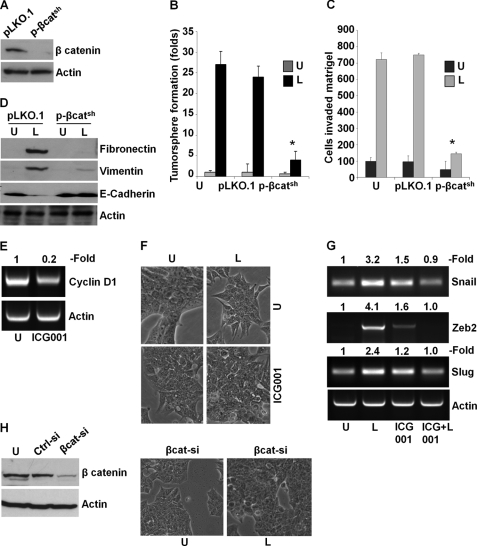
We found that leptin induces β-catenin via concomitant activation of Akt-GSK3β and MTA1-Wnt1-GSK3β axes. To investigate whether β-catenin plays a critical role in oncogenic actions of leptin in breast cancer cells, we used β-cateninshRNA lentivirus and puromycin to select for stable pools of MCF7 cells with endogenous β-catenin depletion. We analyzed pLKO.1 (vector control) and β-cateninshRNA stable MCF7 cell pools for β-catenin protein expression by immunoblot analysis, and we found that β-catenin protein expression was significantly reduced in β-cateninshRNA cells as compared with pLKO.1 control cells (Fig. 6A). Leptin treatment stimulated tumorsphere formation in pLKO.1 control cells, and β-cateninshRNA cells exhibited reduced tumorsphere formation even in the presence of leptin (Fig. 6B). As evident in Fig. 6C, inhibition of β-catenin abrogated leptin-induced invasion potential in comparison with vector control cells. Importantly, β-catenin depletion compromised the ability of leptin to induce mesenchymal markers such as fibronectin and vimentin and to inhibit epithelial markers such as E-cadherin (Fig. 6D). Given our results showing a critical role for β-catenin in mediating oncogenic functions of leptin as well as molecular changes related to mesenchymal as well as epithelial markers, we reasoned that targeted inhibition of this signaling using a small molecule inhibitor might be able to mitigate leptin-induced EMT. To test this, we utilized a peptidomimetic small molecule (ICG-001) that has been shown to inhibit β-catenin signaling (57, 58). Treatment of MCF7 cells with ICG-001 resulted in 70–80% inhibition of the β-catenin responsive gene, cyclinD1, indicating inhibition of β-catenin transactivation function (Fig. 6E). Showing the importance of β-catenin signaling in leptin function, ICG-001 treatment inhibited leptin-induced EMT of breast cancer cells. Leptin-treated MCF7 cells exhibited fibroblast-like appearance with increased pseudopodia formation, whereas cells co-treated with leptin and ICG-001 showed typical epithelial phenotype (Fig. 6F). Our findings showed that exposure of MCF7 cells to leptin resulted in up-regulation of mesenchymal markers, including Snail, Slug, and Zeb2 (Fig. 1 and supplemental Fig. S1). We further investigated if ICG-001 treatment can inhibit leptin-mediated increased expression of Snail, Slug, and Zeb2 in MCF7 cells. Consistent with the effect of ICG-001 on leptin-induced EMT, we observed inhibition of Snail, Slug, and Zeb2 expression in cells cotreated with ICG-001 and leptin as compared with only leptin-treated cells (Fig. 6G). Next, we utilized the siRNA approach to effectively silence β-catenin (Fig. 6H) followed by leptin treatment for 3 days, and we observed the morphological changes. Abrogation of β-catenin expression inhibited leptin-induced EMT in breast cancer cells (Fig. 6H). Based on our studies, we propose a model in which β-catenin activation is critical for leptin-induced epithelial-mesenchymal transition of breast cancer cells.
FIGURE 6.
Depletion of β-catenin or inhibition of β-catenin activity using ICG-001 abrogates leptin-mediated tumorsphere formation, invasion, and epithelial-mesenchymal transition of breast cancer cells. A, β-catenin was depleted in MCF7 cells using lentiviral β-catenin shRNA constructs and a negative control construct that was created in the same vector system (pLKO.1). Stable pools of β-catenin-depleted (p-β-catsh) and vector control (pLKO.1) cells were used for total protein isolation, and equal amounts of proteins were subjected to immunoblot analysis using β-catenin antibodies. The membranes were re-blotted using actin antibody as control. The blots are representative of multiple independent experiments. B, parental, pLKO.1, and p-β-catsh cells were subjected to tumorsphere formation in the presence (L) or absence (U) of 100 ng/ml leptin. The number of tumorspheres was counted in 10 different views under microscope. The results are presented as fold-change with untreated. *, p < 0.001, compared with untreated controls. Silencing of β-catenin abrogates leptin-induced tumorsphere formation. C, parental, pLKO.1, and p-β-catsh cells were subjected to Matrigel-invasion assay in the presence (L) or absence (U) of 100 ng/ml leptin. The number of cells that invaded through the Matrigel was counted in five different regions. The slides were blinded to remove counting bias. The results show mean of three independent experiments performed in triplicate. *, p < 0.005 compared with untreated controls (U). D, parental, pLKO.1, or p-β-catsh cells were treated with 100 ng/ml leptin (L) or untreated (U). Equal amounts of proteins were subjected to immunoblot analysis using fibronectin, vimentin, and E-cadherin antibodies. The membranes were re-blotted using actin antibody as control. The blots are representative of multiple independent experiments. Depletion of β-catenin abrogates leptin-mediated increase in mesenchymal markers and decrease in epithelial markers. E, MCF7 cells were treated with 5 μm ICG-001 followed by total RNA isolation. ICG-001 treatment reduced cyclin D1 expression. Actin was used as loading control. F, MCF7 cells were untreated (U), treated with 100 ng/ml leptin (L), and 5 μm ICG-001 (ICG001) alone or in combination and observed for morphological changes associated with EMT as shown in phase-contrast images. Leptin-treated cells exhibited spindle-shaped cells, increased intercellular separation, and pseudopodia. These alterations were absent in ICG-001 + leptin-treated cells, which closely resembled the untreated cells. G, MCF7 cells were treated as in E followed by total RNA isolation and RT-PCR using primers specific for Snail, Zeb2, Slug, and Actin. ICG-001 treatment inhibited leptin-induced expression of Snail, Zeb2, and Slug. Actin expression level was used as control. H, MCF7 cells were transiently transfected with si-β-catenin or si-control siRNAs (Invitrogen) using FuGENE (Invitrogen). Immunoblot analysis was performed using β-catenin antibodies, and actin antibodies were used as control. si-β-Catenin inhibits the expression of β-catenin. Breast cancer cells transfected with si-β-catenin were treated with leptin and followed for morphological changes. Leptin treatment did not induce any morphological changes in cells lacking β-catenin expression.
Leptin Administration Modulates EMT Markers As Well As Important Components of Akt/GSK3β and MTA1/Wnt1 Pathways in Vivo
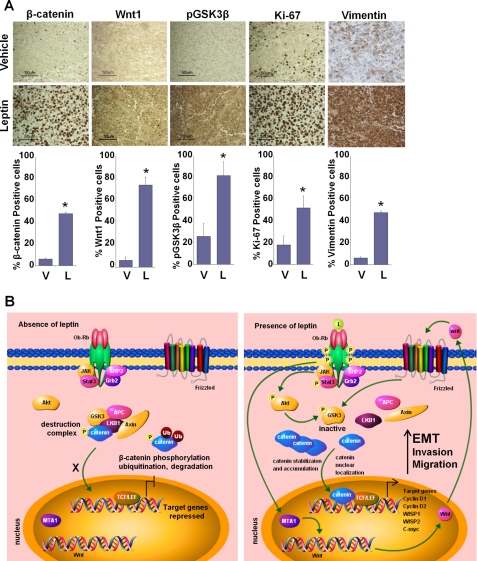
We have shown earlier that growth of MDA-MB-231 breast cancer cell xenografts in female athymic nude mice is stimulated significantly by intraperitoneal leptin administration (dosage of 5 mg/kg, 5 days a week for 6 weeks) (6). In this study, the average tumor volume in the leptin-treated group was 2.5–3.0-fold higher than the vehicle-treated mice after 6 weeks of leptin administration (6). We utilized tumor tissue from the same experiment to determine the effect of leptin treatment on the expression of EMT markers as well as important signaling molecules in vivo (Fig. 7A). Vimentin protein expression was very low in the tumors from control group mice. Leptin administration caused an up-regulation of vimentin expression. Increased expression of Wnt1, pGSK3β, and increased nuclear accumulation of β-catenin was observed in tumor tissues from the leptin-treated group, but the control group showed low to no expression of these signaling molecules (Fig. 7A). Results from immunohistochemical analysis of tumor samples corroborated our in vitro findings. Collectively, the findings presented here suggest that leptin induces EMT in breast cancer cells and provide in vitro as well as in vivo evidence for involvement of MTA1-Wnt1-β-catenin and Akt-GSK3β-β-catenin signaling in oncogenic actions of leptin (Fig. 7B).
FIGURE 7.
In vivo evidence for leptin-mediated alterations in EMT markers as well as Wnt1, GSK3β, β-catenin, vimentin, and Ki-67 expression levels. A, MDA-MB-231 cell-derived tumors were developed in nude mice and treated with vehicle (V) or leptin (L) for 6 weeks. Tumors were collected at the end of 6 weeks and subjected to immunohistochemical analysis using GSK3β, Wnt1, β-catenin, vimentin, and Ki-67 antibodies. Bar diagrams show quantitation of protein expression in tumors from vehicle and leptin-treated mice. Columns, mean (n = 8); bar, S.D. * indicates significantly different (p < 0.005) compared with control. B, schematic model of leptin-stimulated Akt/GSK3β and MTA1/Wnt1 axes activating β-catenin leading to epithelial-mesenchymal transition in breast cancer cells. Under basal conditions, in the absence of leptin, unphosphorylated GSK3-β forms a destruction complex with LKB1, Axin, and APC leading to phosphorylation, ubiquitination, and degradation of β-catenin resulting in repression of target genes. Leptin stimulation leads to Akt phosphorylation, which in turn phosphorylates GSK3-β rendering it inactive, which leads to disintegration of destruction complex. Leptin also stimulates MTA1 overexpression leading to increased Wnt1 expression that phosphorylates GSK3-β. These events lead to accumulation, nuclear translocation of β-catenin, and activation of target genes. Leptin stimulates β-catenin activation via Akt/GSK3-dependent and MTA1/Wnt1-dependent pathways.
DISCUSSION
EMT, an essential normal physiological process for embryonic development, tissue remodeling, and wound healing, has recently been implicated in cancer progression. Epithelium-derived tumors switch to a mesenchymal phenotype that facilitates migration and invasion potential resulting in increased tumor aggressiveness, recurrence, and overall poor prognosis. Many studies have examined the possible role of EMT in breast cancer (38, 59). Previous studies from our laboratory and others have shown that leptin increases motility and invasion of breast cancer cells (1, 6, 20, 21, 60). We hypothesized that EMT was the likely mechanism by which leptin increased breast cancer cell migration and invasion. Our findings present a pivotal role of leptin in acquisition of mesenchymal characteristics and aggressive behavior in breast cancer. The key mechanism that we elucidate to account for this important function of leptin is that it increases β-catenin stabilization and nuclear translocation. We further show that leptin-mediated stabilization of β-catenin is achieved by concomitant activation of Akt/GSK3 and MTA1/Wnt1 signaling leading to destruction of the LKB1-GSK3β-Axin complex. We also provide molecular evidence supporting the regulatory role of MTA1 in leptin-induced Wnt1 up-regulation, β-catenin stabilization, and its nuclear translocation. The significance of our in vitro results is strengthened by the fact that key components of this leptin-mediated pathway we describe are detected in tumors treated with leptin.
The dual-action molecule β-catenin enhances cell-cell adhesion when bound to cadherin complexes and functions as a transcriptional coactivator upon nuclear translocation (53). Loss of the E-cadherin-β-catenin complex and nuclear import of β-catenin are important steps in EMT progression (49, 50). Our data show that leptin treatment enhances nuclear translocation of β-catenin leading to increased recruitment of β-catenin at TCF/LEF-binding sites. Leptin-induced hyperactive β-catenin signaling results in elevated coactivation function as evident by higher levels of TCF/LEF-responsive genes (cyclin D1 and fibronectin). Elevated fibronectin levels have been implicated in acquisition of EMT (27, 38, 46). Nuclear β-catenin induces expression of genes that support tumor invasion and transition to mesenchymal phenotype (38, 50) via recruiting transcriptional coactivators, cyclic AMP-response element-binding protein, or its closely related homolog p300. β-Catenin is an intracellular signaling protein that has been generally considered “nontargetable” because of the absence of any enzymatic activities. Recent studies, however, show that other aspects of this pathway such as β-catenin activation or transcriptional activities may be targeted to inhibit its downstream effects. We hypothesized that disruption of the β-catenin-mediated transcription complex assembly could be an effective strategy to inhibit leptin-induced EMT. In this regard, recent studies have identified a peptidomimetic small molecule, ICG-001, that selectively inhibits coactivation function of β-catenin (57, 58). ICG-001 treatment suppresses expression of fibronectin, collagen I, collagen III, fibroblast-specific protein-1, and Slug in human kidney tubular epithelial cells (61). We found that ICG-001 treatment inhibited leptin-induced Snail, Zeb2, and Slug expression in breast cancer cells. Consistently, ICG-001 treatment or knockdown of β-catenin also inhibited leptin-induced morphological changes associated with epithelial-mesenchymal transition of breast cancer cells. Therefore, specific targeting of leptin-induced hyperactive β-catenin signaling with a small molecule inhibitor could represent a novel and effective strategy for therapeutic intervention of leptin-induced EMT.
Our data show that leptin induces canonical Wnt1 signal pathway functioning through β-catenin-dependent mechanisms. Several signaling kinases, including ERK-p90-RSK and Akt, phosphorylate GSK3β and regulate the function of the Wnt pathway (62). We show that leptin stimulates expression of Wnt1, and silencing of Wnt1 inhibits leptin-induced phosphorylation of GSK3β. Recent studies investigating transcriptional regulation of Wnt1 show the interplay between the Six3 corepressor and MTA1 (54, 55). Wnt1 expression is known to be coregulated by Six3 homeodomain protein that interacts with the Groucho family of corepressors recruiting histone deacetylases (63, 64). MTA1 has been recently identified as an important upstream modifier of Wnt1 transcription and function via inhibition of Six3 expression leading to derepression of the Wnt1 gene. Our studies report that leptin increases expression of MTA1 in breast cancer cells. Silencing of MTA1 not only inhibits leptin-induced Wnt1 expression but also abrogates phosphorylation of GSK3β. In addition, MTA1 silencing inhibits leptin-induced β-catenin expression and nuclear localization. One of the important findings of our studies is that MTA1 is a novel target of leptin that plays an important role in mediating its biological actions.
Leptin has been shown as a potent growth-stimulating factor for breast cancer that induces breast cancer cell migration and invasion. Here, we have shown that leptin stimulates epithelial-mesenchymal transition of breast cancer cells. Therefore, blocking leptin activity might prove useful to inhibit breast cancer progression and metastatic potential. Circulating levels of leptin can be neutralized with soluble leptin receptors that bind free leptin in circulation. High levels of leptin receptors have been reported in breast cancer cells that can be blocked by the leptin antagonist that binds to leptin receptors but does not activate them, thus inhibiting the intracellular signaling network. In addition, specific anti-leptin receptor monoclonal antibodies that bind to the receptor prevent leptin signaling or anti-leptin antibodies. Anti-leptin receptor monoclonal antibodies have high molecular mass ensuring a longer half-life in circulation and good affinity for the receptor. But these mouse-generated antibodies need to be humanized to eliminate potential immunogenicity. Yet another approach is the development of nanobodies that target leptin receptor and block leptin-induced conformational changes without interfering with the leptin-leptin receptor interaction. These nanobodies are easily modifiable and can selectively inhibit peripheral activity of leptin as they do not cross the blood-brain barrier. Leptin muteins with antagonistic properties and other proteins blocking leptin activities are also under development (65). To date, these promising reagents directly targeting leptin and the leptin receptor are under initial stages of development. Therefore, understanding the molecular network of leptin signaling and identifying critical molecules for biological functions of leptin can be extremely useful as this understanding can allow us to utilize existing effective agents to inhibit highly active leptin network in breast cancer.
Here, we report a novel function of leptin as an inducer of EMT in breast cancer cells providing in vitro as well as in vivo evidence of some novel and important players of the leptin network. Based on our findings, we also present a model for leptin-induced EMT in breast cancer. We propose that leptin transmits signals via both MTA1-Wnt1 and Akt pathways that cause the phosphorylation and hence inactivation of GSK3β leading to dissociation of LKB1-GSK3β-Axin complex. Inactivation of the destruction complex allows accumulation and nuclear translocation of β-catenin, alteration of responsive gene expression, and ultimately inducing the epithelial-mesenchymal transition of breast cancer cells in response to leptin treatment (Fig. 7B). Furthermore, consistent with critical role of β-catenin in leptin-mediated EMT, inhibition of β-catenin attenuates leptin-induced morphological as well as genetic changes. Collectively, this study gives insight into the specific pathways that are required for leptin-induced EMT and highlights the importance of leptin levels in breast cancer.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants K01DK076742 and R03DK089130 (to N. K. S.) and R01CA131294 (to D. S.).

This article contains supplemental Figs. 1–5.
- LEPR
- leptin receptor
- EMT
- epithelial-mesenchymal transition
- MMTV
- murine mammary tumor virus
- APC
- adenomatous polyposis coli
- TCF
- T-cell factor
- LEF
- lymphoid-enhancing factor.
REFERENCES
- 1. Cirillo D., Rachiglio A. M., la Montagna R., Giordano A., Normanno N. (2008) Leptin signaling in breast cancer. An overview. J. Cell. Biochem. 105, 956–964 [DOI] [PubMed] [Google Scholar]
- 2. Grossmann M. E., Ray A., Nkhata K. J., Malakhov D. A., Rogozina O. P., Dogan S., Cleary M. P. (2010) Obesity and breast cancer. Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 29, 641–653 [DOI] [PubMed] [Google Scholar]
- 3. Guo S., Gonzalez-Perez R. R. (2011) Notch, IL-1, and leptin cross-talk outcome (NILCO) is critical for leptin-induced proliferation, migration, and VEGF/VEGFR-2 expression in breast cancer. PLoS One 6, e21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ray A., Cleary M. P. (2010) Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin. Ther. Targets 14, 443–451 [DOI] [PubMed] [Google Scholar]
- 5. Artac M., Altundag K. (2011) Leptin and breast cancer: an overview. Med. Oncol., in press [DOI] [PubMed] [Google Scholar]
- 6. Knight B. B., Oprea-Ilies G. M., Nagalingam A., Yang L., Cohen C., Saxena N. K., Sharma D. (2011) Survivin up-regulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr. Relat. Cancer 18, 413–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garofalo C., Surmacz E. (2006) Leptin and cancer. J. Cell. Physiol. 207, 12–22 [DOI] [PubMed] [Google Scholar]
- 8. Ahima R. S., Osei S. Y. (2004) Leptin signaling. Physiol. Behav. 81, 223–241 [DOI] [PubMed] [Google Scholar]
- 9. Ishikawa M., Kitayama J., Nagawa H. (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 10, 4325–4331 [DOI] [PubMed] [Google Scholar]
- 10. Garofalo C., Koda M., Cascio S., Sulkowska M., Kanczuga-Koda L., Golaszewska J., Russo A., Sulkowski S., Surmacz E. (2006) Increased expression of leptin and the leptin receptor as a marker of breast cancer progression. Possible role of obesity-related stimuli. Clin. Cancer Res. 12, 1447–1453 [DOI] [PubMed] [Google Scholar]
- 11. Tessitore L., Vizio B., Jenkins O., De Stefano I., Ritossa C., Argiles J. M., Benedetto C., Mussa A. (2000) Leptin expression in colorectal and breast cancer patients. Int. J. Mol. Med. 5, 421–426 [DOI] [PubMed] [Google Scholar]
- 12. Cleary M. P., Phillips F. C., Getzin S. C., Jacobson T. L., Jacobson M. K., Christensen T. A., Juneja S. C., Grande J. P., Maihle N. J. (2003) Genetically obese MMTV-TGF-α/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat. 77, 205–215 [DOI] [PubMed] [Google Scholar]
- 13. Cleary M. P., Juneja S. C., Phillips F. C., Hu X., Grande J. P., Maihle N. J. (2004) Leptin receptor-deficient MMTV-TGF-α/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. 229, 182–193 [DOI] [PubMed] [Google Scholar]
- 14. Chua S. C., Jr., Liu S. M., Li Q., Sun A., DeNino W. F., Heymsfield S. B., Guo X. E. (2004) Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am. J. Physiol. Endocrinol. Metab. 286, E384–E392 [DOI] [PubMed] [Google Scholar]
- 15. Park J., Kusminski C. M., Chua S. C., Scherer P. E. (2010) Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am. J. Pathol. 177, 3133–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dogan S., Hu X., Zhang Y., Maihle N. J., Grande J. P., Cleary M. P. (2007) Effects of high fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-α mice. Breast Cancer Res. 9, R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nunez N. P., Perkins S. N., Smith N. C., Berrigan D., Berendes D. M., Varticovski L., Barrett J. C., Hursting S. D. (2008) Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr. Cancer 60, 534–541 [DOI] [PubMed] [Google Scholar]
- 18. Zheng Q., Dunlap S. M., Zhu J., Downs-Kelly E., Rich J., Hursting S. D., Berger N. A., Reizes O. (2011) Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr. Relat. Cancer 18, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saxena N. K., Vertino P. M., Anania F. A., Sharma D. (2007) Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J. Biol. Chem. 282, 13316–13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saxena N. K., Sharma D., Ding X., Lin S., Marra F., Merlin D., Anania F. A. (2007) Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 67, 2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saxena N. K., Taliaferro-Smith L., Knight B. B., Merlin D., Anania F. A., O'Regan R. M., Sharma D. (2008) Bidirectional cross-talk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 68, 9712–9722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez R. R., Leavis P. (2001) Leptin up-regulates αβ3-integrin expression and interleukin-1β, up-regulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine 16, 21–28 [DOI] [PubMed] [Google Scholar]
- 23. Pinteaux E., Inoue W., Schmidt L., Molina-Holgado F., Rothwell N. J., Luheshi G. N. (2007) Leptin induces interleukin-1β release from rat microglial cells through a caspase 1-independent mechanism. J. Neurochem. 102, 826–833 [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez R. R., Devoto L., Campana A., Bischof P. (2001) Effects of leptin, interleukin-1α, interleukin-6, and transforming growth factor-β on markers of trophoblast invasive phenotype. Integrins and metalloproteinases. Endocrine 15, 157–164 [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez R. R., Cherfils S., Escobar M., Yoo J. H., Carino C., Styer A. K., Sullivan B. T., Sakamoto H., Olawaiye A., Serikawa T., Lynch M. P., Rueda B. R. (2006) Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J. Biol. Chem. 281, 26320–26328 [DOI] [PubMed] [Google Scholar]
- 26. Fusco R., Galgani M., Procaccini C., Franco R., Pirozzi G., Fucci L., Laccetti P., Matarese G. (2010) Cellular and molecular cross-talk between leptin receptor and estrogen receptor-α in breast cancer. Molecular basis for a novel therapeutic setting. Endocr. Relat. Cancer 17, 373–382 [DOI] [PubMed] [Google Scholar]
- 27. Thiery J. P. (2002) Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2, 442–454 [DOI] [PubMed] [Google Scholar]
- 28. Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 [DOI] [PubMed] [Google Scholar]
- 29. Vernon A. E., LaBonne C. (2004) Tumor metastasis. A new twist on epithelial-mesenchymal transitions. Curr. Biol. 14, R719–R721 [DOI] [PubMed] [Google Scholar]
- 30. Hajra K. M., Chen D. Y., Fearon E. R. (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 62, 1613–1618 [PubMed] [Google Scholar]
- 31. Cano A., Pérez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 [DOI] [PubMed] [Google Scholar]
- 32. Li J., Zhou B. P. (2011) Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT-associated cancer stem cell-like characters. BMC Cancer 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanwar S. S., Yu Y., Nautiyal J., Patel B. B., Majumdar A. P. (2010) The Wnt/β-catenin pathway regulates growth and maintenance of colonospheres. Mol. Cancer 9, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang A. D., Camp E. R., Fan F., Shen L., Gray M. J., Liu W., Somcio R., Bauer T. W., Wu Y., Hicklin D. J., Ellis L. M. (2006) Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 66, 46–51 [DOI] [PubMed] [Google Scholar]
- 35. Sharma D., Wang J., Fu P. P., Sharma S., Nagalingam A., Mells J., Handy J., Page A. J., Cohen C., Anania F. A., Saxena N. K. (2010) Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 52, 1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taliaferro-Smith L., Nagalingam A., Zhong D., Zhou W., Saxena N. K., Sharma D. (2009) LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28, 2621–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma D., Fondell J. D. (2002) Ordered recruitment of histone acetyltransferases and the TRAP-Mediator complex to thyroid hormone-responsive promoters in vivo. Proc. Natl. Acad. Sci. U.S.A. 99, 7934–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomaskovic-Crook E., Thompson E. W., Thiery J. P. (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 11, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hugo H., Ackland M. L., Blick T., Lawrence M. G., Clements J. A., Williams E. D., Thompson E. W. (2007) Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J. Cell. Physiol. 213, 374–383 [DOI] [PubMed] [Google Scholar]
- 40. Mani S. A., Guo W., Liao M. J., Eaton E. N., Ayyanan A., Zhou A. Y., Brooks M., Reinhard F., Zhang C. C., Shipitsin M., Campbell L. L., Polyak K., Brisken C., Yang J., Weinberg R. A. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morel A. P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 3, e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santisteban M., Reiman J. M., Asiedu M. K., Behrens M. D., Nassar A., Kalli K. R., Haluska P., Ingle J. N., Hartmann L. C., Manjili M. H., Radisky D. C., Ferrone S., Knutson K. L. (2009) Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 69, 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Creighton C. J., Li X., Landis M., Dixon J. M., Neumeister V. M., Sjolund A., Rimm D. L., Wong H., Rodriguez A., Herschkowitz J. I., Fan C., Zhang X., He X., Pavlick A., Gutierrez M. C., Renshaw L., Larionov A. A., Faratian D., Hilsenbeck S. G., Perou C. M., Lewis M. T., Rosen J. M., Chang J. C. (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. U.S.A. 106, 13820–13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brennan K. R., Brown A. M. (2004) Wnt proteins in mammary development and cancer. J. Mammary Gland. Biol. Neoplasia 9, 119–131 [DOI] [PubMed] [Google Scholar]
- 45. Turashvili G., Bouchal J., Burkadze G., Kolar Z. (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73, 213–223 [DOI] [PubMed] [Google Scholar]
- 46. Lin S. Y., Xia W., Wang J. C., Kwong K. Y., Spohn B., Wen Y., Pestell R. G., Hung M. C. (2000) β-Catenin, a novel prognostic marker for breast cancer. Its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. U.S.A. 97, 4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakopoulou L., Mylona E., Papadaki I., Kavantzas N., Giannopoulou I., Markaki S., Keramopoulos A. (2006) Study of phospho-β-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod. Pathol. 19, 556–563 [DOI] [PubMed] [Google Scholar]
- 48. Candidus S., Bischoff P., Becker K. F., Höfler H. (1996) No evidence for mutations in the α- and β-catenin genes in human gastric and breast carcinomas. Cancer Res. 56, 49–52 [PubMed] [Google Scholar]
- 49. Micalizzi D. S., Farabaugh S. M., Ford H. L. (2010) Epithelial-mesenchymal transition in cancer. Parallels between normal development and tumor progression. J. Mammary Gland. Biol. Neoplasia 15, 117–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moon R. T., Bowerman B., Boutros M., Perrimon N. (2002) The promise and perils of Wnt signaling through β-catenin. Science 296, 1644–1646 [DOI] [PubMed] [Google Scholar]
- 51. Jope R. S., Johnson G. V. (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 52. Kimelman D., Xu W. (2006) β-Catenin destruction complex. Insights and questions from a structural perspective. Oncogene 25, 7482–7491 [DOI] [PubMed] [Google Scholar]
- 53. Cadigan K. M., Liu Y. I. (2006) Wnt signaling. Complexity at the surface. J. Cell Sci. 119, 395–402 [DOI] [PubMed] [Google Scholar]
- 54. Kumar R., Balasenthil S., Pakala S. B., Rayala S. K., Sahin A. A., Ohshiro K. (2010) Metastasis-associated protein 1 short form stimulates Wnt1 pathway in mammary epithelial and cancer cells. Cancer Res. 70, 6598–6608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kumar R., Balasenthil S., Manavathi B., Rayala S. K., Pakala S. B. (2010) Metastasis-associated protein 1 and its short form variant stimulates Wnt1 transcription through promoting its derepression from Six3 corepressor. Cancer Res. 70, 6649–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singh R. R., Kumar R. (2007) MTA family of transcriptional metaregulators in mammary gland morphogenesis and breast cancer. J. Mammary Gland. Biol. Neoplasia 12, 115–125 [DOI] [PubMed] [Google Scholar]
- 57. Emami K. H., Nguyen C., Ma H., Kim D. H., Jeong K. W., Eguchi M., Moon R. T., Teo J. L., Oh S. W., Kim H. Y., Moon S. H., Ha J. R., Kahn M. (2004) A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. U.S.A. 101, 12682–12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Henderson W. R., Jr., Chi E. Y., Ye X., Nguyen C., Tien Y. T., Zhou B., Borok Z., Knight D. A., Kahn M. (2010) Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. U.S.A. 107, 14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Creighton C. J., Chang J. C., Rosen J. M. (2010) Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J. Mammary Gland. Biol. Neoplasia 15, 253–260 [DOI] [PubMed] [Google Scholar]
- 60. McMurtry V., Simeone A. M., Nieves-Alicea R., Tari A. M. (2009) Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clin. Exp. Metastasis 26, 197–204 [DOI] [PubMed] [Google Scholar]
- 61. Hao S., He W., Li Y., Ding H., Hou Y., Nie J., Hou F. F., Kahn M., Liu Y. (2011) Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22, 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McManus E. J., Sakamoto K., Armit L. J., Ronaldson L., Shpiro N., Marquez R., Alessi D. R. (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signaling defined by knock-in analysis. EMBO J. 24, 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lagutin O. V., Zhu C. C., Kobayashi D., Topczewski J., Shimamura K., Puelles L., Russell H. R., McKinnon P. J., Solnica-Krezel L., Oliver G. (2003) Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 17, 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu C. C., Dyer M. A., Uchikawa M., Kondoh H., Lagutin O. V., Oliver G. (2002) Six3-mediated autorepression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development 129, 2835–2849 [DOI] [PubMed] [Google Scholar]
- 65. Gertler A. (2006) Development of leptin antagonists and their potential use in experimental biology and medicine. Trends Endocrinol. Metab. 17, 372–378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.