Background: H-ficolin is a pattern recognition molecule of the human innate immune system.
Results: The ligand specificity of H-ficolin is explored, and a purification procedure is developed.
Conclusion: H-ficolin binds to patterns of acetyl groups and activates complement upon binding.
Significance: The findings expand and emphasize the differences of the specificities of the three human ficolins (H-, L-, and M-ficolin).
Keywords: Carbohydrate-binding Protein, Complement System, Innate Immunity, Pathogen-associated Molecular Pattern (PAMP), Pattern Recognition Receptor, Protein Purification
Abstract
Ficolins are pattern recognition molecules of the innate immune system. H-ficolin is found in plasma associated with mannan-binding lectin-associated serine proteases (MASPs). When H-ficolin binds to microorganisms the MASPs are activated, which in turn activate the complement system. H-ficolin is the most abundant ficolin in humans, yet its ligand binding characteristics and biological role remain obscure. We examined the binding of H-ficolin to Aerococcus viridans as well as to a more defined artificial target, i.e. acetylated bovine serum albumin. A strict dependence for calcium ions and inhibition at high NaCl concentration was found. The binding to acetylated bovine serum albumin was inhibited by acetylsalicylic acid and sodium acetate as well as by N-acetylated glucosamine and galactosamine (GlcNAc and GalNAc) and glycine (GlyNAc). The binding to A. viridans was sensitive to the same compounds, but, importantly, higher concentrations were needed for inhibition. N-Acetylated cysteine was also inhibitory, but this inhibition was parallel with reduction in the oligomerization of H-ficolin and thus represents structural changes of the molecule. Based on our findings, we developed a procedure for the purification of H-ficolin from serum, involving PEG precipitation, affinity chromatography on Sepharose derivatized with acetylated serum albumin, ion exchange chromatography, and gel permeation chromatography. The purified H-ficolin was observed to elute at 700 kDa, similar to what we find for H-ficolin in whole serum. MASP-2 was co-purified with H-ficolin, and the purified H-ficolin·MASP-2 complex could activate complement as measured by cleavage of complement factor C4. This study extends our knowledge of the specificity of this pattern recognition molecule, and the purified product will enable further studies.
Introduction
Recognition in innate immunity relies on the ability of pattern-recognition molecules (PRMs)2 to bind foreign markers, the so-called pathogen-associated molecular patterns (PAMPs). One group of PRMs is able to initiate activation of the complement system upon binding. The group is composed of mannan-binding lectin (MBL) and the three ficolins (H-, L-, and M-ficolin) (1, 2). Both MBL and ficolins recognize specific structures presented in an organized pattern on the surface of pathogens but also structures on apoptotic or necrotic cells.
MBL and ficolins are found in complexes with three serine proteases, MASP-1, MASP-2, and MASP-3, as well as two nonenzymatic fragments MAp19 and MAp44 (1, 3–10). Upon binding of ficolin·MASP or MBL·MASP complexes to PAMPs, MASP-2 is activated and is then able to cleave C4 and C2 to generate the C3 convertase, C4bC2a (5, 9, 11, 12). The function and substrate specificity of MASP-1 and MASP-3 remain to be determined, although MASP-1 seems to possess a promiscuous proteolytic activity and can cleave C2 and factor D (13). Therefore, MASP-1 may serve a role in generating C3 convertase (14). When C3b is deposited on the C4bC2a complex C2a attains C5 convertase activity. C5a is a potent inflammatory mediator, whereas C5b initiates the formation of a membrane attack complex, which is expanded through deposition of C6–C9. This complex eventually forms a hole in the cell membrane of bacteria, for example, which leads to their destruction. The deposited components C4b and C3b also function as molecular tags, facilitating phagocytosis. The functions of MASP-3, MAp19, and MAp44 remain unknown, but each of these proteins has been suggested to act as regulators of complement activation, and recently it was suggested that MASP-3 took part in activation of factor B and factor D of the complement system (15).
The ficolins and MBL are oligomers of a basic structural subunit. Each subunit consists of three identical polypeptide chains with an N-terminal cysteine-containing region, a collagen-like region, and three C-terminal ligand-binding domains (1, 16). In MBL the ligand-binding domain is a calcium-dependent carbohydrate recognition domain (C-type) (17, 18), whereas the ficolins make use of fibrinogen-like domains (19). The subunits associate into higher order oligomers linked by intersubunit disulfide bonds and display clusters of ligand binding domains. This structural organization of many recognition domains displayed in one molecule results in high avidity interactions with suitable PAMPs even though each single ligand binding is of low affinity.
L-ficolin (gi: 166214934) and M-ficolin (gi: 8051584) are paralogs with an amino acid sequence identity of 80%, and the respective genes are located in close proximity on chromosome 9. L-ficolin is synthesized primarily in the liver, and M-ficolin is synthesized exclusively by monocytes and granulocytes (6, 20–23). Both proteins show binding activity toward a variety of N-acetylated structures (5, 24).
Although similar in structure, H-ficolin (gi: 27754776) is quite different at the amino acid sequence level as compared with L-ficolin and M-ficolin with a sequence identity of only 45 and 46%, respectively. H-ficolin is synthesized by hepatocytes and bile duct epithelial cells where the protein is then secreted into the bloodstream and bile, respectively (25, 26). H-ficolin is also found in the mucosa of the lung where it originates from bronchial epithelial cells and alveolar type II epithelial cells (25). The mean serum concentration of H-ficolin is 18.4 μg/ml, as compared with 3.3 and 1.07 μg/ml for L- and M-ficolin, respectively, and H-ficolin is thus the most abundant PRM of the lectin pathway (2, 27). H-ficolin has only been characterized in humans, but based on data base sequence alignment, orthologs of the human H-ficolin gene (FCN3, chromosome 1p36.11) are identified in both lower and higher primates as well as in other lower species such as mouse, rat, and cat (28, 29). The mouse and rat orthologs of the H-ficolin gene have been identified as pseudogenes, indicating that the gene has been inactivated after branching of the rodent lineage (29).
The search for patterns that are recognized by H-ficolin has only revealed a few likely physiologically relevant candidates. Although L- and M-ficolin binds to a variety of bacteria, H-ficolin has only been observed to bind to Aerococcus viridans. The H-ficolin·MASP complex activates complement upon binding to A. viridans or to polysaccharides purified from A. viridans (9, 30). One reports describe binding of H-ficolin to lipopolysaccharide (LPS) isolated from Salmonella typhimurium (31). We have not been able to demonstrate binding to the corresponding intact bacteria. Recently, binding to LPS from Hafnia alvei was demonstrated (32). With regard to endogenous ligands, H-ficolin reportedly binds to apoptotic cells and may thus serve a homeostatic role (33, 34).
Like the other ficolins, H-ficolin has been reported to bind N-acetylated compounds such as GalNAc and GlcNAc and acetylated amino acids or acetylated proteins (34, 35), but detailed studies of the specificity of such binding are lacking.
We describe here in more detail the specificity of the binding of H-ficolin to an artificially acetylated surface as well as to a natural polysaccharide ligand. We document that affinity chromatography on acetylated surfaces may be used for purification of H-ficolin from serum. The presented method does not require large amounts of monoclonal anti-H-ficolin antibody, as was used in a method described previously (26), and selects for actively binding forms of H-ficolin.
EXPERIMENTAL PROCEDURES
TRIFMA for H-ficolin Binding to A. viridians and AcBSA
Binding of H-ficolin to ligand was tested by TRIFMA. A. viridans strains 86965 and Ring 44 were isolated from mice and kindly provided by Fredrik Dagnaes-Hansen et al. (36). The A. viridans strains were grown in L-broth medium (Q-Biogene, Carlsbad, CA) overnight at 37 °C and fixed by adding formaldehyde to the broth cultures to a final concentration of 1% (w/v). This treatment stabilizes the cells but does not alter the polysaccharide antigens (24). The wells of FluoroNunc microtiter plates (Nunc, Kamstrup, Denmark) were coated with 1 μg of AcBSA (Sigma) in 100 μl of 15 mm Na2CO3, 35 mm NaHCO3, 1.5 mm NaN3, pH 9.6 (coating buffer), or with 109 A. viridans in 100 μl of coating buffer by incubation overnight at 4 °C. An absorbance of 1.0 at 600 nm corresponds to ∼1.8 × 109 bacteria/ml (27). Residual protein-binding sites were blocked by incubation with TBS (10 mm Tris, 145 mm NaCl, pH 7.4) with 0.1% (w/v) human serum albumin (HSA). Samples were tested in duplicate, and each successive step was followed by washing three times in TBS with 0.05% (v/v) Tween 20 and 5 mm CaCl2 (TBS/tw/Ca2+). A human serum sample with 40 μg of H-ficolin/ml was diluted 500-fold in TBS/tw/Ca2+ or in the same buffer containing 10 mm EDTA instead of calcium (TBS/tw/EDTA) with increasing concentrations of NaCl (36.5–1000 mm) and incubated in AcBSA-coated microtiter wells. Similarly, purified H-ficolin (see below) was diluted 1000-fold in the same buffers to 118 ng/ml and incubated in the wells. After washing, bound H-ficolin was detected using 100 ng of biotinylated mouse monoclonal (mAb) anti-H-ficolin antibody 4H5 (34) (Hycult Biotechnology, Uden, The Netherlands) in 100 μl of TBS/tw/Ca2+. After 2 h of incubation, bound antibody was detected by adding 10 ng of europium-labeled streptavidin (PerkinElmer Life Sciences) in 100 μl of TBS with 0.05% (v/v) Tween 20 and 25 μm EDTA, pH 7.4. After washing, 200 μl of enhancement solution (PerkinElmer Life Sciences) was added, and the released and captured europium was quantified by time-resolved fluorometry on a Victor® fluorometer (PerkinElmer Life Sciences). We found that the capacity of A. viridans to bind H-ficolin decreased upon storage of the formalin-fixed bacteria at 4 °C. Using freshly prepared bacteria, 107/wells were sufficient for binding.
pH Dependence of H-ficolin Binding
Microtiter wells were coated with AcBSA or with A. viridians and blocked with TBS/tw. Seventeen ng of purified H-ficolin (see below) was mixed with 100 μl of the following buffers: 100 mm glycine, pH 1.5, pH 2.5, pH 4.0, and pH 5.0; 100 mm BisTris, pH 6.0; 100 mm Tris-HCl, pH 7.0 and pH 8.0; 100 mm diethylamine, pH 8.5; 100 mm ethylamine, pH 9.0 and pH 10.0. The pH was adjusted using HCl or NaOH. After incubation for 2 h at room temperature, bound H-ficolin was detected as described above.
Inhibition of H-ficolin Binding
Microtiter wells were with 1 μg of AcBSA in 100 μl of coating buffer; 107 A. viridans in 100 μl of coating buffer; or 1 μg of mannan prepared from Saccharomyces cerevisiae (37) in 100 μl of PBS. Residual binding sites were blocked with HSA in TBS, and samples were incubated and washed as above. Human serum was diluted 700-fold in TBS/tw/Ca2+ with increasing concentrations of putative inhibitors (final concentrations of 0.82–200 mm), and 100-μl samples were added to each well. The following molecules were tested: d-glucose (Glc), l-glucose, d-mannose (Man), l-fucose, d-fucose, d-galactose (Gal), N-acetylglucosamine (GlcNAc), N-acetylmannosamine (ManNAc), N-acetylgalactosamine (GalNAc), glucosamine (GlcN), mannosamine (ManN), galactosamine (GalN), glycine (Gly), N-acetylated glycine (GlyNAc), l-cysteine (Cys), N-acetylcysteine (CysNAc), acetylcholine, acetylsalicylic acid, sodium citrate, and sodium acetate, all from Sigma. The samples were added to the wells, and bound H-ficolin was detected as above. Inhibition of MBL binding was tested in the same manner on the mannan-coated wells, and bound MBL was quantified using europium-labeled monoclonal anti-MBL antibodies as described previously (38, 39).
Binding of Ficolins to Neoglycoproteins
Wells of FluoroNunc microtiter plates were coated with 1 μg of neoglycoproteins: l-fucose BSA (Fuc36BSA), N-acetylglucose BSA (GlcNAc36BSA), N-acetylgalactose BSA (GalNAc28BSA), d-galactose BSA (Gal32BSA), d-mannose BSA (Man41BSA), all from Dextra Laboratories, UK, in 100 μl of coating buffer and subsequently blocked with TBS/tw. The subscript number refers to the average number of sugar residues per protein molecule. Purified H-ficolin (see below) and purified L-ficolin (24) as well as recombinant M-ficolin (produced in CHO cells and kindly provided by Anne Trommelholt Jepsen, University of Southern Denmark, Denmark) were added to the wells in 100 μl of TBS/tw/Ca2+ and incubated overnight at 4 °C. Bound H-ficolin was detected as described above, whereas bound L- and M-ficolin were detected using a biotinylated anti-L-ficolin mAb (GN5, Hycult Biotechnology) or biotinylated anti-M-ficolin mAb (7G1) (2), respectively, followed by europium-labeled streptavidin as above.
Quantification of Ficolins and MBL
Microtiter wells were coated with antibody by incubation with 100 ng of anti-H-ficolin antibody (4H5) in 100 μl of PBS overnight at 4 °C. The wells were blocked with HSA in TBS. Samples were diluted in 20 mm Tris-HCl, 1 m NaCl, 0.05% Triton X-100, 10 mm CaCl2, 1 mg HSA/ml, pH 7.4 (assay buffer), and 100 μl was added to each well and incubated overnight at 4 °C. Bound H-ficolin was detected with 100 ng of biotinylated anti-H-ficolin (4H5) in 100 μl of TBS/tw/Ca2+ followed by europium-labeled streptavidin as described above. The concentration of H-ficolin was read from a calibration curve constructed using serial dilutions of a local standard serum pool (27). The concentrations of MBL and M-ficolin were quantified in a similar manner using anti-MBL and anti-M-ficolin antibodies, respectively (2, 40).
Assay for C4b Deposition on AcBSA
When the H-ficolin·MASP complex was bound to target, MASP-2 becomes activated and cleaves the complement component C4 leading to the covalent deposition of C4b on the surface. By measuring the amount of deposited C4b, the functional activity of the purified H-ficolin·MASP complex can be evaluated. The C4 cleaving capacity was quantified as described by Petersen et al. (41) using coating with AcBSA instead of mannan. Briefly, samples were incubated in AcBSA-coated wells followed by incubation with purified C4. Deposited C4b was detected by biotinylated anti-C4 antibody, and the amount of antibody bound was estimated with europium-labeled streptavidin as described above.
Purification of H-ficolin
HSA was coupled to CNBr-activated Sepharose beads (GE Healthcare), and residual reactive sites were blocked with ethanolamine. This resulted in 37 mg of HSA per ml of hydrated beads. Some of the HSA-Sepharose beads were acetylated; the beads were washed three times with methanol followed by incubation in 10% (v/v) acetic acid anhydride (Sigma) in 40% (v/v) methanol for 30 min at room temperature. The acetylated HSA-Sepharose beads (AcHSA-Sepharose) were washed in TBS and packed into a column. Similarly, nonacetylated HSA-Sepharose beads were packed into a column, and both columns were washed with 100 mm glycine, pH 2.5, and equilibrated in TBS/tw/Ca2+.
Citrated plasma from one blood donor (18 μg of H-ficolin/ml) was coagulated by the addition of 1 m CaCl2 to 10 mm and incubated until complete coagulation, and the serum was collected after centrifugation at 2,000 × g. Preliminary experiments showed that the bulk of H-ficolin was precipitated with 8% PEG 6000, although only small amounts precipitated at 4%. A stock of 16% (w/v) PEG in TBS was added to 205 ml of serum to achieve a final PEG concentration of 4% and stirred for 30 min at room temperature. The mixture was centrifuged at 5000 × g for 10 min and the supernatant collected. PEG was added to the supernatant to a final concentration of 8% and incubated for 30 min with stirring before centrifugation at 2000 × g for 10 min at room temperature. The precipitated proteins were washed in 9% PEG, spun at 2000 × g for 10 min at room temperature, dissolved in 80 ml of TBS/tw/Ca2+, and centrifuged for 5 min at 3000 × g to remove aggregates. At 4 °C, the supernatant was passed through 10 ml of an HSA-Sepharose column (this removes most L-ficolin), and the effluent was loaded onto a 10-ml AcHSA-Sepharose column to bind H-ficolin. The column was washed with 80 ml of TBS/tw/Ca2+ followed by 15 ml of TBS/tw/Ca2+ with 200 mm GlcNAc, pH 7.5, which elute MBL and L-ficolin bound to the beads. H-ficolin was eluted from the beads in two successive steps, first with 15 ml of 1 m sodium acetate, pH 7.5, followed by 15 ml of10 mm diethylamine, pH 11.5. The column was washed with 15 ml of TBS/tw/Ca2+ between each step. The eluates were collected in 2-ml fractions, and the diethylamine eluate was neutralized with 1 m Tris, pH 7.5. Fractions with H-ficolin were pooled and concentrated by ion exchange chromatography (IEC). After dialysis overnight at 4 °C against 10 mm Tris-HCl, 5 mm CaCl2, 20 mm NaCl, 0.01% Tween 20, pH 7.5 (buffer A), the sample was centrifuged at 3,000 × g for 10 min at room temperature. At room temperature, the supernatant was loaded onto an anion exchange column (1-ml Resource Q column, GE Healthcare) equilibrated in buffer A. After washing with 5 ml of buffer A, bound proteins were eluted with 10 mm Tris-HCl, 5 mm CaCl2, 1 m NaCl, 0.01% (v/v) Tween 20, pH 7.5 (buffer B). The H-ficolin-containing fractions were pooled, and a 500-μl sample subjected to GPC on a Superose 6 HR 10/30 column (GE Healthcare). The column buffer was TBS with 0.01% Tween 20 and 5 mm CaCl2, pH 7.5, and the column was run at a flow rate of 1 ml/min with 250-μl fractions being collected in Tween-treated microtiter wells. A serum sample of 100 μl containing 20 μg of H-ficolin/ml was subjected to GPC under identical conditions. The H-ficolin in each fraction was quantified as described above, and the ability to mediate deposition of C4 fragments onto AcBSA-coated microtiter wells was also tested.
SDS-PAGE and Western Blotting
SDS-PAGE was performed at standard conditions using a discontinuous buffer system on 4–12% polyacrylamide gradient gels (Criterion XT BisTris gels; Bio-Rad). For silver staining, performed according to the method described by Nesterenko (42), the molecular weight markers used were Precision Plus ProteinTM unstained standards (Bio-Rad), and Precision Plus ProteinTM standards, all blue (Bio-Rad), were used for Western blotting. For Western blotting, the proteins were transferred onto a nitrocellulose membrane (HybondTM ECLTM, GE Healthcare). H-ficolin was detected using goat anti-human H-ficolin antibody (AF2367, R&D Systems, Minneapolis, MN) diluted to 0.1 μg/ml in TBS with 0.05% (v/v) Tween 20, 1 mm EDTA, 1 mg of HSA, and 100 μg of normal human immunoglobulin per ml. After overnight incubation, the membrane was washed in TBS/tw without NaN3 and incubated for 1.5 h with horseradish peroxidase (HRP)-conjugated rabbit anti-goat antibody (P0449, Dako, Denmark) diluted 3000-fold in the same buffer with 1 mm EDTA and 100 μg normal human Ig/ml. After washing, a chemiluminescent substrate (SuperSignal® West Pico Chemiluminescent Substrate, 34080, Pierce) was added, and light emission was detected by CCD photon image capture. MASP-2 and L-ficolin were detected similarly with primary biotinylated anti-MASP-2 antibody (6G12 (43)) and anti-L-ficolin antibody (GN5, Hycult Biotechnology) diluted at 1 μg/ml. Following incubation, the primary antibodies were detected using either HRP-conjugated streptavidin (P0397, Dako) or HRP-conjugated rabbit anti-mouse antibodies (P0260, Dako), respectively; both diluted 3000-fold.
H-ficolin after Treatment with Reducing Compounds
To test their effect on H-ficolin, β-mercaptoethanol, cysteine, and acetylcysteine at 10 and 100 mm were added to 100 ng of H-ficolin in TBS. As a control, 100 ng of H-ficolin was reduced with 60 mm DTT. The samples were analyzed by Western blotting.
RESULTS
Calcium and pH Dependence of H-ficolin Binding
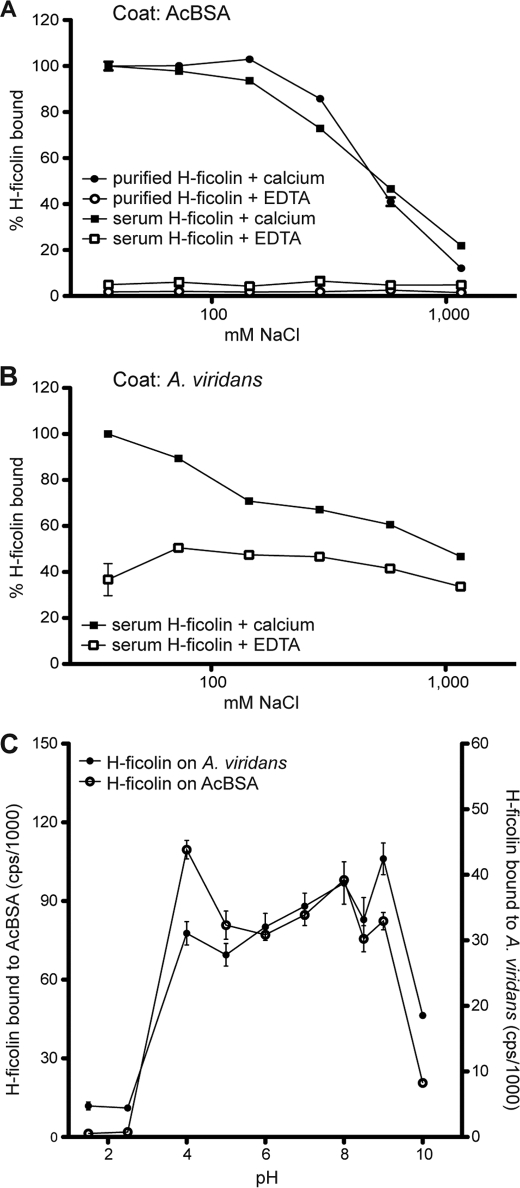
H-ficolin bound avidly to surfaces coated with A. viridans or AcBSA (Fig. 1). The binding of H-ficolin to AcBSA was abolished by the presence of EDTA instead of calcium (Fig. 1A), indicating that H-ficolin is strictly dependent on divalent metal ions for ligand binding. The binding of H-ficolin was also influenced by the NaCl concentration, i.e. at 1 m NaCl essentially no binding was observed (Fig. 1A). Identical results were obtained for purified H-ficolin and for H-ficolin present in serum. As illustrated in Fig. 1B, the requirements for binding of H-ficolin in serum to A. viridans are somewhat different from AcBSA. H-ficolin binding to A. viridans was only partially inhibited by EDTA. In the presence of calcium the binding increased at hypotonic salt concentration, and only a small decrease (34%) in binding was observed at higher concentration of NaCl. The binding of H-ficolin to AcBSA as well as to A. viridans-coated wells was also analyzed in relation to pH. The results illustrated in Fig. 1C show that the binding of H-ficolin to both surfaces was abrogated at both low (below 4) and high pH (above 9).
FIGURE 1.
Influence of buffer composition on H-ficolin binding. H-ficolin binding to AcBSA-coated microtiter wells (A) and to A. viridans-coated microtiter wells (B) was studied in the presence of calcium (5 mm) or EDTA (5 mm) with increasing NaCl concentrations. Bound H-ficolin is depicted in percentage (y axis) in relation to the binding seen at 36.5 mm NaCl (100%). C, binding of H-ficolin to AcBSA and A. viridans analyzed in buffers of various pH. Bound H-ficolin was detected with anti-H-ficolin antibody and is given as counts/s on the y axis. On the left side the counts obtained from the binding to AcBSA are given, and on the right side the counts/s from binding to A. viridians are given.
Inhibition of H-ficolin Binding
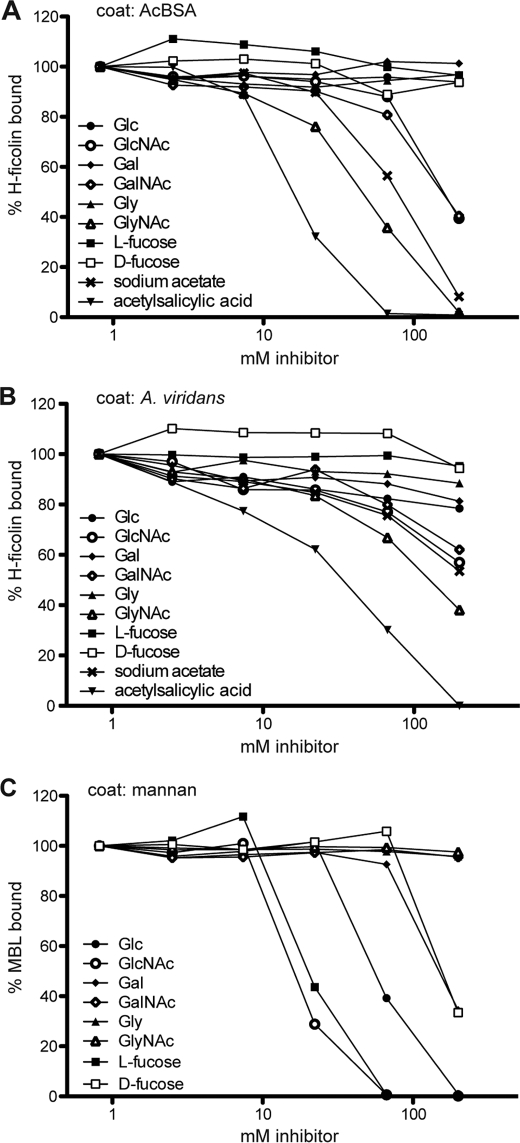
Several compounds were tested for their influence on the binding of H-ficolin. Fig. 2 shows the binding of H-ficolin to AcBSA- or A. viridans-coated wells (Fig. 2, A and B, respectively) in the presence of increasing concentrations of different compounds. For comparison and as a control for the procedure, we similarly tested the inhibition of the binding of MBL to mannan (Fig. 2C). Table 1 summarizes the inhibition data of the molecules we tested. By and large the same molecules inhibit the binding to both surfaces, although in several cases the binding of H-ficolin to A. viridans-coated wells was more difficult to inhibit than the binding to AcBSA. In general, N-acetylated molecules showed inhibitory activity for the binding to AcBSA, both sugars and amino acids and acetylsalicylic acid.
FIGURE 2.
Inhibition of the binding of H-ficolin. The ability of various compounds to inhibit the binding of H-ficolin to AcBSA (A) and A. viridans (B) and of MBL to mannan (C) was examined. Serum was diluted in TBS/tw/Ca2+ with increasing concentrations of different inhibitors (0.82–200 mm) followed by incubation on coated microtiter wells. Bound H-ficolin was detected with anti-H-ficolin antibody, and MBL was detected with anti-MBL. The amount of binding is expressed as percentage as compared with buffer with no inhibitor.
TABLE 1.
Inhibition of H-ficolin binding
The concentration (mm) needed to reach 70% of maximal signal (I70) for H-ficolin binding to AcBSA or A. viridians was determined graphically from inhibition curves such as shown in Fig. 2. NI means not inhibitory, i.e. resulting in less than 30% inhibition at 200 mm.
| Inhibitor | H-ficolin on AcBSA, I70 (mm) | H-ficolin on A. viridans, I70 (mm) |
|---|---|---|
| Glucose | NI | NI |
| Glucosamine | NI | NI |
| N-Acetylglucosamine | 115 | 115 |
| Galactose | NI | NI |
| Galactosamine | NI | NI |
| N-Acetylgalactosamine | 100 | 137 |
| Mannose | NI | NI |
| Mannosamine | NI | NI |
| N-Acetylmannosamine | NI | NI |
| Glycine | NI | NI |
| N-Acetylglycine | 29 | 56 |
| Cysteine | 4 | 7 |
| N-Acetylcysteine | 4 | 7 |
| Acetylsalicylic acid | 12 | 15 |
| Acetylcholine | NI | NI |
| l-fucose | NI | NI |
| d-fucose | NI | NI |
| Sodium acetate | 47 | 93 |
| Sodium citrate | NI | NI |
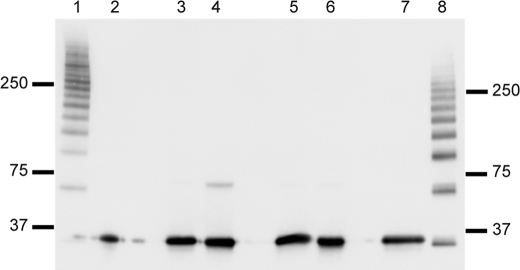
As reported previously (24), N-acetylcysteine strongly inhibited the binding of H-ficolin. However, this may be secondary to its reductive capability as it was found that cysteine showed identical inhibitory capability. Western blot analysis of H-ficolin incubated with different reductive compounds showed that oligomeric H-ficolin was broken down to slightly smaller oligomers at 10 mm N-acetylcysteine (Fig. 3, lane 8) and to single polypeptides when incubated with 100 mm N-acetylcysteine (Fig. 3, lane 7). These lanes should be compared with the nonreduced H-ficolin in Fig. 3, lane 1. The disruption of disulfide bonds may impair the binding capacity of H-ficolin as oligomerization is needed for high avidity binding. As shown in Fig. 3, dithiothreitol, cysteine, and β-mercaptoethanol all lead to reduction of single polypeptide chains at 10 mm.
FIGURE 3.
Analysis by Western blotting of H-ficolin treated with reducing agents. H-ficolin was incubated with dithiothreitol, β-mercaptoethanol, cysteine, or N-acetylcysteine and subsequently analyzed by Western blotting. Lane 1 represents an unreduced sample, and lane 2 is a sample reduced with 60 mm DTT. Lanes 3 and 4 represent samples reduced with 100 and 10 mm β-mercaptoethanol, respectively. Similarly, lanes 5 and 6 represent samples reduced with 100 and 10 mm cysteine, respectively, and lanes 7 and 8 represent samples reduced with CysNAc at a concentration of 100 and 10 mm, respectively. Approximately 100 ng of purified H-ficolin was applied in each well of the gel. The blot was developed with anti-H-ficolin antibody. The positions of the molecular mass markers (kDa) are indicated.
Binding of Ficolins to Neoglycoproteins
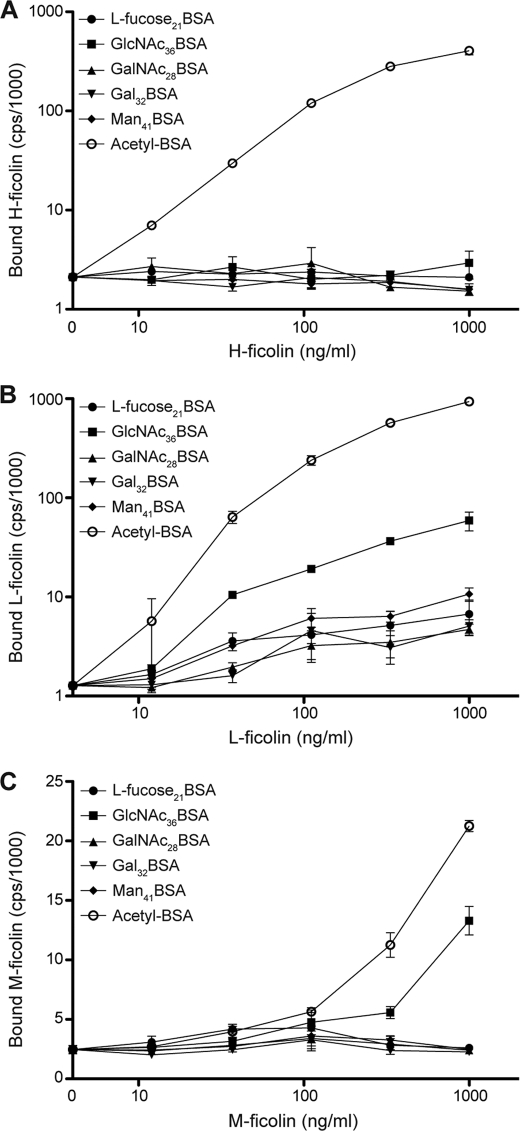
To further explore the ability of carbohydrates to bind to ficolins, we presented a pattern of carbohydrates, i.e. neoglycoproteins coated in wells, to the ficolins. All three ficolins bound avidly to wells coated with AcBSA (Fig. 4, A–C). H-ficolin did not bind to any of the neoglycoproteins tested, although M-and L-ficolin exhibited a similar binding pattern with binding to the GlcNAc-BSA-coated surface and no apparent binding to the others. In both cases more binding to AcBSA-coated wells was seen.
FIGURE 4.
Binding of ficolins to neoglycoproteins. H-ficolin, L-ficolin, or M-ficolin were incubated in microtiter wells coated with 1 μg of the neoglycoproteins or acetylated BSA as indicated on the figure. Each of the three ficolins was diluted in TBS/tw/Ca2+ to the concentrations given on the x axis. A, binding of H-ficolin; B, binding of L-ficolin; and C, binding of M-ficolin. The vertical bars indicate the S.D. In most cases the symbols cover the bars.
Purification of H-ficolin
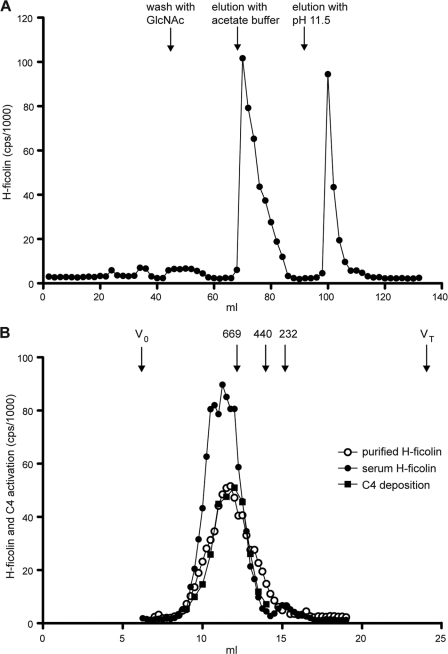
We developed a procedure for the purification of H-ficolin from human serum. We have previously demonstrated that most of the H-ficolin is precipitated by 8% PEG and more than 90% remain in the supernatant at 4% PEG. Most MBL and C1q are precipitated at 4% (24). We chose to make a 4–8% PEG cut of 205 ml of serum as a first step in the purification of H-ficolin. Approximately 83% of the H-ficolin was recovered (Table 2). The precipitate from the 4–8% PEG cut was dissolved in TBS/tw/Ca2+ and passed through an HSA-Sepharose pre-column to remove nonspecific proteins. Much of the residual amount of L-ficolin and MBL, which was still left in the 4–8% PEG cut, will bind to this column. For L-ficolin, this occurs due to its ability to bind CNBr-treated and -inactivated Sepharose (44) and MBL due to its propensity for binding to Sepharose when other interfering serum proteins have been removed (45). The effluent was collected and passed through an acetyl-HSA-Sepharose column. Fig. 5A illustrates the chromatographic profile obtained when loading the PEG cut onto a 10-ml acetyl-HSA-Sepharose column. All of the H-ficolin was bound to the column. The small amounts of attached MBL and L-ficolin were eluted when the column was subsequently washed with 200 mm GlcNAc in TBS/tw/Ca2+ (data not shown). The binding of H-ficolin is less influenced by this procedure, as is also evident when comparing the amount of GlcNAc needed to reach half-maximal inhibition of binding to AcBSA ∼200 mm (Fig. 2A) and the ∼20 mm GlcNAc needed to inhibit MBL binding to mannan (Fig. 2C). Bound H-ficolin was eluted in two successive steps as follows: first with sodium acetate at neutral pH and second with diethylamine at high pH. The H-ficolin-containing fractions from the sodium acetate eluate were combined, as were the fractions from the diethylamine eluate, and the H-ficolin was further purified and concentrated by IEC. Dialysis against the low salt starting buffer (buffer A) for the IEC, followed by centrifugation, resulted in precipitation of a substantial amount of the H-ficolin in each pool. After removal of the precipitate by centrifugation, we found the supernatant from the sodium acetate pool and the diethylamine pool to contain 52 and 31% of the H-ficolin measured before dialysis, respectively. The quantitative aspects of the purification scheme are outlined in Table 2. Only the purification scheme obtained using elution from AcHSA-Sepharose with sodium acetate is shown because IEC of the diethylamine supernatant resulted in a very low recovery (less than 7%). In comparison, 82% of the H-ficolin was recovered when the sodium acetate supernatant was subjected to IEC. To further purify the H-ficolin, the sample eluted from the Resource Q column was subjected to GPC. Fig. 5B shows the H-ficolin content in GPC fractions of the purified H-ficolin compared with the profile obtained when measuring H-ficolin in fractions from a similar separation of serum. The size of the purified H-ficolin was found to be ∼700 kDa and similar to the size observed for H-ficolin in serum. The serum-derived fractions revealed a small peak of H-ficolin eluting at 230 kDa, likely representing lower oligomeric forms.
TABLE 2.
H-ficolin recovery during purification
The amount of H-ficolin in each purification step was determined by TRIFMA as described under “Experimental Procedures.” The recovery (%) was determined by dividing the total amount of H-ficolin at a given purification step with the total amount of H-ficolin in the starting material (serum).
| Purification step | Volume | H-ficolin | Total H-ficolin | Recovery |
|---|---|---|---|---|
| ml | μg/ml | μg | % | |
| Serum | 205 | 17 | 3385 | 100 |
| 4–8% PEG cut | 80 | 35 | 2822 | 83 |
| Effluent from HSA-Sepharose | 80 | 35 | 2817 | 83 |
| Sodium acetate eluate | 17 | 101 | 1665 | 49 |
| Sodium acetate eluate + dialysis and centrifugation | 22 | 39 | 861 | 25 |
| Eluate from Resource Q column | 0.75 | 942 | 707 | 21 |
| GPC | 4 | 118 | 472 | 14 |
FIGURE 5.
Purification of H-ficolin from serum. A, purification of H-ficolin on an AcHSA column. A 4–8% PEG cut from serum was run through an HSA-coupled Sepharose column in TBS/tw/Ca2+, and the effluent was loaded onto an AcHSA-Sepharose column. The column was washed with GlcNAc to elute any remaining L-ficolin and H-ficolin and was subsequently eluted as follows: first with 1 m sodium acetate, pH 7.5, and next with 10 mm diethylamine, pH 11.5. B, H-ficolin containing fractions from A were pooled and concentrated and then subjected to GPC on a Superose 6 column. A serum sample was subjected to GPC on the same GPC column. The amount of H-ficolin in each fraction was quantified by TRIFMA, and was analyzed for the ability to activate and deposit C4 fragments onto an AcBSA surface. The elution volume of thyroglobulin (669 kDa), ferritin (440 kDa), and catalase (232 kDa) as well as the void volume (V0) and the total volume (VT) are given at top of figure.
Analysis of Purified H-ficolin by SDS-PAGE and Western Blotting
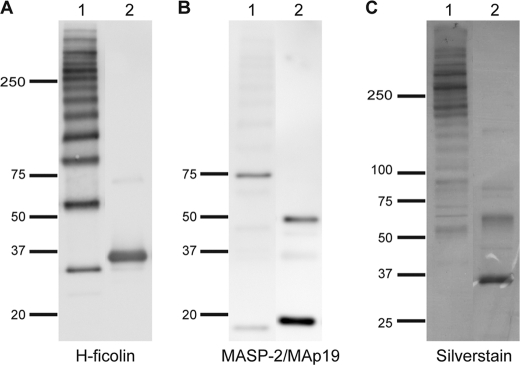
Analysis by Western blotting of the fractions from the GPC peak is shown in Fig. 6. The blot was developed with two different antibodies as follows: first with anti-H-ficolin antibody and subsequently with anti-MASP-2 antibody. The anti-H-ficolin antibody revealed a band of ∼35 kDa after reduction, corresponding to the single polypeptide chain of H-ficolin (Fig. 6A, lane 2). Under nonreducing conditions, several bands appeared (Fig. 6A, lane 1). The bands represent H-ficolin oligomers, apart from the band at ∼35 kDa representing single, noncovalently associated H-ficolin peptide chains. The distribution of oligomers at the beginning of the peak is quite similar to what is seen at the end of the peak (data not shown). When MASP-2 becomes activated, it is cleaved into two disulfide-linked chains, an A- and a B-chain. When the blot was developed with an antibody reacting with the N terminus of MASP-2 as well as with MAp19, we observe under reducing conditions two distinct bands, one at ∼50 kDa corresponding to the A-chain of MASP-2 and one at ∼19 kDa corresponding to MAp19 (Fig. 6B, lane 2). Under nonreducing conditions, two bands were seen at ∼75 and at ∼19 kDa corresponding to full-length MASP-2 and MAp19, respectively (Fig. 6B, lane 1). No bands were visible when the blot was developed with an anti-L-ficolin antibody (data not shown), and no signal was obtained if the fractions were tested for the presence of L-ficolin and MBL by TRIFMA. The peak fractions from the GPC were pooled and subjected to SDS-PAGE. The purity of the H-ficolin preparation is illustrated in Fig. 6C. Similarly to what we found on Western blots, we found H-ficolin in the nonreduced sample to be separated into a ladder of bands (Fig. 6C, lane 1). When analyzed under reducing conditions, the oligomers were disrupted, and we only observed the monomer polypeptide chain of H-ficolin (Fig. 6C, lane 2). We estimated the H-ficolin to represent at least 90% of the purified protein. The content of MASP-2 and MAp19 in the preparation was too low to be visible by silver staining. The lower oligomers are much more pronounced on the Western blot than on the silver-stained gel probably due to more efficient transfer to the membrane.
FIGURE 6.
Analysis of purified H-ficolin by SDS-PAGE and Western blotting. A, purified H-ficolin sample (pool from GPC, Fig. 5B) was run under nonreducing (lane 1) and reducing (lane 2) conditions, and the blot was developed with anti-H-ficolin antibody. The positions of the molecular mass markers (kDa) are indicated. B, same blot as in A developed with an anti-MASP-2/MAp19 antibody. C, H-ficolin containing fractions from the GPC were pooled and analyzed by SDS-PAGE and silver staining. Lane 1 was run under nonreducing conditions and lane 2 in reducing conditions.
Purified H-ficolin Complex and Activation of C4
When H-ficolin·MASP complexes bind to ligand, MASP-2 becomes activated. The ability of the purified H-ficolin·MASP complexes to cleave C4 was tested by adding purified C4 to AcBSA-coated microtiter wells, which had been preincubated with fractions from the GPC. The deposition of C4b was measured using anti-C4 antibody, and the ability to deposit activated C4 fragments on the acetylated BSA surface was found to coincide with the elution of the purified H-ficolin as seen in Fig. 5B.
DISCUSSION
H-ficolin is a PRM that may serve a function in the first line of defense against microorganisms (33). It also recognize apoptotic cells and may thus contribute to the maintenance of tissue homeostasis (34). It is of importance to decipher in detail the specificity of such recognition molecules.
Early studies found that H-ficolin was able to agglutinate human erythrocytes coated with lipopolysaccharides (LPS) from S. typhimurium and more weakly LPS from Salmonella minnesota. The agglutination could be inhibited with GlcNAc and GalNAc monosaccharides (31). We have not been able to show binding of H-ficolin to the whole bacteria corresponding to the LPS used in these studies (data not shown). H-ficolin has been shown to bind to A. viridans (27), and H-ficolin could inhibit the growth of A. viridans in serum (30). Binding to a capsular polysaccharide-rich component of A. viridans (PSA for polysaccharide) but with no inhibition by carbohydrate structures was examined (34, 46). Two studies have indicated binding of H-ficolin to protozoan parasites (47, 48), but also in these cases no specific inhibition by carbohydrates was examined for H-ficolin.
We wanted to perform a thorough study of the specificity of H-ficolin and started by examining the ability of H-ficolin to bind acetyl group containing patterns. We found this recognition to be calcium-dependent for the binding to AcBSA and that this binding was markedly dependent on salt concentration (Fig. 1). The binding of M-ficolin to AcBSA-coated surfaces is similarly abrogated by EDTA and inhibited at high salt concentrations (data not shown). A dependence of calcium for binding of ficolins to ligand has previously been suggested but no data presented (5, 49). The requirement for calcium may be understood from the crystal structure of the recognition domain of H- and M-ficolin as a Ca2+-binding site is found in the vicinity of the ligand-binding site (50, 51). The removal of the Ca2+ ion could change the structure of the ligand-binding site resulting in abrogation of ligand binding. The strong influence of increased NaCl concentration in the environment suggests that ionic interactions are important in the interaction with patterns of acetyl groups. It is important to note that at physiological NaCl concentrations full binding is still seen.
We next examined the specificity of the binding of serum H-ficolin to A. viridans. In the presence of calcium, binding was reduced by ∼50% when increasing the NaCl to hypertonic conditions. Chelating calcium only reduced the binding by 30%, and the dependence on ionic strength disappeared when no calcium was present. This indicates that the interaction with this microbial target is of a quite different nature than simply a pattern of acetyl groups, and it is remarkable how much the conditions for binding of H-ficolin differ depending on the target. H-ficolin has previously been suggested to agglutinate sheep erythrocytes in a calcium-independent manner (31), and it is possible that H-ficolin binding to this surface as well as to A. viridans is more complex than to AcBSA. Hence, the binding of H-ficolin to A. viridans could represent both the calcium-dependent binding observed to AcBSA and a calcium-independent interaction of H-ficolin with a component of the bacterial capsule.
Similar to what is observed for L-ficolin and M-ficolin (5, 24, 52), we found that N-acetylated molecules but not their nonacetylated counterparts could prevent the binding of H-ficolin, in this case the binding to A. viridans and to AcBSA (Fig. 2). This is in agreement with the observation that GalNAc and GlcNAc hinder agglutination of sheep erythrocytes by H-ficolin (31). N-Acetylated noncarbohydrate compounds (GlyNAc and CysNAc) as well as acetylsalicylic acid, sodium acetate, and cysteine also inhibited the binding of H-ficolin. CysNAc strongly inhibited the binding of H-ficolin, but this seemed to be due to its reductive capability rather than to a specific inhibition, since as little as 10 mm CysNAc affected the oligomerization of H-ficolin (Fig. 3). In contrast to L-ficolin and M-ficolin (5, 24), acetylcholine did not interfere with H-ficolin binding. Therefore, O-acetylated compounds may not inhibit H-ficolin. Interestingly, the binding to A. viridans was inhibited to a much lesser degree than the binding to AcBSA. The molecular patterns on the A. viridans surface are more heterogeneous when compared with the uniform AcBSA surface, and H-ficolin may bind to the A. viridans surface in a more complex manner. In terms of inhibitory potential, we found that acetylsalicylic acid was the most potent inhibitor followed by GlyNAc, sodium acetate, and GalNAc and GlcNAc displaying almost identical inhibitory profiles. Such a difference may be explored in the future with the use of compounds varying in positions of the acetyl groups and densities of acetyl groups in the pattern recognized.
The fibrinogen-like domain of H-ficolin has been crystallized in complex with d-fucose and galactose (50), and d-fucose has been shown to inhibit the agglutination of human erythrocytes coated with LPS from S. typhimurium (31). Recombinant H-ficolin has been demonstrated to bind galactosylated BSA using surface plasmon resonance spectroscopy (49). However, in the same study no significant interaction of H-ficolin with galactose epitopes on glycan arrays could be detected. In marked contrast to Sugimoto et al. (31), we could not inhibit the binding of H-ficolin to AcBSA or to A. viridans by d-fucose. Galactose did not show any effect (Fig. 2 and Table 1). We also analyzed the binding of H-ficolin to neoglycoproteins coated in microtiter wells and did not observe binding to Gal32BSA (Fig. 4). However, we also did not observe binding to GalNAc28BSA. We have previously observed that the binding of MBL (another soluble PRM) is very weak to such neoglycoproteins as compared with binding to surface structures from yeasts (data not shown). Taken together, this emphasizes that the three-dimensional organization and the ligand density are important determinants for binding of ficolins when compared with the very weak binding of monomeric ligands such as the ones used in crystallography. The difference between the data we present here and previous results requires further comparisons of the techniques used for studies of interactions between oligomeric molecules with polymeric patterns.
On the basis of the experience gained from the studies presented above, we developed a specific and affordable method for the purification of H-ficolin from serum. The key step of the process is affinity chromatography on AcHSA-derivatized Sepharose. The procedure resulted in the purification of oligomeric and functionally active H-ficolin molecules in complex with MASP-2. The mean yield of the process is 14% of the total H-ficolin in serum. No MBL and L-ficolin was co-purified. The purified H-ficolin was very similar to the appearance of H-ficolin in serum as evident from Western blotting and when examined by GPC. This procedure will thus enable specific purification of H-ficolin in a natural configuration for future studies of its ability to recognize microbial targets and indeed to study its ability to interact with the complement system.
Redundancy in receptor-ligand interactions is characteristic of many different biological settings and may be seen for ficolins. However, there is a fine balance for this to have a physiological meaning. We describe in this report similarities and differences between the three ficolins in terms of recognition of PAMPs.
Acknowledgment
We thank Annette Hansen for expert technical assistance.
The support from the Danish Council for Independent Research and the Novo Nordisk Foundation is appreciated.
- PRM
- pattern recognition molecule
- MBL
- mannan-binding lectin
- MASP
- MBL-associated serine protease
- AcBSA
- acetylated bovine serum albumin
- C4
- complement factor C4
- PAMP
- pathogen-associated molecular pattern
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- IEC
- ion exchange chromatography
- TRIFMA
- time-resolved immunofluorometric assay
- tw
- Tween 20
- HSA
- human serum albumin
- GPC
- gel permeation chromatography.
REFERENCES
- 1. Thiel S. (2007) Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins, and associated proteins. Mol. Immunol. 44, 3875–3888 [DOI] [PubMed] [Google Scholar]
- 2. Wittenborn T., Thiel S., Jensen L., Nielsen H. J., Jensenius J. C. (2010) Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J. Innate Immun. 2, 167–180 [DOI] [PubMed] [Google Scholar]
- 3. Skjoedt M. O., Hummelshoj T., Palarasah Y., Honore C., Koch C., Skjodt K., Garred P. (2010) A novel mannose-binding lectin/ficolin-associated protein is highly expressed in heart and skeletal muscle tissues and inhibits complement activation. J. Biol. Chem. 285, 8234–8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Degn S. E., Hansen A. G., Steffensen R., Jacobsen C., Jensenius J. C., Thiel S. (2009) MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J. Immunol. 183, 7371–7378 [DOI] [PubMed] [Google Scholar]
- 5. Frederiksen P. D., Thiel S., Larsen C. B., Jensenius J. C. (2005) M-ficolin, an innate immune defense molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 62, 462–473 [DOI] [PubMed] [Google Scholar]
- 6. Liu Y., Endo Y., Iwaki D., Nakata M., Matsushita M., Wada I., Inoue K., Munakata M., Fujita T. (2005) Human M-ficolin is a secretory protein that activates the lectin complement pathway. J. Immunol. 175, 3150–3156 [DOI] [PubMed] [Google Scholar]
- 7. Matsushita M., Fujita T. (1992) Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176, 1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsushita M., Endo Y., Fujita T. (2000) Cutting edge. Complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 164, 2281–2284 [DOI] [PubMed] [Google Scholar]
- 9. Matsushita M., Kuraya M., Hamasaki N., Tsujimura M., Shiraki H., Fujita T. (2002) Activation of the lectin complement pathway by H-ficolin (Hakata antigen). J. Immunol. 168, 3502–3506 [DOI] [PubMed] [Google Scholar]
- 10. Sørensen R., Thiel S., Jensenius J. C. (2005) Mannan-binding lectin-associated serine proteases, characteristics and disease associations. Springer Semin. Immunopathol. 27, 299–319 [DOI] [PubMed] [Google Scholar]
- 11. Lynch N. J., Roscher S., Hartung T., Morath S., Matsushita M., Maennel D. N., Kuraya M., Fujita T., Schwaeble W. J. (2004) L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 172, 1198–1202 [DOI] [PubMed] [Google Scholar]
- 12. Vorup-Jensen T., Jensenius J. C., Thiel S. (1998) MASP-2, the C3 convertase generating protease of the MBLectin complement-activating pathway. Immunobiology 199, 348–357 [DOI] [PubMed] [Google Scholar]
- 13. Takahashi M., Ishida Y., Iwaki D., Kanno K., Suzuki T., Endo Y., Homma Y., Fujita T. (2010) Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J. Exp. Med. 207, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsushita M., Thiel S., Jensenius J. C., Terai I., Fujita T. (2000) Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J. Immunol. 165, 2637–2642 [DOI] [PubMed] [Google Scholar]
- 15. Iwaki D., Kanno K., Takahashi M., Endo Y., Matsushita M., Fujita T. (2011) The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J. Immunol. 187, 3751–3758 [DOI] [PubMed] [Google Scholar]
- 16. Holmskov U., Thiel S., Jensenius J. C. (2003) Collections and ficolins. Humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21, 547–578 [DOI] [PubMed] [Google Scholar]
- 17. Holmskov U., Malhotra R., Sim R. B., Jensenius J. C. (1994) Collectins. Collagenous C-type lectins of the innate immune defense system. Immunol. Today 15, 67–74 [DOI] [PubMed] [Google Scholar]
- 18. Drickamer K. (1988) Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 263, 9557–9560 [PubMed] [Google Scholar]
- 19. Matsushita M., Endo Y., Hamasaki N., Fujita T. (2001) Activation of the lectin complement pathway by ficolins. Int. Immunopharmacol. 1, 359–363 [DOI] [PubMed] [Google Scholar]
- 20. Teh C., Le Y., Lee S. H., Lu J. (2000) M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-d-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology 101, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu J., Le Y., Kon O. L., Chan J., Lee S. H. (1996) Biosynthesis of human ficolin, an Escherichia coli-binding protein, by monocytes. Comparison with the synthesis of two macrophage-specific proteins, C1q and the mannose receptor. Immunology 89, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsushita M., Endo Y., Taira S., Sato Y., Fujita T., Ichikawa N., Nakata M., Mizuochi T. (1996) A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem. 271, 2448–2454 [DOI] [PubMed] [Google Scholar]
- 23. Kjaer T. R., Hansen A. G., Sørensen U. B., Nielsen O., Thiel S., Jensenius J. C. (2011) Investigations on the pattern recognition molecule M-ficolin. Quantitative aspects of bacterial binding and leukocyte association. J. Leukocyte Biol. 90, 425–437 [DOI] [PubMed] [Google Scholar]
- 24. Krarup A., Thiel S., Hansen A., Fujita T., Jensenius J. C. (2004) L-ficolin is a pattern recognition molecule specific for acetyl groups. J. Biol. Chem. 279, 47513–47519 [DOI] [PubMed] [Google Scholar]
- 25. Akaiwa M., Yae Y., Sugimoto R., Suzuki S. O., Iwaki T., Izuhara K., Hamasaki N. (1999) Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J. Histochem. Cytochem. 47, 777–786 [DOI] [PubMed] [Google Scholar]
- 26. Yae Y., Inaba S., Sato H., Okochi K., Tokunaga F., Iwanaga S. (1991) Isolation and characterization of a thermolabile β2-macroglycoprotein (“thermolabile substance” or “Hakata antigen”) detected by precipitating (auto)antibody in sera of patients with systemic lupus erythematosus. Biochim. Biophys. Acta 1078, 369–376 [DOI] [PubMed] [Google Scholar]
- 27. Krarup A., Sørensen U. B., Matsushita M., Jensenius J. C., Thiel S. (2005) Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect. Immun. 73, 1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garred P., Honoré C., Ma Y. J., Rørvig S., Cowland J., Borregaard N., Hummelshøj T. (2010) The genetics of ficolins. J. Innate Immun. 2, 3–16 [DOI] [PubMed] [Google Scholar]
- 29. Endo Y., Liu Y., Kanno K., Takahashi M., Matsushita M., Fujita T. (2004) Identification of the mouse H-ficolin gene as a pseudogene and orthology between mouse ficolins A/B and human L-/M-ficolins. Genomics 84, 737–744 [DOI] [PubMed] [Google Scholar]
- 30. Tsujimura M., Miyazaki T., Kojima E., Sagara Y., Shiraki H., Okochi K., Maeda Y. (2002) Serum concentration of Hakata antigen, a member of the ficolins, is linked with inhibition of Aerococcus viridans growth. Clin. Chim. Acta 325, 139–146 [DOI] [PubMed] [Google Scholar]
- 31. Sugimoto R., Yae Y., Akaiwa M., Kitajima S., Shibata Y., Sato H., Hirata J., Okochi K., Izuhara K., Hamasaki N. (1998) Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J. Biol. Chem. 273, 20721–20727 [DOI] [PubMed] [Google Scholar]
- 32. Swierzko A., Lukasiewicz J., Cedzynski M., Maciejewska A., Jachymek W., Niedziela T., Matsushita M., Lugowski C. (2012) New functional ligands for ficolin-3 among lipopolysaccharides of Hafnia alvei. Glycobiology 22, 267–280 [DOI] [PubMed] [Google Scholar]
- 33. Kuraya M., Ming Z., Liu X., Matsushita M., Fujita T. (2005) Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology 209, 689–697 [DOI] [PubMed] [Google Scholar]
- 34. Honoré C., Hummelshoj T., Hansen B. E., Madsen H. O., Eggleton P., Garred P. (2007) The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 56, 1598–1607 [DOI] [PubMed] [Google Scholar]
- 35. Thomsen T., Schlosser A., Holmskov U., Sorensen G. L. (2011) Ficolins and FIBCD1. Soluble and membrane-bound pattern recognition molecules with acetyl group selectivity. Mol. Immunol. 48, 369–381 [DOI] [PubMed] [Google Scholar]
- 36. Dagnaes-Hansen F., Kilian M., Fuursted K. (2004) Septicemia associated with an Aerococcus viridans infection in immunodeficient mice. Lab. Anim. 38, 321–325 [DOI] [PubMed] [Google Scholar]
- 37. Nakajima T., Ballou C. E. (1974) Characterization of the carbohydrate fragments obtained from Saccharomyces cerevisiae mannan by alkaline degradation. J. Biol. Chem. 249, 7679–7684 [PubMed] [Google Scholar]
- 38. Thiel S., Bjerke T., Hansen D., Poulsen L. K., Schiotz P. O., Jensenius J. C. (1995) Ontogeny of human mannan-binding protein, a lectin of the innate immune system. Pediatr. Allergy Immunol. 6, 20–23 [DOI] [PubMed] [Google Scholar]
- 39. Christiansen O. B., Kilpatrick D. C., Souter V., Varming K., Thiel S., Jensenius J. C. (1999) Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand. J. Immunol. 49, 193–196 [DOI] [PubMed] [Google Scholar]
- 40. Thiel S., Møller-Kristensen M., Jensen L., Jensenius J. C. (2002) Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology 205, 446–454 [DOI] [PubMed] [Google Scholar]
- 41. Petersen S. V., Thiel S., Jensen L., Steffensen R., Jensenius J. C. (2001) An assay for the mannan-binding lectin pathway of complement activation. J. Immunol. Methods 257, 107–116 [DOI] [PubMed] [Google Scholar]
- 42. Nesterenko M. V., Tilley M., Upton S. J. (1994) A simple modification of Blum's silver stain method allows for 30-minute detection of proteins in polyacrylamide gels. J. Biochem. Biophys. Methods 28, 239–242 [DOI] [PubMed] [Google Scholar]
- 43. Møller-Kristensen M., Jensenius J. C., Jensen L., Thielens N., Rossi V., Arlaud G., Thiel S. (2003) Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J. Immunol. Methods 282, 159–167 [DOI] [PubMed] [Google Scholar]
- 44. Le Y., Tan S. M., Lee S. H., Kon O. L., Lu J. (1997) Purification and binding properties of a human ficolin-like protein. J. Immunol. Methods 204, 43–49 [DOI] [PubMed] [Google Scholar]
- 45. Tan S. M., Chung M. C., Kon O. L., Thiel S., Lee S. H., Lu J. (1996) Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem. J. 319, 329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsujimura M., Ishida C., Sagara Y., Miyazaki T., Murakami K., Shiraki H., Okochi K., Maeda Y. (2001) Detection of serum thermolabile β2-macroglycoprotein (Hakata antigen) by enzyme-linked immunosorbent assay using polysaccharide produced by Aerococcus viridans. Clin. Diagn. Lab. Immunol. 8, 454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cestari Idos S., Krarup A., Sim R. B., Inal J. M., Ramirez M. I. (2009) Role of early lectin pathway activation in the complement-mediated killing of Trypanosoma cruzi. Mol. Immunol. 47, 426–437 [DOI] [PubMed] [Google Scholar]
- 48. Evans-Osses I., Ansa-Addo E. A., Inal J. M., Ramirez M. I. (2010) Involvement of lectin pathway activation in the complement killing of Giardia intestinalis. Biochem. Biophys. Res. Commun. 395, 382–386 [DOI] [PubMed] [Google Scholar]
- 49. Gout E., Garlatti V., Smith D. F., Lacroix M., Dumestre-Pérard C., Lunardi T., Martin L., Cesbron J. Y., Arlaud G. J., Gaboriaud C., Thielens N. M. (2010) Carbohydrate recognition properties of human ficolins. Glycan array screening reveals the sialic acid binding specificity of M-ficolin. J. Biol. Chem. 285, 6612–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garlatti V., Belloy N., Martin L., Lacroix M., Matsushita M., Endo Y., Fujita T., Fontecilla-Camps J. C., Arlaud G. J., Thielens N. M., Gaboriaud C. (2007) Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 26, 623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garlatti V., Martin L., Gout E., Reiser J. B., Fujita T., Arlaud G. J., Thielens N. M., Gaboriaud C. (2007) Structural basis for innate immune sensing by M-ficolin and its control by a pH-dependent conformational switch. J. Biol. Chem. 282, 35814–35820 [DOI] [PubMed] [Google Scholar]
- 52. Le Y., Lee S. H., Kon O. L., Lu J. (1998) Human L-ficolin. Plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 425, 367–370 [DOI] [PubMed] [Google Scholar]