Abstract
Ability in various cognitive domains is often assessed by measuring task performance, such as the accuracy of a perceptual categorization. A similar analysis can be applied to metacognitive reports about a task to quantify the degree to which an individual is aware of his or her success or failure. Here, we review the psychological and neural underpinnings of metacognitive accuracy, drawing on research in memory and decision-making. These data show that metacognitive accuracy is dissociable from task performance and varies across individuals. Convergent evidence indicates that the function of the rostral and dorsal aspect of the lateral prefrontal cortex (PFC) is important for the accuracy of retrospective judgements of performance. In contrast, prospective judgements of performance may depend upon medial PFC. We close with a discussion of how metacognitive processes relate to concepts of cognitive control, and propose a neural synthesis in which dorsolateral and anterior prefrontal cortical subregions interact with interoceptive cortices (cingulate and insula) to promote accurate judgements of performance.
Keywords: metacognition, confidence, conflict, prefrontal cortex, functional magnetic resonance imaging, individual differences
I am not yet able, as the Delphic inscription has it, to know myself, so it seems to me ridiculous, when I do not yet know that, to investigate irrelevant things.
Plato's Phaedrus, 229E
1. Introduction
The notion that accurate self-knowledge has value, and is something to strive for, has preoccupied thinkers since Socrates. But, as the quotation from Plato illustrates, self-knowledge is not always (or even often) evident, and at best tends to be a noisy and inaccurate impression of one's mental milieu [1]. Empirical work in the psychological sciences has thrown up counterintuitive examples of self-knowledge being confabulated, dissociated from reality or otherwise inaccurate [2,3]. To take one striking case, when decisions about facial attractiveness or supermarket goods are surreptitiously reversed, subjects are often unaware of these reversals, and go on to confabulate explanations of why they chose options they had in fact rejected [4,5]. Furthermore, self-assessments of personality and cognitive biases tend to be poorer than similar assessments applied to others, leading to an ‘introspection illusion’ [6]. Such subjective inaccuracy perhaps accounts for the demise of an introspectionist method in the late nineteenth century: if verbal reports vary from setting to setting, and can be contradicted from trial to trial, then what hope is there for an objective science of the subjective? [7].
The very notion that an individual can turn his or her mental faculties inward was considered logically incoherent by Comte, who thought it paradoxical that the mind might divide into two to permit self-observation [8]. We now understand the brain as a network of regions working in concert, and thus, it is perhaps unsurprising that one set of regions (such as the prefrontal cortex: PFC) might process, hierarchically, information arising from lower levels (such as primary sensory regions). Indeed, several recent models of local and large-scale brain function rely on hierarchy as a principal organizing factor [9,10]. That self-knowledge, and its accuracy, is under neural control is supported by mounting evidence in the neuropsychological literature, some of which will be reviewed later in this article. For example, in cases of traumatic injury to the frontal lobes, individuals may have deficits in self-knowledge of altered cognition and personality, as measured by the discrepancy between reports from the patient and family members [11]. Such studies have focused on alterations in self-related, or autonoetic, metacognition [12], but analogous discrepancies can be measured in assessments of task performance in healthy individuals.
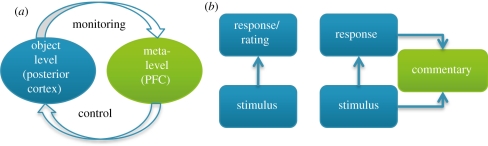
By focusing on self-reports about memory performance—metacognitive reports—Flavell provided a systematic framework for the study of self-knowledge in healthy individuals [13]. Here, the metacognitive report is treated as an object of study in its own right, and the accuracy of such reports (as dissociated from accuracy, or performance, on the task itself) provide an empirical scaffold upon which to build studies of self-knowledge [14,15]. An influential model of metacognition was developed to account for behavioural dissociations between the ‘object’ level—cognition, or, more correctly, task performance—and the ‘meta’ level, conceptualized as both monitoring and controlling the object level (figure 1; [17]). This approach shares similarities with an influential model of executive function [18]. The two-level framework has been extended to study monitoring of perception [19,20], decision-making [21,22], sense of agency [23] and learning [24]. To the extent that the meta level imperfectly monitors the object level, self-reports about cognition will be inaccurate, perhaps manifesting as a lack of awareness of the object level [25].
Figure 1.
(a) A schematic adapted from Shimamura [16] showing how the levels of Nelson and Narens' cognitive psychology model of metacognition can be naturally mapped onto a hierarchical brain structure. (b) The left panel shows a first-order process, such as a simple visual discrimination, that may occur in the absence of metacognitive report. The right panel shows the same discrimination, this time with the information available for a second-order commentary about the decision.
Despite progress in the definition and measurement of metacognition, the psychological and neural underpinnings of metacognitive accuracy remain ill understood [16,26]. In this paper, we review different approaches to eliciting metacognitive reports and quantifying their accuracy, and consider psychological and computational explanations for dissociations between metacognitive accuracy and task performance. We go on to consider recent studies that apply convergent neuroscience methodologies—functional and structural magnetic resonance imaging (MRI), transcranial magnetic stimulation (TMS) and neuropsychological approaches—to reveal cortical substrates mediating differences in metacognitive accuracy both between and within individuals. We end with a discussion of how metacognitive processes relate to neuroscientific notions of cognitive control, and propose a synthesis wherein dorsolateral and anterior prefrontal cortical subregions interact with interoceptive cortices (cingulate and insula) to promote metacognitive accuracy.
2. Measurement of metacognition
There are several flavours of metacognitive report, but all share the elicitation of subjective beliefs about cognition—how much do I know (viz. what can I report) about ongoing task performance? In this section, we review the behavioural methods available to the researcher interested in metacognition, focusing primarily on measures employed in the cognitive neuroscience studies that are discussed in subsequent sections.
A first distinction is that judgements can either be prospective, occurring prior to performance of a task, or retrospective, occurring after task completion (table 1). In metamemory research, prospective judgements include feelings of knowing (FOK) and judgements of learning (JOL). A JOL elicits a belief during learning about how successful recall will be for a particular item on subsequent testing [27]. In contrast, an FOK is a judgement about a different aspect of memory, namely that of knowing the answer to a particular question despite being unable to explicitly recall it [28]. FOKs are usually studied by first asking the participants to recall answers to general knowledge questions, and, for answers they cannot recall, to predict whether they might be able to recognize the answer from a list of alternatives. Related to FOKs are tip-of-the-tongue states, in which an item cannot be recalled despite a feeling that retrieval is possible [29].
Table 1.
Summary of metacognitive measures classified by domain and time of elicitation. We note that a more general class of prospective judgements is also possible that refers to cognitive abilities not tied to a particular task.
| timing | object-level domain |
||
|---|---|---|---|
| memory | decision-making | sensory | |
| prospective | judgement of learning; feeling of knowing | performance estimate | n.a. |
| retrospective | confidence | confidence, wager | visibility rating, confidence |
Retrospective reports can be similarly elicited by asking the subject to give an additional report or commentary over and above their initial forced-choice response. For example, Peirce & Jastrow [30] asked observers to rate their degree of confidence in a perceptual judgement using the following scale:
0 denoted absence of any preference for one answer over its opposite, so that it seemed nonsensical to answer at all. ‘1’ denoted a distinct leaning to one alternative. ‘2’ denoted some little confidence of being right. ‘3’ denoted as strong a confidence as one would have about such sensations.
Since this seminal work, asking for confidence-in-accuracy has become a standard tool for eliciting judgements of performance in a variety of settings [24,31]. One potential problem with eliciting subjective confidence is that of reliability: why should the subject be motivated to reveal his or her true confidence, when there is little incentive to do so [32]? In addition, the necessarily subjective instructions given when eliciting reports of confidence preclude the use of these measures in non-human animal species. To address these concerns, Kunimoto and colleagues introduced wagers contingent on the correctness of the decision as an intuitive measure of retrospective confidence [33,34]. In the simplest form of post-decision wagering (PDW), a participant is asked to gamble on whether their response was correct. If the decision is correct, the wager amount is kept; if it is incorrect, the amount is lost. The size of the chosen gamble is assumed to reflect a subject's confidence in his or her decision. In the same spirit as PDW, the Lottery Rule aims to elicit true underlying decision confidence [35], and is similar to the Becker–DeGroot–Marschak procedure used to elicit item values in behavioural economics [36].
Once a metacognitive judgement is elicited, how might we assess its accuracy? Again, several, often complementary, methods are available. Metacognitive accuracy is defined by how closely metacognitive judgements track ongoing task performance. Crucially, therefore, all measures require that an independent measure of the object level—task performance—is acquired, in order to quantify the relationship between the meta and object levels (figure 1). For example, after asking for an FOK judgement, we might assess whether the proportion of times a participant is indeed able to recognize the correct, but hitherto unrecalled, item from a list of alternatives. Then, by plotting the strength of the JOL or FOK against objective memory performance (actual recall success for JOLs, and recognition performance for FOKs), a measure of metacognitive accuracy can be derived from the associated correlation score [15]. Similar confidence-accuracy correlations can be computed for retrospective confidence judgements. If the metacognitive report bears some relation to task performance, then these correlation coefficients will be significantly non-zero [37].
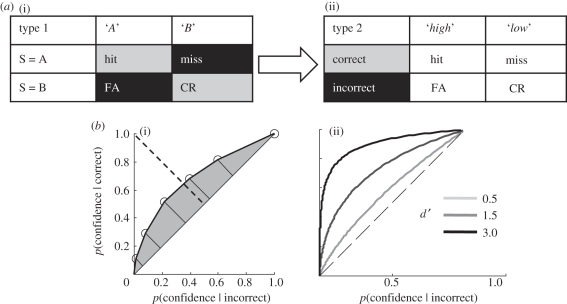
A related approach quantifies the accuracy of metacognitive assessments using the logic of signal detection theory (SDT), which assesses how faithfully an organism separates signal from noise [38,39]. In standard applications of SDT (type 1), sensitivity is defined by how well an observer can discriminate an objective state of the world (e.g. the presence or absence of a stimulus; figure 2a). By applying similar logic to metacognitive reports, the objective state of the world becomes the subject's trial-by-trial task performance (correct or incorrect; figure 2a) and the subjective report is now a judgement of that performance [40,41]. An advantage of the SDT approach is that it dissociates bias from sensitivity: in other words, measures of metacognitive accuracy are relatively unaffected by an observer's overall tendency to use higher or lower confidence ratings (figure 2b; although see [42,43]). Further, it naturally connects a process-level characterization of the relationship between the object (type 1) and meta level (type 2) to measures of behaviour, and this relationship can be taken into account to provide an unbiased measure of metacognitive accuracy [44]. This generative aspect of SDT will be discussed further in a following section.
Figure 2.
(a) Contingency tables for (i) type 1 SDT, and (ii) type 2 SDT. Rows correspond to objective states of the world; columns correspond to subjects' reports about the world; FA, false alarm; CR, correct rejection. In the type 2 table, ‘high’ and ‘low’ refer to decision confidence. The linking arrow and colour scheme indicates that ‘correct’ and ‘incorrect’ states of the world for the type 2 analysis are derived from averaging particular type 1 outcomes. (b) (i) Example of a type 2 receiver operating characteristic (ROC) function for a single subject in a perceptual decision task where performance is held constant using a staircase procedure. The shaded area indicates the strength of the relationship between performance and confidence. (ii) Theoretical type 2 ROC functions for different levels of type 1 d′ (assuming neutral type 1 response criteria) demonstrating that metacognitive accuracy is predicted to increase as task performance increases.
Before closing our discussion on measures of metacognition, we note that a separate line of research has assessed the extent to which humans and other species use, or represent, uncertainty about the consequences of their actions to optimize decision-making (see [45,46] for reviews). To highlight one example, Barthelme & Mamassian showed that when human observers are allowed to choose between pairs of visual stimuli upon which to carry out a task, they systematically chose the less uncertain, thus improving their performance [47]. Related work has demonstrated that subjects use knowledge of uncertainty to optimally bias decision-making in perceptual [48,49] and motor [50] tasks, and that species as diverse as dolphins, pigeons and monkeys can use an ‘opt-out’ response to improve their reward rate when decisions are uncertain [51]. Recent single-neuron recording studies have begun to outline candidate mechanisms for a representation of uncertainty in the decision system [52,53]. However, and crucially for the purposes of the present paper, use-of-uncertainty measures do not dissociate metacognition from task performance on a trial-by-trial basis, and thus cannot be used to study mechanisms underlying beliefs about performance. For example, on each trial of the ‘opt-out’ paradigm, the animal either chooses to complete the task, or opt-out. On trials where the animal opts-out (uses a ‘metacognitive’ response), we are unable to measure performance, as no task is completed. On trials where the animal does not opt-out, performance measures are all we have. Thus, measures of metacognitive accuracy cannot be computed based on pairwise correlations between the two response types [54].
3. Psychological determinants of metacognitive accuracy
In healthy individuals, metacognitive judgements are usually predictive of subsequent or past task performance [55]. What, then, underlies this ability to know that we know? On a direct-access view, metamemorial judgements are based upon a survey of memory contents, and thus draw upon the same information as a subsequent recognition or recall phase [28]. In contrast, inferential accounts suggest that JOL, FOK and confidence judgements draw upon various mnemonic cues that may only be partially related to the target [56] (see [57] for a review). Such cues include the fluency or ease with which information is processed [58,59], the accessibility or relatedness of cue information to the target [60] and, for retrospective confidence judgements, the speed of a previous decision [17,61]. Because the available cues may only be indirectly related to the target, inferential accounts naturally accommodate dissociations between memory performance and metacognitive accuracy; in contrast, direct-access accounts predict a tight relationship between subjective and objective indices of knowledge.
A complementary perspective on the antecedents of metacognitive reports is provided by type 2 SDT. Consider a perceptual decision task where post-decision wagers are elicited to tap knowledge of task performance. Optimal wagering behaviour requires computing the conditional probability of being correct given a previous choice [p(correct|choice)] to decide whether to wager high or low. There are various proposals as to how this might be achieved [43,62]. In an echo of direct-access accounts of metamemory discussed above, most involve tracking the strength of the underlying evidence entering into the choice. Galvin and colleagues [41] showed that the conditional probability of being correct or incorrect for a given decision signal is a simple linear transformation of type 1 probability distributions. Similarly, in a dynamic situation, Vickers [31] proposed that decision confidence could be derived from the absolute distance between the winning and losing integrators in an evidence accumulation framework (see also [52]). Confidence, therefore, is equated with the difficulty of the decision in these approaches [63,64]. Two corollaries arise from this ‘direct translation hypothesis’ [65]. First, given that confidence is equated with choice probability (as derived from information governing choice), direct-translation approaches cannot accommodate dissociations between the object and meta level. Second, if both performance and metacognitive judgements draw upon the same information, metacognitive accuracy or the ability to discriminate correct from incorrect decisions, always increases as task performance itself increases. Importantly, both these hypotheses have been empirically falsified: for the same level of task performance, judgement confidence may differ considerably between conditions [66–68], and, when performance is held constant using a staircase procedure, metacognitive accuracy varies across individuals [21], and can be dissociated from performance through pharmacological [69], neural [20] and task-based [70] manipulations (figure 3).
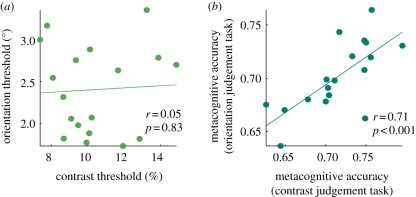
Figure 3.
Data from a visual decision task demonstrating a dissociation of metacognitive accuracy from task performance. Subjects made a visual decision (either an orientation or contrast judgement) and then provided a retrospective confidence rating. A measure of metacognitive accuracy was derived from these ratings by calculating the area under the type 2 ROC function. Performance on the orientation judgement task did not predict task performance on the contrast judgement task (a). However, metacognitive accuracy was strongly correlated between tasks (b), suggesting that it is both independent of task performance and stable within individuals. Reproduced with permission from Song et al. [70].
Empirical dissociations between first-order and second-order components of decision-making have prompted a search for models that can accommodate such findings [71]. Recent models have been couched in an ‘evidence accumulation’ framework, in which samples of data are accumulated over time in order to model the temporal evolution of a decision [19,72,73]. Del Cul et al. [19] proposed a dual-route evidence accumulation framework in which evidence for behaviour (a forced-choice report of stimulus identity) and evidence for subjective report (visibility) were accumulated separately. The fit of this model could account for the observed decoupling of subjective reports from performance in patients with damage to the PFC (see the study of Maniscalco & Lau [74] for an alternative account). In a related approach, Pleskac & Busemeyer [72] devised an evidence accumulation scheme that could account for a wide range of empirical regularities governing the relationship between choice and confidence ratings. The solution here was to allow accumulation to continue beyond the time at which the first-order decision is made. The same noisy accumulator is then accessed to form the confidence judgement at a later timepoint. Interestingly, this model makes strong predictions about post-decision neural activity in the parietal and frontal cortices previously associated with pre-decision evidence accumulation [75], and recent developments of PDW methods in non-human primates may allow this and related hypotheses to be tested [76].
Despite being dissociable, metacognitive accuracy does generally scale with task performance [33,77–80]. Note that this regularity differs conceptually from the fact that trial-by-trial judgements of confidence tend to correlate with performance; such scaling is, after all, what measures of metacognitive accuracy attempt to capture. Instead, it is the fact that, between sessions, or individuals, metacognitive accuracy itself covaries with performance on the task (figure 2b). A tied relationship between performance and metacognition presents a particular problem for studies of the neural correlates of metacognitive ability: how are we to disentangle brain systems involved in metacognition from those involved in performing the task itself (cf. [81])? In the following section, we keep this confound of performance in mind, and consider the extent to which it is addressed by studies of the neural basis of metacognitive accuracy.
4. Neural basis of metacognitive accuracy
(a). Studies of metamemory
Initial evidence regarding the neural basis of metacognition was obtained from neuropsychological cases [82]. Hirst and colleagues suggested that metamemory might be impaired in patients with Korsakoff's syndrome, a neurological disorder characterized by severe anterograde amnesia that occurs as a result of chronic alcohol abuse and nutritional deficiency [83]. Structural brain changes in Korsakoff's include increases in cerebrospinal fluid and severe volume loss in the orbitofrontal cortices and thalamus [84]. Shimamura & Squire [85] found that Korsakoff's patients have a selective impairment in the accuracy of FOK judgements compared with an amnesic control group, despite being equated on recognition memory performance. These findings suggested that metamemory impairment is due to damage in brain regions other than medial temporal lobe and diencephalic midline structures associated with amnesia. In line with this hypothesis, subsequent studies found that non-amnesic patients with frontal lobe damage also exhibit poor metamemory accuracy (e.g. [86]; see [87] for a review).
While implicating frontal lobe structures in metacognitive accuracy, these early studies lacked anatomical specificity. Using lesion overlap measurements, Schnyer and colleagues found that damage to the right ventromedial prefrontal cortex (VMPFC) was associated with decreased FOK accuracy but intact confidence judgements, suggesting a possible dissociation between brain systems supporting different classes of metamemorial judgements [88] (table 1). Patients in Schnyer et al.'s study also showed deficits in memory performance, but impairment in FOK accuracy could not be explained by these changes in performance alone. In support of a selective role for medial PFC in FOK judgements, patients with lesion overlap in the dorsal anterior cingulate cortex (ACC) who were matched in recognition performance to a control group showed a selective FOK deficit, despite intact confidence judgements [79]. The reverse dissociation was reported by Pannu et al. [89], who found that deficits in retrospective confidence judgements were predominantly associated with lateral frontal lesions. As we discuss below, together this evidence suggests that prospective judgements are supported by medial PFC function, whereas retrospective judgements depend on lateral PFC.
Complementary functional brain imaging studies have shown that regions in the medial and lateral PFC are active during metamemorial judgements, with activity in PFC modulated by both prospective and retrospective confidence judgements [90–94]. VMPFC (peak Montreal Neurological Institute coordinate: −3, 30, −18) showed greater activity during accurate FOK judgements, and increased connectivity with medial temporal lobe memory structures in the FOK condition compared with a low-level control task [95]. Complementing this work, individual differences in metacognitive accuracy for prospective JOLs correlated with VMPFC activity (peak: −11, 42, −26) on accurate, but not inaccurate, prediction trials [78]; these differences were not explained by individual differences in memory performance.
(b). Retrospective confidence judgements in psychophysics
Other studies have begun to harness the methods of psychophysics to tightly clamp or adjust for differences in performance while simultaneously studying metacognition and its neural substrates (figure 4).
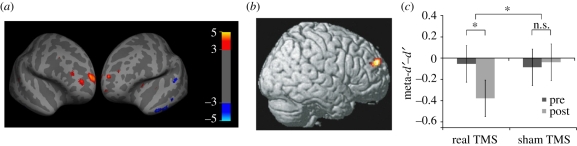
Figure 4.
Convergent evidence for a role of rostrolateral PFC in metacognitive accuracy. (a) Across individuals, grey matter volume in rlPFC was found to positively correlate (hot colours) with metacognitive accuracy (type 2 ROC area) after controlling for differences in task performance [21]. (b) In a complementary study, BOLD signal in right posterior-lateral BA10 was positively correlated with metacognitive accuracy (gamma) but not differences in task performance [96]. (c) The necessity of lateral PFC for metacognitive accuracy was confirmed by combining TMS with SDT: following repetitive TMS to bilateral dlPFC, subjects exhibited reduced meta-d′ (the type 2 d′ expected from a given level of type 1 sensitivity) despite intact task performance [20]. Panels reproduced with permission from [21,96,20].
As an example of this approach, Lau and Passingham matched performance between two visual masking conditions, but found differences in threshold for metacognitive commentaries about the stimulus (‘seen’ responses) that were associated with activity in left dorsolateral PFC [67] (dlPFC; peak: −46, 48, 14). Confirming a causal role for PFC in subjective report threshold, patients with lesions to rostrolateral prefrontal cortex (rlPFC, BA10) have an increased threshold for producing metacognitive commentaries about a stimulus compared with controls, despite objective performance being matched between groups [19]. The peak correlation between lesion and decrease in subjective report threshold was seen in left BA10 (peak: −32, 54, −6).
Taking an individual differences approach, Fleming et al. [21] constrained perceptual decision performance to be near-threshold (71%) through use of a staircase procedure, while collecting retrospective confidence ratings. Considerable variation in metacognitive accuracy (using type 2 SDT analysis) was found despite task performance remaining constant across individuals. Through use of structural brain imaging, this variance in metacognitive accuracy was shown to positively correlate with grey matter volume in right rlPFC (BA10; peak: 24, 65, 18; figure 4a), and greater metacognitive accuracy was associated with increased white matter integrity (fractional anisotropy) in a region of the corpus callosum known to project to the rlPFC [97]. Such findings are consistent with individual differences in localized brain structure affecting a region's functional properties [98]. In a complementary study using functional MRI, subjects performed a visual working-memory test and provided retrospective confidence ratings. Metacognitive accuracy as determined by the gamma statistic correlated with the level of activity in right posterior-lateral BA10 [96] (peak: 16, 56, 28), despite being uncorrelated with task performance (figure 4b).
While correlational analyses can reveal candidate brain regions mediating metacognitive accuracy, confirmation of their necessity ultimately requires intervention studies. By applying repetitive TMS to temporarily inactivate bilateral dlPFC, Rounis et al. [20] selectively decreased metacognitive accuracy while leaving performance on a perceptual task unaffected. Further, by explicitly modelling the link between type 1 and type 2 responses [44], they were able to show that dlPFC TMS decreased metacognitive accuracy below that expected from a direct-translation account alone (figure 4c). Taken together, these studies provide convergent evidence that rostrolateral aspects of PFC (BA10/46) play a mediating role in the accuracy of retrospective commentaries.
A role for rlPFC in metacognition is consistent with its anatomical position at the top of the cognitive hierarchy, receiving information from other prefrontal cortical regions, cingulate and anterior temporal cortex [99]. Further, compared with non-human primates, rlPFC has a sparser spatial organization that may support greater interconnectivity [100]. The contribution of rlPFC to metacognitive commentary may be to represent task uncertainty in a format suitable for communication to others, consistent with activation here being associated with evaluating self-generated information [101,102], and attention to internal representations [103]. Such a conclusion is supported by recent evidence from structural brain imaging that ‘reality monitoring’ and metacognitive accuracy share a common neural substrate in anterior PFC [104]. In contrast, dlPFC may maintain information about a previous decision, consistent with its role in working memory [105,106]. However, in comparison with, for example, parietal cortex [107], reliable cytoarchitectonic boundaries are not yet established for human rlPFC [108]. Indeed, activations ascribed to either lateral rlPFC or dlPFC in this review cluster around a transition zone between BA10 and BA46 [96,109]; thus, it is unclear whether they arise from a single functional region, or multiple subregions subserving different functions. Single-subject analyses [110] may aid in solving this puzzle.
(c). Nature of individual differences
Harnessing individual differences can provide leverage on the neural correlates of metacognitive accuracy [21,78,96]. Such studies implicitly assume intrapersonal stability of metacognitive capacity. However, in the metamemory literature, evidence for a stable metacognitive ability is surprisingly weak [111,112]. Given the interdependence of metacognition and performance discussed above, one explanation for this null result might be methodological in nature, as a performance-confidence relationship is naturally harder to quantify than performance itself. A similar line of thought led Keleman et al. to speculate that ‘stable metacognitive performance might be detected using very large numbers of trials’ [112]. In support of this view, Fleming et al. showed good split-half reliability (r = 0.69) in a perceptual decision task with hundreds of trials [21], and metacognitive accuracy has been shown to be stable across two perceptual tasks (r = 0.71), despite performance itself being uncorrelated (r = 0.05; figure 3) [70]. An important unanswered question is whether metacognitive accuracy is stable across domain (e.g. memory and decision-making), as might be predicted by their overlapping neural substrates [113].
(d). Summary
There is now considerable evidence that damage to the PFC selectively affects the accuracy of metacognitive reports while leaving task performance relatively intact. Intriguingly, there is some evidence for a lateral–medial separation between neural systems supporting retrospective confidence judgements and prospective judgements of performance, respectively. The role of ventromedial PFC in prospective judgements of performance may be explained by its strong connections with medial temporal lobe memory structures and its role in imagination of the future [114,115]. In contrast, the role of anterior and dorsolateral PFC in retrospective judgements of confidence may be more closely aligned to that of a performance monitor, integrating and maintaining information pertaining to the immediately preceding decision to facilitate accurate metacognitive commentary. In the next section, we focus in greater detail on performance-monitoring functions to illustrate connections between metacognition and a separate but substantial literature on the neuroscience of cognitive control.
5. Relationship between metacognition and cognitive control
An influential suggestion is that decision-making systems should be sensitive to the current level of conflict between possible responses to mobilize additional ‘cognitive control’ resources in an adaptive fashion [116]. Activity in ACC and anterior insula is increased during heightened response conflict (see [117,118] for reviews), whereas lateral PFC activity correlates with behavioural adjustments, such as increased caution, following high-conflict trials [119,120]. Further, the ACC is suggested to recruit lateral PFC to increase levels of control when conflict occurs [117]. This proposal for a cognitive control loop shares obvious similarities with concepts of monitoring and control in metacognition research (figure 1); indeed, a previous review proposed metacognition might be commensurate with cognitive control [121]. However, such a view would predict that any system with the capacity for monitoring and control has metacognitive representations, which is not usually held to be the case. Instead, philosophers have discussed and debated two ‘levels’ of metacognition [122]: one involving declarative (conscious) meta-representation [123]; the other low-level, based on non-verbal epistemic feelings of uncertainty [124,125]. For present purposes, we consider monitoring processes as metacognitive to the extent they are consciously reportable, and thus available for deployment outside of a ‘closed-loop’ optimization of the task at hand (see also [126]). Such reports can be empirically dissociated from monitoring and control: for example, skilled typists show subtle post-error adjustments in the absence of awareness, and yet accept blame for errors that are surreptitiously inserted by the experimenters on the screen [127]. Interestingly, subjective effects of heightened decision conflict may themselves be reportable in the absence of awareness of antecedents of this conflict [128], and thus it is not always simple to decide whether performance monitoring involves meta-representation.
What might govern the accessibility of performance-monitoring information to awareness? We suggest that rlPFC is particularly important for the representation of information pertaining to a previous decision in a globally accessible frame of reference. In a direct comparison of confidence judgements following mnemonic and perceptual decisions, both ACC and right dlPFC activity increased with decreasing confidence [113]; however, only right dlPFC encoded confidence independent of changes in reaction time, leading the authors to suggest that while ACC responds to online decision conflict, dlPFC activity underlies the selection of metacognitive responses. Furthermore, a recent study found that activity in rlPFC both increases during metacognitive reports and correlates with reported confidence [109]. Thus, the accuracy of metacognitive commentaries, as dissociated from adjustments in performance, might be governed by the fidelity with which rlPFC integrates and maintains information from cingulate and insula involved in online adjustments in task performance, consistent with reciprocal anatomical connections between these regions [129].
If only a subset of nodes in this network is present, one might find effective performance monitoring in the absence of metacognition. This pattern of results was observed in a patient with a large left prefrontal cortical lesion, who displayed intact performance adjustments in the Stroop task, without being able to report changes in the subjective sense of effort while performing the task [130]. As the patient displayed intact conflict-related N2 event-related potential responses during the Stroop task, the authors suggested that (implicit) monitoring and control is maintained by an intact right ACC, while a subjective feeling of effort would normally be mediated by the damaged lateral PFC. Such a conclusion is supported by recent evidence that lateral PFC activity is higher in subjects with a strong tendency to avoid cognitively demanding decisions [131]. Importantly for our hypothesis, if lateral PFC receives input from non-conscious monitoring loops, the reverse dissociation would not be predicted: we might be able to control objects we cannot report, but should not be able report upon objects we cannot (cognitively) control.
The respective roles of nodes in this network remain to be determined, but there is initial evidence for division of labour. TMS to dlPFC impairs metacognition following correct but not incorrect decisions, suggesting a role in representing confidence rather than monitoring for errors [20]. In contrast, reporting of response errors has been linked to the error-related positivity [132] with a possible source in insula cortex [118]. Indeed, accurate metacognitive commentaries about performance require access to information about both beliefs and responses. For example, just after hitting a shot in tennis, you might have high confidence (low uncertainty) that the spot you chose to aim at is out of reach of your opponent (your belief), but low confidence in correctly executing the shot (your response). Thus, for commentaries to integrate information both about a belief and response, the ‘frame of reference’ in which information is encoded is crucial. If information is maintained in segregated sensorimotor loops, performance adjustments could be made based on deviations from an expected trajectory without this information being more generally available for, say, verbal report. It remains an open question as to the extent to which decision-making relies on ‘embodied’ or domain-general circuitry [133], but a role for the PFC in the abstract encoding of decision-related information, independent of response modality, has been found using fMRI conjunction analyses [134,135]. It will be of interest to test whether this same activity is involved in metacognition.
6. Conclusions
Cognitive psychology has developed a rich theoretical framework and empirical tools for studying self-assessments of cognition. A crucial variable of interest is the accuracy of metacognitive reports with respect to their object-level targets: in other words, how well do we know our own minds? We now understand metacognition to be under segregated neural control, a conclusion that might have surprised Comte, and one that runs counter to an intuition that we have veridical access to the accuracy of our perceptions, memories and decisions. A detailed, and eventually mechanistic, account of metacognition at the neural level is a necessary first step to understanding the failures of metacognition that occur following brain damage [87] and psychiatric disorder [136]. In this paper, we summarized a variety of behavioural approaches for measuring the accuracy of metacognitive assessments, and reviewed the possible neural substrates of metacognitive accuracy in humans. We conclude that there are potentially separable brain systems for prospective and retrospective judgements of performance, and our synthesis of recent neuropsychological and brain imaging findings implicates the rostrolateral PFC as crucial in mediating retrospective judgements of cognition. In this model, the rostrolateral PFC receives input from interoceptive cortex involved in ‘closed-loop’ monitoring and control, generating a metacognitive representation of the state of the system that can be deployed or reported outside of the current task at hand.
We close with a number of open questions we hope will be addressed by future studies:
— To what extent does metacognitive accuracy (and its associated neural correlates) generalize across different object-level domains?
— To what extent does metacognition rely on abstract (response-independent) decision variables?
— Are the neural correlates of error-monitoring and confidence separable [71]?
— Do dlPFC (∼BA46) and rlPFC (∼BA10) make differential contributions to metacognition?
— If task performance can be monitored and corrected in the absence of metacognitive report, what is the functional role of metacognitive (in)accuracy?
Acknowledgements
Preparation of this article was supported by Wellcome Trust Programme grant 078865/Z/05/Z to R.J.D., and a Sir Henry Wellcome Fellowship to S.M.F. We thank Matt Dixon, Chris Frith, Tali Sharot and Jon Simons for helpful comments on a previous draft of this manuscript.
References
- 1.Carruthers P. 2011. The opacity of mind: an integrative theory of self-knowledge. New York, NY: Oxford University Press [Google Scholar]
- 2.Nisbett R. E., Wilson T. D. 1977. Telling more than we can know: verbal reports on mental processes. Psychol. Rev. 84, 231. 10.1037/0033-295X.84.3.231 (doi:10.1037/0033-295X.84.3.231) [DOI] [Google Scholar]
- 3.Wilson T. D., Dunn E. W. 2004. Self-knowledge: its limits, value, and potential for improvement. Ann. Rev. Psychol. 55, 493–518 10.1146/annurev.psych.55.090902.141954 (doi:10.1146/annurev.psych.55.090902.141954) [DOI] [PubMed] [Google Scholar]
- 4.Johansson P., Hall L., Sikström S., Olsson A. 2005. Failure to detect mismatches between intention and outcome in a simple decision task. Science 310, 116–119 10.1126/science.1111709 (doi:10.1126/science.1111709) [DOI] [PubMed] [Google Scholar]
- 5.Hall L., Johansson P., Tärning B., Sikström S., Deutgen T. 2010. Magic at the marketplace: choice blindness for the taste of jam and the smell of tea. Cognition 117, 54–61 10.1016/j.cognition.2010.06.010 (doi:10.1016/j.cognition.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 6.Pronin E. 2007. Perception and misperception of bias in human judgment. Trends Cogn. Sci. 11, 37–43 10.1016/j.tics.2006.11.001 (doi:10.1016/j.tics.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 7.Boring E. 1953. A history of introspection. Psychol. Bull. 50, 169–189 10.1037/h0090793 (doi:10.1037/h0090793) [DOI] [PubMed] [Google Scholar]
- 8.James W. 1950. The principles of psychology, vol. 1 New York, NY: Dover Publications [Google Scholar]
- 9.Friston K. 2005. A theory of cortical responses. Phil. Trans. R. Soc. B 360, 815–836 10.1098/rstb.2005.1622 (doi:10.1098/rstb.2005.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koechlin E., Hyafil A. 2007. Anterior prefrontal function and the limits of human decision-making. Science 318, 594–598 10.1126/science.1142995 (doi:10.1126/science.1142995) [DOI] [PubMed] [Google Scholar]
- 11.Schmitz T. W., Rowley H. A., Kawahara T. N., Johnson S. C. 2006. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia 44, 762–773 10.1016/j.neuropsychologia.2005.07.012 (doi:10.1016/j.neuropsychologia.2005.07.012) [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe J., Van Snellenberg J. X., DeRosse P., Balsam P., Malhotra A. K. 2012. Judgments of agency in schizophrenia: an impairment in autonoetic metacognition. Phil. Trans. R. Soc. B 367, 1391–1400 10.1098/rstb.2012.0006 (doi:10.1098/rstb.2012.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavell J. 1979. Metacognition and cognitive monitoring: a new area of cognitive-developmental inquiry. Am. Psychol. 34, 906–911 10.1037/0003-066X.34.10.906 (doi:10.1037/0003-066X.34.10.906) [DOI] [Google Scholar]
- 14.Ericsson K., Simon H. 1980. Verbal reports as data. Psychol. Rev. 87, 215–251 10.1037/0033-295X.87.3.215 (doi:10.1037/0033-295X.87.3.215) [DOI] [Google Scholar]
- 15.Nelson T. 1984. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychol. Bull. 95, 109–133 10.1037/0033-2909.95.1.109 (doi:10.1037/0033-2909.95.1.109) [DOI] [PubMed] [Google Scholar]
- 16.Shimamura A. P. 2008. A neurocognitive approach to metacognitive monitoring and control. In Handbook of memory and metamemory: essays in honor of Thomas O. Nelson (eds Dunlosky J., Bjork R.), pp. 373–390 New York, NY: Psychology Press [Google Scholar]
- 17.Nelson T. O., Narens L. 1990. Metamemory: a theoretical framework and new findings. Psychol. Learn. Motivation: Adv. Res. Theory 26, 125–173 10.1016/S0079-7421(08)60053-5 (doi:10.1016/S0079-7421(08)60053-5) [DOI] [Google Scholar]
- 18.Shallice T., Burgess P. 1996. The domain of supervisory processes and temporal organization of behaviour. Phil. Trans. R. Soc. B 351, 1405–1411; discussion 1411–1412 10.1098/rstb.1996.0124 (doi:10.1098/rstb.1996.0124) [DOI] [PubMed] [Google Scholar]
- 19.Del Cul A., Dehaene S., Reyes P., Bravo E., Slachevsky A. 2009. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain 132, 2531. 10.1093/brain/awp111 (doi:10.1093/brain/awp111) [DOI] [PubMed] [Google Scholar]
- 20.Rounis E., Maniscalco B., Rothwell J., Passingham R., Lau H. 2010. Theta-burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cogn. Neurosci. 1, 165–175 10.1080/17588921003632529 (doi:10.1080/17588921003632529) [DOI] [PubMed] [Google Scholar]
- 21.Fleming S. M., Weil R. S., Nagy Z., Dolan R. J., Rees G. 2010. Relating introspective accuracy to individual differences in brain structure. Science 329, 1541–1543 10.1126/science.1191883 (doi:10.1126/science.1191883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marti S., Sackur J., Sigman M., Dehaene S. 2010. Mapping introspection's blind spot: reconstruction of dual-task phenomenology using quantified introspection. Cognition 115, 303–313 10.1016/j.cognition.2010.01.003 (doi:10.1016/j.cognition.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 23.Morsella E., Wilson L. E., Berger C. C., Honhongva M., Gazzaley A., Bargh J. A. 2009. Subjective aspects of cognitive control at different stages of processing. Atten. Percept. Psychophys. 71, 1807–1824 10.3758/APP.71.8.1807 (doi:10.3758/APP.71.8.1807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dienes Z. 2008. Subjective measures of unconscious knowledge. Prog. Brain Res. 168, 49–64 10.1016/S0079-6123(07)68005-4 (doi:10.1016/S0079-6123(07)68005-4) [DOI] [PubMed] [Google Scholar]
- 25.Schooler J. W. 2002. Re-representing consciousness: dissociations between experience and meta-consciousness. Trends Cogn. Sci. 6, 339–344 10.1016/S1364-6613(02)01949-6 (doi:10.1016/S1364-6613(02)01949-6) [DOI] [PubMed] [Google Scholar]
- 26.Schwartz B., Bacon E. 2008. Metacognitive neuroscience. In Handbook of memory and metamemory: essays in honor of Thomas O. Nelson (eds Dunlosky J., Bjork R.), pp. 355–371 New York, NY: Psychology Press [Google Scholar]
- 27.Arbuckle T. 1969. Discrimination of item strength at time of presentation. J. Exp. Psych. 8, 126–131 10.1037/h0027455 (doi:10.1037/h0027455) [DOI] [Google Scholar]
- 28.Hart J. 1965. Memory and the feeling-of-knowing experience. J. Educ. Psychol. 56, 208–216 10.1037/h0022263 (doi:10.1037/h0022263) [DOI] [PubMed] [Google Scholar]
- 29.Brown A. S. 1991. A review of the tip-of-the-tongue experience. Psychol. Bull. 109, 204–223 10.1037/0033-2909.109.2.204 (doi:10.1037/0033-2909.109.2.204) [DOI] [PubMed] [Google Scholar]
- 30.Peirce C. S., Jastrow J. 1885. On small differences in sensation. Memoir. Natl Acad. Sci. 3, 73–83 [Google Scholar]
- 31.Vickers D. 1979. Decision processes in visual perception. New York, NY: Academic Press [Google Scholar]
- 32.Eriksen C. W. 1960. Discrimination and learning without awareness: a methodological survey and evaluation. Psychol. Rev. 67, 279–300 10.1037/h0041622 (doi:10.1037/h0041622) [DOI] [PubMed] [Google Scholar]
- 33.Kunimoto C. 2001. Confidence and accuracy of near-threshold discrimination responses. Conscious. Cogn. 10, 294–340 10.1006/ccog.2000.0494 (doi:10.1006/ccog.2000.0494) [DOI] [PubMed] [Google Scholar]
- 34.Persaud N., McLeod P., Cowey A. 2007. Post-decision wagering objectively measures awareness. Nat. Neurosci. 10, 257–261 10.1038/nn1840 (doi:10.1038/nn1840) [DOI] [PubMed] [Google Scholar]
- 35.Hollard G., Massoni S., Vergnaud J. C. 2010. Subjective belief formation and elicitation rules: experimental evidence. Working paper no. 10088, Centre d'Economie, Université Panthéon-Sorbonne, Paris, France [Google Scholar]
- 36.Becker G. M., DeGroot M. H., Marschak J. 1964. Measuring utility by a single-response sequential method. Behav. Sci. 9, 226–232 10.1002/bs.3830090304 (doi:10.1002/bs.3830090304) [DOI] [PubMed] [Google Scholar]
- 37.Dienes Z., Altmann G., Kwan L. 1995. Unconscious knowledge of artificial grammars is applied strategically. J. Exp. Psychol. Learn. Mem. Cogn. 21, 1322–1338 10.1037/0278-7393.21.5.1322 (doi:10.1037/0278-7393.21.5.1322) [DOI] [Google Scholar]
- 38.Macmillan N., Creelman C. 2005. Detection theory: a user's guide. New York, NY: Lawrence Erlbaum [Google Scholar]
- 39.Green D., Swets J. 1966. Signal detection theory and psychophysics. New York, NY: Wiley [Google Scholar]
- 40.Clarke F., Birdsall T., Tanner W. 1959. Two types of ROC curves and definition of parameters. J. Acoust. Soc. Am. 31, 629–630 10.1121/1.1907764 (doi:10.1121/1.1907764) [DOI] [Google Scholar]
- 41.Galvin S. J., Podd J. V., Drga V., Whitmore J. 2003. Type 2 tasks in the theory of signal detectability: discrimination between correct and incorrect decisions. Psychol. Bull. Rev. 10, 843–876 10.3758/BF03196546 (doi:10.3758/BF03196546) [DOI] [PubMed] [Google Scholar]
- 42.Evans S., Azzopardi P. 2007. Evaluation of a ‘bias-free’ measure of awareness. Spatial Vision 20, 61–77 10.1163/156856807779369742 (doi:10.1163/156856807779369742) [DOI] [PubMed] [Google Scholar]
- 43.Fleming S. M., Dolan R. J. 2010. Effects of loss aversion on post-decision wagering: implications for measures of awareness. Conscious. Cogn. 19, 352–363 10.1016/j.concog.2009.11.002 (doi:10.1016/j.concog.2009.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maniscalco B., Lau H. In press A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious. Cogn. [DOI] [PubMed] [Google Scholar]
- 45.Kersten D., Mamassian P., Yuille A. 2004. Object perception as Bayesian inference. Ann. Rev. Psychol. 55, 271–304 10.1146/annurev.psych.55.090902.142005 (doi:10.1146/annurev.psych.55.090902.142005) [DOI] [PubMed] [Google Scholar]
- 46.Kording K. 2007. Decision theory: what ‘should’ the nervous system do? Science 318, 606–610 10.1126/science.1142998 (doi:10.1126/science.1142998) [DOI] [PubMed] [Google Scholar]
- 47.Barthelmé S., Mamassian P. 2009. Evaluation of objective uncertainty in the visual system. PLoS Comput. Biol. 5, e1000504 (doi:10.1371/journal.pcbi.1000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landy M., Goutcher R., Trommershäuser J., Mamassian P. 2007. Visual estimation under risk. J. Vis. 7, 4. 10.1167/7.6.4 (doi:10.1167/7.6.4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteley L., Sahani M. 2008. Implicit knowledge of visual uncertainty guides decisions with asymmetric outcomes. J. Vis. 8, 2. 10.1167/8.3.2 (doi:10.1167/8.3.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trommershauser J., Maloney L., Landy M. 2003. Statistical decision theory and trade-offs in the control of motor response. Spatial Vision 16, 255–275 10.1163/156856803322467527 (doi:10.1163/156856803322467527) [DOI] [PubMed] [Google Scholar]
- 51.Smith J. D., Couchman J. J., Beran M. J. 2012. The highs and lows of theoretical interpretation in animal-metacognition research. Phil. Trans. R. Soc. B 367, 1297–1309 10.1098/rstb.2011.0366 (doi:10.1098/rstb.2011.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kepecs A., Uchida N., Mainen H. A., Zariwala Z. F. 2008. Neural correlates, computation and behavioural impact of decision confidence. Nature 455, 227–231 10.1038/nature07200 (doi:10.1038/nature07200) [DOI] [PubMed] [Google Scholar]
- 53.Kiani R., Shadlen M. 2009. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324, 759–764 10.1126/science.1169405 (doi:10.1126/science.1169405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kepecs A., Mainen Z. F. 2012. A computational framework for the study of confidence in humans and animals. Phil. Trans. R. Soc. B 367, 1322–1337 10.1098/rstb.2012.0037 (doi:10.1098/rstb.2012.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz B., Metcalfe J. 1996. Methodological problems and pitfalls in the study of human metacognition. In Metacognition: knowing about knowing (eds Metcalfe J., Shimamura A.). Cambridge, MA: MIT Press [Google Scholar]
- 56.Koriat A. 1997. Monitoring one's own knowledge during study: a cue-utilization approach to judgments of learning. J. Exp. Psych. Gen. 126, 349–370 10.1037/0096-3445.126.4.349 (doi:10.1037/0096-3445.126.4.349) [DOI] [Google Scholar]
- 57.Koriat A. 2007. Metacognition and consciousness. In The Cambridge handbook of consciousness (eds Zelazo P. D., Moscovitch M., Thompson E.), pp. 289–325, Cambridge, UK: Cambridge University Press [Google Scholar]
- 58.Alter A. L., Oppenheimer D. M. 2009. Uniting the tribes of fluency to form a metacognitive nation. Personality Soc. Psychol. Rev. 13, 219–235 10.1177/1088868309341564 (doi:10.1177/1088868309341564) [DOI] [PubMed] [Google Scholar]
- 59.Busey T. A., Tunnicliff J., Loftus G. R., Loftus E. F. 2000. Accounts of the confidence-accuracy relation in recognition memory. Psychol. Bull. Rev. 7, 26–48 10.3758/BF03210724 (doi:10.3758/BF03210724) [DOI] [PubMed] [Google Scholar]
- 60.Koriat A. 1993. How do we know that we know? The accessibility model of the feeling of knowing. Psychol. Rev. 100, 609–639 10.1037/0033-295X.100.4.609 (doi:10.1037/0033-295X.100.4.609) [DOI] [PubMed] [Google Scholar]
- 61.Baranski J. V., Petrusic W. M. 1998. Probing the locus of confidence judgments: experiments on the time to determine confidence. J. Exp. Psych. Hum. Percept. Perform. 24, 929–945 10.1037/0096-1523.24.3.929 (doi:10.1037/0096-1523.24.3.929) [DOI] [PubMed] [Google Scholar]
- 62.Clifford C., Arabzadeh E., Harris J. 2008. Getting technical about awareness. Trends Cogn. Sci. 12, 54–58 10.1016/j.tics.2007.11.009 (doi:10.1016/j.tics.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 63.Insabato A., Pannunzi M., Rolls E. T., Deco G. 2010. Confidence-related decision making. J. Neurophys. 104, 539–547 10.1152/jn.01068.2009 (doi:10.1152/jn.01068.2009) [DOI] [PubMed] [Google Scholar]
- 64.Rolls E. T., Grabenhorst F., Deco G. 2010. Choice, difficulty, and confidence in the brain. NeuroImage 53, 694–706 10.1016/j.neuroimage.2010.06.073 (doi:10.1016/j.neuroimage.2010.06.073) [DOI] [PubMed] [Google Scholar]
- 65.Higham P. A., Perfect T. J., Bruno D. 2009. Investigating strength and frequency effects in recognition memory using type-2 signal detection theory. J. Exp. Psychol. Learn. Mem. Cogn. 35, 57–80 10.1037/a0013865 (doi:10.1037/a0013865) [DOI] [PubMed] [Google Scholar]
- 66.Busey T. A., Arici A. 2009. On the role of individual items in recognition memory and metacognition: challenges for signal detection theory. J. Exp. Psychol. Learn. Mem. Cogn. 35, 1123–1136 10.1037/a0016646 (doi:10.1037/a0016646) [DOI] [PubMed] [Google Scholar]
- 67.Lau H. C., Passingham R. E. 2006. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc. Natl Acad. Sci. USA 103, 18 763–18 768 10.1073/pnas.0607716103 (doi:10.1073/pnas.0607716103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilimzig C., Tsuchiya N., Fahle M., Einhäuser W., Koch C. 2008. Spatial attention increases performance but not subjective confidence in a discrimination task. J. Vis. 8, 7.1–10 10.1167/8.5.7 (doi:10.1167/8.5.7) [DOI] [PubMed] [Google Scholar]
- 69.Izaute M., Bacon E. 2005. Specific effects of an amnesic drug: effect of lorazepam on study time allocation and on judgment of learning. Neuropsychopharmacology 30, 196–204 10.1038/sj.npp.1300564 (doi:10.1038/sj.npp.1300564) [DOI] [PubMed] [Google Scholar]
- 70.Song C., Kanai R., Fleming S. M., Weil R. S., Schwarzkopf D. S., Rees G. 2011. Relating inter-individual differences in metacognitive performance on different perceptual tasks. Conscious. Cogn. 20, 1787–1792 10.1016/j.concog.2010.12.011 (doi:10.1016/j.concog.2010.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeung N., Summerfield C. 2012. Metacognition in human decision-making: confidence and error monitoring. Phil. Trans. R. Soc. B 367, 1310–1321 10.1098/rstb.2011.0416 (doi:10.1098/rstb.2011.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pleskac T. J., Busemeyer J. R. 2010. Two-stage dynamic signal detection: a theory of choice, decision time, and confidence. Psychol. Rev. 117, 864–901 10.1037/a0019737 (doi:10.1037/a0019737) [DOI] [PubMed] [Google Scholar]
- 73.Ratcliff R., Starns J. J. 2009. Modeling confidence and response time in recognition memory. Psychol. Rev. 116, 59–83 10.1037/a0014086 (doi:10.1037/a0014086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maniscalco B., Lau H. 2009. Evaluating signal detection models of perceptual decision confidence. Front. Syst. Neurosci. Conf. Abstract: Computational and systems neuroscience, 2009. 10.3389/conf.neuro.06.2009.03.335 (doi:10.3389/conf.neuro.06.2009.03.335) [DOI] [Google Scholar]
- 75.Gold J., Shadlen M. 2007. The neural basis of decision making. Ann. Rev. Neurosci. 30, 535–574 10.1146/annurev.neuro.29.051605.113038 (doi:10.1146/annurev.neuro.29.051605.113038) [DOI] [PubMed] [Google Scholar]
- 76.Middlebrooks P. G., Sommer M. A. 2011. Metacognition in monkeys during an oculomotor task. J. Exp. Psychol. Learn. Mem. Cogn. 37, 325–337 10.1037/a0021611 (doi:10.1037/a0021611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kruger J., Dunning D. 1999. Unskilled and unaware of it: how difficulties in recognizing one's own incompetence lead to inflated self-assessments. J. Pers. Soc. Psychol. 77, 1121–1134 [DOI] [PubMed] [Google Scholar]
- 78.Kao Y. C., Davis E. S., Gabrieli J. D. E. 2005. Neural correlates of actual and predicted memory formation. Nat. Neurosci. 8, 1776–1783 10.1038/nn1595 (doi:10.1038/nn1595) [DOI] [PubMed] [Google Scholar]
- 79.Modirrousta M., Fellows L. K. 2008. Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia 46, 2958–2965 10.1016/j.neuropsychologia.2008.06.011 (doi:10.1016/j.neuropsychologia.2008.06.011) [DOI] [PubMed] [Google Scholar]
- 80.Morgan M., Mason A. 1997. Blindsight in normal subjects? Nature 385, 401–402 10.1038/385401b0 (doi:10.1038/385401b0) [DOI] [PubMed] [Google Scholar]
- 81.Lau H. 2010. Are we studying consciousness yet? In Frontiers of consciousness: Chichele lectures (eds Weiskrantz L., Davies M.). Oxford, UK: Oxford University Press [Google Scholar]
- 82.Shimamura A. P. 2000. Toward a cognitive neuroscience of metacognition. Conscious. Cogn. 9, 313–323 10.1006/ccog.2000.0450 (doi:10.1006/ccog.2000.0450) [DOI] [PubMed] [Google Scholar]
- 83.Hirst W. 1982. The amnesic syndrome: descriptions and explanations. Psychol. Bull. 91, 435–460 10.1037/0033-2909.91.3.435 (doi:10.1037/0033-2909.91.3.435) [DOI] [PubMed] [Google Scholar]
- 84.Zahr N., Kaufman K. 2011. Clinical and pathological features of alcohol-related brain damage. Nat. Rev. Neurol. 7, 284–294 10.1038/nrneurol.2011.42 (doi:10.1038/nrneurol.2011.42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimamura A. P., Squire L. R. 1986. Memory and metamemory: a study of the feeling-of-knowing phenomenon in amnesic patients. J. Exp. Psychol. Learn. Mem. Cogn. 12, 452–460 10.1037/0278-7393.12.3.452 (doi:10.1037/0278-7393.12.3.452) [DOI] [PubMed] [Google Scholar]
- 86.Janowsky J. S., Shimamura A. P., Kritchevsky M., Squire L. R. 1989. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav. Neurosci. 103, 548. 10.1037/0735-7044.103.3.548 (doi:10.1037/0735-7044.103.3.548) [DOI] [PubMed] [Google Scholar]
- 87.Pannu J., Kaszniak A. 2005. Metamemory experiments in neurological populations: a review. Neuropsychol. Rev. 15, 105–130 10.1007/s11065-005-7091-6 (doi:10.1007/s11065-005-7091-6) [DOI] [PubMed] [Google Scholar]
- 88.Schnyer D. M., Verfaellie M., Alexander M. P., LaFleche G., Nicholls L., Kaszniak A. W. 2004. A role for right medial prefontal cortex in accurate feeling-of-knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia 42, 957–966 10.1016/j.neuropsychologia.2003.11.020 (doi:10.1016/j.neuropsychologia.2003.11.020) [DOI] [PubMed] [Google Scholar]
- 89.Pannu J., Kaszniak A., Rapcsak S. 2005. Metamemory for faces following frontal lobe damage. J. Int. Neuropsychol. Soc. 11, 668–676 [DOI] [PubMed] [Google Scholar]
- 90.Kikyo H., Ohki K., Miyashita Y. 2002. Neural correlates for feeling-of-knowing. Neuron 36, 177–186 10.1016/S0896-6273(02)00939-X (doi:10.1016/S0896-6273(02)00939-X) [DOI] [PubMed] [Google Scholar]
- 91.Chua E. F., Schacter D. L., Rand-Giovannetti E., Sperling R. A. 2006. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. NeuroImage 29, 1150–1160 10.1016/j.neuroimage.2005.09.058 (doi:10.1016/j.neuroimage.2005.09.058) [DOI] [PubMed] [Google Scholar]
- 92.Chua E. F., Schacter D. L., Sperling R. A. 2009. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. J. Cogn. Neurosci. 21, 1751–1765 10.1162/jocn.2009.21123 (doi:10.1162/jocn.2009.21123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim H., Cabeza R. 2007. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J. Neurosci. 27, 12190. 10.1523/JNEUROSCI.3408-07.2007 (doi:10.1523/JNEUROSCI.3408-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moritz S., Gläscher J., Sommer T., Büchel C., Braus D. F. 2006. Neural correlates of memory confidence. NeuroImage 33, 1188–1193 10.1016/j.neuroimage.2006.08.003 (doi:10.1016/j.neuroimage.2006.08.003) [DOI] [PubMed] [Google Scholar]
- 95.Schnyer D. M., Nicholls L., Verfaellie M. 2005. The role of VMPC in metamemorial judgments of content retrievability. J. Cogn. Neurosci. 17, 832–846 10.1162/0898929053747694 (doi:10.1162/0898929053747694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yokoyama O., et al. 2010. Right frontopolar cortex activity correlates with reliability of retrospective rating of confidence in short-term recognition memory performance. Neurosci. Res. 68, 199–206 10.1016/j.neures.2010.07.2041 (doi:10.1016/j.neures.2010.07.2041) [DOI] [PubMed] [Google Scholar]
- 97.Park H. J., Kim J. J., Lee S. K., Seok J. H., Chun J., Kim D. I., Lee J. D. 2008. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapping 29, 503–516 10.1002/hbm.20314 (doi:10.1002/hbm.20314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanai R., Rees G. 2011. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 12, 231–242 10.1038/nrn3000 (doi:10.1038/nrn3000) [DOI] [PubMed] [Google Scholar]
- 99.Ramnani N., Owen A. M. 2004. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5, 184–194 10.1038/nrn1343 (doi:10.1038/nrn1343) [DOI] [PubMed] [Google Scholar]
- 100.Semendeferi K., Teffer K., Buxhoeveden D. P., Park M. S., Bludau S., Amunts K., Travis K., Buckwalter J. 2011. Spatial organization of neurons in the frontal pole sets humans apart from Great Apes. Cereb. Cortex 21, 1485–1497 10.1093/cercor/bhq191 (doi:10.1093/cercor/bhq191) [DOI] [PubMed] [Google Scholar]
- 101.Simons J. S., Henson R. N. A., Gilbert S. J., Fletcher P. C. 2008. Separable forms of reality monitoring supported by anterior prefrontal cortex. J. Cogn. Neurosci. 20, 447–457 10.1162/jocn.2008.20036 (doi:10.1162/jocn.2008.20036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshida W., Ishii S. 2006. Resolution of uncertainty in prefrontal cortex. Neuron 50, 781–789 10.1016/j.neuron.2006.05.006 (doi:10.1016/j.neuron.2006.05.006) [DOI] [PubMed] [Google Scholar]
- 103.Gilbert S. J., Spengler S., Simons J. S., Frith C. D., Burgess P. W. 2006. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain-behavior associations. Cereb. Cortex 16, 1783–1789 10.1093/cercor/bhj113 (doi:10.1093/cercor/bhj113) [DOI] [PubMed] [Google Scholar]
- 104.Buda M., Fornito A., Bergström Z. M., Simons J. S. 2011. A specific brain structural basis for individual differences in reality monitoring. J. Neurosci. 31, 14 308–14 313 10.1523/JNEUROSCI.3595-11.2011 (doi:10.1523/JNEUROSCI.3595-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curtis C., D'Esposito M. 2003. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7, 415–423 10.1016/S1364-6613(03)00197-9 (doi:10.1016/S1364-6613(03)00197-9) [DOI] [PubMed] [Google Scholar]
- 106.Sakai K., Rowe J. B., Passingham R. E. 2002. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat. Neurosci. 5, 479–484 10.1038/nn846 (doi:10.1038/nn846) [DOI] [PubMed] [Google Scholar]
- 107.Scheperjans F., Hermann K., Eickhoff S. B., Amunts K., Schleicher A., Zilles K. 2008. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb. Cortex 18, 846–867 10.1093/cercor/bhm116 (doi:10.1093/cercor/bhm116) [DOI] [PubMed] [Google Scholar]
- 108.John J. P., Yashavantha B. S., Gado M., Veena R., Jain S., Ravishankar S., Csernansky J. G. 2007. A proposal for MRI-based parcellation of the frontal pole. Brain Struct. Funct. 212, 245–253 10.1007/s00429-007-0157-x (doi:10.1007/s00429-007-0157-x) [DOI] [PubMed] [Google Scholar]
- 109.Fleming S. M., Huijgen J., Dolan R. J. Submitted Prefrontal mechanisms for awareness of task performance. [Google Scholar]
- 110.Smith R., Keramatian K., Christoff K. 2007. Localizing the rostrolateral prefrontal cortex at the individual level. NeuroImage 36, 1387–1396 10.1016/j.neuroimage.2007.04.032 (doi:10.1016/j.neuroimage.2007.04.032) [DOI] [PubMed] [Google Scholar]
- 111.Thompson W. B., Mason S. E. 1996. Instability of individual differences in the association between confidence judgments and memory performance. Mem. Cogn. 24, 226–234 10.3758/BF03200883 (doi:10.3758/BF03200883) [DOI] [PubMed] [Google Scholar]
- 112.Kelemen W. L., Frost P. J., Weaver C. A. 2000. Individual differences in metacognition: evidence against a general metacognitive ability. Mem. Cogn. 28, 92–107 10.3758/BF03211579 (doi:10.3758/BF03211579) [DOI] [PubMed] [Google Scholar]
- 113.Fleck M. S., Daselaar S. M., Dobbins I. G., Cabeza R. 2006. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb. Cortex 16, 1623–1630 10.1093/cercor/bhj097 (doi:10.1093/cercor/bhj097) [DOI] [PubMed] [Google Scholar]
- 114.Sharot T., Riccardi A., Raio C. 2007. Neural mechanisms mediating optimism bias. Nature 450, 102–105 10.1038/nature06280 (doi:10.1038/nature06280) [DOI] [PubMed] [Google Scholar]
- 115.Hassabis D., Maguire E. 2007. Deconstructing episodic memory with construction. Trends Cogn. Sci. 11, 299–306 10.1016/j.tics.2007.05.001 (doi:10.1016/j.tics.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 116.Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., Cohen J. D. 2001. Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652 10.1037/0033-295X.108.3.624 (doi:10.1037/0033-295X.108.3.624) [DOI] [PubMed] [Google Scholar]
- 117.Ridderinkhof K. R., Ullsperger M., Crone E. A., Nieuwenhuis S. 2004. The role of the medial frontal cortex in cognitive control. Science 306, 443–447 10.1126/science.1100301 (doi:10.1126/science.1100301) [DOI] [PubMed] [Google Scholar]
- 118.Ullsperger M., Harsay H. A., Wessel J. R., Ridderinkhof K. R. 2010. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 214, 629–643 10.1007/s00429-010-0261-1 (doi:10.1007/s00429-010-0261-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacDonald A. W., Cohen J. D., Stenger V. A., Carter C. S. 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838 10.1126/science.288.5472.1835 (doi:10.1126/science.288.5472.1835) [DOI] [PubMed] [Google Scholar]
- 120.Kerns J. G., Cohen J. D., MacDonald A. W., Cho R. Y., Stenger V. A., Carter C. S. 2004. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 10.1126/science.1089910 (doi:10.1126/science.1089910) [DOI] [PubMed] [Google Scholar]
- 121.Fernandez-Duque D., Baird J. A., Posner M. I. 2000. Executive attention and metacognitive regulation. Conscious. Cogn. 9, 288–307 10.1006/ccog.2000.0447 (doi:10.1006/ccog.2000.0447) [DOI] [PubMed] [Google Scholar]
- 122.Arango-Muñoz S. 2010. Two levels of metacognition. Philosophia 39, 71–82 10.1007/s11406-010-9279-0 (doi:10.1007/s11406-010-9279-0) [DOI] [Google Scholar]
- 123.Carruthers P. 2009. How we know our own minds: the relationship between mindreading and metacognition. Behav. Brain Sci. 32, 121–138 10.1017/S0140525X09000545 (doi:10.1017/S0140525X09000545) [DOI] [PubMed] [Google Scholar]
- 124.Proust J. 2007. Metacognition and metarepresentation: is a self-directed theory of mind a precondition for metacognition? Syntheses 159, 271–295 10.1007/s11229-007-9208-3 (doi:10.1007/s11229-007-9208-3) [DOI] [Google Scholar]
- 125.Evans J. 2008. Dual-processing accounts of reasoning, judgment, and social cognition. Annu. Rev. Psychol. 59, 255–278 10.1146/annurev.psych.59.103006.093629 (doi:10.1146/annurev.psych.59.103006.093629) [DOI] [PubMed] [Google Scholar]
- 126.Shea N., Heyes C. 2010. Metamemory as evidence of animal consciousness: the type that does the trick. Biol. Philos. 25, 95–110 10.1007/s10539-009-9171-0 (doi:10.1007/s10539-009-9171-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Logan G. D., Crump M. J. C. 2010. Cognitive illusions of authorship reveal hierarchical error detection in skilled typists. Science 330, 683–686 10.1126/science.1190483 (doi:10.1126/science.1190483) [DOI] [PubMed] [Google Scholar]
- 128.Wenke D., Fleming S. M., Haggard P. 2010. Subliminal priming of actions influences sense of control over effects of action. Cognition 115, 26–38 10.1016/j.cognition.2009.10.016 (doi:10.1016/j.cognition.2009.10.016) [DOI] [PubMed] [Google Scholar]
- 129.Medalla M., Barbas H. 2010. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. J. Neurosci. 30, 16 068–16 081 10.1523/JNEUROSCI.1773-10.2010 (doi:10.1523/JNEUROSCI.1773-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naccache L., Dehaene S., Cohen L., Habert M.-O., Guichart-Gomez E., Galanaud D., Willer J.-C. 2005. Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia 43, 1318–1328 10.1016/j.neuropsychologia.2004.11.024 (doi:10.1016/j.neuropsychologia.2004.11.024) [DOI] [PubMed] [Google Scholar]
- 131.McGuire J. T., Botvinick M. M. 2010. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc. Natl Acad. Sci. USA 107, 7922–7926 10.1073/pnas.0910662107 (doi:10.1073/pnas.0910662107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nieuwenhuis S., Ridderinkhof K. R., Blom J., Band G. P. H., Kok A. 2001. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38, 752–760 10.1111/1469-8986.3850752 (doi:10.1111/1469-8986.3850752) [DOI] [PubMed] [Google Scholar]
- 133.Freedman D. J., Assad J. A. 2011. A proposed common neural mechanism for categorization and perceptual decisions. Nat. Neurosci. 14, 143–146 10.1038/nn.2740 (doi:10.1038/nn.2740) [DOI] [PubMed] [Google Scholar]
- 134.Heekeren H., Marrett S., Ruff D., Bandettini P., Ungerleider L. 2006. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc. Natl Acad. Sci. USA 103, 10 023–10 028 10.1073/pnas.0603949103 (doi:10.1073/pnas.0603949103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ho T. C., Brown S., Serences J. T. 2009. Domain general mechanisms of perceptual decision making in human cortex. J. Neurosci. 29, 8675–8687 10.1523/JNEUROSCI.5984-08.2009 (doi:10.1523/JNEUROSCI.5984-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.David A. S., Bedford N., Wiffen B., Gilleen J. 2012. Failures of metacognition and lack of insight in neuropsychiatric disorders. Phil. Trans. R. Soc. B 367, 1379–1390 10.1098/rstb.2012.0002 (doi:10.1098/rstb.2012.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]