Background: Self-association of the HRD complex is important for its function in ER quality control, but the oligomeric state of the complex is still unclear.
Results: The luminal component Yos9 dimerizes independently.
Conclusion: Dimerization of Yos9 suggests a dimeric state of the HRD complex.
Significance: The assembly of a functional HRD complex oligomer is further elucidated on a structural level.
Keywords: ER-associated Degradation, ERAD, Protein Degradation, Protein Folding, Protein Processing, Protein Structure, X-ray Crystallography, Yeast
Abstract
In yeast, the membrane-bound HMG-CoA reductase degradation (HRD) ubiquitin-ligase complex is a key player of the ER-associated protein degradation pathway that targets misfolded proteins for proteolysis. Yos9, a component of the luminal submodule of the ligase, scans proteins for specific oligosaccharide modifications, which constitute a critical determinant of the degradation signal. Here, we report the crystal structure of the Yos9 domain that was previously suggested to confer binding to Hrd3, another component of the HRD complex. We observe an αβ-roll domain architecture and a dimeric assembly which are confirmed by analytical ultracentrifugation of both the crystallized domain and full-length Yos9. Our binding studies indicate that, instead of this domain, the N-terminal part of Yos9 including the mannose 6-phosphate receptor homology domain mediates the association with Hrd3 in vitro. Our results support the model of a dimeric state of the HRD complex and provide first-time evidence of self-association on its luminal side.
Introduction
The accumulation of misfolded proteins compromises the survival of cells and, ultimately, of the entire organism. In addition to chaperones, which assist proteins in adopting their proper fold, a quality control mechanism in the endoplasmic reticulum (ER)4 identifies and eliminates misfolded proteins in a process frequently referred to as ER-associated protein degradation (1–3). At the very end of this process, proteins are degraded by the ubiquitin-proteasome system in the cytosol.
As a prerequisite for their elimination, misfolded proteins have to be identified. This process is best understood for glycoproteins. Quality control mechanisms distinguish between proteins that are still in the productive folding process and those that are terminally misfolded (4). Although both species expose hydrophobic patches on their surface, only terminally misfolded proteins carry particular oligosaccharide structures, which are generated by ER-resident mannosidases (5–7). The degradation signal is thus bipartite: one part is defined by unfolded regions within the polypeptide chain, the other is a specific sugar structure on the substrate (8–11). In yeast, Htm1/Mnl1 was recently found to act as a mannosidase that catalyzes the last cleavage step before a glycan can function as a degradation signal (12, 13). Yos9 acts downstream of Htm1/Mnl1 by binding to those oligosaccharides that are produced by Htm1/Mnl1 cleavage, in particular to the terminal α-1,6-mannose residue (14, 15).
A linkage between substrate recognition in the ER lumen and ubiquitylation in the cytosol is formed by the HMG-CoA reductase degradation (HRD) complex at the ER membrane. The yeast complex consists of at least five core components (4): the RING-finger E3 ubiquitin ligase Hrd1 and its binding partner Hrd3 (16), Der1, which is recruited by Usa1 (17), and Yos9. The recognition factor Yos9 resides on the luminal side of the complex, where it binds to the luminal domain of the membrane-anchored Hrd3 (10). Hrd3 interacts with ER-associated protein degradation substrates regardless of their glycosylation state, whereas Yos9 scans bound substrates for their glycans (9, 10). Deletion of Yos9 leads to a slower degradation of several ER-associated protein degradation substrates (8, 18, 19). In addition to the core components of the HRD complex mentioned above, the E2 ubiquitin-conjugating enzyme Ubc7 is recruited by the membrane anchor Cue1 (20), and the AAA ATPase Cdc48 is recruited by Ubx2 (21).
Recently, it has been revealed that self-association plays a role in the functionality of the HRD ligase (22, 23). Usa1 and Hrd1 have been identified as self-associating proteins, with Usa1 promoting oligomerization of Hrd1 (22, 23). Although Usa1 and Hrd1 have been shown to interact in a 1:1 ratio (22), the oligomeric state of the HRD complex has not been unequivocally determined until now. Data from sucrose gradient centrifugation (17, 22) and size exclusion chromatography experiments (22) hint at a dimeric composition of the HRD complex.
Yos9 is a 542-amino acid protein that contains an N-terminal signal sequence for ER import, the C-terminal ER retrieval sequence HDEL, and a predicted mannose 6-phosphate receptor homology (MRH) domain roughly located between the amino acid positions 90–250 (18). The MRH domain is supposed to bind to oligosaccharides, as has been shown for the MRH domain of the mammalian ortholog OS-9 (15). The C-terminally adjacent 170 residues of Yos9 were found to be necessary for interaction with Hrd3 and were thus proposed to represent the Hrd3 interaction site (10).
Here, we have determined the structure of this proposed Hrd3 interaction domain. Because the crystal structure revealed a dimeric arrangement, we denote this domain as the dimerization domain (DD). Contrary to previous findings, additional in vitro studies revealed that the Yos9 DD does not confer binding to the HRD complex. Instead, the Yos9 N-terminal part including the MRH domain was found to be necessary and sufficient to establish the Hrd3 interaction. Furthermore, we present biophysical data that both full-length Yos9 and its isolated DD form dimers in solution, too. The identity of the dimer interface in the crystal structure and in solution is demonstrated by mutagenesis studies. Finally, we propose that this additional dimerization interface on the luminal side of the HRD complex contributes to the overall self-association of the complex (supplemental Tables S2 and S3).
EXPERIMENTAL PROCEDURES
Cloning and Protein Preparation
DNA coding for the desired parts of the protein was amplified from genomic Saccharomyces cerevisiae DNA and inserted into the pQLinkH vector (24). Site-directed mutagenesis was performed following a two-step QuikChange mutagenesis protocol as described in Ref. 25 with minor modifications.
Genes were expressed as N-terminally tagged oligohistidine fusion proteins in Escherichia coli RosettaTM 2(DE3) (Novagen, Darmstadt, Germany). Cells were grown in TB medium at 37 °C until A600 = 2.0 and induced with 0.5 mm isopropyl-β-d-thiogalactopyranoside at 17 °C overnight. A selenomethionine derivative was prepared by inhibiting the methionine biosynthesis of the expression strain as described in Ref. 26.
Soluble protein was purified from cell lysate by immobilized metal ion (Ni2+) affinity chromatography followed by size exclusion chromatography with a buffer containing 250 mm NaCl and 20 mm Hepes, pH 7.5. Insoluble protein was purified and refolded as described in Ref. 14 with minor modifications. The oligohistidine tag was removed from wild-type Yos9266–424 for crystallization and analytical ultracentrifugation by cleavage with tobacco etch virus protease, leaving a Gly-Ser overhang at the N terminus.
Crystallization and Structure Determination
Yos9266–424 was crystallized at 20 °C by vapor diffusion in hanging drops. An equal amount of mother liquor (0.55 m NaH2PO4, 0.55 m KH2PO4, 0.1 m Tris, pH 8.5, adjusted to a final pH of 5.05) was added to 3-μl drops of protein with a concentration of 8.6 mg/ml. For data collection, crystals were briefly soaked in mother liquor with additional 20% (v/v) glycerol and flash-frozen in liquid nitrogen.
Diffraction experiments were performed at beamline 14.1 (27) of the Helmholtz-Zentrum Berlin für Materialien und Energie and Freie Universität Berlin at BESSY. Selenomethionine derivative crystals were used for phasing by multiple-wavelength anomalous diffraction. Data were processed with the program XDS (28). Determination of the heavy atom substructure, density modification, autobuilding of an initial model, and refinement were done with Phenix (29). The model was manually extended and rebuilt in the program COOT (30). TLS groups were assigned using the TLSMD web server (31). Model quality was evaluated by Molprobity statistics (32). The model was refined to crystallographic R factors of 18.1% (Rwork) and 22.0% (Rfree) respectively. 97.3% of the residues in the final model are located in the favored regions of the Ramachandran diagram and additional 2.7% in the allowed regions.
Structure Evaluation
The overall fold of the protein was classified using the CATH web server (33). Interfaces present in the crystal were analyzed using the web service ePISA (34). Molecular graphics were prepared in PyMOL (35). TopDraw was used for drawing the topology scheme (36).
Analytical Ultracentrifugation
Sedimentation experiments were performed using a Beckman Optima XL-A analytical ultracentrifuge equipped with absorption optics. Six-sector Epon cells were filled with 70 μl of protein solution and buffer in dialysis equilibrium in sample and reference sector, respectively. Buffers were composed of 250 mm NaCl, 20 mm Hepes, pH 7.5, for Yos9266–424, and 150 mm NaCl, 1 mm CaCl2, 10% glycerol, 10 mm Hepes, pH 7.5, for Yos924–539. Sedimentation equilibrium of the wild-type proteins was reached after 2 h of overspeed followed by about 24 h of equilibrium speed (Yos9266–424, 28,000/24,000 rpm; Yos924–539, 24,000/20,000 rpm). Sedimentation equilibrium experiments of mutant Yos9266–424 were performed at three rotor speeds of 18,000, 21,000, and 24,000 rpm for 24–30 h each. Rotor temperature for all experiments was 10 °C. Approach to equilibrium was followed by absorbance scans at appropriate wavelengths using radial increments of 0.001 cm and 9–10 replicates. Molecular masses were derived from the radial absorption profiles using the program POLYMOLE (37).
Cross-linking Assay
In a total volume of 7.0 μl, 0.15–2.95 μg of Yos9266–424 was incubated with a 50× molar excess of bis[sulfosuccinimidyl] suberate cross-linking reagent (Pierce) for 30 min at room temperature. Protein and reaction buffer contained 250 mm NaCl and 20 mm Hepes, pH 7.5. The reaction was stopped by addition of 3 μl of 4× SDS sample buffer, and samples were separated on a 15% SDS-polyacrylamide gel immediately.
Co-immunoprecipitation
Preparation of microsomal extracts and co-immunoprecipitation were performed as described in Ref. 38. Yeast cells from a Yos9 knock-out strain containing an HA-tagged version of Hrd3 (6xHA-HRD3, Δyos9::HIS3, prc1-1, trp1-1 (am), his3-Δ200, leu2-3,−112, lys2-801, ura3-52) were harvested in logarithmic growth phase and washed with water including 1 mm PMSF. The cells were disrupted by vortexing with glass beads in IP buffer (50 mm Hepes-NaOH, 50 mm NaCl, 125 mm potassium acetate, 2 mm MgCl2, 1 mm EDTA, 10 μm CaCl2, 3% glycerol at pH 7.5) supplemented with 1 mm PMSF. Cellular debris was removed by centrifugation for 5 min at 1,000 × g. Microsomes were sedimented at 20,000 × g for 20 min and solubilized in 1 ml of IP buffer plus 0.5% Nonidet P-40. Insoluble material was removed by centrifugation for 20 min at 20,000 × g.
Microsomal extracts from 100 OD cells were incubated overnight at 4 °C with 15 μl of protein A-Sepharose beads (GE Healthcare), 1 μl of monoclonal anti-HA antibody (Sigma-Aldrich), and 10 μg of recombinant oligohistidine-tagged Yos9. Control samples contained buffer instead of microsomal extracts. Beads were washed three times with IP buffer plus 0.5% Nonidet P-40. Bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE and immunoblotting with Penta-His HRP conjugate (Qiagen) and monoclonal anti-HA antibody.
Additional methods are described in supplemental Experimental Procedures.
RESULTS
Yos9 DD Exhibits αβ-Roll Architecture
Along with other truncation variants of Yos9 (Fig. 1), we purified the proposed Hrd3 binding domain (10), Yos9266–424. The protein was crystallized, and the structure was determined at 2.5-Å resolution by multiple-wavelength anomalous dispersion phasing (Protein Data Bank ID 2YMA). Data collection and refinement statistics are listed in Table 1.
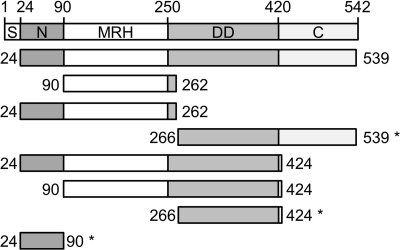
FIGURE 1.
Constructs of Yos9 used in this study. Domain organization of the full-length Yos9 protein (top) and its truncated variants (bottom) are shown. Numbers indicate the position of the respective amino acid. S, signal sequence for ER import; N, N-terminal region; C, C-terminal domain. The signal sequence and the last three residues were omitted from the protein hereinafter considered as full-length Yos9 (Yos924–539). Constructs that are solubly expressed in E. coli are marked with an asterisk.
TABLE 1.
Data collection and refinement statistics
Values in parentheses refer to the higest resolution shell. Rms, root mean square.
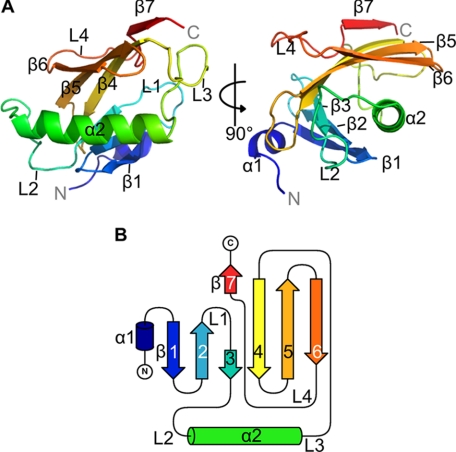
The overall fold of the domain can be classified according to CATH as an αβ-roll architecture, comprising two α-helices and seven β-strands (Fig. 2). In detail, two antiparallel β-sheets, which meet at a short parallel connection, curve around the hydrophobic side of the amphipathic α2-helix (Fig. 2A). The first β-sheet bears three β-strands (β1–β3) and is arranged in an up-and-down topology. The second β-sheet comprises four β-strands (β4–β7) that are arranged in a C-type Greek key topology (39). Four extended loop regions (L1–L4) can be identified in the structure. Loops L1 and L4 connect strands within the β-sheets, and loops 2 and 3 connect helix α2 to the β-sheets (Fig. 2B).
FIGURE 2.
Structure of the dimerization domain of Yos9. A, schematic representation of structure (Yos9266–424) from two different viewpoints related by 90° rotation around the vertical axis. Coloring follows the primary structure from blue (N terminus) to red (C terminus). α-Helices, β-strands, and extended loops are depicted as α1/2, β1–7, and L1–4, respectively. B, topology of Yos9 DD. Coloring corresponds to A.
The structure shows a highly compact domain fold with an evenly charged surface (supplemental Fig. S1). Two molecules related by noncrystallographic symmetry are located in the asymmetric unit of the crystal, hinting to a possible dimeric arrangement of this domain. Thus, and anticipating further results, we hereafter denote this domain as the Yos9 DD.
The N-terminal helix α1 is present in only one of the molecules of the asymmetric unit, whereas in the other, the corresponding residues are stretched out to contact neighboring Yos9 DD molecules in the crystal lattice by inserting into a hydrophobic groove between α2 and strands β4–β6 within the second β-sheet (supplemental Fig. S1). The noncrystallographic symmetry was used as a restraint in structure refinement, leading to a final root mean square deviation of about 0.3 Å between equivalent α-carbon positions of both protein chains, excluding the N-terminal region.
The C-terminal 25–27 amino acids of the protein are not visible in the electron density, suggesting a high flexibility. Large solvent channels in the crystal lattice could easily accommodate this unmodeled part of the protein. The integrity of the crystallized domain was confirmed by dissolving a crystal and subsequent SDS-PAGE analysis (data not shown).
Dimerization Domain Contributes to Self-association of Yos9
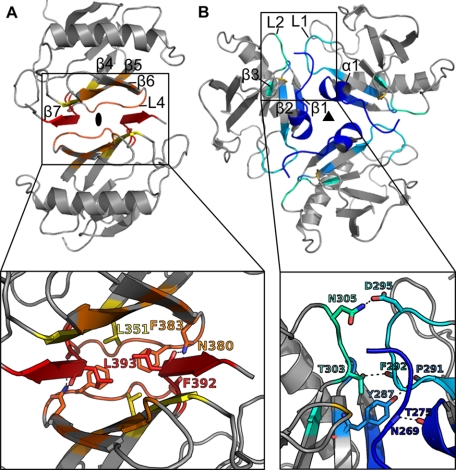
Besides the N-terminal stretch, several other contact areas are present between the protein molecules in the crystal. The properties of possible oligomerization interfaces were analyzed using the ePISA server (34), revealing that the main assemblies are the dimer in the asymmetric unit (Fig. 3A) and the trimer, which arranges around the 3-fold axis in the crystal (Fig. 3B). In the dimer interface, the second β-sheet forms a β-sandwich with additional contacts in the Greek key loop L4, whereas the trimer interface extends over helix α1, the adjacent loops L1 and L2, and the first β-sheet.
FIGURE 3.
Possible assemblies of Yos9266–424 in crystal structure. Coloring of the interfacing residues corresponds to Fig. 2, noninterfacing residues are grayed out. Upper, assemblies; lower, close-up view of interfaces. A, dimer of Yos9266–424 as present in the asymmetric unit. The extended N terminus of the upper molecule was omitted for clarity. The Greek key loop L4 covers the bottom of the β-sandwich. Side chains of mutated residues (Asn380, Leu393) are depicted in stick representation along with side chains of contacting residues. B, trimeric assembly at the crystallographic 3-fold axis. Side chains involved in hydrogen-bonding interactions are shown.
Although both interfaces cover a surface area of about 10% of the total surface and contain a large fraction of hydrophobic residues, they differ in the number of polar contacts. In contrast to 20 hydrogen bonds and four salt bridges in the dimer interface, the trimer interface contains only four hydrogen bonds. As a result, only the dimer assembly is calculated to be thermodynamically stable (supplemental Table S1).
Previously, recombinantly expressed and refolded full-length Yos9 has been reported to run on a size exclusion column at an elution volume corresponding approximately to the theoretical size of the trimer (14). However, the shape of a molecule has a significant impact on its hydrodynamic radius, thereby potentially distorting the molecular mass estimate derived from size exclusion chromatography experiments. Therefore, other methods were employed to investigate the oligomeric state of both Yos9 DD and the full-length protein.
As a first approach, we cross-linked Yos9 DD at different concentrations and analyzed the products by SDS-PAGE (Fig. 4A). At the highest protein concentration tested, a significant amount was captured in a dimeric state. The rest of the protein was present as monomeric and higher oligomeric species. Thus, the cross-linking approach suggests that the dimer represents the predominant oligomerization state of Yos9 DD in solution.
FIGURE 4.
Yos9 self-association experiments. A, cross-linking of wild-type Yos9266–424. The protein was cross-linked at concentrations ranging from 1 to 20 μm with a 50-fold molar excess of bis[sulfosuccinimidyl] suberate (BS3). An untreated protein sample was run in the leftmost lane. B, sedimentation equilibrium results. The mean apparent molecular mass (Mapp) divided by the monomer molecular mass (Mmono) is plotted against the concentration. Crosses, Yos924–539; triangles, wild-type Yos9266–424; circles, Yos9266–424 L393A; squares, Yos9266–424 N380A.
A more accurate assessment of the oligomeric state of a protein in solution can be achieved by analytical ultracentrifugation. We performed sedimentation equilibrium experiments to analyze the oligomeric state of Yos9. Within the investigated concentration range, the mean apparent molecular masses of both the full-length protein and the Yos9 DD correspond to the molecular mass of the dimer (Fig. 4B). Therefore, it can be concluded that Yos9 forms a dimer in solution, and furthermore, that the DD is involved in dimerization of Yos9.
To test whether the dimerization interface observed in the crystal structure corresponds to the dimerization interface in solution, we mutated several interfacing residues in the Yos9 DD and analyzed the proteins by analytical ultracentrifugation. Prior to that, we recorded circular dichroism spectra, confirming a similar fold of the mutant Yos9 DD compared with the wild-type protein (supplemental Fig. S2).
Two mutants, L393A and N380A, were found to have a mean apparent molecular mass of approximately the size of the monomer throughout the investigated concentration range (Fig. 4B). The residues are located at the end of strand β6 (Asn380) and at the beginning of β7 (Leu393), respectively, as depicted in Fig. 3A. The amide group of the Asn380 side chain forms hydrogen bonds to the backbone carbonyl of Leu393 and Phe392, thus providing four of a total of 20 hydrogen bonds in the interface. The side chain of Leu393 contacts the side chains of residues Leu351 and Phe383, thus contributing to the dimer formation by hydrophobic interactions. The switch from dimer to monomer upon mutation to alanine indicates that both residues are crucial for the dimerization of the protein. Because they are not involved in any other interface found in the crystal lattice, the dimer interface in the asymmetric unit of the crystal most likely represents the dimerization interface in solution.
Yos9 DD Is Not Involved in Association with Hrd3
In previous in vivo experiments, deletion of the Yos9 DD led to abrogation of its interaction with Hrd3 (10). Therefore, it was originally proposed that this region is responsible for binding between Yos9 and Hrd3, by which Yos9 would be brought into the vicinity of the substrate for scanning its oligosaccharide structures (9, 10).
To address the binding capability of the isolated Yos9 DD to Hrd3 in vitro, we co-immunoprecipitated purified truncation variants of Yos9 with yeast cell lysate from a Yos9 knock-out strain expressing HA-tagged Hrd3 (Fig. 5). SDS-PAGE analysis and CD spectra of the truncated proteins are represented in supplemental Fig. S3. In contrast to the full-length Yos924–539, the DD (Yos9266–424) was not co-immunoprecipitated along with Hrd3. Only variants bearing both the very N terminus of Yos9 (amino acids 24–90) and the MRH domain (amino acids 90–262) were co-immunoprecipitated with HA-Hrd3. To confirm this observation, His7-tagged full-length Yos924–539 and the DD (Yos9266–424) purified from recombinant E. coli cells were bound to a Co2+ affinity matrix (Talon) and incubated with a microsomal extract from the Yos9-knock-out strain expressing Hrd3 as HA fusion protein (supplemental Fig. S4). The pulldown experiment showed Hrd3 binding by the full-length Yos9 but not by the Yos9 DD. Thus, we propose that Hrd3 and Yos9 interact via the N-terminal region of Yos9 comprising amino acids 24–262.
FIGURE 5.
Co-immunoprecipitation of Yos9 truncation variants. Microsomal extracts from a Yos9 knock-out strain expressing HA-tagged Hrd3 were immunoprecipitated in the presence of oligohistidine-tagged Yos9 truncation constructs. Co-immunoprecipitated Yos9 was detected by immunoblotting with anti-His antibody. Amino acid boundaries and domains included in the constructs are depicted on top of the corresponding lanes. Expected masses for the His7 fusion proteins are: Yos924–539, 62 kDa; Yos990–262, 23 kDa; Yos924–262, 30 kDa; Yos924–424, 49 kDa; Yos990–424, 41 kDa; Yos9266–539, 34 kDa; Yos9266–424, 21 kDa; and Yos924–90, 11 kDa.
DISCUSSION
The human ortholog of Yos9, OS-9, relies on an intact MRH domain to bind to the Hrd3 ortholog SEL1L (40). In agreement with this observation, our experiments suggest that the N-terminal part of Yos9 including the MRH domain (amino acids 24–260) is necessary for binding to Hrd3. However, the binding might actually be mediated by amino acids 24–90 in a manner where the interaction can only be detected when the MRH domain stabilizes the N-terminal domain. We cannot be sure that the isolated N terminus (Yos924–90) is folded properly because CD spectra predict 38% random coil structure (supplemental Fig. S3).
In our hands, the Yos9 DD does not establish the interaction with Hrd3 directly. In previous experiments, Yos91–420 immunoprecipitated with Hrd3 whereas Yos91–250 did not (10). Here, we report that Hrd3 indeed binds to Yos924–262 but not to Yos9266–424. One possible explanation for this discrepancy is the instability of Yos91–250 in vivo, which was speculated to be caused by the misfolding of the protein (10). Misfolding of Yos91–250 could have included the Hrd3 binding site, thereby preventing co-immunoprecipitation.
Based on the assembly of molecules in the crystal structure of the Yos9 DD, we studied the self-association of Yos9 in solution. Yos9, previously suspected to arrange in a trimeric fashion (14), was found to form dimers in analytical ultracentrifugation. Mutational analysis confirmed that the dimer interface in the asymmetric unit of the crystal structure is consistent with the dimer interface in solution. However, we were unable to detect a direct Yos9-Yos9 interaction in immunoprecipitation experiments from yeast cell extracts containing different epitope-tagged variants of Yos9 (data not shown). This could indicate an intrinsic instability of the Yos9 dimer within the assembled HRD ligase that is not observed with the isolated protein.
We cannot formally exclude the possibility that the Yos9 dimerization observed in the crystals of Yos9 DD and in solution substitutes for interactions with a missing partner present in the HRD complex. Cases of this kind have been described, e.g. in the transport protein particle TRAPP, whose subunits Bet3 (41, 42) and Tpc6 (43) are homodimeric when recombinantly produced in isolation, whereas they form heterodimers after co-expression of their genes (44, 45) or within larger subcomplexes of TRAPP (46). This heterodimerization, however, is facilitated by sequence and structure conservation between Bet3 and Tpc6 that permits intermolecular interactions across conserved protein surfaces. Because no other subunit of the HRD complex shares significant sequence similarity with Yos9, this type of interaction involving Yos9 appears rather unlikely in the context of the HRD complex.
Self-association within the HRD complex has so far been reported for Derlin1, the mammalian homolog of Der1 (47, 48) and for the cytosolic domains of Usa1 and Hrd1 (22, 23). Hrd1 is able to oligomerize on its own when overexpressed, but its oligomerization is dependent on Usa1 at endogenous levels (23). Although the question of the oligomeric state of the HRD complex has not been answered definitely yet, sucrose gradient centrifugation (17, 22) and size exclusion chromatography experiments (22) with the HRD complex suggest a dimeric assembly. Furthermore, a dimeric assembly of Hrd1 would be in good agreement with the finding that other RING-finger ubiquitin ligases form homodimers (49). Our analytical ultracentrifugation analysis indicates that the luminal component Yos9 dimerizes in vitro, thereby providing further evidence for a dimeric assembly of the HRD complex.
We did not observe any effect on the interaction between Yos9 and Hrd3 upon destabilization of the dimer interface, nor did we observe a reduced clearance of the model substrate CPY* (supplemental Fig. S5). The function of the HRD complex has been shown to rely on its oligomerization state, but because the self-association seems to be mainly driven by Usa1 (22, 23), the disruption of a dimer formed by a peripheral component like Yos9 is not expected to exert much influence on the assembly of the whole complex. Furthermore, additional dimerization sites might be present in Yos9.
Taken together, we propose a model in which self-association of the HRD complex is driven mainly by Usa1, but intrinsic self-association properties of additional components contribute to the formation of the oligomer, as represented in Fig. 6. Because Usa1-induced self-association is crucial for the functionality of the E3 ligase (23), it seems plausible that it represents the main driving force for the oligomerization of the complex. However, because many weak interactions stabilize a complex more efficiently than just a few stronger ones, a multisite self-association could provide a higher stability for the oligomer as a whole. Furthermore, it has been proposed that substrate binding to the HRD complex counteracts Usa1-induced oligomerization (23). The lack of detectable Yos9 dimerization under our in vivo conditions may be explained by a dynamic assembly and disassembly of the HRD complex dimer. Nevertheless, autonomous self-association of peripheral components of the complex could allow for a much faster reassembly of the HRD complex dimer despite a relatively weak dimerization of individual components.
FIGURE 6.
Schematic model of self-association sites within the HRD complex. Brown arrows indicate association between two proteins, black arrows indicate self-association of the respective component. Self-associating proteins and known self-associating domains therein are highlighted by coloring. H/U, segments within the N-terminal cytosolic domain of Usa1 that are associated with Hrd1 interaction (H) and self-association (U), respectively (23).
Also for Hrd3, which is known to be present in a 1:1 stoichiometry to Hrd1 (16), an additional self-association tendency is to be expected but still remains to be shown. A quantification of the contribution of each of the self-associating components to the oligomerization of the HRD complex and further studies regarding the influence on substrate binding and processing are future challenges to provide further insights into the function of the HRD ligase.
Very recently it was demonstrated that Yos9 is also involved in the degradation of certain nonglycosylated proteins (50) which would be consistent with a direct binding of these substrates to Yos9. In this context we recall the presence of a conspicuous hydrophobic surface groove of Yos9 (see supplemental Fig. S1) that accommodates a peptide fragment of a neighboring molecule in the crystal lattice. It is tempting to speculate that this surface of Yos9 may mediate direct interactions with substrate proteins in a similar manner, but proof of this assumption requires further experiments.
Supplementary Material
Acknowledgments
We thank Marcel Jurk and Otto Ristau for assistance with analytical ultracentrifugation and data analysis, Yvette Roske and Oliver Daumke for assistance with diffraction data collection, Ulrich Gohlke for reviewing the structure determination, and Ernst Jarosch for fruitful discussions and advice. We thank Janett Tischer and Tracy Dornblut for excellent technical assistance and acknowledge Uwe Müller and the beamline support from the staff of Helmholtz-Zentrum Berlin für Materialien und Energie at BESSY.
This work was supported by Deutsche Forschungsgemeinschaft grants (to U. H. and T. S.) and by Sonderforschungsbereich 740.

This article contains supplemental Experimental Procedures and additional references, Figs. S1–S5, and Tables S1–S3.
The atomic coordinates and structure factors (code 2YMA) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- ER
- endoplasmic reticulum
- DD
- dimerization domain
- HRD
- HMG-CoA reductase degradation
- MRH
- mannose 6-phosphate receptor homology.
REFERENCES
- 1. Brodsky J. L., McCracken A. A. (1997) ER-associated and proteasome-mediated protein degradation: how two topologically restricted events came together. Trends Cell Biol. 7, 151–156 [DOI] [PubMed] [Google Scholar]
- 2. Hammond C., Helenius A. (1995) Quality control in the secretory pathway. Curr. Opin. Cell Biol. 7, 523–529 [DOI] [PubMed] [Google Scholar]
- 3. Werner E. D., Brodsky J. L., McCracken A. A. (1996) Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc. Natl. Acad. Sci. U.S.A. 93, 13797–13801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch C., Gauss R., Horn S. C., Neuber O., Sommer T. (2009) The ubiquitylation machinery of the endoplasmic reticulum. Nature 458, 453–460 [DOI] [PubMed] [Google Scholar]
- 5. Aebi M., Bernasconi R., Clerc S., Molinari M. (2010) N-Glycan structures: recognition and processing in the ER. Trends Biochem. Sci. 35, 74–82 [DOI] [PubMed] [Google Scholar]
- 6. Hebert D. N., Garman S. C., Molinari M. (2005) The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 15, 364–370 [DOI] [PubMed] [Google Scholar]
- 7. Jakob C. A., Burda P., Roth J., Aebi M. (1998) Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J. Cell Biol. 142, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhamidipati A., Denic V., Quan E. M., Weissman J. S. (2005) Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol. Cell 19, 741–751 [DOI] [PubMed] [Google Scholar]
- 9. Denic V., Quan E. M., Weissman J. S. (2006) A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126, 349–359 [DOI] [PubMed] [Google Scholar]
- 10. Gauss R., Jarosch E., Sommer T., Hirsch C. (2006) A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat. Cell Biol. 8, 849–854 [DOI] [PubMed] [Google Scholar]
- 11. Xie W., Kanehara K., Sayeed A., Ng D. T. (2009) Intrinsic conformational determinants signal protein misfolding to the Hrd1/Htm1 endoplasmic reticulum-associated degradation system. Mol. Biol. Cell 20, 3317–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clerc S., Hirsch C., Oggier D. M., Deprez P., Jakob C., Sommer T., Aebi M. (2009) Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J. Cell Biol. 184, 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gauss R., Kanehara K., Carvalho P., Ng D. T., Aebi M. (2011) A complex of Pdi1p and the mannosidase Htm1p initiates clearance of unfolded glycoproteins from the endoplasmic reticulum. Mol. Cell 42, 782–793 [DOI] [PubMed] [Google Scholar]
- 14. Quan E. M., Kamiya Y., Kamiya D., Denic V., Weibezahn J., Kato K., Weissman J. S. (2008) Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol. Cell 32, 870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh T., Chen Y., Hu D., Hanashima S., Yamamoto K., Yamaguchi Y. (2010) Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol. Cell 40, 905–916 [DOI] [PubMed] [Google Scholar]
- 16. Gardner R. G., Swarbrick G. M., Bays N. W., Cronin S. R., Wilhovsky S., Seelig L., Kim C., Hampton R. Y. (2000) Endoplasmic reticulum degradation requires lumen to cytosol signaling: transmembrane control of Hrd1p by Hrd3p. J. Cell Biol. 151, 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carvalho P., Goder V., Rapoport T. A. (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373 [DOI] [PubMed] [Google Scholar]
- 18. Szathmary R., Bielmann R., Nita-Lazar M., Burda P., Jakob C. A. (2005) Yos9 protein is essential for degradation of misfolded glycoproteins and may function as lectin in ERAD. Mol. Cell 19, 765–775 [DOI] [PubMed] [Google Scholar]
- 19. Kim W., Spear E. D., Ng D. T. (2005) Yos9p detects and targets misfolded glycoproteins for ER-associated degradation. Mol. Cell 19, 753–764 [DOI] [PubMed] [Google Scholar]
- 20. Biederer T., Volkwein C., Sommer T. (1997) Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278, 1806–1809 [DOI] [PubMed] [Google Scholar]
- 21. Neuber O., Jarosch E., Volkwein C., Walter J., Sommer T. (2005) Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat. Cell Biol. 7, 993–998 [DOI] [PubMed] [Google Scholar]
- 22. Horn S. C., Hanna J., Hirsch C., Volkwein C., Schütz A., Heinemann U., Sommer T., Jarosch E. (2009) Usa1 functions as a scaffold of the HRD-ubiquitin ligase. Mol. Cell 36, 782–793 [DOI] [PubMed] [Google Scholar]
- 23. Carvalho P., Stanley A. M., Rapoport T. A. (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheich C., Kümmel D., Soumailakakis D., Heinemann U., Büssow K. (2007) Vectors for co-expression of an unrestricted number of proteins. Nucleic Acids Res. 35, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W., Malcolm B. A. (1999) Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques 26, 680–682 [DOI] [PubMed] [Google Scholar]
- 26. Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. (1993) Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 [DOI] [PubMed] [Google Scholar]
- 27. Heinemann U., Büssow K., Mueller U., Umbach P. (2003) Facilities and methods for the high-throughput crystal structural analysis of human proteins. Acc. Chem. Res. 36, 157–163 [DOI] [PubMed] [Google Scholar]
- 28. Kabsch W. (2010) XDS. Acta Crystallogr. D 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of COOT. Acta Crystallogr. D 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Painter J., Merritt E. A. (2006) TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 39, 109–111 [Google Scholar]
- 32. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cuff A. L., Sillitoe I., Lewis T., Redfern O. C., Garratt R., Thornton J., Orengo C. A. (2009) The CATH classification revisited: architectures reviewed and new ways to characterize structural divergence in superfamilies. Nucleic Acids Res. 37, D310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krissinel E., Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 35. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrödinger, LLC, New York [Google Scholar]
- 36. Bond C. S. (2003) TopDraw: a sketchpad for protein structure topology cartoons. Bioinformatics 19, 311–312 [DOI] [PubMed] [Google Scholar]
- 37. Behlke J., Ristau O., Schönfeld H. J. (1997) Nucleotide-dependent complex formation between the Escherichia coli chaperonins GroEL and GroES studied under equilibrium conditions. Biochemistry 36, 5149–5156 [DOI] [PubMed] [Google Scholar]
- 38. Gauss R., Sommer T., Jarosch E. (2006) The Hrd1p ligase complex forms a linchpin between ER-luminal substrate selection and Cdc48p recruitment. EMBO J. 25, 1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hutchinson E. G., Thornton J. M. (1993) The Greek key motif: extraction, classification and analysis. Protein Eng. 6, 233–245 [DOI] [PubMed] [Google Scholar]
- 40. Christianson J. C., Shaler T. A., Tyler R. E., Kopito R. R. (2008) OS-9 and GRP94 deliver mutant α1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat. Cell Biol. 10, 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim Y. G., Sohn E. J., Seo J., Lee K. J., Lee H. S., Hwang I., Whiteway M., Sacher M., Oh B. H. (2005) Crystal structure of bet3 reveals a novel mechanism for Golgi localization of tethering factor TRAPP. Nat. Struct. Mol. Biol. 12, 38–45 [DOI] [PubMed] [Google Scholar]
- 42. Turnbull A. P., Kümmel D., Prinz B., Holz C., Schultchen J., Lang C., Niesen F. H., Hofmann K. P., Delbrück H., Behlke J., Müller E. C., Jarosch E., Sommer T., Heinemann U. (2005) Structure of palmitoylated BET3: insights into TRAPP complex assembly and membrane localization. EMBO J. 24, 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kümmel D., Müller J. J., Roske Y., Misselwitz R., Büssow K., Heinemann U. (2005) The structure of the TRAPP subunit TPC6 suggests a model for a TRAPP subcomplex. EMBO Rep. 6, 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim M. S., Yi M. J., Lee K. H., Wagner J., Munger C., Kim Y. G., Whiteway M., Cygler M., Oh B. H., Sacher M. (2005) Biochemical and crystallographic studies reveal a specific interaction between TRAPP subunits Trs33p and Bet3p. Traffic 6, 1183–1195 [DOI] [PubMed] [Google Scholar]
- 45. Kümmel D., Müller J. J., Roske Y., Henke N., Heinemann U. (2006) Structure of the Bet3-Tpc6B core of TRAPP: two Tpc6 paralogs form trimeric complexes with Bet3 and Mum2. J. Mol. Biol. 361, 22–32 [DOI] [PubMed] [Google Scholar]
- 46. Kim Y. G., Raunser S., Munger C., Wagner J., Song Y. L., Cygler M., Walz T., Oh B. H., Sacher M. (2006) The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 127, 817–830 [DOI] [PubMed] [Google Scholar]
- 47. Lilley B. N., Ploegh H. L. (2005) Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. U.S.A. 102, 14296–14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ye Y., Shibata Y., Kikkert M., van Voorden S., Wiertz E., Rapoport T. A. (2005) Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. U.S.A. 102, 14132–14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knipscheer P., Sixma T. K. (2007) Protein-protein interactions regulate Ubl conjugation. Curr. Opin. Struct. Biol. 17, 665–673 [DOI] [PubMed] [Google Scholar]
- 50. Jaenicke L. A., Brendebach H., Selbach M., Hirsch C. (2011) Yos9p assists in the degradation of certain nonglycosylated proteins from the endoplasmic reticulum. Mol. Biol. Cell 22, 2937–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.