Background: RasGRP4 is one member of the RasGRP family and is highly expressed in mast cells.
Results: FcϵRI signaling, mast cell function, and thymocyte development are severely impaired by RasGRP1 and RasGRP4 deficiency.
Conclusion: This study indicated that the RasGRP family is important in mast cells and T cells.
Significance: This work demonstrates immune cells employ multiple members of the RasGRP family to activate the Ras-Erk pathway.
Keywords: Immunology, Mast Cell, Ras, Signal Transduction, T Cell, Allergic Response, Immunoreceptor, Thymocyte Development
Abstract
The RasGRP (Ras guanine nucleotide-releasing protein) family proteins are guanine nucleotide exchange factors that activate Ras GTPases, ultimately leading to MAPK activation and many cellular processes. The RasGRP family has four members. Published studies demonstrate that RasGRP1, RasGRP2, and RasGRP3 play critical roles in T cells, platelets, and B cells, respectively. RasGRP4 is highly expressed in mast cells. Although previous data suggest that it is important in mast cell development and function, the role of RasGRP4 in mast cells and allergic responses has not been clearly demonstrated. In this study, we generated RasGRP4−/− mice to examine the function of RasGRP4. Analyses of these mice showed that mast cells were able to develop normally in vivo and in vitro. Despite high levels of RasGRP4 expression in mast cells, RasGRP4 deficiency led to only a modest reduction in FcϵRI-mediated degranulation and cytokine production. Interestingly, mast cells deficient in both RasGRP1 and RasGRP4 had a much more severe block in FcϵRI-mediated signaling and mast cell function. We also made the unexpected finding that RasGRP4 functions during thymocyte development. Our data suggest that after the engagement of immunoreceptors, immune cells likely employ multiple members of the RasGRP family to transduce critical signals.
Introduction
Ras guanine nucleotide-releasing protein (RasGRP)2 is one type of GEF (GTP/GDP exchange factor) responsible for the activation of Ras. There are four members in the RasGRP family, termed RasGRP1–4. Each protein contains the same domains, a Ras exchange motif to interact with Ras, CDC25 (Ras activator), EF hands (Ca2+ binding), and C1 domain (DAG binding) (1, 2). In addition, RasGRP1 has a unique tail region (∼180 residues) at its C terminus, which is absent in other members of this family.
The function of RasGRP proteins has been under intense investigation in recent years. They are expressed in different leukocytes and regulate their activation. RasGRP1 is predominantly expressed in T lymphocytes. RasGRP1−/− mice have a marked deficiency in the development of single positive (SP) thymocytes. TCR-mediated Erk activation is totally blocked in RasGRP1−/− thymocytes (3) and in a Jurkat cell line that is deficient in RasGRP1 protein (4), thus demonstrating that RasGRP1 links TCR engagement to Ras-Erk activation. Moreover, published data suggest that RasGRP1 most likely activates Ras at the Golgi apparatus rather than at the plasma membrane (5, 6). Interestingly, RasGRP1 is primarily localized to the Golgi in thymocytes during positive selection, whereas it is present at the plasma membrane during negative selection (7). Although RasGRP1 is very important in T cells, RasGRP2 has been shown to be critical in platelet activation (8, 9). On the other hand, RasGRP3 is preferentially expressed in B cells and is required for optimal activation of Erk upon B cell receptor (BCR) cross-linking (10, 11).
Different from other members of the RasGRP family, RasGRP4 is highly expressed in mast cells and other myeloid cells (12, 13). Its function in mast cells was suggested from studies with C3H/HeJ mice, which are hyporesponsive to methacholine stimulation via the airway (14). These mice express a dysfunctional RasGRP4 protein that lacks a DAG-binding domain (C1 domain) because of aberrant splicing. Interestingly, substantial amounts of non-functional forms of the RasGRP4 transcript were also found in patients with asthma or mastocytosis (13). These results demonstrate that abnormal allergic responses are associated with expression of non-functional RasGRP4. However, these studies do not exclude the possibility that other genes are also involved.
The importance of RasGRP4 in mast cells was also demonstrated in a human mast cell line (HMC-1). HMC-1 cells were initially established from a patient with mast cell leukemia. They are deficient in RasGRP4, FcϵRI, and other proteins, such as prostaglandin D2 (PGD2) synthase. As a result, they are characterized as immature mast cells (15). Reintroduction of RasGRP4 into HMC-1 cells restores the expression of PGD2 synthase. These data suggest that RasGRP4 may be required for mast cell development and maturation. In addition, microarray analysis of HMC-1 cells indicates that RasGRP4 regulates the expression of hundreds of genes, although the mechanism is unclear (16).
Although the above studies suggest that RasGRP4 might be important in mast cell maturation, gene expression, and allergic responses, the role of RasGRP4 in FcϵRI-mediated signaling in mast cells is not yet known. In this study, we generated RasGRP4-deficient mice to study its function in mast cell signaling and function in vivo and in vitro. In contrast to previous studies, we found that RasGRP4 deficiency did not affect mast cell development and maturation. However, RasGRP4−/− mice were hyporesponsive to stimulation from the FcϵRI. We also analyzed mast cells deficient in both RasGRP1 and RasGRP4. Our data indicated that RasGRP4 is important in FcϵRI-mediated signaling and mast cell function. In addition to its role in mast cells, RasGRP4 protein also functions during thymocyte development.
EXPERIMENTAL PROCEDURES
Mice
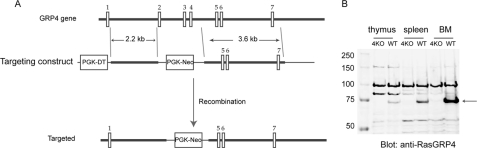
RasGRP4 knockout mice were generated by replacing exons 2, 3, and 4 of the rasgrp4 gene with the PGK neo cassette (Fig. 1A). ES cells were targeted and injected into 129/Sv blastocysts to generate chimeric mice. These mice were backcrossed with C57BL/6 mice for at least 10 generations before analysis. RasGRP1−/− mice were kindly provided by Dr. Jim Stone (3). All mice were used in accordance with the National Institutes of Health guidelines. The experiments described in this study were reviewed and approved by the Duke University Institutional Animal Care Committee. Mice were housed in specific pathogen-free conditions.
FIGURE 1.
Generation of RasGRP4−/− mice. A, targeting strategy. Exons 2 to 4 were replaced by the PGK neo cassette. B, absence of RasGRP4 protein. Postnuclear lysates from thymocytes, splenocytes, and bone marrow cells from RasGRP4−/− (4KO) and WT mice were analyzed by Western blotting with anti-RasGRP4 anti-serum. An arrow indicates where RasGRP4 protein migrated.
Antibodies and Flow Cytometry Analysis
The following antibodies were used for Western blotting: rabbit anti-RasGRP4 and anti-LAT, anti-pTyr (4G10, Millipore), anti phospho-PLC-γ1, pSyk/Zap, pAkt, Akt, pErk, pp38, p38, pJnk (Cell Signaling), and anti-Erk2, Syk, Jnk1 (Santa Cruz Biotechnology). Antibodies used in FACS analysis were the following: APC-Cy7-conjugated anti-CD8, APC-anti-B220, PE-Cy7-anti-CD4, PE-anti-c-Kit, APC-anti-TCRβ (Biolegend), and FITC-anti-FcϵRIα (eBioscience). Flow cytometry was performed using the FACSCanto (BD Biosciences) and analyzed by the FlowJo software.
BMMC Culture and Activation
Mast cells were derived from bone marrow cells from RasGRP4−/− and WT mice in IMDM supplemented with 10% fetal bovine serum and recombinant IL-3 (5 ng/ml). After being cultured in IL-3 medium for 3 weeks, the purity of cells was analyzed by FACS analysis of FcϵRIα and c-Kit expression. For biochemical analysis, BMMCs (2–5 × 106/ml) were preloaded with anti-DNP IgE (1 μg/ml, SPE-7 mAb, Sigma) in IMDM without IL-3 for 4–6 h. Cells were washed with IMDM and then stimulated with DNP-HSA (100 ng/ml) for the indicated time points. A total of 1 × 107 cells were lysed in 500 μl of ice-cold radioimmune precipitation assay lysis buffer (1% Triton, 0.5% sodium deoxycholic acid, 0.1% SDS, 25 mm Tris-Cl (pH 7.6), 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4). Degranulation of BMMCs was determined by measuring the release of β-hexosaminidase as previously described (17).
Ca2+ Flux
BMMCs (2–5 × 106/ml) were preloaded with anti-DNP IgE (1 μg/ml) in IMDM without IL-3 for 4 h. Cells were washed twice with IMDM and then loaded with 5 μm Fluo-4, AM (Molecular Probes) for 30 min. Cells were washed again and further incubated in IMDM for 30 min. DNP-HSA (100 ng/ml) and ionomycin (1 μm) were used to induce calcium flux in these cells. Ca2+ flux was analyzed by FACS.
Western Blotting
Lysates were resolved on SDS-PAGE and transferred to nitrocellulose membranes. After incubation with primary antibodies, membranes were washed three times and probed with either anti-mouse or rabbit Ig conjugated to Alexa Fluor 680 or IRDye800. Membranes were then visualized with the LI-COR Bioscience Odyssey system (LI-COR).
Passive Systemic Anaphylaxis
Mice were first sensitized with 2 μg of anti-DNP-IgE by intravenous injection for 20–24 h. They were then injected intravenously with 500 μg of DNP-HSA for 1.5 min. Mice were euthanized with CO2, and blood was immediately collected by cardiac puncture. The histamine concentration in serum was determined using a competitive histamine enzyme-linked immunosorbent assay kit (Immunotech).
Detection of Cytokine Production
BMMCs were sensitized in 1 μg/ml anti-DNP IgE for 4–6 h followed by stimulation with 100 ng/ml of DNP-HSA for 1 h. Total RNAs were isolated with the TRIzol reagent (Invitrogen). cDNAs were synthesized with the Super Script reverse transcriptase (Invitrogen) using oligo(dT) as the primer. Quantification of cytokine RNAs was performed by real-time PCR with SYBR Green Super mix (Bio-Rad). The primers used to amplify cytokine cDNAs were described previously (17).
RESULTS
Generation of RasGRP4 Knockout Mice
As mentioned previously, RasGRP4 is highly expressed in mast cells and other myeloid cells (12, 13). Its functional importance in mast cells was suggested from studies with C3H/HeJ mice and a RasGRP4-deficient human mast cell line (15). To investigate RasGRP4 function in mast cells in vivo, we generated RasGRP4 knockout mice by replacing exons 2, 3, and 4 of the Rasgrp4 gene with the PGK neo cassette (Fig. 1A). To reduce variations in the genetic background of RasGRP4−/− mice, we backcrossed them with C57BL/6 mice for 10 generations. The success of gene targeting was confirmed by anti-RasGRP4 Western blotting. RasGRP4 protein was clearly seen in thymocytes, splenocytes, and bone marrow cells from WT mice and was not detected in cells from RasGRP4−/− mice (Fig. 1B). This result indicated that RasGRP4 protein is clearly absent in cells from RasGRP4−/− mice. In addition, RasGRP4 is expressed in other cell types in which its expression has not been analyzed, such as thymocytes.
RasGRP4 in Mast Cell Degranulation and Cytokine Production
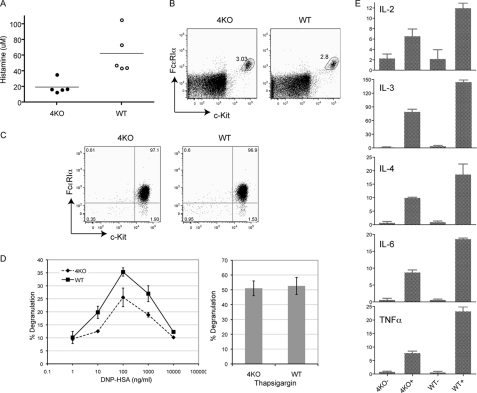
We first examined whether FcϵRI-mediated mast cell degranulation is affected by RasGRP4 deficiency in vivo by performing a systemic anaphylaxis assay. RasGRP4−/− and WT mice were first intravenously injected with monoclonal anti-DNP IgE. At 20–24 h after injection, mice were challenged with DNP-HSA to induce anaphylaxis. At 1.5 min after the challenge, blood was collected to measure histamine concentration by ELISA. As shown in Fig. 2A, histamine concentration in RasGRP4−/− mice was reduced to ∼1/3 of that in WT mice. The reduced histamine release was not due to a developmental block in mast cell development. Similar percentages of mast cells were detected in the cells isolated from the peritoneal cavities of these mice (RasGRP4−/−, 2.9 ± 0.5%; WT, 3.4 ± 0.6%). RasGRP4−/− mast cells expressed comparable levels of FcϵRI and c-Kit (Fig. 2B). In addition, comparable numbers of mast cells were detected in the ear skin dermis by toluidine blue staining (RasGRP4−/−, 228 ± 38 cells/cm2; WT, 236 ± 44 cells/cm2). The differences between the numbers of mast cells in these mice were not statistically significant.
FIGURE 2.
RasGRP4 in mast cell development and function. A, RasGRP4 function in passive systemic anaphylaxis. 4KO and WT mice were sensitized with 2 μg of anti-DNP IgE for 20–24 h. Anaphylaxis was induced by injection of 500 μg of DNP-HSA. Histamine concentration in the blood was determined by ELISA (n = 5 for both groups of mice). The horizontal bars indicate mean ± S.D. B, expression of c-Kit and FcϵRI on mast cells from the peritoneal cavities of 4KO and WT mice. C, expression of c-Kit and FcϵRI on WT and 4KO BMMCs. D, FcϵRI-mediated degranulation. BMMCs were sensitized with 1 μg/ml of anti-DNP IgE and then stimulated with various concentrations of DNP-HSA for 10 min (left panel) or stimulated with thapsigargin (right panel). Degranulation data were expressed as percentages of the released versus total β-hexosaminidase activity. Data shown are representative of four independent experiments. E, cytokine RNA expression. Sensitized BMMCs were stimulated with 100 ng/ml DNP-HSA for 1 h (+) or left untreated (-) before RNA isolation. RNAs were used in real-time PCR to quantitate RNA levels of different cytokines. Data shown were normalized by GAPDH and are representative of three independent experiments.
To study RasGRP4 function in mast cells in vitro, mast cells (BMMCs) were derived from WT and RasGRP4−/− bone marrow cells. After growing in medium with IL-3 for 3 weeks, more than 95% of cultured cells were c-Kit+ and FcϵRI+. RasGRP4−/− BMMCs expressed similar levels of c-Kit and FcϵRI as WT cells (Fig. 2C), further indicating that RasGRP4 is dispensable for mast cell development. We first tested whether RasGRP4−/− BMMCs could undergo normal degranulation after activation through the FcϵRI. BMMCs were first sensitized with anti-DNP IgE and then stimulated with different concentrations of DNP-HSA to induce degranulation, which was assayed by measuring the release of β-hexosaminidase. As shown in Fig. 2D, in agreement with the in vivo data, FcϵRI-evoked degranulation by RasGRP4−/− BMMCs was decreased compared with WT BMMCs. As controls, thapsigargin-induced degranulation, which bypasses FcϵRI-mediated proximal signaling events, was similar between these BMMCs.
As an important outcome of FcϵRI engagement is the production of different cytokines, we next analyzed the effect of RasGRP4 deficiency on cytokine production. BMMCs were sensitized with anti-DNP IgE and cross-linked with DNP-HSA for 1 h. Total RNAs were then prepared from resting or stimulated WT and RasGRP4−/− cells. Cytokine RNA levels were quantitated by real-time PCR. As shown in Fig. 2E, after FcϵI engagement, RasGRP4−/− mast cells expressed less IL-2, IL-3, Il-4, IL-6, and TNFα RNAs than WT cells. Together, our in vivo and in vitro data indicated that although RasGRP4 is not required in mast cell development, it is important in FcϵRI-mediated mast cell systemic anaphylaxis, degranulation, and cytokine production.
RasGRP4 in FcϵRI-mediated Proximal Signaling
Because RasGRP4 deficiency impaired mast cell degranulation and cytokine production, we next investigated whether FcϵRI-evoked signaling events are affected in RasGRP4−/− mast cells. BMMCs were sensitized with anti-DNP IgE and activated with DNP-HSA for 0, 2, 5, 10, 30, and 60 min. Total lysates were prepared and analyzed by Western blotting with different antibodies as indicated in Fig. 3. Overall tyrosine phosphorylation of proteins was relatively normal in RasGRP4−/− cells compared with WT cells. However, FcϵRI-mediated Erk and p38 phosphorylation was reduced in RasGRP4−/− cells. In contrast, Jnk activation was relatively normal, although the phosphorylation of the lower band was slightly reduced in RasGRP4−/− cells. We ensured that a similar amount of lysates was loaded in each lane by reblotting with antibodies against non-phosphorylated Erk, Jnk, and p38. These data indicated that RasGRP4 is required for optimal Erk and p38 activation after FcϵRI engagement.
FIGURE 3.
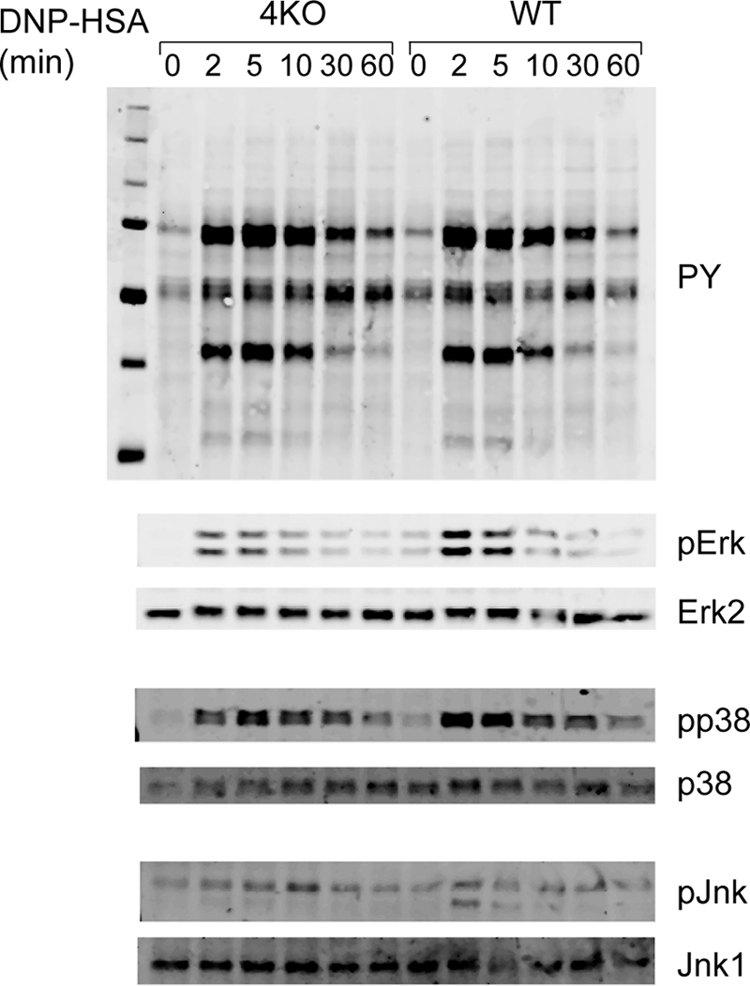
The effect of RasGRP4 deficiency on FcϵRI-mediated signaling. After sensitization with anti-DNP IgE, BMMCs were stimulated with DNP-HSA (100 ng/ml) for the indicated time points. Whole cell lysates were blotted with antibodies against pTyr, pErk, pp38, and pJNK. The same membranes were also reblotted with antibodies against the pan forms of MAPKs.
Both RasGRP1 and RasGRP4 Are Required for Mast Cell Function
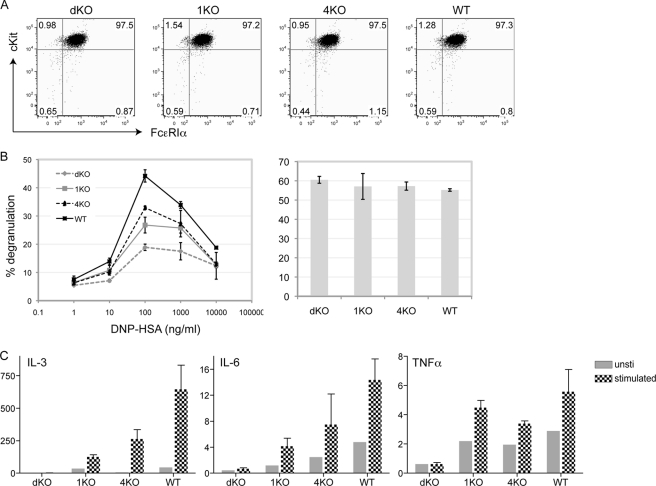
Previous studies have shown that RasGRP1 is critical in mast cell function (18). To assess whether RasGRP1 and RasGRP4 have redundant roles in mast cells, we generated RasGRP double-deficient mice (dKO) by crossing RasGRP1−/− (1KO) with RasGRP4−/− (4KO) mice. Mast cells were derived from the bone marrow cells of WT, 1KO, 4KO, and dKO mice. Analyses of these BMMCs showed that they expressed similar levels of FcϵRIα and c-Kit (Fig. 4A), suggesting that RasGRP4 and RasGRP1 are likely dispensable for mast cell differentiation and maturation in vitro. This result was supported by the data that similar numbers of mast cells were found in the ear skin dermis of dKO mice (dKO, 213 ± 26 cells/cm2; WT, 236 ± 44 cells/cm2). As shown in Fig. 4, B and C, single deficiency of RasGRP (1KO or 4KO) impaired FcϵRI-mediated degranulation and production of IL-3, IL-6, and TNF-α. Deficiency of both RasGRP proteins (dKO) further decreased degranulation and cytokine production, suggesting that both RasGRP1 and RasGRP4 play important roles in mast cell function.
FIGURE 4.
The effect of RasGRP1 and RasGRP4 deficiency on mast cell function. A, surface expression of FcϵRIα and c-Kit. BMMCs were derived from the bone marrow of RasGRP1−/−RasGRP4−/− (dKO), RasGRP1−/− (1KO), RasGRP4−/− (4KO), and WT mice. B, FcϵRI-mediated degranulation. BMMCs were sensitized with anti-DNP IgE and then stimulated with various concentrations of DNP-HSA for 10 min (left panel). Thapsigargin-induced degranulation is shown in the right panel. Data shown are representative of four independent experiments. C, FcϵRI-induced cytokine RNA synthesis. Sensitized BMMCs were stimulated with 100 ng/ml DNP-HSA for 1 h. RNAs were isolated from sensitized BMMCs before and after cross-linking with DNP-HSA. RNAs were used in RT-PCR followed by real-time PCR. Data shown were normalized by GAPDH and are representative of three independent experiments.
RasGRP1 and RasGRP4 in FcϵRI-mediated Proximal Signaling
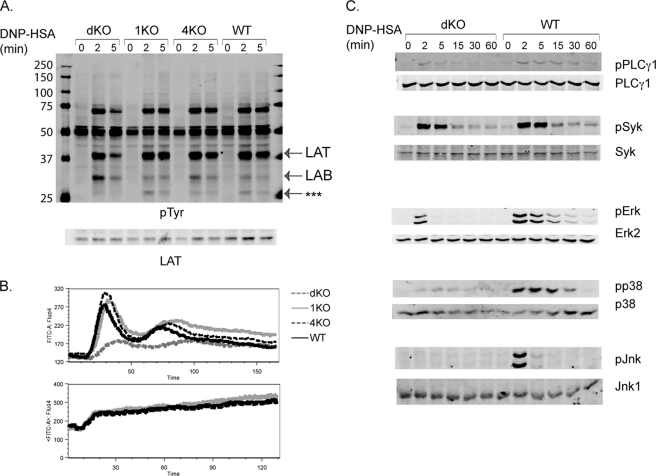
To investigate which signaling events are affected in BMMCs that are deficient in both RasGRP proteins, BMMCs were sensitized with anti-DNP IgE and activated with DNP-HSA for 0, 2, and 5 min. Total lysates were prepared and analyzed by Western blotting. As shown in Fig. 5A, overall tyrosine phosphorylation of proteins was relatively normal in dKO cells compared with that in 1KO, 4KO, and WT cells. However, we consistently observed reduced LAT phosphorylation and increased LAB phosphorylation in dKO cells. Moreover, the phosphorylation of a protein with a molecular mass of ∼27 kDa was diminished in dKO cells (Fig. 5A, ***). Previous studies indicate that LAT functions positively in FcϵRI-mediated MAPK activation, calcium mobilization, and mast cell function through interacting with Grb2, Gads, and PLC-γ1/2 and that LAB negatively regulates this pathway (17). Thus, the reduced degranulation and cytokine production by dKO cells could be caused by reduced LAT phosphorylation and increased LAB phosphorylation.
FIGURE 5.
The effect of RasGRP1 and RasGRP4 deficiency on FcϵRI-mediated proximal signaling. A, tyrosine phosphorylation of proteins. After sensitization with anti-DNP IgE, BMMCs were stimulated with DNP-HSA (100 ng/ml) for the indicated time points. Whole cell lysates were blotted with an anti-pTyr antibody and then reblotted with anti-LAT antisera. B, calcium flux. BMMCs were sensitized with anti-DNP IgE and then loaded with Fluo-4 AM. DNP-HSA was used to induce Ca2+ mobilization (top panel). For controls, ionomycin (1 μg/ml) was used (bottom panel). C, activation of PLCγ1, Syk, Erk, p38, and Jnk. WT and dKO cell lysates were blotted with antibodies against the phosphorylated and non-phosphorylated forms of PLCγ1, Syk, Erk, p38, and Jnk.
Because LAT and LAB phosphorylation was affected, we then examined FcϵRI-mediated calcium flux by FACS analysis. Mast cells were sensitized with anti-DNP IgE, loaded with Fluo-4, and activated with DNP-HSA. Compared with WT cells, 1KO and 4KO cells showed similar levels of calcium flux after the engagement of the FcϵRI (Fig. 5B). In contrast, dKO cells showed a considerable reduction in calcium flux. These BMMCs had similar levels of calcium flux after ionomycin stimulation (Fig. 5B, bottom panel).
We further analyzed the effect of RasGRP double deficiency on other signaling events. Because phosphorylation of LAT and LAB was affected, we examined activation of Lyn and Syk, two PTKs that initiate FcϵRI signaling cascades. Phosphorylation of Lyn and Syk were similar in dKO and WT cells (Fig. 5C and data not shown). Another molecule we examined was PLC-γ, which is activated after interacting with LAT and functions in FcϵRI-mediated calcium flux. Phosphorylation of PLC-γ1 was slightly reduced in dKO cells, which could account for impaired calcium flux in these cells. We further analyzed activation of three MAPKs using anti-phospho Erk, p38, and Jnk antibodies. As shown in Fig. 5C, deficiency of both RasGRP1 and RasGRP4 impaired activation of Erk, p38, and Jnk. Together, our results indicated that RasGRP1 and RasGRP4 might have an overlapping function in FcϵR-mediated calcium flux and MAPK activation. Deficiency in both of these proteins significantly impaired FcϵRI-mediated signaling and mast cell function.
RasGRP4 in T Cell Development
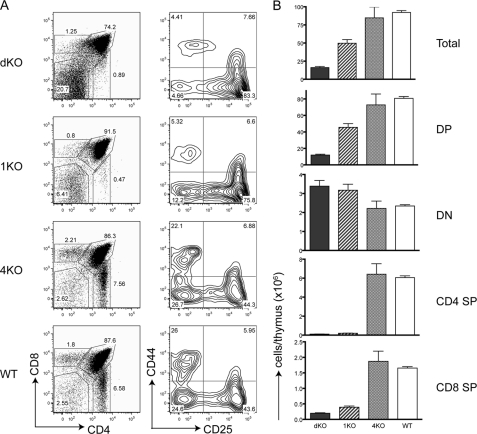
Previous studies have shown that RasGRP1 is important during thymocyte development and T cell activation. In RasGRP1−/− mice, thymocyte development is severely blocked at the DP stage (3). Because our data in Fig. 1B showed that RasGRP4 protein was detected in both thymocytes and splenocytes, we next investigated whether RasGRP4 functions during T cell development. FACS analysis showed that the percentages (Fig. 6A) and total numbers (B) of different thymocyte subsets in 4KO mice were similar to those in WT mice, indicating that disruption of the rasgrp4 gene alone had no obvious effect on T cell development. For 1KO mice, the percentages of SP thymocytes were reduced significantly, and total numbers of DP and SP cells were also reduced, as described previously (3). Despite the fact that RasGRP4 deficiency alone had no obvious effect, deficiency in both RasGRP proteins had a much more severe consequence on thymocyte development in dKO mice. Thymi from dKO mice appeared much smaller than those from 1KO mice. As shown in Fig. 6B, the number of total thymocytes in dKO mice was only ∼1/5 of that in WT mice. Further analysis of different subsets showed that the percentage of DP cells was reduced to ∼74.2% of thymocytes. However, the percentage of DN cells was increased to ∼20.7%. The percentages of CD4 and CD8 SP in dKO mice were still reduced, similar to those in 1KO mice. Analysis of the DN population indicated that thymocyte development was partially blocked at the DN3 stage in 1KO and dKO mice. There was an increased percentage of DN3 thymocytes in dKO mice compared with 1KO mice (83% versus 76%.), indicating that RasGRP proteins are important in pre-TCR signaling.
FIGURE 6.
A severe block of thymocyte development by RasGRP1 and RasGRP4 double deficiency. A, thymocyte development. CD4 and CD8 expression of total thymocytes from dKO, 1KO, 4KO, and WT mice was analyzed by FACS. CD25 and CD44 expression in DN thymocytes from these mice was also analyzed. The figure shown is one representative of four mice analyzed. B, total numbers of different thymocyte populations. Thymocytes from eight 2-month-old mice of each genotype were used for the calculation.
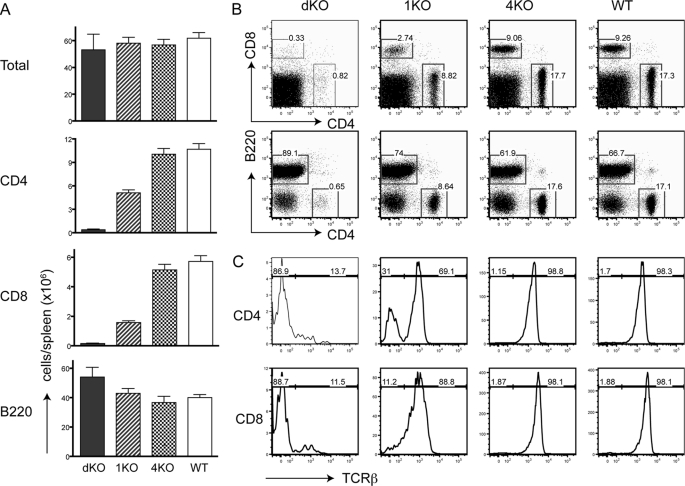
We further examined the lymphoid compartments in the periphery of RasGRP dKO, 1KO, and 4KO mice. At 5 weeks old, total numbers of splenocytes in these mice were similar (Fig. 7A). 4KO mice had normal numbers and percentages of CD4+ and CD8+ cells in comparison with WT mice (Fig. 7, A and B). Because of the partial block in thymocyte development, the numbers of CD4+ and CD8+ cells in 1KO mice were decreased by ∼50%. Interestingly, the numbers of CD4+ and CD8+ cells in dKO spleens were drastically decreased. The percentages of CD4+ and CD8+ cells were only 0.8% and 0.3%, respectively. Further analysis of these small numbers of CD4+ and CD8+ cells indicated that most of them were not T cells as they were TCRβ− (Fig. 7C) and Thy1.2− (data not shown). They were likely dendritic cells that expressed either CD4 or CD8. Thus, there were only very few mature T cells in mice deficient in both RasGRP1 and RasGRP4. Together, these data indicate that although RasGRP1 plays a dominant role, RasGRP4 is also important during thymocyte development.
FIGURE 7.
Very few T cells in mice deficient in both RasGRP1 and RasGRP4. A, the numbers of splenic T and B cells. Spleens from eight 2-month-old mice of each genotype were used in the calculation. B, expression of CD4, CD8, and B220 on splenocytes. C, the percentages of TCRβ+ cells. TCRβ expression was analyzed on CD4+ or CD8+ cells from spleens from dKO, 1KO, 4KO, and WT mice.
DISCUSSION
Although previous studies suggested that RasGRP4 is likely to be important during mast cell development, the role of RasGRP4 in mast cell function remained unclear. A human mast cell line deficient in RasGRP4 has a characteristic of immature mast cells and fails to express the FcϵRI and other proteins, such as prostaglandin D2 (PGD2) synthase. Interestingly, transfection of RasGRP4 into these cells restores the expression of PGD2 synthase (15). In addition, RasGRP4 regulates the expression of hundreds of genes (16). Therefore, we generated RasGRP4−/− mice to more clearly assess the role of RasGRP4 in mast cells. Our data from analyses of RasGRP4−/− mice indicated that RasGRP4 deficiency had no effect on mast cell development and maturation. Normal numbers of mast cells, which were identified by the expression of FcϵRI and c-Kit, were found in the peritoneal cavity and skin of RasGRP4−/− mice. RasGRP4−/− BMMCs also expressed normal levels of FcϵRI and c-Kit. Upon stimulation with thapsigargin, they had normal granule release as assayed by β-hexosaminidase activity, implying that they likely have normal granule formation. Together, data from our in vivo and in vitro analyses indicated that RasGRP4 is dispensable during mast cell development and maturation. It is not clear why RasGRP4 deficiency had a more significant impact on human mast cells. It is possible that, in addition to RasGRP4, these cells lack another protein(s) required for proper mast cell development and maturation.
Our functional analysis of RasGRP4−/− mast cells showed that FcϵRI-mediated degranulation in vivo and in vitro was reduced. These results are in agreement with previous studies using C3H/HeJ mice. These mice express a dysfunctional RasGRP4 protein that lacks a DAG-binding domain. Consequently, they are hyporesponsive to methacholine stimulation (14). We further analyzed why these RasGRP4−/− mast cells are hyporesponsive to stimulation and found that FcϵRI-mediated Erk and p38 activation was reduced, whereas activation of other signaling events was not largely affected. Even though RasGRP4 is highly expressed in mast cells, RasGRP4−/− mast cells are still capable of activating Erk. In contrast, Erk activation in RasGRP1−/− T cells is abolished. Thus, RasGRP4 does not play an analogous role in mast cells as to RasGRP1 in T cells.
Because the Ras-MAPK pathway could still be activated in RasGRP4−/− cells, it is likely that other RasGRP proteins or GEFs can compensate for the loss of RasGRP4. Indeed, FcϵRI-mediated signaling and effector function were more severely blocked in mast cells deficient in both RasGRP1 and RasGRP4. dKO mast cells were able to develop normally; however, their degranulation and cytokine production were drastically impaired compared with 1KO cells. Moreover, deficiency of both proteins had more of an impact on proximal signaling events following FcϵRI engagement. In dKO mast cells, phosphorylation of LAT, a critical adaptor protein that couples FcϵRI engagement to Erk activation and calcium flux, was reduced. Consequently, calcium flux was also reduced in dKO cells. Interestingly, phosphorylation of LAB, an adaptor that negatively regulates FcϵRI signaling, was increased. In addition, phosphorylation of an unknown 27-kDa phosphorylated protein was reduced. We have yet to identify this 27-kDa protein or determine the mechanism by which RasGRP double deficiency affects the phosphorylation of LAT, LAB, and this unidentified protein. LAT and LAB are known substrates of Syk. However, we did not detect obvious changes in Syk phosphorylation (Fig. 5C). Lyn phosphorylation was also unaffected (not shown). Thus, how RasGRP proteins, which normally function downstream of LAT and LAB phosphorylation and calcium flux, regulate these signaling events is not clear. We speculate that FcϵRI-mediated MAPK activation might provide a positive feedback on LAT phosphorylation and function. In T cells, LAT is also phosphorylated on Ser/Thr residues. Phosphorylation of LAT Thr-155 by Erk provides a negative feedback on TCR activation (19). However, another study suggests that phosphorylation of LAT serine residues is important for its function in T cell activation. Mutations of conserved serine residues impair TCR-mediated LAT tyrosine phosphorylation, Erk activation, and calcium flux (20). Thus, it is possible that LAT is also a substrate of Erk in mast cells. In dKO mast cells, LAT phosphorylation on serine residues may be reduced because of defective Ras-Erk activation, leading to reduced LAT tyrosine phosphorylation and impaired LAT function in FcϵRI-mediated signaling.
RasGRP1 and RasGRP4 double deficiency caused a drastic reduction in the activation of Erk, p38, and Jnk, indicating the importance of these proteins in the activation of the Ras-MAPK pathway. Although the effect of RasGRP deficiency on Erk activation was expected, we were surprised to see that Jnk and p38 activation was also affected. It is likely that the impaired Jnk and p38 activation is an indirect consequence of RasGRP deficiency. Reduced tyrosine phosphorylation of LAT in dKO cells could affect recruitment of Gads and SLP-76 to LAT, which is responsible for activation of Rac1 and Cdc42. These two small G proteins are important in the activation of the JNK pathway through MEKK1-SEK1/MKK7 in mast cells (21). How FcϵRI aggregation induces p38 activation in mast cells is not clear. However, the TAK1-MKK6-p38 pathway has been well described in other immune effector cells (22). It is possible that impaired LAT phosphorylation in dKO mast cells may also affect activation of the TAK1-MKK6-p38 pathway.
Previous studies have clearly demonstrated that RasGRP1 plays an important role during thymocyte development and TCR-mediated Ras-Erk activation (3, 4). Thymocyte development in RasGRP1−/− mice is partially blocked at the DP to SP transition. Although these data indicate that RasGRP1 plays a predominant role in TCR-mediated Erk activation, it is possible that other members of the RasGRP family compensate for the loss of RasGRP1 in T cells. Our analysis showed that thymocyte development in RasGRP4−/− mice was relatively normal, suggesting that RasGRP4 does not have a major role during thymocyte development. However, mice deficient in both RasGRP1 and RasGRP4 had a much more severe block in thymocyte development than RasGRP1−/− mice. Thymic cellularity was reduced to 1/5 of that of WT thymi and had increased DN populations. Consequently, there were very few mature T cells in the periphery. These data suggested that RasGRP4 also functions during thymocyte development, especially in the absence of RasGRP1. Because a small number of DP thymocytes and mature T cells were still present in dKO mice, it is possible that RasGRP2 and/or RasGRP3 can also function in pre-TCR or TCR-mediated signaling. This possibility can be tested in the future by generating mice deficient in three or four members of the RasGRP family.
Acknowledgments
We thank the Duke University Cancer Center Flow Cytometry, DNA Sequencing, and Transgenic Mouse facilities for excellent services and all laboratory members for valuable discussions and editing.
This study was supported, in whole or in part, by National Institutes of Health Grants AI048674 and AI056156.
- RasGRP
- Ras guanine nucleotide-releasing protein
- DAG
- diacylglycerol
- TCR
- T cell receptor
- SP
- single positive
- DP
- double positive
- HMC
- human mast cells
- IMDM
- Iscove's modified Dulbecco's medium
- BMMC
- bone marrow-derived mast cells
- DNP
- dinitrophenol
- HSA
- human serum albumin
- LAT
- Linker for activation of T cells
- LAB
- Linker for activation of B cells.
REFERENCES
- 1. Ebinu J. O., Bottorff D. A., Chan E. Y., Stang S. L., Dunn R. J., Stone J. C. (1998) RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 [DOI] [PubMed] [Google Scholar]
- 2. Springett G. M., Kawasaki H., Spriggs D. R. (2004) Non-kinase second-messenger signaling. New pathways with new promise. BioEssays 26, 730–738 [DOI] [PubMed] [Google Scholar]
- 3. Dower N. A., Stang S. L., Bottorff D. A., Ebinu J. O., Dickie P., Ostergaard H. L., Stone J. C. (2000) RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1, 317–321 [DOI] [PubMed] [Google Scholar]
- 4. Roose J. P., Mollenauer M., Gupta V. A., Stone J., Weiss A. (2005) A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25, 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bivona T. G., Pérez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. (2003) Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424, 694–698 [DOI] [PubMed] [Google Scholar]
- 6. Mor A., Philips M. R. (2006) Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 24, 771–800 [DOI] [PubMed] [Google Scholar]
- 7. Daniels M. A., Teixeiro E., Gill J., Hausmann B., Roubaty D., Holmberg K., Werlen G., Holländer G. A., Gascoigne N. R., Palmer E. (2006) Compartmentalized Ras/MAPK signaling. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444, 724–729 [DOI] [PubMed] [Google Scholar]
- 8. Dupuy A. J., Morgan K., von Lintig F. C., Shen H., Acar H., Hasz D. E., Jenkins N. A., Copeland N. G., Boss G. R., Largaespada D. A. (2001) Activation of the Rap1 guanine nucleotide exchange gene, CalDAG-GEF I, in BXH-2 murine myeloid leukemia. J. Biol. Chem. 276, 11804–11811 [DOI] [PubMed] [Google Scholar]
- 9. Eto K., Murphy R., Kerrigan S. W., Bertoni A., Stuhlmann H., Nakano T., Leavitt A. D., Shattil S. J. (2002) Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc. Natl. Acad. Sci. U.S.A. 99, 12819–12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teixeira C., Stang S. L., Zheng Y., Beswick N. S., Stone J. C. (2003) Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102, 1414–1420 [DOI] [PubMed] [Google Scholar]
- 11. Oh-hora M., Johmura S., Hashimoto A., Hikida M., Kurosaki T. (2003) Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198, 1841–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reuther G. W., Lambert Q. T., Rebhun J. F., Caligiuri M. A., Quilliam L. A., Der C. J. (2002) RasGRP4 is a novel Ras activator isolated from acute myeloid leukemia. J. Biol. Chem. 277, 30508–30514 [DOI] [PubMed] [Google Scholar]
- 13. Yang Y., Li L., Wong G. W., Krilis S. A., Madhusudhan M. S., Sali A., Stevens R. L. (2002) RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J. Biol. Chem. 277, 25756–25774 [DOI] [PubMed] [Google Scholar]
- 14. Li L., Yang Y., Wong G. W., Stevens R. L. (2003) Mast cells in airway hyporesponsive C3H/HeJ mice express a unique isoform of the signaling protein Ras guanine nucleotide releasing protein 4 that is unresponsive to diacylglycerol and phorbol esters. J. Immunol. 171, 390–397 [DOI] [PubMed] [Google Scholar]
- 15. Li L., Yang Y., Stevens R. L. (2003) RasGRP4 regulates the expression of prostaglandin D2 in human and rat mast cell lines. J. Biol. Chem. 278, 4725–4729 [DOI] [PubMed] [Google Scholar]
- 16. Katsoulotos G. P., Qi M., Qi J. C., Tanaka K., Hughes W. E., Molloy T. J., Adachi R., Stevens R. L., Krilis S. A. (2008) The diacylglycerol-dependent translocation of Ras guanine nucleotide-releasing protein 4 inside a human mast cell line results in substantial phenotypic changes, including expression of interleukin 13 receptor α2. J. Biol. Chem. 283, 1610–1621 [DOI] [PubMed] [Google Scholar]
- 17. Zhu M., Liu Y., Koonpaew S., Granillo O., Zhang W. (2004) Positive and negative regulation of FcϵRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 200, 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y., Zhu M., Nishida K., Hirano T., Zhang W. (2007) An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J. Exp. Med. 204, 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuda S., Miwa Y., Hirata Y., Minowa A., Tanaka J., Nishida E., Koyasu S. (2004) Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT. EMBO J. 23, 2577–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez-Florensa M., Garcia-Blesa A., Yelamos J., Munoz-Suano A., Dominguez-Villar M., Valdor R., Alonso A., Garcia-Cozar F., Aparicio P., Malissen B., Aguado E. (2011) Serine residues in the LAT adaptor are essential for TCR-dependent signal transduction. J. Leukocyte Biol. 89, 63–73 [DOI] [PubMed] [Google Scholar]
- 21. Ishizuka T., Terada N., Gerwins P., Hamelmann E., Oshiba A., Fanger G. R., Johnson G. L., Gelfand E. W. (1997) Mast cell tumor necrosis factor α production is regulated by MEK kinases. Proc. Natl. Acad. Sci. U.S.A. 94, 6358–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]