Background: Mast cells contain tryptase·heparin complexes.
Results: Fibrinogen is preferentially cleaved by these complexes.
Conclusion: A major way mast cells prevent the generation of fibrin and fibrin-platelet clots in inflammatory sites is by their exocytosed tryptase·heparin complexes proteolytically destroying fibrinogen.
Significance: The use of recombinant tryptase·heparin complexes might be an effective way to prevent blood coagulation in the clinic.
Keywords: Blood Coagulation Factors, Fibrin, Fibrinogen, Heparin, Immunology, Serine Protease, Coagulation, Tryptase, hTryptase-β, mMCP-6
Abstract
The mouse and human TPSB2 and TPSAB1 genes encode tetramer-forming tryptases stored in the secretory granules of mast cells (MCs) ionically bound to heparin-containing serglycin proteoglycans. In mice these genes encode mouse MC protease-6 (mMCP-6) and mMCP-7. The corresponding human genes encode a family of serine proteases that collectively are called hTryptase-β. We previously showed that the α chain of fibrinogen is a preferred substrate of mMCP-7. We now show that this plasma protein also is highly susceptible to degradation by hTryptase-β· and mMCP-6·heparin complexes and that Lys575 is a preferred cleavage site in the protein α chain. Because cutaneous mouse MCs store substantial amounts of mMCP-6·heparin complexes in their secretory granules, the passive cutaneous anaphylaxis reaction was induced in the skin of mMCP-6+/mMCP-7− and mMCP-6−/mMCP-7− C57BL/6 mice. In support of the in vitro data, fibrin deposits were markedly increased in the skin of the double-deficient mice 6 h after IgE-sensitized animals were given the relevant antigen. Fibrinogen is a major constituent of the edema fluid that accumulates in tissues when MCs degranulate. Our discovery that mouse and human tetramer-forming tryptases destroy fibrinogen before this circulating protein can be converted to fibrin changes the paradigm of how MCs hinder fibrin deposition and blood coagulation internally. Because of the adverse consequences of fibrin deposits in tissues, our data explain why mice and humans lack a circulating protease inhibitor that rapidly inactivates MC tryptases and why mammals have two genes that encode tetramer-forming serine proteases that preferentially degrade fibrinogen.
Introduction
Stored in abundance in the secretory granules of mouse mast cells (MCs)3 ionically bound to heparin-containing serglycin proteoglycans (SGPGs) (1, 2) are the tetramer-forming tryptases known as mouse MC protease 6 (mMCP-6) (3, 4) and mMCP-7 (5) whose respective Tpsb2 and Tpsab1 genes reside back-to-back on chromosome 17A3.3 (6). The corresponding human TPSB2 and TPSAB1 genes reside on chromosome 16p13.3. The initial hTPSAB1- and hTPSB2-derived cDNAs isolated by Miller et al. (11) and Vanderslice et al. (10) encoded enzymes they designated as hTryptase-β1 and -β2, respectively. These proteases had the same substrate specificities (7–9) and amino acid sequences except for residue 132 (10, 11). Numerous point mutations in the human TPSAB1 and TPSB2 genes have been identified in recent years that result in the expression of >25 different protein isoforms of each tetramer-forming enzyme.4 Because the functional significance of these allelic isoforms has not been deduced, investigators generally refer to the enzymatically active products of the human TPSAB1 and TPSB2 genes collectively as hTryptase-β (12).
WT C57BL/6 (B6) mice constitutively lack mMCP-7 due to a splice site mutation in the Tpsab1 gene (13, 14). In contrast, the heparin+ MCs in the connective tissues of all examined mouse strains contain appreciable amounts of enzymatically active mMCP-6, and transgenic B6 mice that lack this tryptase cannot combat bacterial (15) and helminth (16) infections efficiently. Despite their beneficial roles in immunity, mMCP-6 has proinflammatory activity in different disease models (17–23).
Numerous in vitro studies have been carried out in attempts to identify those endogenous and exogenous proteins that are preferentially cleaved by mouse and human MC tryptases. Although many candidates have been identified, the studies performed in the 1980s and 1990s are difficult to interpret today because of the substantial allelic variations of the human TSPAB1 and TPSB2 genes, the identification of splice variants that encode functionally distinct hTryptase-β isoforms that are enzymatically active even as monomers (24), and the questionable purity of some of the lung-derived tryptase preparations used in those earlier studies. The failure to appreciate the existence of tryptase redundancy (17) also hindered our understanding of the importance of these MC-restricted tetramer-forming enzymes in different disease states.
Sodium cromoglycate prevents the degranulation of mammalian MCs, and Samoszuk and Corwin (25) noted that tumor-bearing BALB/c mice that were given sodium cromoglycate intraperitoneally contained lakes of clotted blood in and around their tumors. These data suggested the presence of anti-coagulation factors in the secretory granules of the animal's constitutive safranin+ MCs. One of these anti-coagulation factors was shown to be mMCP-7 (26). Unexplained was how the α chains of the circulating 340-kDa fibrinogen dimer could fit inside the small pocket in the tryptase donut-shaped tetramer unit. More important, the biologic significance of the data were not apparent due to the fact that WT B6 mice constitutively lack mMCP-7 (13, 14) yet have no noticeable defect in fibrinogen metabolism in tissues during a MC-dependent anaphylaxis reaction.
Nevertheless, in support of the above mMCP-7 data, Thomas et al. (27) reported that the α chain of hFibrinogen was susceptible to an undefined hTryptase-β·heparin complex enriched from human lung biopsies. Problematically, the authors concluded that their enzyme preparation preferentially cleaved the hFibrinogen α chain at Arg591, which resides in a 14-mer sequence missing in the fibrinogens from many species, including the mouse. Another deficiency of that study and an earlier study that also used an undefined hTryptase-β·heparin complex purified from human lung (28) was that no in vivo experiment was carried out to determine the physiologic relevance of the in vitro data. The lack of an mMCP-6-null mouse also hindered progress.
We now show that the α chains of human and mouse fibrinogen are highly susceptible to mMCP-6· and hTryptase-β·heparin complexes at neutral pH, cleaving the plasma protein further upstream than previously reported. Our findings, therefore, change the paradigm as to how MCs hinder blood coagulation and fibrin deposition in inflammatory tissue sites.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant mMCP-6 (29) and hTryptase-β (8, 9) were generated in High Five™ insect cells and purified from the serum-free conditioned medium, as previously described. To obtain active enzyme, each purified bioengineered zymogen was exposed to enterokinase (Biozyme Lab, San Diego, CA) at a >100:1 pro-EK-tryptase:enterokinase ratio in the presence of CsCl density gradient-purified heparin glycosaminoglycan (amount approximately equal to that of the recombinant tryptase). A recombinant, enzymatically active hTryptase-β·heparin complex (30) also was obtained from Promega (Madison, WI). Mouse and human fibrinogen were obtained from Sigma as well as pancreatic trypsin and the spectrophotometric substrate tosyl-Gly-Pro-Lys-p-nitroanilide. Anti-fibrinogen antibody ab83477 was obtained from Abcam (Cambridge, MA). This rabbit antibody was generated against a 50-mer synthetic peptide that corresponds to residues 576–625 in the C-terminal domain of the α chain of hFibrinogen. It recognizes human and mouse fibrinogen. A homologous recombination approach was used to create the 6−/7− B6 mouse strain (15) that was used in the passive cutaneous anaphylaxis (PCA) reactions noted below. These mice and their littermate control 6+/7− B6 mice were housed at the same animal facility at The University of Texas M. D. Anderson Cancer Center.
Preferential Proteolysis of α Chain of hFibrinogen by hTryptase-β·Heparin Complexes in Presence and Absence of Serum
For determination of the preferred cleavage site(s) in hFibrinogen, the purified plasma protein (15–50 μg) was incubated with 0.1–0.4 μg of different recombinant hTryptase-β·heparin complexes at 37 °C for 1 min to 3 h. At the end of each digestion, an equal volume of 2× SDS-PAGE buffer (2% SDS, 0.01% bromphenol blue, and 63 mm Tris-HCl, pH 6.8, with or without 5% β-mercaptoethanol) was added. The samples were boiled for 5 min and then loaded onto preformed 12% Tris-HCl polyacrylamide gels (Bio-Rad). β-Mercaptoethanol was included in the SDS-PAGE sample buffer to disrupt the intrachain and interchain disulfide bonds in fibrinogen. After electrophoresis at ∼105 V, one gel in each experiment was stained with Coomassie Blue for visualization of its digestion products. The duplicate gel was used for immunoblotting. This gel was soaked in transfer buffer (15% methanol, 25 mm Tris-HCl, and 200 mm glycine, pH 8.5), and then the SDS-PAGE-resolved proteins were transferred at 4 °C onto an Immun-Blot PVDF membrane (Bio-Rad) for ∼2 h at ∼110 V. The resulting protein blots were placed in Tween 20-supplemented Tris-buffered saline (TBST) additionally containing 5% nonfat milk for 2 h; they were then washed with TBST. This was followed by a 2-h incubation with a 1:1000 dilution of rabbit anti-fibrinogen ab83477 antibody in TBST buffer containing 5% nonfat milk. After 3 washes in TBST, each antibody-treated blot was incubated in the dark for 1 h with affinity-purified goat anti-rabbit IgG horseradish peroxidase conjugate (1:500 dilution) (Bio-Rad). The resulting protein blot was washed three times with TBST buffer and incubated for 1 min with Amersham Biosciences (GE Healthcare) enhanced chemiluminescence (ECL) detection reagents. Amersham Biosciences hyperfilm ECL was used for development of the immunoblot.
To evaluate the possible consequences of circulating protease inhibitors, hFibrinogen (15 μg) was incubated with trypsin (0.05 μg) or recombinant hTryptase-β·heparin complexes (0.4 μg) for 15 min at 37 °C before and after exposure to 0.2% human serum. The amounts of trypsin and hTryptase-β used in these experiments were enzymatically equivalent based on their ability to cleave tosyl-Gly-Pro-Lys-p-nitroanilide. Equal volumes of 2× SDS-PAGE buffer were added, and the samples were analyzed by SDS-PAGE. The ability of the endogenous protease inhibitors in human serum to inactivate a recombinant mMCP-6·heparin complex also was evaluated.
Identification of Preferred hTryptase-β Susceptible Site in α Chain of hFibrinogen
hFibrinogen (30 μg) was incubated with recombinant hTryptase-β (0.4 μg) for 1 or 15 min at 37 °C in the presence of heparin. The digestion products were subjected to SDS-PAGE, and electrophoresis was carried out on longer preparative gels to optimally resolve the three chains of the protein and their digestion products. The resulting gels were weakly stained with Coomassie Blue. The main, large-sized fragment of the α chain of hFibrinogen generated in each instance was excised from the gel and washed twice with 50% acetonitrile in water. After discarding the supernatant, the sample was subjected to C-terminal amino acid sequence determination by exhaustive proteolysis with three separate enzymes and orthogonal mapping of the resulting sequences by nanoelectrospray tandem mass spectrometry (LC-MS/MS).
Peptide sequence analysis of each digestion mixture also was performed using microcapillary reversed phase high performance liquid chromatography coupled with MS/MS on an LTQ-Orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA). The Orbitrap repetitively surveyed an m/z range from 395 to 1600, while data-dependent MS/MS spectra on the 20 most abundant ions in each survey scan were acquired in the linear ion trap. MS/MS spectra were acquired with relative collision energy of 30%, 2.5-Da isolation width, and recurring ions were dynamically excluded for 60 s. Preliminary sequencing of the peptides was facilitated with the SEQUEST algorithm with a 30-ppm mass tolerance against the MGI human subset of the Uniprot Knowledgebase, concatenated to a reverse decoy data base. Peptides were accepted with mass error <2.5 ppm and score thresholds to attain an estimated false discovery rate of ∼1%. Data sets for all digest results were combined in silico, culled of minor contaminating keratin or autolytic peptide spectra, and re-searched with SEQUEST against the GenBankTM reference hFibrinogen sequence without taking into account enzyme specificity. The mapping of hFibrinogen C-terminal peptides and subsequent manual confirmation of their MS/MS spectra were facilitated with in-house versions of Proteomics Browser Suite (ThermoFisher Scientific).
A 7-kDa peptide was occasionally seen in the hFibrinogen digests. To purify and identify this fragment, a large scale digestion reaction was performed in which 300 μg of hFibrinogen was incubated 15 min at 37 °C with 8 μg of a recombinant hTryptase-β·heparin complex. The resulting digest was subjected to a spin-column filtration step (4 °C, 20 min, 4,000 × g) using a Centricon 10-kDa cutoff membrane (Millipore, Billercia, MA). The low molecular weight peptides in the filtrate were concentrated by a 20% trichloroacetic acid precipitation step. The pellet was washed twice with acetone and subjected to SDS-PAGE. The resulting purified 7-kDa peptide was then characterized by MS/MS analysis.
Preferential Proteolysis of α Chain of mFibrinogen by hTryptase-β· and mMCP-6·Heparin Complexes
The ability of recombinant hTryptase-β· and mMCP-6·heparin complexes to cleave mFibrinogen was deduced as described above for evaluating the proteolytic susceptibility of hFibrinogen. Confirmatory data were obtained using 6+/7− B6 mouse bone marrow-derived MCs (mBMMCs). For the latter experiments, 6+/7− B6 mBMMCs were generated by culturing their hematopoietic progenitors for 4 weeks in IL-3-enriched WEHI-3 cell-conditioned medium as previously described (31). The resulting immature MCs were then cultured for an additional 2 weeks in the presence of 10 ng/ml recombinant mouse IL-33 (R&D Systems, Minneapolis, MN) as the latter cytokine induces this population of MCs to markedly increase its granule accumulation of naturally occurring mMCP-6·SGPG complexes (32). Approximately 10 million of the resulting mBMMCs in 200 μl of PBS were sonicated on ice. Samples of mFibrinogen were incubated for 5 min at 37 °C with varying amounts of boiled and non-boiled 6+/7− mBMMC lysate.
The spectrophotometric method of Svendsen et al. (33) was used to quantitate the levels of enzymatically active mMCP-6 in each mBMMC lysate. For these experiments, samples of 6+/7− mBMMC lysate were placed in assay buffer (50 mm Tris-HCl and 0.1 m NaCl, pH 7.5) containing 0.5 mm tosyl-Gly-Pro-Lys-p-nitroanilide. After 5–15 min of incubation at room temperature, the change in optical density at 405 nm was determined. The amount of enzymatically active mMCP-6 in each mBMMC lysate was then calculated using a standard curve based on known amounts of pancreatic trypsin. The resulting treated mFibrinogen samples were placed in an equal volume of 2× SDS-PAGE buffer containing β-mercaptoethanol and subjected to SDS-PAGE. Visualization of the fragments of mFibrinogen created by the naturally occurring mMCP-6·SGPG complexes in the mBMMC lysates was achieved by staining with Coomassie Blue and by SDS-PAGE immunoblotting with anti-fibrinogen antibody ab83477.
In Vitro Anticoagulant Activities of mMCP-6· and hTryptase-β·Heparin Complexes
A standard fibrinogenolysis assay (26) was used to evaluate the anticoagulant activities of mMCP-6· and hTryptase-β·heparin complexes as well as the same heparin preparation used to create these complexes. For these studies sodium citrate-treated human plasma (100 μl/assay) was incubated at 37 °C with heparin alone (100 μg) or with heparin bound to recombinant hTryptase-β (3.3 and 6.6 μg) or mMCP-6 (10 μg) for 30–60 min. The time required for thrombin to clot the samples relative to untreated plasma was then determined using a fibrometer.
PCA Reaction
The PCA reaction was carried out on 6+/7− and 6−/7− B6 mice as previously described (15). Briefly, 100 ng of anti-2,4-dinitrophenol IgE (Sigma) in 20 μl of PBS was injected intradermally into the ears. Two days later, 20–100 μg of 2,4-dinitrophenol human serum albumin (Sigma) in 200 μl of PBS was injected into the tail vein of each sensitized animal. The animals were sacrificed 6 h later. The ears were removed, embedded in Tissue-Tek optimum cutting temperature compound (Sakura Finetek, Torrance, CA), and frozen in isopentane mixed with dry ice. They were kept at −70 °C until the analyses were performed. For immunohistochemistry, 5-μm frozen sections were air-dried for 30 min at room temperature, rehydrated with PBS, and incubated for 1 h at 37 °C with a solution containing Texas Red-avidin (Invitrogen) to label heparin, fluorescein-labeled goat anti-mFibrinogen/mFibrin IgG (Nordic Immunology, Eindhoven, The Netherlands) to evaluate fibrin deposits, and Hoechst 33342 (Invitrogen) to label nuclei. Sections were then viewed with an Eclipse 80i microscope (Nikon Instruments Inc., Melville, NY). Pictures were acquired with a Hamamatsu C10800 digital camera and HC Image software 1.1.3.1 (Hamamatsu Corp., Bridgewater, NJ).
RESULTS
Preferential Proteolysis of α Chain of hFibrinogen by hTryptase-β·Heparin Complexes in Presence and Absence of Serum
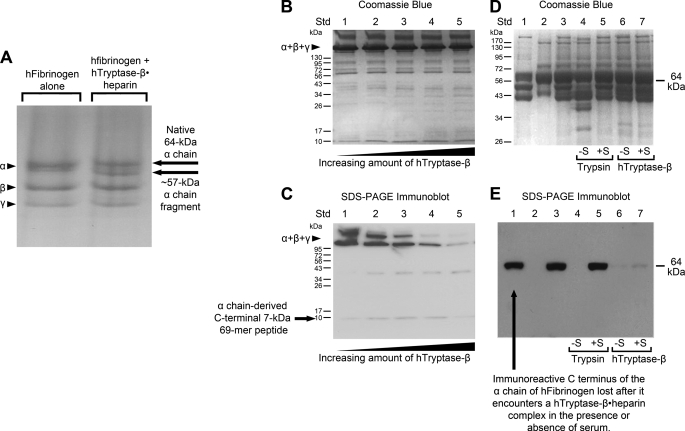
We previously showed that the α chains of mouse and human fibrinogen are highly susceptible to recombinant and naturally occurring mMCP-7 in vitro and in vivo (26). In this study, we discovered that the hFibrinogen α chain also was preferentially cleaved when this plasma protein was exposed in vitro at neutral pH to varying amounts of a recombinant hTryptase-β·heparin complex (Fig. 1) or when exposed to a small amount of the complex for varying periods of time (data not shown). The resulting hFibrinogen digestion products were evaluated by SDS-PAGE in the presence (Fig. 1A) and absence (Fig. 1B) of β-mercaptoethanol.
FIGURE 1.
The α chain of hFibrinogen is a preferred target of hTryptase-β·heparin complexes. In A, hFibrinogen was incubated with a recombinant hTryptase-β·heparin complex (∼500:1 substrate:enzyme ratio, 37 °C, 1 min), and the resulting digest was analyzed by SDS-PAGE in the presence of β-mercaptoethanol to disrupt the protein disulfide bonds. The position of the protein native α chain and its prominent, slightly smaller fragment is highlighted. In B and C, hFibrinogen was incubated with increasing amounts of a recombinant hTryptase-β·heparin complex (3500:1 to 250:1 substrate:enzyme ratio, 37 °C, 10 min). In these instances the resulting digests were analyzed by SDS-PAGE in the absence of β-mercaptoethanol. The SDS-PAGE gel in B was stained with Coomassie Blue. In C, a protein blot of the separated proteins in the duplicate gel was stained with the rabbit anti-fibrinogen antibody ab83477. In D and E, hFibrinogen was incubated with trypsin (lanes 4 and 5) or hTryptase-β·heparin (lanes 6 and 7) in the presence (lanes 5 and 7) or absence (lanes 4 and 6) of 0.2% normal human serum. For controls, lanes 1, 2, and 3 in the gel contained hFibrinogen alone, serum alone, and both, respectively. The samples were electrophoresed in the presence of β-mercaptoethanol. One gel (D) was stained by Coomassie Blue. In the duplicate gel (E), the separated proteins were transferred, and the resulting protein blot was probed with anti-fibrinogen antibody ab83477.
The subtle change in molecular weight of the treated protein (especially when the analysis was carried out in the absence of β-mercaptoethanol) indicated that proteolysis was limited when the substrate:enzyme ratio was >100:1 and that cleavage preferentially occurred near the N and/or C terminus of the protein α chain. The rabbit antibody ab83477 was generated against a 50-mer synthetic peptide that corresponds to residues 576–625 in the C-terminal domain of the 644-mer α chain of hFibrinogen. As expected, untreated hFibrinogen was recognized by antibody ab83477 (Fig. 1C). The loss of immunoreactivity that occurred after exposure to the hTryptase-β·heparin complex indicated that cleavage preferentially occurred somewhere in the exposed C-terminal portion of the protein α chain. An ∼7-kDa immunoreactive band that corresponded to the liberated 69-mer peptide from the protein α chain is seen in Fig. 1C. However, its amount varied from experiment to experiment apparently because hTryptase-β also cleaves at least one other site in this peptide, thereby proteolytically destroying the antigen recognized by the antibody. Two immunoreactive bands are seen in the untreated protein (Fig. 1C, lane 1), which presumably are hFibrinogen monomer and dimer.
No protease inhibitor is present in normal human blood, plasma, or serum that rapidly inactivates hTryptase-β (34). In Fig. 1, D and E, hFibrinogen was incubated with trypsin or hTryptase-β·heparin in the presence or absence of normal human serum. The samples were then electrophoresed in the presence of β-mercaptoethanol. As can be seen in these figures, trypsin cannot digest hFibrinogen in the presence of serum due to its diverse array of protease inhibitors that negatively affect this serine protease. In contrast, hFibrinogen remains susceptible to hTryptase-β when the digestion reaction is carried out in the presence of serum because there is no protease inhibitor in blood that rapidly inactivates this MC-restricted protease.
The large-sized fragment(s) of hFibrinogen α chain that formed after a 15-min (Fig. 2) or 1-min (supplemental Fig. S1) incubation with the hTryptase-β·heparin complex was purified by SDS-PAGE and analyzed by MS/MS and C-terminal amino acid sequence analyses. The obtained data revealed that the N terminus of the hFibrinogen α chain was not highly susceptible to the serine protease. In contrast, cleavage preferentially occurred at Lys575, which resides 69 amino acids from the C terminus of the protein α chain. In support of this finding, a ∼7-kDa peptide was occasionally seen in the SDS-PAGE immunoblot analysis of the digests that was recognized by antibody ab83477 (Fig. 1C). MS/MS analysis of this purified peptide revealed that it was indeed derived from the C terminus of the α chain of hFibrinogen (supplemental Figs. S2). Despite this finding, more peptides that correspond to residues 576–625 in the α chain were found in the digests of the hFibrinogen sample that encountered the hTryptase-β·heparin complex for 1 min (supplemental Fig. S1) than for 15 min (Fig. 2). Moreover, most of the immunoreactivity seen in the intact protein was quickly lost after hFibrinogen encountered the hTryptase-β·heparin complex for 15 min or longer (Fig. 1, C and E). The accumulated data indicated that the 69-mer C terminus of the fibrinogen α chain was rapidly cleaved at Lys575 and at least one other site in most instances.
FIGURE 2.
MS/MS and C-terminal amino acid sequence analysis. The prominent fragment of the hFibrinogen α chain that formed after this plasma protein was incubated with a recombinant hTryptase-β·heparin complex for 15 min (75:1 enzyme:substrate ratio) was purified by SDS-PAGE and then subjected to C-terminal amino acid sequence analysis by LC-MS/MS. Shown are the susceptible peptides that were identified in the analyses. The entire fragment was mapped. The 19-mer sequence highlighted in pink corresponds to the protein N-terminal signal peptide of the protein that was not present in the analyzed protein as it is removed in the endoplasmic reticulum before the protein is secreted from its expressing cell. The vertical red line highlights Lys575, which is the preferred site of cleavage by the hTryptase-β·heparin complex.
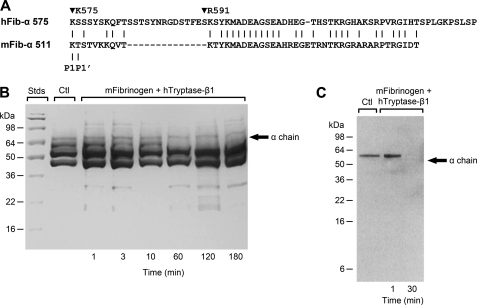
C Terminus of α Chain of mFibrinogen Also Is Highly Susceptible to hTryptase-β· and mMCP-6·Heparin Complexes
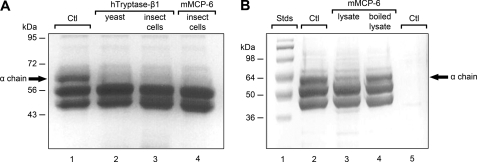
Although Thomas et al. (27) reported that the α chain of hFibrinogen was highly susceptible to an undefined hTryptase-β·heparin complex purified from human lung biopsies in their in vitro study, these investigators concluded that proteolysis preferentially occurred at Arg591. As noted in Fig. 3A, Arg591 resides in a 14-mer sequence that is not present in the α chain of mFibrinogen. It is possible that the human lung preparation used in the Thomas et al. (27) study contained an allelic isoform or splice variant of hTryptase-β that had a novel substrate specificity. However, if proteolysis occurred at this non-conserved amino acid sequence in vivo during a PCA reaction, the α chain of mFibrinogen should not be efficiently cleaved by hTryptase-β or its mouse ortholog mMCP-6 when either enzyme is ionically bound to heparin. Not only was mFibrinogen rapidly cleaved by recombinant hTryptase-β generated in yeast (Fig. 3, B and C), this mouse protein also was rapidly cleaved by recombinant hTryptase-β and mMCP-6 generated in insect cells (Fig. 4A). The recombinant mMCP-6·heparin complex also cleaved fibrinogen in the presence of the protease inhibitors in 0.2% human serum (data not shown).
FIGURE 3.
The α chain of mFibrinogen is susceptible to hTryptase-β·heparin complexes despite the fact that its C terminus is different from that in hFibrinogen. The amino acid sequences of the C termini of the α chains of reference human (GenBankTM accession number NP_068657) and mouse (GenBankTM accession number NP_034326) fibrinogen are shown in A. As noted, the P1-P1′ cleavage site in the α chain of fibrinogen in the two species is nearly identical. mFibrinogen was incubated with a hTryptase-β·heparin complex for 1–180 min, and the resulting digests were subjected to SDS-PAGE. The gel shown in B was stained with Coomassie Blue. As seen in B, the α chain of mFibrinogen is preferentially cleaved by the hTryptase-β·heparin complex. In C, a SDS-PAGE immunoblot was prepared of mFibrinogen that was untreated or had been exposed to an hTryptase-β·heparin complex for 1 or 30 min. The resulting blot was stained with rabbit anti-fibrinogen antibody ab83477. Like what occurred when hFibrinogen encountered the enzyme complex (Fig. 1E), the antigen in the C terminus of mFibrinogen that was recognized by the antibody was quickly lost.
FIGURE 4.
The α chain of mFibrinogen is susceptible to recombinant mMCP-6·heparin glycosaminoglycan complexes and naturally occurring mMCP-6·SGPG complexes. A, mFibrinogen was incubated for 10 min at 37 °C in the absence (lane 1) or presence of recombinant hTryptase-β·heparin glycosaminoglycan (lanes 2 and 3) or mMCP-6·heparin glycosaminoglycan (lane 4) complexes. The recombinant proteases used in this experiment were generated in yeast (lane 2) or insect cells (lanes 3 and 4), and the substrate:enzyme ratio was 30:1 or higher. The α chain of mFibrinogen is indicated by the arrow. Because similar data were obtained if the recombinant tryptase was expressed in insect cells or yeast, the active factor in the preparations was not a contaminant that originated from the expressing cell. B, mFibrinogen (lane 2) was exposed to non-boiled (lane 3) and boiled (lane 4) mBMMC lysate that contained naturally occurring mMCP-6·SGPG complexes. Molecular weight markers are shown in lane 1. Shown in lane 5 is the low amount of protein present in the lysate used in this experiment to document that the substrate:enzyme ratio was >100:1. The loss of most of the α chain of fibrinogen in lane 3 indicates that this plasma protein is also susceptible to naturally occurring mMCP-6.
hTryptase-β and mMCP-6 are packaged in the secretory granules of MCs ionically bound to SGPGs rather than glycosaminoglycans. Moreover, both naturally occurring mammalian enzymes contain complex-type N-linked glycans. Proteins are glycosylated in mammalian cells differently than in insect cells and yeast. Recombinant hTryptase-β·glycosaminoglycan complexes were used in the above experiments that were generated in yeast and insect cells (Fig. 1–3, and supplemental Fig. S1) rather than endogenous hTryptase-β· and mMCP-6·SGPG complexes from MCs. Thus, we next evaluated whether or not the α chain of mFibrinogen also was susceptible to the native mMCP-6·SGPG complexes present in IL-3/IL-33-developed 6+/7− mBMMCs. As noted in Fig. 4B, mFibrinogen α chain was rapidly cleaved by a heat-sensitive neutral protease in these cells, as occurs with a recombinant mMCP-6·heparin complex (Fig. 4A).
The two major proteases in WT B6 mBMMCs are mMCP-6 (4) and the chymase/elastase family member mMCP-5 (35). In support of the Fig. 4B data, lysates of mMCP-5-null B6 mBMMCs also rapidly degraded the α chain of mFibrinogen (data not shown), thereby confirming that the relevant protease in these MCs was mMCP-6.
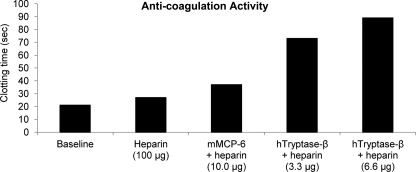
Anticoagulant Activities of mMCP-6· and hTryptase-β·Heparin Complexes and Importance of mMCP-6·Heparin Complexes in Fibrin Deposition in the PCA Reaction of B6 Mice
The findings that the α chains of mouse and human fibrinogen were rapidly cleaved by mMCP-6· and hTryptase-β·heparin complexes in vitro even if the digestion reactions were carried out in the presence of the protease inhibitors in 0.2% normal serum raised the possibility that these MC-restricted granule mediators might effectively hinder thrombin-mediated coagulation of plasma. The ability of thrombin to clot plasma was markedly delayed if the plasma samples were initially exposed to a mouse or human tryptase·heparin complex (Fig. 5). Although the heparin preparation used to create the tryptase complexes in the Fig. 5 experiment had weak anti-coagulant activity, that was done intentionally to demonstrate that the primary anti-coagulant factor in each protease·heparin complex was mMCP-6 or hTryptase-β rather than heparin glycosaminoglycan. In a repeat experiment, the evaluated heparin preparation once again did not cause a significant delay in clotting time even when used at 100 μg/ml. In contrast, pre-exposure of this second plasma sample to a recombinant hTryptase-β·heparin complex (4 μg/ml) resulted in a 1.9-fold increase in clotting time relative to untreated plasma. Based on these data, we concluded that the active factor in these complexes was the tryptase rather than the heparin cofactor as the latter glycosaminoglycan used to create the complexes had relatively poor anticoagulant activity at the dose used.
FIGURE 5.
Anticoagulant activities of recombinant mMCP-6· and hTryptase-β·heparin complexes. In the depicted experiment, sodium citrate-treated human plasma (100 μl/assay) was incubated at 37 °C with heparin alone (100 μg) or with heparin bound to recombinant hTryptase-β (3.3 and 6.6 μg) or mMCP-6 (10 μg) for 60 min. The time required for thrombin to clot the treated samples relative to untreated plasma (base line) was determined using a fibrometer.
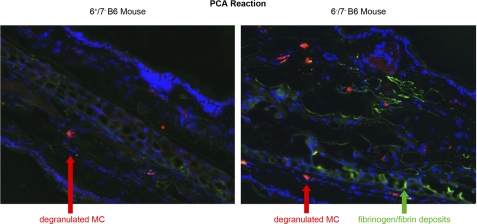
In support of our in vitro data, more fibrin deposits accumulated in the skin of 6−/7− B6 mice after these animals had been subjected to the PCA reaction (Fig. 6, right) than in the skin of similarly treated 6+/7− B6 mice (Fig. 6, left). The presence of these complexes outside of the activated MCs and the presence of edema, which results from exocytosed histamine in these experiments, confirmed that the cutaneous MCs in both animal populations had degranulated. Six hours after the induction of the PCA reaction, the ear of the mMCP-6-null B6 mouse contained noticeably more fibrin than the corresponding ear of the treated WT B6 mouse. Similar data were obtained in another IgE-sensitized 6−/7− B6 mouse that received the same amount of antigen as well as two other mice that received 5-fold less antigen (data not shown).
FIGURE 6.
Fibrin immunohistochemistry after a PCA reaction. Anti-2,4-dinitrophenol-IgE was injected into the ears of a 6+/7− B6 mouse (left panel) and a 6−/7− B6 mouse (right panel). 2,4-Dinitrophenol albumin was then injected into the tail veins of both sensitized animals. Six hours later the animals were sacrificed, and processed for immunohistochemistry using fluorescein-labeled anti-mFibrinogen/fibrin antibody (green; arrow). The tissue sections also were stained with Hoechst 33342, which is a dye that binds the DNA in the nucleus of cells (blue), as well as with Texas Red-labeled avidin (red; arrows), which recognizes heparin to identify MCs and their exocytosed protease·heparin complexes.
DISCUSSION
We previously noted that mFibrinogen is a preferred target of recombinant and naturally occurring mMCP-7 in vitro and in vivo (26). We now demonstrate that mFibrinogen also is a preferred target of mMCP-6 when this MC-restricted tryptase is ionically bound to heparin glycosaminoglycan or SGPG (Fig. 4). We additionally show that mMCP-6 has a prominent role in preventing the internal accumulation of fibrin deposits when the MCs in the skin of the B6 mouse degranulate in the PCA reaction (Fig. 6). The findings that the α chains of mouse (Fig. 3) and human (Figs. 1 and 2 and supplemental Figs. S1 and S2) fibrinogen are similarly cleaved by recombinant hTryptase-β·heparin complexes at neutral pH suggest that the in vivo mouse data are relevant to what occurs in the inflammatory sites of humans when their tissue MCs degranulate in the PCA reaction.
The formation of intravascular fibrin deposits and fibrin·platelet clots can have life-threatening consequences. When the MCs in the skin and other tissue sites degranulate, these immune cells quickly release the contents of their secretory granules, which include histamine and tryptase·heparin SGPG complexes. Histamine is the major vasopermeability factor that induces the edema that occurs at sites where tissue MCs degranulate. This causes an influx of plasma fibrinogen into the inflammatory site.
Fibrinogen is a major constituent of blood where its concentration is 2–4 g/liter. The prominent isoform of fibrinogen in human and mouse blood and plasma is a head-to-head dimer of two monomers that in each instance consists of three peptide chains that are linked by disulfide bonds. Although we now show that mouse and human MC tryptases readily cleave the C terminus of the fibrinogen α chain, no evidence for proteolysis of the N terminus of the chain was found (Fig. 2, supplemental Figs. S1 and S2). Due to the spatial constraints of the two fibrinogen monomers in the circulating dimer unit (36), it is likely that the N terminus of the protein α chain has difficulty entering the central pore of the tryptase tetramer (37). The C terminus of the longer α chain is not covalently bound to the protein β and γ chains via disulfide bonds. It, therefore, is more exposed than the rest of the 3-chained plasma protein. Presumably, this structural feature is the reason why the C terminus of the fibrinogen α chain is more susceptible to mouse and human MC tryptases than the protein β and γ chains.
Factor Xa converts inactive pro-thrombin to active thrombin, and some low molecular weight fragments of heparin from porcine MCs have anti-Factor Xa activity. Once formed, thrombin is negatively regulated by anti-thrombin-III/serpin C1. In the absence of heparin or heparan sulfate, the efficiency of the latter thrombin·serpin C1 interaction is poor. However, serpin C1 undergoes a conformational change that increases its affinity for thrombin >1000-fold when this protease inhibitor encounters a highly sulfated pentasaccharide sequence present in the heparin chains of SGPG and the heparan sulfate chains of syndecan-4 proteoglycan. The MCs in human and mouse skin have SGPGs that contain heparin chains. Fibrin deposits are rare in the edema sites that form when cutaneous MCs degranulate, as noted in our WT B6 mice that have been subjected to the PCA reaction (Fig. 6, left). It, therefore, has been proposed that the relevant anticoagulant factor released from activated MCs is heparin, which hinders the formation of fibrin by preventing the Factor Xa-dependent generation of thrombin and then by catalyzing the serpin C1-dependent inactivation of any thrombin that is able to accumulate in the edema site.
Although MC-derived porcine heparin is used pharmacologically to prevent thrombin-dependent coagulation in vivo and in vitro, it is now believed that the anticoagulant heparan sulfate chains of the endogenous syndecan-4 proteoglycans on the surfaces of endothelial cells are the physiologically relevant glycosaminoglycans (38). In support of this conclusion, no more than a third of the heparin chains attached to the SGPGs in mouse and human MCs are able to activate serpin C1, and many heparin preparations by themselves have poor anticoagulant activity as noted in the preparation we used to create our tryptase·heparin complexes (Fig. 5). In addition, we and others showed that MC-derived heparin is covalently attached to the protease-resistant peptide core of serglycin and that the primary function of the resulting SGPG is to package numerous neutral proteases in the MC secretory granules (1, 2). When the MCs in varied connective tissue sites are activated, their exocytosed protease·heparin SGPG complexes stay intact in extracellular matrices for hours (39). Most of these complexes eventually are endocytosed by macrophages (40) and other nearby cells (41, 42) where they are destroyed in lysosomes. Thus, very little, if any, MC-derived heparin glycosaminoglycan is available in normal circumstances to bind to serpin C1, Factor Xa, or even thrombin when the MCs in tissues degranulate. The low molecular fragments of porcine heparin that are used pharmacologically to prevent blood coagulation also have not been detected in any mouse or human edema site. It, therefore, remains to be determined how MCs physiologically prevent blood coagulation and fibrin deposition in edema tissue sites.
Pejler and Karlström (43) reported that thrombin can be slowly destroyed by the MC chymase family member mMCP-4 in vitro if the reaction was carried out in the absence of plasma or serum. This finding raised the possibly that the chymase-dependent destruction of thrombin might be an alternate way that MCs hinder blood coagulation and fibrin formation in vivo. We previously showed that the MCs in our 6−/7− mice have no defect in mMCP-4 expression (15). The discovery that fibrin deposits accumulate in the skin of 6−/7− B6 mice that have been subjected to the PCA reaction (Fig. 6), therefore, suggests that the mMCP-4-dependent destruction of thrombin is not the primary way MCs prevent coagulation and fibrin formation in edema sites, at least in the B6 mouse during a PCA reaction.
In the alternate MC-dependent anti-coagulation mechanism we uncovered, the cell tetramer-forming tryptases proteolytically destroy fibrinogen before this plasma protein can be converted to fibrin by thrombin. Our data suggest that mMCP-6· and hTryptase-β·heparin complexes initially cleave the exposed C terminus of the fibrinogen α chain (Figs. 1–4, supplemental Figs. S1 and S2) as previously shown for mMCP-7 (26). In support of our in vitro data, a noticeable increase in the amount of fibrin was detected in the skin of our 6−/7− B6 mice 6 h after the induction of a PCA reaction (Fig. 6).
We show that hTryptase-β·heparin complexes preferentially cleave the α chain of hFibrinogen at Lys575 (Fig. 2, supplemental Figs. S1 and S2). Although Thomas et al. (27) reported that an undefined hTryptase-β·heparin complex purified from human lung also cleaves the α chain of hFibrinogen in their in vitro study, these investigators concluded that proteolysis preferentially occurs at Arg591, which resides in a 14-mer sequence not present in the fibrinogens of many species, including the mouse (Fig. 3A). The discovery that human and mouse MC tryptase·heparin complexes rapidly cleave the α chain of mFibrinogen (Figs. 3 and 4) revealed that these complexes must normally degrade fibrinogen at a more conserved site that presumably resides upstream of Arg591. The finding that the liberated peptides were only weakly recognized by anti-fibrinogen antibody ab83477 (Figs. 1 and 3) indicated that hTryptase-β·heparin complexes eventually cleave the 69-mer C-terminal domain of fibrinogen α chain at more than one site.
To explain the SDS-PAGE immunoblot (Fig. 1) and MS/MS C-terminal amino acid sequencing data of the digests (Fig. 2, supplemental Figs. S1 and S2), hTryptase-β·heparin complexes could initially cleave the fibrinogen α chain at Lys575 and then quickly cleave the newly generated 69-mer peptide at multiple sites including Arg591. Alternately, hTryptase-β could progressively nibble the C terminus of the fibrinogen α chain, eventually stopping at Lys575. These possibilities are not mutually exclusive events, and experimental evidence was obtained for both (e.g. see Figs. 2, supplemental Figs. S1 and S2). Recombinant mMCP-6·heparin complexes preferentially cleave phage-display peptides at Lys-Thr sequences (29) like the Lys575-Thr576 sequence in mFibrinogen (Fig. 3). However, if the nibbling scenario is the major way endogenous hTryptase-β· and mMCP-6·heparin complexes attack the α chain of fibrinogen in vivo, it is possible that cleavage stops at Lys575 due to a steric problem in inserting the remaining C-terminal domain of the α chain into the central pore of the tryptase donut-shaped tetramer unit.
The findings that mMCP-6, mMCP-7, and hTryptase-β are more effective anticoagulants than many heparin preparations on a weight basis (Fig. 5) have important clinical implications and change the paradigm as to how mouse and human MCs prevent blood coagulation and fibrin accumulation when these immune cells degranulate. Formation of fibrin deposits and fibrin·platelet clots internally could have devastating consequences in vivo. Thus, our data provide an explanation why no hTryptase-β-null human has been identified (44) and why mammals possess two genes that encode MC-restricted serine proteases that recognize fibrinogen. The findings also account for the apparent strong evolutionary pressure to prevent the expression of circulating protease inhibitors that could efficiently inactivate mouse and human MC tryptases. Our discovery that fibrin deposits do not accumulate in the skin of the 6+/7− B6 mouse when this WT animal is subjected to the PCA reaction (Fig. 6) (in contrast to what occurs in 6−/7− B6 mice (Fig. 6) emphasizes the importance of using transgenic mice that lack both tetramer-forming tryptases in disease models, as we previously noted in one of our arthritis studies (17).
Finally, our data explain why some pediatric mastocytosis patients who have an excess of hTryptase-β+ MCs have excessive bleeding of their skin and gastrointestinal tract (45) and why some women have menstrual-like bleeding shortly after they experience a severe anaphylactic event.5 The finding that mMCP-6, mMCP-7, and hTryptase-β are potent anticoagulants raises the possibility that the next generation of tryptase inhibitors that are more specific than those currently available might be useful in hindering the bleeding abnormalities that sometimes occur in patients with systemic mastocytosis and/or anaphylaxis. The use of the recombinant hTryptase-β·heparin complexes we created (8, 9) also might be a more effective way to prevent blood coagulation in the clinic than by using heparin alone.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants AI065858 and AI059746. This work was also supported by Cancer Prevention and Research Institute of Texas Grant RP110166 and by a research fellowship grant from the Harvard Club of Australia Foundation.

This article contains supplemental Figs. S1 and S2.
As of 28 Dec. 2011, the Human Genome Project identified point mutations in the human TPSAB1 gene (see www.ncbi.nlm.nih.gov) that alter its 275-mer translated product at residues 15, 18, 23, 26, 28, 29, 33, 44, 50–53, 76, 78, 115, 116, 118, 126, 129, 132, 133, 136, 141, 151, 162, 168, 170, 205, 215, 216, 221, 244, and 245. Likewise, mutations in the human TPSB2 gene (see ncbi.nlm.nih.gov) were identified that alter its 275-mer translated product at residues 15, 18, 23, 28, 29, 51, 115, 116, 118, 132, 133, 136, 141, 151, 162, 168, 170, 205, 215, 216, 221, and 245. Due to differential splicing, functionally distinct isoforms of the translated proteins from the human TPSAB1 and TPSB2 genes also have been identified (24). Because of these genomic and mRNA data, it is virtually impossible to interpret functional data obtained using hTryptase-β preparations purified from any human tissue, especially if pool tissue from more than one individual was used in the study.
M. Castells, unpublished data.
- MC
- mast cell
- SGPG
- serglycin proteoglycan
- 6+/7+
- mMCP-6+/mMCP-7+
- B6
- C57BL/6
- mBMMC
- mouse bone marrow-derived MCs
- mMCP
- mouse MC protease
- PCA
- passive cutaneous anaphylaxis.
REFERENCES
- 1. Humphries D. E., Wong G. W., Friend D. S., Gurish M. F., Qiu W. T., Huang C., Sharpe A. H., Stevens R. L. (1999) Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400, 769–772 [DOI] [PubMed] [Google Scholar]
- 2. Forsberg E., Pejler G., Ringvall M., Lunderius C., Tomasini-Johansson B., Kusche-Gullberg M., Eriksson I., Ledin J., Hellman L., Kjellén L. (1999) Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400, 773–776 [DOI] [PubMed] [Google Scholar]
- 3. Reynolds D. S., Stevens R. L., Lane W. S., Carr M. H., Austen K. F., Serafin W. E. (1990) Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc. Natl. Acad. Sci. U.S.A. 87, 3230–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds D. S., Gurley D. S., Austen K. F., Serafin W. E. (1991) Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J. Biol. Chem. 266, 3847–3853 [PubMed] [Google Scholar]
- 5. McNeil H. P., Reynolds D. S., Schiller V., Ghildyal N., Gurley D. S., Austen K. F., Stevens R. L. (1992) Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc. Natl. Acad. Sci. U.S.A. 89, 11174–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong G. W., Yasuda S., Morokawa N., Li L., Stevens R. L. (2004) Mouse chromosome 17A3.3 contains 13 genes that encode functional tryptic-like serine proteases with distinct tissue and cell expression patterns. J. Biol. Chem. 279, 2438–2452 [DOI] [PubMed] [Google Scholar]
- 7. Harris J. L., Niles A., Burdick K., Maffitt M., Backes B. J., Ellman J. A., Kuntz I., Haak-Frendscho M., Craik C. S. (2001) Definition of the extended substrate specificity determinants for β-tryptases I and II. J. Biol. Chem. 276, 34941–34947 [DOI] [PubMed] [Google Scholar]
- 8. Huang C., Li L., Krilis S. A., Chanasyk K., Tang Y., Li Z., Hunt J. E., Stevens R. L. (1999) Human tryptases α and β/II are functionally distinct due in part to a single amino acid difference in one of the surface loops that forms the substrate binding cleft. J. Biol. Chem. 274, 19670–19676 [DOI] [PubMed] [Google Scholar]
- 9. Huang C., De Sanctis G. T., O'Brien P. J., Mizgerd J. P., Friend D. S., Drazen J. M., Brass L. F., Stevens R. L. (2001) Evaluation of the substrate specificity of human mast cell tryptase β I and demonstration of its importance in bacterial infections of the lung. J. Biol. Chem. 276, 26276–26284 [DOI] [PubMed] [Google Scholar]
- 10. Vanderslice P., Ballinger S. M., Tam E. K., Goldstein S. M., Craik C. S., Caughey G. H. (1990) Human mast cell tryptase. Multiple cDNAs and genes reveal a multigene serine protease family. Proc. Natl. Acad. Sci. U.S.A. 87, 3811–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller J. S., Moxley G., Schwartz L. B. (1990) Cloning and characterization of a second complementary DNA for human tryptase. J. Clin. Invest. 86, 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz L. B., Lewis R. A., Austen K. F. (1981) Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 256, 11939–11943 [PubMed] [Google Scholar]
- 13. Ghildyal N., Friend D. S., Freelund R., Austen K. F., McNeil H. P., Schiller V., Stevens R. L. (1994) Lack of expression of the tryptase mouse mast cell protease 7 in mast cells of the C57BL/6J mouse. J. Immunol. 153, 2624–2630 [PubMed] [Google Scholar]
- 14. Hunt J. E., Stevens R. L., Austen K. F., Zhang J., Xia Z., Ghildyal N. (1996) Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J. Biol. Chem. 271, 2851–2855 [DOI] [PubMed] [Google Scholar]
- 15. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 16. Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., Lee D. M. (2008) Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNeil H. P., Shin K., Campbell I. K., Wicks I. P., Adachi R., Lee D. M., Stevens R. L. (2008) The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 58, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 18. Shin K., Nigrovic P. A., Crish J., Boilard E., McNeil H. P., Larabee K. S., Adachi R., Gurish M. F., Gobezie R., Stevens R. L., Lee D. M. (2009) Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 182, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tremaine W. J., Brzezinski A., Katz J. A., Wolf D. C., Fleming T. J., Mordenti J., Strenkoski-Nix L. C., Kurth M. C. (2002) Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059). An open-label pilot study. Aliment. Pharmacol. Ther. 16, 407–413 [DOI] [PubMed] [Google Scholar]
- 20. Isozaki Y., Yoshida N., Kuroda M., Handa O., Takagi T., Kokura S., Ichikawa H., Naito Y., Okanoue T., Yoshikawa T. (2006) Anti-tryptase treatment using nafamostat mesilate has a therapeutic effect on experimental colitis. Scand. J. Gastroenterol. 41, 944–953 [DOI] [PubMed] [Google Scholar]
- 21. Hamilton M. J., Sinnamon M. J., Lyng G. D., Glickman J. N., Wang X., Xing W., Krilis S. A., Blumberg R. S., Adachi R., Lee D. M., Stevens R. L. (2011) Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. U.S.A. 108, 290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J., Sun J., Lindholt J. S., Sukhova G. K., Sinnamon M., Stevens R. L., Adachi R., Libby P., Thompson R. W., Shi G. P. (2011) Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ. Res. 108, 1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh S. W., Pae C. I., Lee D. K., Jones F., Chiang G. K., Kim H. O., Moon S. H., Cao B., Ogbu C., Jeong K. W., Kozu G., Nakanishi H., Kahn M., Chi E. Y., Henderson W. R., Jr. (2002) Tryptase inhibition blocks airway inflammation in a mouse asthma model. J. Immunol. 168, 1992–2000 [DOI] [PubMed] [Google Scholar]
- 24. Jackson N. E., Wang H. W., Bryant K. J., McNeil H. P., Husain A., Liu K., Tedla N., Thomas P. S., King G. C., Hettiaratchi A., Cairns J., Hunt J. E. (2008) Alternate mRNA splicing in multiple human tryptase genes is predicted to regulate tetramer formation. J. Biol. Chem. 283, 34178–34187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samoszuk M., Corwin M. A. (2003) Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int. J. Cancer 107, 159–163 [DOI] [PubMed] [Google Scholar]
- 26. Huang C., Wong G. W., Ghildyal N., Gurish M. F., Sali A., Matsumoto R., Qiu W. T., Stevens R. L. (1997) The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J. Biol. Chem. 272, 31885–31893 [DOI] [PubMed] [Google Scholar]
- 27. Thomas V. A., Wheeless C. J., Stack M. S., Johnson D. A. (1998) Human mast cell tryptase fibrinogenolysis. Kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry 37, 2291–2298 [DOI] [PubMed] [Google Scholar]
- 28. Schwartz L. B., Bradford T. R., Littman B. H., Wintroub B. U. (1985) The fibrinogenolytic activity of purified tryptase from human lung mast cells. J. Immunol. 135, 2762–2767 [PubMed] [Google Scholar]
- 29. Huang C., Friend D. S., Qiu W. T., Wong G. W., Morales G., Hunt J., Stevens R. L. (1998) Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 160, 1910–1919 [PubMed] [Google Scholar]
- 30. Niles A. L., Maffitt M., Haak-Frendscho M., Wheeless C. J., Johnson D. A. (1998) Recombinant human mast cell tryptase β. Stable expression in Pichia pastoris and purification of fully active enzyme. Biotechnol. Appl. Biochem. 28, 125–131 [PubMed] [Google Scholar]
- 31. Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. (1984) Interleukin 3. A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J. Immunol. 132, 1479–1486 [PubMed] [Google Scholar]
- 32. Kaieda S., Shin K., Nigrovic P. A., Seki K., Lee R. T., Stevens R. L., Lee D. M. (2010) Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1). J. Biol. Chem. 285, 21478–21486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Svendsen L., Blombäck B., Blombäck M., Olsson P. I. (1972) Substrates for determination of trypsin, thrombin, and thrombin-like enzymes. Folia Haematol. Int. Mag. Klin. Morphol. Blutforsch. 98, 446–454 [PubMed] [Google Scholar]
- 34. Alter S. C., Kramps J. A., Janoff A., Schwartz L. B. (1990) Interactions of human mast cell tryptase with biological protease inhibitors. Arch. Biochem. Biophys. 276, 26–31 [DOI] [PubMed] [Google Scholar]
- 35. McNeil H. P., Austen K. F., Somerville L. L., Gurish M. F., Stevens R. L. (1991) Molecular cloning of the mouse mast cell protease-5 gene. A novel secretory granule protease expressed early in the differentiation of serosal mast cells. J. Biol. Chem. 266, 20316–20322 [PubMed] [Google Scholar]
- 36. Kollman J. M., Pandi L., Sawaya M. R., Riley M., Doolittle R. F. (2009) Crystal structure of human fibrinogen. Biochemistry 48, 3877–3886 [DOI] [PubMed] [Google Scholar]
- 37. Pereira P. J., Bergner A., Macedo-Ribeiro S., Huber R., Matschiner G., Fritz H., Sommerhoff C. P., Bode W. (1998) Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature 392, 306–311 [DOI] [PubMed] [Google Scholar]
- 38. Shworak N. W., Kojima T., Rosenberg R. D. (1993) Isolation and characterization of ryudocan and syndecan heparan sulfate proteoglycans, core proteins, and cDNAs from a rat endothelial cell line. Haemostasis 23, 161–176 [DOI] [PubMed] [Google Scholar]
- 39. Ghildyal N., Friend D. S., Stevens R. L., Austen K. F., Huang C., Penrose J. F., Sali A., Gurish M. F. (1996) Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis. Prolonged retention of exocytosed mMCP-6 in connective tissues and rapid accumulation of enzymatically active mMCP-7 in the blood. J. Exp. Med. 184, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fabian I., Bleiberg I., Aronson M. (1978) Increased uptake and desulphation of heparin by mouse macrophages in the presence of polycations. Biochim. Biophys. Acta 544, 69–76 [DOI] [PubMed] [Google Scholar]
- 41. Atkins F. M., Metcalfe D. D. (1983) Degradation of the heparin matrix of mast cell granules by cultured fibroblasts. J. Immunol. 131, 1420–1425 [PubMed] [Google Scholar]
- 42. Atkins F. M., Friedman M. M., Metcalfe D. D. (1985) Biochemical and microscopic evidence for the internalization and degradation of heparin-containing mast cell granules by bovine endothelial cells. Lab. Invest. 52, 278–286 [PubMed] [Google Scholar]
- 43. Pejler G., Karlström A. (1993) Thrombin is inactivated by mast cell secretory granule chymase. J. Biol. Chem. 268, 11817–11822 [PubMed] [Google Scholar]
- 44. Trivedi N. N., Tamraz B., Chu C., Kwok P. Y., Caughey G. H. (2009) Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J. Allergy Clin. Immunol. 124, 1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kettelhut B. V., Metcalfe D. D. (1991) Pediatric mastocytosis J. Invest. Dermatol. 96, Supplemental 15S–18S [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.