Background: The mycobacterial proteasomal ATPase Mpa recruits substrates modified with Pup (prokaryotic ubiquitin-like protein) for degradation. Interestingly, Mpa itself is also a pupylation target.
Results: Pupylation of Mpa prevents its interaction with the proteasome and leads to reversible Mpa deoligomerization and inactivation.
Conclusion: Pupylation of Mpa reversibly modulates its activity.
Significance: Like ubiquitination, pupylation may play a regulatory role in addition to targeting proteins for degradation.
Keywords: ATP-dependent Protease, ATPases, Mycobacterium tuberculosis, Proteasome, Protein Degradation, Ubiquitination, Mpa, Post-translational Modification, Prokaryotic Ubiquitin-like Protein, Pupylation
Abstract
Pupylation is a bacterial post-translational modification of target proteins on lysine residues with prokaryotic ubiquitin-like protein Pup. Pup-tagged substrates are recognized by a proteasome-interacting ATPase termed Mpa in Mycobacterium tuberculosis. Mpa unfolds pupylated substrates and threads them into the proteasome core particle for degradation. Interestingly, Mpa itself is also a pupylation target. Here, we show that the Pup ligase PafA predominantly produces monopupylated Mpa modified homogeneously on a single lysine residue within its C-terminal region. We demonstrate that this modification renders Mpa functionally inactive. Pupylated Mpa can no longer support Pup-mediated proteasomal degradation due to its inability to associate with the proteasome core. Mpa is further inactivated by rapid Pup- and ATPase-driven deoligomerization of the hexameric Mpa ring. We show that pupylation of Mpa is chemically and functionally reversible. Mpa regains its enzymatic activity upon depupylation by the depupylase Dop, affording a rapid and reversible activity control over Mpa function.
Introduction
Proteasomes present in Mycobacterium tuberculosis (Mtb)4 and other actinobacteria recruit their substrates by a pathway functionally similar but chemically distinct from eukaryotic ubiquitination (1–3). The small protein Pup (prokaryotic ubiquitin-like protein) is attached to substrate lysine residues by the enzyme PafA (3). In this reaction, the C-terminal glutamate of Pup is activated by phosphorylation of the γ-carboxyl group (4, 5). Nucleophilic attack by the ϵ-amino group of the substrate lysine then results in isopeptide bond formation between Pup and the substrate. In mycobacteria, Pup is encoded with a C-terminal glutamine residue that must first be converted to a glutamate by the enzyme Dop (deamidase of Pup), making pupylation in these organisms a two-step pathway with the sequential action of the deamidase Dop and the Pup ligase PafA (3, 6). In addition to its deamidation activity, Dop has been shown to catalyze the removal of Pup from Pup-modified lysine residues acting as a “depupylase of Pup-protein conjugates” (7, 8). Hence, pupylation, like ubiquitination in eukaryotes, is a reversible process suited to serve regulatory functions in the cell in addition to targeting substrates to the proteasome.
Analysis of the mycobacterial proteome for pupylated substrates under standard in vitro culture conditions by three different groups revealed roughly 750 pupylation target proteins of which approximately 100 have been confirmed by identification of the modified lysine(s) (9–11). Potential and confirmed pupylation targets fall into a large variety of functional categories. Interestingly, enzymes involved in the Pup-proteasome system are themselves also proposed (proteasomal core subunits α and β, Dop, PafA) or confirmed (mycobacterial proteasomal ATPase Mpa) targets of pupylation, suggesting an element of autoregulation in this system. Mpa has previously been shown to accumulate in a Pup ligase knock-out strain, indicating that pupylation of Mpa leads to its degradation (12).
The proteasomal ATPase Mpa belongs to the AAA protein family and forms hexameric rings with a central pore (13, 14), which can interact with the 20 S core particle (15). The Mpa ring recruits pupylated substrates to the proteasome by binding Pup to its N-terminal coiled-coil domains located on the ring face distal to the proteasome core particle (16, 17). Substrates are then translocated through the Mpa pore into the core particle, where they are degraded (18). The unstructured N terminus of Pup, which is not itself involved in binding to the coiled-coil domains (16), serves as the site of translocation initiation, allowing the pupylated substrate to be threaded through the Mpa ring (18, 19). It has been shown that Mpa can also enhance the depupylation activity of Dop toward pupylated proteins (7, 8), suggesting that the unfoldase activity of Mpa might improve accessibility of the modification site for Dop.
Here, we investigate the effect of pupylation on the function of the proteasomal ATPase Mpa. Using the reconstituted pupylation system of Mtb, we show that pupylation of Mpa occurs predominantly on one target lysine. Pupylation at this position prevents interaction of Mpa-Pup with the proteasome core. Ultimately, pupylation leads to deoligomerization of the Mpa-hexamer driven by the unfolding activity of Mpa, thereby rendering Mpa-Pup fully inactive. We further demonstrate that pupylation is a reversible process and that depupylated Mpa regains its enzymatic activities.
EXPERIMENTAL PROCEDURES
Cloning and Protein Purification
Untagged Mpa was previously cloned into pET20 (Novagen) via NdeI and BamHI restriction sites to generate the pET20-mpa-overexpressing plasmid (18). The plasmid coding for the pupylation site variant MpaK591A was generated from pET20-mpa by site-directed mutagenesis.
Dop-His6, PafA-His6, PupE (a Pup Q64E variant) (3), as well as Mpa, PanB-Strep, Pup-GFP, open-gate proteasome (18) (all from Mtb), and GroEL-trap (GroEL D87K) (20) were expressed and purified as described. PupL39SL40S (17) was derived from PupE by site-directed mutagenesis and expressed and purified accordingly. It is referred to as Pup39S40S.
All protein concentrations were determined spectrometrically via their absorbance at 280 nm. Mpa concentrations are provided in terms of hexamer regardless of the true assembly state. The concentration of open gate proteasome refers to the assembled 28-mer, and PanB concentrations refer to concentration of the protomer.
Mpa Pupylation and Depupylation
In vitro conjugation of PupE (12 μm) to Mpa (1 μm), MpaK591A (1 μm), or PanB-Strep (6 μm) with PafA-His6 (1 μm) was carried out at 23 °C in buffer R (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 20 mm MgCl2, 10% glycerol, 1 mm DTT) supplemented with 5.5 mm ATP, 100 mm phosphocreatine (Sigma), and 2.1 units/ml creatine phosphokinase. The reaction was started by addition of PafA and was complete after 10 h. PafA was then removed by Ni2+-affinity chromatography, and the flow-through was concentrated by ultrafiltration (Amicon spin column, 30,000 MWCO). The pupylated Mpa was further purified by size-exclusion chromatography (Superose 6), the pupylated PanB by multiple rounds of ultrafiltration (18), and both were stored in buffer S (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 1 mm EDTA, and 1 mm DTT). Preparative pupylation with Pup39S40S (18 μm) was carried out under the same conditions except that PafA-His6 concentration was increased (5.25 μm) to obtain a fully pupylated sample. A mock pupylation and purification for Mpa was carried out as control under the same conditions except without addition of PupE and PafA-His6.
Analytical Gel Filtration
The elution behavior of Mpa, Mpa-Pup, and Mpa-Pup39S40S in size exclusion chromatography was examined on a Superdex 200 column (20-ml column volume; GE Healthcare). The column was equilibrated in buffer S at a flow rate of 0.5 ml/min at 23 °C, then 200 μl of mock pupylated Mpa (0.2 μm), Mpa-Pup (0.2 μm), or Mpa-Pup39S40S (0.2 μm) was loaded. The absorption profile was recorded at 230 nm. Size markers ribonuclease A (13.7 kDa), catalase (232 kDa), ferritin (440 kDa), and blue dextran 2000 (2 MDa) (Amersham Biosciences) were loaded under the same conditions. Apparent molecular masses were estimated by correlating the relative elution positions of the marker proteins in comparison to the void volume and column volume with the logarithm of their molecular mass. Estimates were then calculated by linear interpolation.
Interaction of mock pupylated Mpa or Mpa-Pup39S40S with Δ7CP (open-gate proteasomal core particle) (18) (all 0.6 μm) was analyzed by size exclusion chromatography on a Superose 6 column (24-ml column volume; GE Healthcare). The column was equilibrated at 4 °C in buffer R. Sample volume was 100 μl and had been complemented with 5 mm ATP. Flow was set to 0.5 ml/min.
Electron Microscopy
Negative stain electron microscopy images were recorded to assess the assembly state of native or pupylated Mpa complexes. Specimens for electron microscopy were prepared by applying 20 μl aliquots of 60 nm Mpa or pupylated Mpa in 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 20 mm MgCl2, 10% (v/v) glycerol, and 5 mm ATP on freshly hydrophilized carbon-coated 300 mesh copper grids (Quantifoil) for 30 s. Specimens were washed three times with water, stained with 1% (w/v) uranyl acetate, and were then imaged in a FEI Morgagni 268 transmission electron microscope operating at 100 kV with a magnification of 32,000- or 24,000-fold.
Radioactive ATPase Assay
The ATPase activity of Mpa was determined using a radiometric assay (21). Mock-pupylated Mpa, MpaK591A, Mpa-Pup, Mpa-Pup39S40S (all 12.4 nm), or Mpadepup (7.0 nm), respectively, was incubated in buffer R at 23 °C with 1.5 mm ATP spiked with [γ-32P]ATP (8.88 MBq (240 μCi)/mmol) (Hartmann Analytic, specific activity: 185 TBq (5000 Ci)/mmol). Enzymatic activities were monitored over the course of 1 h by drawing samples at 10-min intervals. All samples were processed as described in Ref. 21, and the amount of liberated γ-32Pi was determined by measuring Cerenkov radiation in a liquid scintillation counter (Packard Tri_carb 1500). All series were background-subtracted by a blank measurement and scaled relative to the total amount of radioactivity in the ATP stock.
Each measurement series (time course) was performed at least three times, and the mean hydrolyzed ATP concentration for each time point was calculated. The data points were scaled to a protein concentration of 10 nm to enhance visual comparability. A linear fit was applied to the data and the ATP hydrolysis rate calculated from the slope. The kcat (per hexamer) was calculated dividing the ATP hydrolysis rate by the Mpa concentration.
Pup-GFP Unfolding Assay
Pup-GFP unfolding was monitored as described previously (18). Briefly, Pup-GFP (1 μm) was incubated at 23 °C with GroEL trap (3 μm) and mock-pupylated Mpa, MpaK591A, Mpa-Pup, Mpa-Pup39S40S, or Mpadepup (all 0.2 μm) in buffer R supplemented with 5 mm ATP, 25 mm phosphocreatine, and 1 unit/ml phosphocreatine kinase. Unfolding was initiated by addition of Mpa, and fluorescence intensity was monitored at 510 nm (excitation at 400 nm) on a fluorescence spectrometer (PTI). Data set was normalized globally.
Pup-GFP Degradation Assay
Pup-GFP degradation was performed analogous to Pup-GFP unfolding without addition of GroEL trap but in the presence of open-gate proteasome (Δ7CP) instead, as described in Ref. 18. Concentration of Mpa or its variants was 0.1 μm, open-gate proteasome 0.2 μm, and GFP-Pup 1 μm.
PanB-Pup Degradation Assay
PanB-Pup (1 μm) was incubated with mock-pupylated Mpa, Mpa-Pup, Mpa-Pup39S40S, or Mpadepup (all 0.1 μm) and 0.1 μm open-gate proteasome at 30 °C in buffer R supplemented with 5 mm ATP, 25 mm phosphocreatine, and 1 units/ml phosphocreatine kinase. The reaction was terminated at the indicated time points by addition of SDS-sample buffer and analyzed by Coomassie-stained SDS-PAGE.
RESULTS
Pup Ligase PafA Pupylates Mpa Predominantly on a Single Lysine Residue
Determination of the pupylated proteome (pupylome) of both Mtb and Mycobacterium smegmatis under standard culture conditions showed that the proteasomal ATPase Mpa can be pupylated in vivo (9–11). A pupylation site was identified as lysine residue 591 for the Mtb protein (11). This residue is located near the C terminus on the face of the Mpa ring that interacts with the proteasomal core particle.
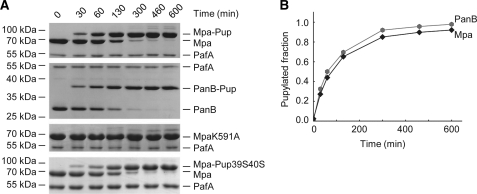
By carrying out in vitro pupylation assays using the recombinantly produced Mtb Pup ligase PafA, we assessed the pupylation efficiency and site specificity for Mpa as pupylation substrate. Recombinantly produced Mpa was incubated with the Pup ligase PafA and PupE (a PupQ64E variant) in the presence of ATP, and samples were drawn at various time intervals to follow the production of Mpa-Pup conjugates by SDS-PAGE (Fig. 1A, top gel). Over the time course of 10 h the Mpa band disappeared concomitant with the production of one main Mpa-Pup conjugate band (Fig. 1B, black trace), indicating that Mpa is converted predominantly into the monopupylated form. At later time points, when most of the Mpa had been converted, additional faint bands could be detected above the Mpa-Pup band (Fig. 1A, top gel), likely representing trace amounts of Mpa pupylated at more than one lysine. Comparison of the pupylation time course for Mpa as substrate with an experiment using an equivalent concentration of the known pupylation substrate PanB (12) (Fig. 1, A and B, second gel and gray trace) establishes that both proteins are pupylated with similar rates. This is in agreement with our previous finding that activation of Pup by phosphorylation is rate-limiting when pupylation substrates are present at saturating concentrations (4). When the pupylation site variant MpaK591A was used in the pupylation reaction (Fig. 1A, third gel), no significant amount of Mpa-Pup conjugate was formed within the same time frame, indicating that the monopupylated Mpa produced during the reaction with wild-type Mpa is homogeneously modified at lysine residue 591.
FIGURE 1.
Mpa is pupylated predominantly on a single lysine residue, and pupylation occurs on a time scale similar to that for the proteasomal substrate PanB. A, Pup conjugation to Mpa (1 μm hexamer, gel), PanB (6 μm monomer, second gel), the pupylation site variant MpaK591A (1 μm hexamer, third gel), or Mpa (1 μm hexamer) with the Pup39S40S variant (fourth gel) by PafA (1 μm or 5.25 μm for the Pup variant) visualized at various time points by SDS-PAGE and Coomassie staining. B, analysis of Mpa wild-type and PanB pupylation time trace by gel band densitometry (A, first and second gels).
Mpa features Pup binding sites at its N-terminal coiled-coil domains, allowing Mpa-Pup to be recruited to active Mpa pores. To study the effect of Mpa pupylation also under conditions where “self-recruitment” cannot take place, we modified Mpa with a Pup variant (Pup39S40S) unable to form the shared coiled-coil (17) with Mpa (Fig. 1A, bottom gel). This form of modified Mpa will be used in functional assays alongside Mpa-Pup.
Pupylation of Mpa Generates an ATPase-inactive Mpa-Pup Population
Mpa is a member of the AAA protein family and exhibits a basal ATPase activity even in the absence of the proteasomal core (13, 18). We analyzed the production of inorganic phosphate from ATP in the presence of unmodified Mpa or the homogeneously monopupylated Mpa-Pup conjugate employing a radiometric ATPase assay (Fig. 2). The unmodified Mpa sample was subjected to a mock pupylation protocol carried out in the absence of PafA and PupE to allow direct comparison of the two samples. Mpa-Pup no longer exhibited ATPase activity (red trace), whereas the pupylation site variant MpaK591A that underwent the same protocol retained almost all of the ATPase activity of the unmodified enzyme (black and gray traces, respectively). Surprisingly, Mpa modified with Pup39S40S, which prevents self-recruitment, retained the ATPase activity (orange trace), suggesting that self-recruitment might play a role in inactivation.
FIGURE 2.
Pupylation of Mpa reversibly abolishes its ATPase activity. A, release of Pi monitored in a radiometric assay under saturating ATP concentrations. The different species are (all scaled to 10 μm): mock pupylated Mpa (gray pentagons), pupylation site variant Mpa K591A (black hexagons), Mpa-Pup (red star), and Mpa-Pup39S40S (orange squares) conjugates, depupylated Mpa (green circles). Control reaction was carried out in the absence of Mpa (light gray hexagons). Data points are given as the mean of at least three independent measurements. B, bar diagram of the ATPase activities corresponding to the curves in A, calculated per hexamer. All error bars represent 1 standard error.
Pupylation of Mpa Leads to Disassembly of the Hexameric Ring
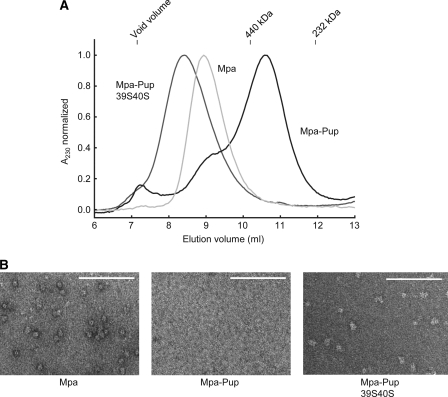
The inability of Mpa-Pup to hydrolyze ATP could be due to a conformational rearrangement after Pup ligation. However, it could also point to a more severe impairment of Mpa which prevents the assembly into hexameric rings in turn resulting in complete loss of ATPase activity. We therefore investigated the assembly state of Mpa-Pup using analytical gel filtration and negative stain electron microscopy (Fig. 3). It has been shown previously that bacterial proteasomal ATPases assemble into hexameric rings with a central pore (13, 14). Accordingly, the unmodified Mpa eluted as a higher order complex before the 440-kDa size marker (Fig. 3A, light gray profile). The excessively high apparent molecular mass of roughly 880 kDa is due to the toroidal shape of the Mpa hexamer featuring long coiled-coil domains extending out from the ring surface. The unusually long retention time is a feature often observed with AAA proteins (14, 22). In comparison, Mpa-Pup eluted much later under identical conditions at an apparent molecular mass of 375 kDa based on the molecular mass standards (Fig. 3A, black profile). The considerable shift in the elution position of Mpa in its pupylated form suggests disassembly of the hexameric ring. To confirm this, we recorded negative stain electron microscopy images of both the pupylated and nonpupylated Mpa sample (Fig. 3B). The micrographs of wild-type Mpa (left image) show uniformly sized ring- or sphere-shaped particles that are evenly distributed across the fields. No such particles could be observed for the pupylated sample (middle image), confirming that the hexameric Mpa rings have been disrupted.
FIGURE 3.
Mpa pupylation and subsequent self-recruitment lead to disassembly of the hexameric ring. A, elution profile (recorded by absorbance at 230 nm) of mock-pupylated Mpa (light gray elution profile) (0.2 μm) and Mpa-Pup (black elution profile) (0.2 μm) as well as Mpa-Pup39S40S (gray elution profile) using a Superdex 200 size exclusion column. B, negative stain electron microscopy images of Mpa (60 nm, left ), Mpa-Pup (60 nm, middle), and Mpa-Pup39S40S (60 nm, right). White scale bars indicate 100 nm.
To test whether self-recruitment is necessary for Pup-driven disassembly, we analyzed the elution behavior of Mpa-Pup39S40S under the same conditions. Mpa-Pup39S40S eluted at a position in agreement with a Pup-modified hexameric complex (Fig. 3A, dark gray trace), indicating that the ability of Mpa-Pup to be recruited and unfolded by the Mpa ring is required for disassembly and stable formation of a disassembled pool of Mpa-Pup. Negative stain electron microscopic images of Mpa-Pup39S40S confirmed that higher oligomers were present (Fig. 3B, right image).
Disassembled Mpa-Pup Can No Longer Unfold Substrate Proteins
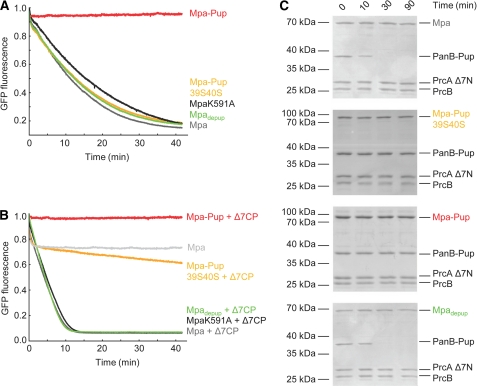
Threading of pupylated substrates through the Mpa pore requires the ATPase-driven up and down movements of loops pointing into the pore and carrying a conserved aromatic-hydrophobic-glycine motif (Ar-φ-Gly), which in case of Mtb Mpa encompasses residues 341FVG343 (18). Using a fluorescent model substrate where Pup is fused N-terminally to GFP, we can follow the unfoldase activity of Mpa (18). No decrease in the fluorescence signal was observed with Mpa-Pup (Fig. 4A, red trace), whereas MpaK591A or unmodified Mpa readily unfolded Pup-GFP within the same time frame (black and gray trace, respectively). This shows that Mpa-Pup has been rendered inactive by post-translational modification with Pup on Lys-591. However, when we used the Pup variant Pup39S40S to modify Mpa, the generated Mpa-Pup39S40S retained its ability to unfold Pup-GFP (Fig. 4A, orange trace). This demonstrates that pupylation of the Mpa hexameric ring in itself does not hinder the unfoldase activity.
FIGURE 4.
Mpa-Pup does not promote proteasomal degradation of pupylated substrates. A, unfolding of Pup-GFP (1 μm) in the presence of GroEL trap (3 μm) by mock-pupylated Mpa (gray trace), MpaK591A (black trace), Mpa-Pup (red trace), Mpa-Pup39S40S (orange trace), or Mpadepup (green trace) (all 0.1 μm) monitored by GFP fluorescence. B, Pup-GFP (1 μm) degradation by open-gate proteasome (Δ7CP, 0.2 μm) followed by GFP fluorescence. Mpa species (all 0.1 μm) share the same color code as in A. A control experiment was conducted in the absence of open-gate proteasome (light gray trace). C, Mpa-Pup (third gel) or Mpa-Pup39S40S (second gel) (both 0.2 μm) are not able to support degradation of PanB-Pup (1 μm) by the open-gate proteasome (0.1 μm) in contrast to mock-treated Mpa (top gel) or depupylated Mpa (Mpadepup, bottom gel) (both 0.2 μm). The degradation progression is visualized by SDS-PAGE and Coomassie staining. PrcA Δ7N refers to the proteasomal α-subunit truncated by the seven N-terminal residues (open-gate), PrcB to the β-subunit, and Δ7CP refers to the assembled open-gate proteasomal core particle.
Mpa-Pup Cannot Support Efficient Proteasomal Degradation of Pupylated Substrates Regardless of Its Assembly State
Our results so far show that the failure of Mpa-Pup to unfold pupylated substrate proteins is due to pupylation-driven disassembly of the Mpa ring. Because ATP-dependent unfolding is required for producing the disassembled, pupylated Mpa species, the question arises whether pupylated, but still assembled Mpa-Pup could still associate with the proteasome core. Furthermore, we cannot exclude that the presence of the proteasomal core particle could lead to reassembly of disassembled Mpa-Pup on top of the proteasomal α-rings. To test whether Mpa-Pup or Mpa-Pup39S40S can support proteasomal degradation of pupylated substrates we performed degradation assays using two substrates: the fluorescent model substrate Pup-GFP, featuring a linear fusion of Pup to GFP, and the known proteasomal substrate PanB-Pup, which we generated in vitro using the Pup ligase PafA (Fig. 4, B and C).
Mpa produces a rapid fluorescence decrease, when added to Pup-GFP in the presence of open-gate proteasome (Δ7CP) and ATP (Fig. 4B, dark gray trace), whereas Mpa-Pup is unfolding-inactive (red trace). The hexameric Mpa-Pup30S40S exhibits a very slow decrease in GFP fluorescence (Fig. 4B, orange trace), indicating that the degradation rate has decreased by at least an order of magnitude. The initial fast drop of GFP fluorescence is independent of degradation, but stems from Mpa-mediated Pup-GFP unfolding and spontaneous refolding of released, nonnative Pup-GFP in the absence of trap (18).
Similarly, Mpa mediates Δ7CP-dependent degradation of the proteasomal substrate PanB-Pup (Fig. 4C, top gel), whereas no degradation activity can be detected in the presence of the Pup-modified form of Mpa (Fig. 4C, third gel), indicating that the assembly defect of Mpa-Pup persists also in the presence of the proteasome core. Mpa-Pup39S40S exhibits a strong defect in degradation activity also toward PanB-Pup (Fig. 4C, second gel). This is in contrast to its barely affected unfoldase activity (Fig. 4A) and strongly indicates a failure of Mpa-Pup39S40S to interact with the proteasome.
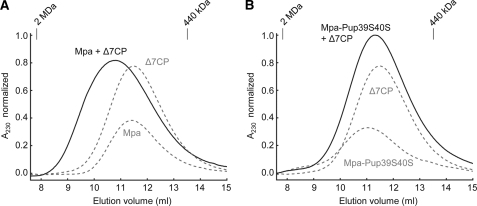
To complement the activity-based experiments with a direct measure of interaction, we examined the migration behavior of Mpa and Mpa-Pup39S40S in the presence of Δ7CP by size exclusion chromatography (Fig. 5). A size shift was observed for Δ7CP with Mpa (Fig. 5A) whereas with Mpa-Pup39S40S no such shift was detectable (Fig. 5B). Considering the size of Δ7CP (800.4 kDa) and Mpa (404.4 kDa), the small shift (Fig. 5A) most likely indicates a rapid equilibrium between free Mpa, free Δ7CP, and the Mpa-Δ7CP complex. The fact that the migration behavior of free Mpa-Pup39S40S is not changed in the presence of Δ7CP indicates that interaction of the ATPase ring with the core particle is perturbed due to the pupylation.
FIGURE 5.
Mpa-Pup39S40S does not interact with open-gate proteasome (Δ7CP). A, gel chromatograms of Mpa (0.6 μm), Δ7CP (0.6 μm), and Mpa together with Δ7CP (both 0.6 μm) and 5 mm ATP. B, similar chromatograms of Mpa-Pup39S40S (0.6 μm) and Mpa-Pup39S40S with Δ7CP (both 0.6 μm). Δ7CP is depicted again from A as visual anchor. Curves in both figures have been normalized globally.
Mpa Pupylation Is Chemically and Functionally Reversible
To test whether Pup modification of Mpa is reversible not only chemically, but also functionally, we preparatively generated Mpa-Pup in vitro using PafA and the deamidated form of the modifier, PupE. After ensuring that the purified Mpa-Pup was functionally inactivated with respect to ATPase, unfoldase and degradation activity, the Pup modification was removed by incubating the sample with the depupylase Dop to produce Mpadepup. The depupylated Mpa regained full ATPase activity (Fig. 2, A and B, green trace), indicating that Mpa upon depupylation had reassembled into active hexamers. Accordingly, the depupylated Mpa enzyme can unfold the fluorescent model substrate Pup-GFP (Fig. 4A, green trace). The recovered unfolding activity of Mpadepup is able to support the degradation of the natural proteasome substrate PanB-Pup as well as the Pup-GFP model substrate in the presence of the proteasome core (Fig. 4, B, green trace, and C, bottom gel).
DISCUSSION
Pupylation is a bacterial post-translational modification that can target substrates for degradation by the proteasome (1–3). Because modification with Pup is reversible (7, 8), pupylation likely also serves regulatory functions.
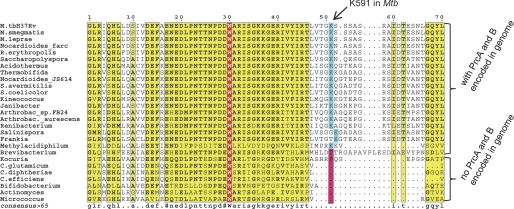
In this study we show that post-translational modification of a target protein with Pup not only renders it a substrate for proteasomal degradation, but simultaneously modulates its enzymatic activities. This is a demonstration of pupylation as an activity-regulating modification. Interestingly, the pupylation substrate in this case is itself a component of the Pup-proteasome degradation pathway, namely the proteasome-interacting ATPase Mpa. Because recognition of pupylated substrates by the Mpa-proteasome complex involves binding of Pup to the coiled-coil domains on the Mpa ring (16, 17), the presence of Mpa is strictly required for the proteasomal degradation of pupylated substrates (18, 19). Alignment of the Mpa amino acid sequences from a large range of actinobacteria shows that Mpa proteins cluster into two groups with respect to the length and sequence of their C-terminal segments (Fig. 6). The pupylation site lysine in Mtb (Lys-591) is well conserved in the one cluster but not in the other. It is striking that the cluster where the pupylation site is conserved also contains the C-terminal GQYL motif. Archaeal and eukaryotic proteasomal ATPases mediate interaction with the core particle by insertion of a similar motif with an aromatic residue at the penultimate position, into pockets on the α-rings (23–25). Our experiments show that pupylation of Mpa prevents docking of Mpa onto the proteasomal α-rings. This allows for a rapid separation of unfoldase from degradation activity. Further shut down via unfolding of Mpa-Pup then leads to a stable pool of disassembled Mpa-Pup. The inactivation could be viewed as a two-step process: initial pupylation abolishes interaction with the proteasome, free Mpa-Pup rings then lead to deoligomerization of Mpa-Pup into an inactive, but readily reactivatable storage pool. Accordingly, all Mpa sequences with a homologous lysine stem from organisms with proteasomal core subunits encoded in their genome. Pupylation of Mpa could thus be a feedback inhibition of the degradation branch to ensure that in those organisms, where the proteasomal degradation branch exists, it is kept in appropriate balance with any degradation-independent function of Pup modification. Those Mpa homologues from bacteria without proteasomal subunit genes do not feature the lysine, like for example the ATPase from Corynebacterium glutamicum. We tested whether the homologue from that organism might be regulated via a different lysine residue. However, the recombinantly produced Mpa-homologue from C. glutamicum could not be pupylated in vitro, and its activity, therefore, appears not to be regulated by the pupylation pathway (supplemental Fig. S1). This strongly suggests that pupylation of Mpa is coupled to regulation of Pup-dependent degradation and is not necessary or beneficial when the degradation pathway is absent. The interruption of Mpa/proteasome interaction by pupylation of Mpa strongly supports this notion.
FIGURE 6.
Alignment of the C-terminal amino acid sequence stretch of Mpa-homologues from a broad selection of actinomycetales. The pupylation site lysine identified for Mtb co-occurs with the proteasomal core subunits α and β (termed PrcA and PrcB in Mtb) and is largely missing in those homologues from organisms without proteasomal subunit genes.
Regulation of large, energy-dependent protease complexes by disassembly of their ATPase components has related examples in the Clp protease system of Gram-positive bacteria, for example the ClpCP protease from Bacillus subtilis (26). Here, control of assembly/disassembly of ClpC is dependent on the presence of so-called adaptor proteins instead of post-translational modification. Only when the adaptor proteins are in complex with ClpC is the ATPase ring formed that interacts with the protease core. This allows fine tuning of the degradation activity to the temporal need for this activity at different developmental stages.
Mpa contributes to the survival strategy of Mtb in the host, as it has been shown that it makes the bacteria more resistant to various forms of stress and that the virulence of the mpa knock-out strain is strongly reduced in the mouse infection model (13, 27). However, the control of Mpa via Pup modification both concerning its in vivo stability (12) as well as its enzymatic activities as demonstrated here, indicates that under certain conditions, Mpa activity has to be reined in. The pupylation-driven disassembly of Mpa might allow this shut-down to take place very rapidly and even in the presence of a high level of other pupylated proteasomal substrates that compete for degradation by the proteasome. In vivo studies will be needed to elucidate which aspect of the Mtb life style requires Mpa to be tightly controlled. For example, it would be very interesting to investigate the behavior of an Mpa pupylation site mutant Mtb strain in the mouse infection model as well as under other stress conditions.
Investigation of the pupylated proteome in Mtb and M. smegmatis (9–11) suggests that Mpa might not be the only member of the Pup-proteasome system that is controlled by pupylation. Although pupylation sites have not been identified yet, this evidence suggests that some form of feedback mechanism could extend to the entire modification pathway, stressing the importance of a carefully tuned pupylation and Pup-driven degradation system.
Supplementary Material
Acknowledgments
We thank the Functional Genomics Center Zurich (FGCZ) for MS and members of the Weber-Ban group and Frank Imkamp for reading the manuscript.
This work was supported by the Swiss National Science Foundation, the National Center for Excellence in Research Structural Biology program of the Swiss National Science Foundation, an ETH research grant, and a Kékulé fellowship by the “Fonds der Chemischen Industrie” (to F. S.).

This article contains supplemental Fig. S1.
- Mtb
- Mycobacterium tuberculosis
- Dop
- deamidase of Pup
- Mpa
- mycobacterial proteasomal ATPase
- Pup
- prokaryotic ubiquitin-like protein.
REFERENCES
- 1. Burns K. E., Liu W. T., Boshoff H. I., Dorrestein P. C., Barry C. E., 3rd (2009) Proteasomal protein degradation in mycobacteria is dependent upon a prokaryotic ubiquitin-like protein. J. Biol. Chem. 284, 3069–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearce M. J., Mintseris J., Ferreyra J., Gygi S. P., Darwin K. H. (2008) Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 322, 1104–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Striebel F., Imkamp F., Sutter M., Steiner M., Mamedov A., Weber-Ban E. (2009) Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nat. Struct. Mol. Biol. 16, 647–651 [DOI] [PubMed] [Google Scholar]
- 4. Guth E., Thommen M., Weber-Ban E. (2011) Mycobacterial ubiquitin-like protein ligase PafA follows a two-step reaction pathway with a phosphorylated pup intermediate. J. Biol. Chem. 286, 4412–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutter M., Damberger F. F., Imkamp F., Allain F. H., Weber-Ban E. (2010) Prokaryotic ubiquitin-like protein (Pup) is coupled to substrates via the side chain of its C-terminal glutamate. J. Am. Chem. Soc. 132, 5610–5612 [DOI] [PubMed] [Google Scholar]
- 6. Imkamp F., Rosenberger T., Striebel F., Keller P. M., Amstutz B., Sander P., Weber-Ban E. (2010) Deletion of dop in Mycobacterium smegmatis abolishes pupylation of protein substrates in vivo. Mol. Microbiol 75, 744–754 [DOI] [PubMed] [Google Scholar]
- 7. Burns K. E., Cerda-Maira F. A., Wang T., Li H., Bishai W. R., Darwin K. H. (2010) “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol. Cell 39, 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imkamp F., Striebel F., Sutter M., Ozcelik D., Zimmermann N., Sander P., Weber-Ban E. (2010) Dop functions as a depupylase in the prokaryotic ubiquitin-like modification pathway. EMBO Rep. 11, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watrous J., Burns K., Liu W. T., Patel A., Hook V., Bafna V., Barry C. E., 3rd, Bark S., Dorrestein P. C. (2010) Expansion of the mycobacterial “PUPylome.” Mol. Biosyst. 6, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poulsen C., Akhter Y., Jeon A. H., Schmitt-Ulms G., Meyer H. E., Stefanski A., Stuhler K., Wilmanns M., Song Y. H. (2010) Proteome-wide identification of mycobacterial pupylation targets. Mol. Syst. Biol. 6, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Festa R. A., McAllister F., Pearce M. J., Mintseris J., Burns K. E., Gygi S. P., Darwin K. H. (2010) Prokaryotic ubiquitin-like protein (Pup) proteome of Mycobacterium tuberculosis [corrected]. PloS One 5, e8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce M. J., Arora P., Festa R. A., Butler-Wu S. M., Gokhale R. S., Darwin K. H. (2006) Identification of substrates of the Mycobacterium tuberculosis proteasome. EMBO J. 25, 5423–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darwin K. H., Lin G., Chen Z., Li H., Nathan C. F. (2005) Characterization of a Mycobacterium tuberculosis proteasomal ATPase homologue. Mol. Microbiol. 55, 561–571 [DOI] [PubMed] [Google Scholar]
- 14. Wolf S., Nagy I., Lupas A., Pfeifer G., Cejka Z., Müller S. A., Engel A., De Mot R., Baumeister W. (1998) Characterization of ARC, a divergent member of the AAA ATPase family from Rhodococcus erythropolis. J. Mol. Biol. 277, 13–25 [DOI] [PubMed] [Google Scholar]
- 15. Wang T., Li H., Lin G., Tang C., Li D., Nathan C., Darwin K. H. (2009) Structural insights on the Mycobacterium tuberculosis proteasomal ATPase Mpa. Structure 17, 1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutter M., Striebel F., Damberger F. F., Allain F. H., Weber-Ban E. (2009) A distinct structural region of the prokaryotic ubiquitin-like protein (Pup) is recognized by the N-terminal domain of the proteasomal ATPase Mpa. FEBS Lett. 583, 3151–3157 [DOI] [PubMed] [Google Scholar]
- 17. Wang T., Darwin K. H., Li H. (2010) Structural insights on the Mycobacterium tuberculosis proteasomal ATPase Mpa. Nat. Struct. Mol. Biol. 17, 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Striebel F., Hunkeler M., Summer H., Weber-Ban E. (2010) The mycobacterial Mpa-proteasome unfolds and degrades pupylated substrates by engaging Pup's N terminus. EMBO J. 29, 1262–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burns K. E., Pearce M. J., Darwin K. H. (2010) Prokaryotic ubiquitin-like protein provides a two-part degron to Mycobacterium proteasome substrates. J. Bacteriol. 192, 2933–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenton W. A., Kashi Y., Furtak K., Horwich A. L. (1994) Residues in chaperonin GroEL required for polypeptide binding and release. Nature 371, 614–619 [DOI] [PubMed] [Google Scholar]
- 21. Bais R. (1975) A rapid and sensitive radiometric assay for adenosine triphosphatase activity using Cerenkov radiation. Anal. Biochem. 63, 271–273 [DOI] [PubMed] [Google Scholar]
- 22. Summer H., Bruderer R., Weber-Ban E. (2006) Characterization of a new AAA+ protein from archaea. J. Struct. Biol. 156, 120–129 [DOI] [PubMed] [Google Scholar]
- 23. Smith D. M., Chang S. C., Park S., Finley D., Cheng Y., Goldberg A. L. (2007) Docking of the proteasomal ATPases' carboxyl termini in the 20 S proteasome's α ring opens the gate for substrate entry. Mol. Cell 27, 731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabl J., Smith D. M., Yu Y., Chang S. C., Goldberg A. L., Cheng Y. (2008) Mechanism of gate opening in the 20 S proteasome by the proteasomal ATPases. Mol. Cell 30, 360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillette T. G., Kumar B., Thompson D., Slaughter C. A., DeMartino G. N. (2008) Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J. Biol. Chem. 283, 31813–31822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirstein J., Schlothauer T., Dougan D. A., Lilie H., Tischendorf G., Mogk A., Bukau B., Turgay K. (2006) Adaptor protein-controlled oligomerization activates the AAA+ protein ClpC. EMBO J. 25, 1481–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darwin K. H., Ehrt S., Gutierrez-Ramos J. C., Weich N., Nathan C. F. (2003) The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302, 1963–1966 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.