Abstract
Various combinations of antibodies directed to cell surface markers have been used to isolate human and rhesus macaque hematopoietic stem cells (HSCs). These protocols result in poor enrichment or require multiple complex steps. Recently, a simple phenotype for HSCs based on cell surface markers from the signaling lymphocyte activation molecule (SLAM) family of receptors has been reported in the mouse. We examined the possibility of using the SLAM markers to facilitate the isolation of highly enriched populations of HSCs in humans and rhesus macaques. We isolated SLAM (CD150+CD48−) and non-SLAM (not CD150+CD48−) cells from human umbilical cord blood CD34+ cells as well as from human and rhesus macaque mobilized peripheral blood CD34+ cells and compared their ability to form colonies in vitro and reconstitute immune-deficient (nonobese diabetic/severe combined immunodeficiency/interleukin-2 γc receptornull, NSG) mice. We found that the CD34+ SLAM population contributed equally or less to colony formation in vitro and to long-term reconstitution in NSG mice compared with the CD34+ non-SLAM population. Thus, SLAM family markers do not permit the same degree of HSC enrichment in humans and rhesus macaques as in mice.
Introduction
The search for hematopoietic stem cell (HSC)-specific surface markers has been a central question for functional stem cell studies and for the development of clinical applications, including transplantation and gene therapy. In mice, using a complex combination of positive and negative selection for 10 to 12 surface markers (Lin−Sca-1+c-kit+Thy-1lo), 1 in 4.9 cells provides long-term reconstitution after intravenous injection.1 However, the use of these complex sets of markers, alone or in combination with the established isolation scheme based on Hoechst dye efflux (side population),2 is incompatible with in situ histologic analyses.
Recently, a simple and broadly applicable method to isolate mouse HSCs has been developed based on expression of cell surface markers, which are members of the signaling lymphocyte activation molecule (SLAM) family, including CD150, CD48, and CD244.1 In transplantation assays, 1 in 4.8 CD150+CD48− bone marrow (BM) cells provides long-term, multilineage reconstitution in recipient mice, similar to the enrichment obtained in the complex Lin−Sca-1+c-kit+Thy-1lo population.1 This same strategy has been successfully applied to enrich HSCs from mouse cyclophosphamide/granulocyte-colony stimulating factor (G-CSF)–mobilized cells,3 mouse fetal liver,4 as well as from the BM of various strains of mice5 and older mice.3 Recent data examining the overlap between SLAM family member expression with the Hoechst dye efflux side population in conjunction with canonical HSC cell surface markers have confirmed the potential of CD150+ selection for mouse HSC enrichment.6 Although it has been suggested that some HSC activity may also be present in the CD150− cell fraction of mouse cells,6 data from multiple groups indicate that there is little or no long-term HSC activity in the CD150− fraction of mouse hematopoietic cells.1,3,4,7–10
In humans, the CD34 cell surface marker is largely used in clinical applications for isolation of HSCs and progenitor cells, and as a predictor of graft HSC content in transplants. The combination CD34+CD38− provides further enrichment (1 in 600 to 1 in 3500),11 but the heterogeneity of this population precludes studies requiring levels of purity achieved in mouse models, such as comparing gene expression profiles or in situ histologic analyses. Given the use of SLAM receptors for identifying long-term repopulating HSCs in mice,1,3,4,7–10 we hypothesized that this strategy may similarly facilitate the isolation of highly enriched populations of HSCs in humans and nonhuman primates. In this study, we isolated SLAM (CD150+CD48−) and non-SLAM (not CD150+CD48−) cells from human umbilical cord blood (UCB) cells as well as human and primate cytokine mobilized peripheral blood (MPB) cells and compared their ability to form colonies in vitro and to reconstitute immune-deficient (nonobese diabetic/severe combined immunodeficiency/interleukin-2 γc receptornull, NSG) mice.
Methods
Collection of hematopoietic cells from healthy donors and from rhesus macaques
Human MPB CD34+ cells were obtained from 3 healthy volunteers (donor 1, 25-year-old woman; donor 2, 21-year-old man; and donor 3, 24-year-old man) after informed consent in accordance with the Declaration of Helsinki, under an institutional review board-approved clinical protocol. The donors received 5 days of G-CSF (filgrastim; Amgen) 10 μg/kg and underwent leukapheresis and CD34+ cell enrichment as previously described.12 The CD34+ cells were cryopreserved before isolation of SLAM (CD150+CD48−) and non-SLAM (not CD150+CD48−) cells.
Human UCB samples were obtained from the National Heart, Lung and Blood Institute Biologic Specimen and Data Repository Coordinating Center. CD34+ cells were collected from 8 pooled UCB samples. Each cryopreserved UCB unit was thawed at 37°C and washed in normal saline buffer containing 5% dextran (Hospira) and 2.5% human serum albumin (Baxter Healthcare). Cells were resuspended in normal saline containing 1% human serum albumin, pooled, and CD34+ cells were enriched as previously described12 before isolation of SLAM and non-SLAM cells.
The rhesus macaque (Macaca mulatta) used in these studies (RQ6566, 4-year-old female) was housed and handled in accordance to guidelines outlined in a protocol approved by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute. Stem cell mobilization and apheresis were as described.13 The CD34+ cells had a purity of 90% and were used without prior cryopreservation for isolation of SLAM and non-SLAM cells.
Isolation of SLAM and non-SLAM cells
Human and rhesus macaque SLAM and non-SLAM cells were isolated using human anti-CD150-fluorescein isothiocyanate (clone A12, eBioscience) and anti-CD48-RPE (clone MEM102, AbD Serotec) monoclonal antibodies. Cell sorting was performed using a FACSVantage cell sorter (BD Biosciences). Gates were defined strictly using isotype controls to eliminate any potential overlap between CD150+ and CD150− cells.
NSG mouse repopulation assay
Sublethally irradiated (300 cGy) 8-week-old NSG mice were transplanted by tail-vein injection with 1 × 103, 1 × 104, 1 × 105, or 1 × 106 human SLAM cells, non-SLAM cells, or unfractionated CD34+ cells. Mice transplanted with less than 1 × 105 cells also received 1 × 105 CD34− carrier cells (99.2% purity). At week 8 after transplantation, mice were killed and BM mononuclear cells were harvested from both femurs and tibias. Aliquots were stained with human CD45-phycoerythrin (PE), CD20-PE, CD3-PE, CD36-PE, and CD34-PE antibodies (all from BD Biosciences) and analyzed on a Coulter FC500 analyzer (Beckman Coulter) for detection of human cell engraftment.
CFU assay
For colony-forming unit (CFU) assays, 2 × 105 transplanted mouse BM cells were seeded in duplicates in 1 mL methylcellulose media (GF+H4435, Stem Cell Technologies) at 37°C in 5% CO2. Colonies of more than 50 cells were scored after 10 to 14 days of incubation.
Results and discussion
Cell surface receptors of the SLAM family, including CD150, CD244, and CD48, were found to be differentially expressed among mouse HSCs (CD150+CD244−CD48−) and more restricted progenitors (CD150−CD244+CD48+).1 Using CFU assays and transplantation studies in NSG mice, we investigated whether SLAM markers could also be used to distinguish human progenitors and more primitive repopulating cells (referred to as human SCID repopulating cells [hSRCs]11).
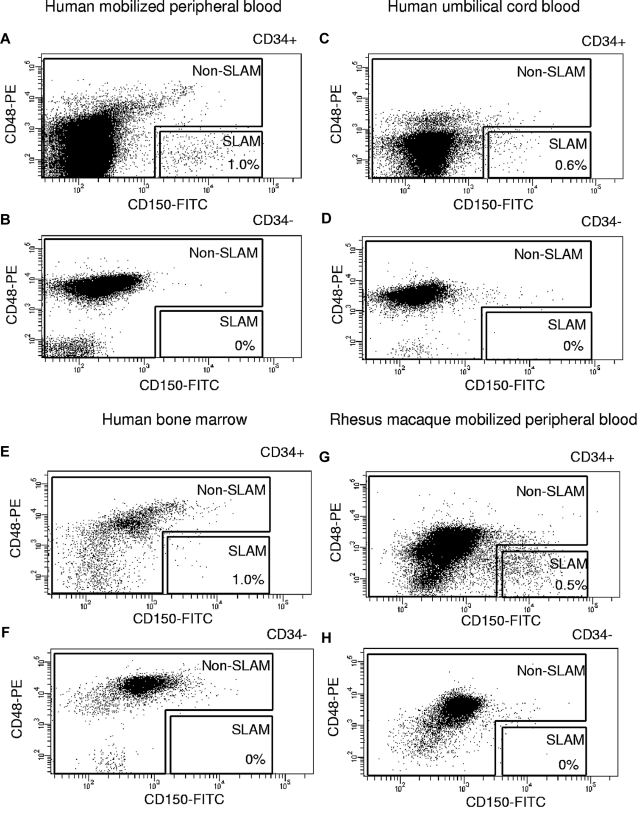
Because human CD150+CD48− cells were uniformly CD244−,1 we omitted the CD244 marker and tested the in vivo repopulating and colony-forming potential of CD150+CD48− cells (SLAM cells) compared with non-SLAM cells, which included all viable cells outside the SLAM gate (not CD150+CD48−). These 2 populations were first sorted from human G-CSF-MPB cells derived from 3 normal volunteers and pre-enriched in CD34+ cells (99% purity) to facilitate sorting of the rare CD150+CD48− cells. Using stringent gates defined with isotype controls, we found that 0.4% to 1.1% of human CD34+ cells were CD150+CD48− (Figure 1A). Variable percentages of the SLAM population were in the CD38− fraction (donor 1, 8%; donor 2, 94%; and donor 3, 87%). No significant numbers of SLAM cells were detected in the CD34− fraction in any donor (Figure 1B). Therefore, the pre-enrichment step in CD34+ cells performed before sorting cells based on the SLAM markers does not result in loss of SLAM cells. Similar results were obtained using human UCB cells (Figure 1C-D), human BM cells (Figure 1E-F), and rhesus macaque MPB cells (Figure 1G-H), indicating a consistent pattern of CD150 and CD48 expression in various sources of hematopoietic cells from large animals.
Figure 1.
Cell sorting experiments of SLAM (CD150+CD48−) and non-SLAM (not CD150+CD48−) populations. Human G-CSF MPB CD34+ cells (A) and CD34− cells (B). Human UCB CD34+ cells (C) and CD34− cells (D). Human BM CD34+ cells (E) and CD34− cells (F). Rhesus macaque MPB CD34+ cells (G) and CD34− cells (H). No significant numbers of SLAM cells are detected in the CD34− population from any hematopoietic source.
In CFU assays, SLAM, non-SLAM, and unfractionated CD34+ cell populations obtained from human G-CSF MPB and human UCB gave rise to colonies of similar size, phenotype, and frequency (supplemental Figure 1A and supplemental Figure 1B, respectively, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) after 10 to 14 days in culture. When rhesus macaque-mobilized CD34+ cells were used in CFU assays, the size, phenotype, and number of colonies obtained from SLAM and non-SLAM populations were also similar to the CFUs derived from unfractionated CD34+ cells (supplemental Figure 1C).
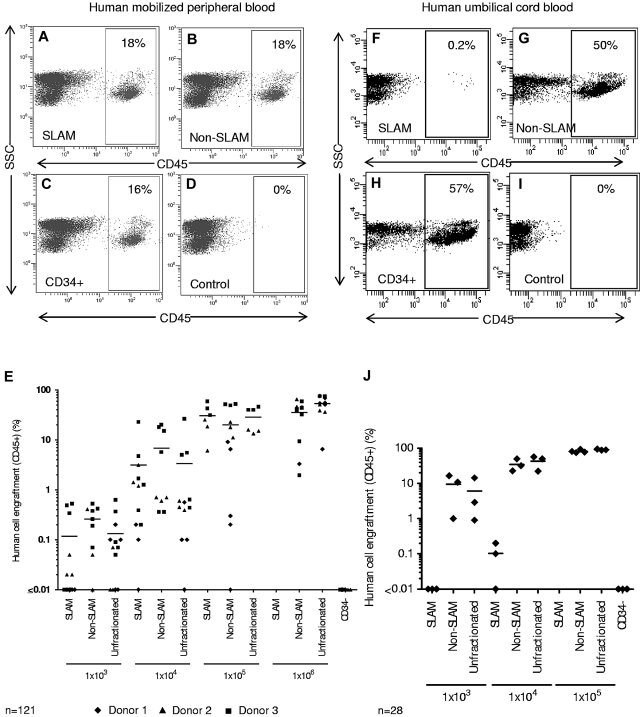
Surprisingly, both SLAM and non-SLAM populations derived from human MPB CD34+ cells from 3 independent donors had in vivo repopulating potential after intravenous transplantation in NSG mice, and levels of human cell engraftment based on CD45 cell surface expression were similar to those obtained with unfractionated CD34+ cells (Figure 2A-E). The human SCID repopulating cell activity in each fraction had a comparable ability to repopulate mice with multiple hematopoietic lineages (data not shown). CD34+ SLAM cells derived from human UCB provided only minimal contribution to long-term hematopoietic reconstitution in NSG mice compared with non-SLAM CD34+ cells and unfractionated CD34+ cells (Figure 2F-J). Rhesus SCID repopulating cells were also detected in both SLAM and non-SLAM populations derived from MPB CD34+ cells, and levels of engraftment were similar to those obtained after transplantation of unfractionated CD34+ cells (supplemental Figure 2). These data confirm that both human and rhesus macaque primitive repopulating cells from various hematopoietic sources are partially contained within the SLAM fraction of cells, but SCID repopulating cells cannot be isolated based only on SLAM family markers.
Figure 2.
Summary of human cell engraftment based on CD45 cell surface expression in the BM of nonobese diabetic/severe combined immunodeficiency/interleukin-2 γc receptornull mice transplanted with SLAM (CD150+CD48−), non-SLAM (not CD150+CD48−), or unfractionated CD34+ cells. Representative flow cytometry analysis after transplantation of 1 × 105 SLAM (A), non-SLAM (B), or unfractionated human CD34+ cells (C) derived from G-CSF MPB. (D) Control mice were injected intravenously with an equal volume of saline. (E) Summary of human cell engraftment after transplantation of G-CSF MPB cells from 3 independent donors in NSG mice. Each symbol represents one mouse, and the horizontal lines indicate the mean levels of human cell engraftment (n = 121 mice). Representative flow cytometry experiment after transplantation of 1 × 104 SLAM (F), non-SLAM (G), or unfractionated human CD34+ cells (H) derived from 8 pooled cord blood samples. (I) Control animals were transplanted with an equal volume of saline. (J) Summary of human cell engraftment after transplantation of cord blood derived cells in NSG mice (n = 28 mice).
To rule out the possibility that these results could be explained by the existence of other post-translationally modified forms of CD150 present on human HSCs not recognized by the antibodies used in this study, we performed real-time reverse-transcriptase polymerase chain reaction to assess CD150 expression on human MPB (n = 3) and UCB (n = 3) CD34+CD38− cells. CD150 expression was 10-fold lower in this population compared with unfractionated mononuclear cells. This contrasts with our ability to detect enrichment of CD150 RNA in mouse HSCs. In addition, using flow cytometry, we were unable to detect increased staining on human CD34+CD38− cells using multiple CD150 antibodies (data not shown).
In contrast to mouse studies, our data in humans and rhesus macaques using UCB and MPB cells indicate that SCID repopulating cells and progenitor cells can be found in both SLAM and non-SLAM populations based on the available antibodies against human and rhesus macaque CD150 and CD48. Therefore, these combinations of antibodies against SLAM markers are not suitable for purification or in situ histologic analyses of HSCs in large mammals. The results derived from the functional studies presented here correlate with results of recently published flow cytometry analyses showing that the patterns of expression of the SLAM family receptors differ between mice and humans.14 Sintes et al14 hypothesized, based on expression patterns, that the most primitive human repopulating cells may be predominantly CD150−CD48+, an observation contrasting with findings in mice1 where HSCs are enriched in the CD150+CD48− population. This observation is not uncommon and is evocative of the CD34 surface marker expression detected on the majority of human HSCs but absent on quiescent mouse HSCs.15–17 It is also reminiscent of the absence of the differentiation marker CD38 on human HSCs11,18–21 and its presence on mouse HSCs.22–26 Additional transplantation studies will be useful to further characterize the repopulating potential of the CD150−CD48+ population in large mammals.
Supplementary Material
Acknowledgments
The authors thank D. Stroncek, J. Procter, M. Sabatino, and S. Leitman for providing human CD34+ cells; Sue Ellen Frodigh and Quyen Chau for processing UCB samples; R. Donahue, M. Metzger, and A. Krouse for mobilization and apheresis of rhesus macaques; D. Adams, M. White, and the University of Michigan Flow Cytometry Core Facility for support; and the animal core facility staff at the National Institutes of Health and the University of Michigan for excellent animal care.
This work was supported in part by the intramural research program of the National Heart, Lung and Blood Institute of the National Institutes of Health and in part by the Howard Hughes Medical Institute and by a Michigan Institute for Clinical and Health Research Pilot Grant.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.L. and M.S. designed and performed the experimental procedures and analyzed the data; A.L. wrote the manuscript; M.S. contributed to the editing; M.W., S.A., and B.I. performed some of the transplantation studies; K.K. sorted the various cell populations; and C.E.D. and S.J.M. designed the experiments and edited the manuscript.
Conflict-of-interest disclosure: S.J.M. is an author on a patent for the use of SLAM family markers for the isolation of mammalian HSCs. The remaining authors declare no competing financial interests.
Correspondence: Cynthia E. Dunbar, National Heart, Lung and Blood Institute, National Institutes of Health, Bldg 10 CRC, Rm 4-5132, 10 Center Dr, MSC-1202, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.
References
- 1.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107(3):924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108(2):737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Ellison FM, Keyvanfar K, et al. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36(10):1236–1243. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111(4):2444–2451. doi: 10.1182/blood-2007-09-115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453(7192):228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 8.Foudi A, Hochedlinger K, Van BD, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27(1):84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiel MJ, Yilmaz OH, Morrison SJ. CD150− cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111(8):4413–4414. doi: 10.1182/blood-2007-12-129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Larochelle A, Vormoor J, Hanenberg H, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2(12):1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 12.Bolan CD, Cecco SA, Wesley RA, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42(7):935–946. doi: 10.1046/j.1537-2995.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- 13.Donahue RE, Kirby MR, Metzger ME, Agricola BA, Sellers SE, Cullis HM. Peripheral blood CD34+ cells differ from bone marrow CD34+ cells in Thy-1 expression and cell cycle status in nonhuman primates mobilized or nonmobilized with granulocyte colony-stimulating factor and/or stem cell factor. Blood. 1996;87(4):1644–1653. [PubMed] [Google Scholar]
- 14.Sintes J, Romero X, Marin P, Terhorst C, Engel P. Differential expression of CD150 (SLAM) family receptors by human hematopoietic stem and progenitor cells. Exp Hematol. 2008;36(9):1199–1204. doi: 10.1016/j.exphem.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3(12):1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 16.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Laver JH, Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells [see comments]. Blood. 1999;94(8):2548–2554. [PubMed] [Google Scholar]
- 18.Novelli EM, Ramirez M, Civin CI. Biology of CD34+. Leuk Lymphoma. 1998;31(3):285–293. doi: 10.3109/10428199809059221. [DOI] [PubMed] [Google Scholar]
- 19.Kerre TC, De Smet G, De Smedt M, et al. Both CD34+38+ and CD34+38− cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J Immunol. 2001;167(7):3692–3698. doi: 10.4049/jimmunol.167.7.3692. [DOI] [PubMed] [Google Scholar]
- 20.Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci U S A. 2002;99(1):413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstegen MMA, van Hennik PB, Terpstra W, et al. Transplantation of human umbilical cord blood cells in macrophage-depleted SCID mice: evidence for accessory cell involvement in expansion of immature CD34+CD38− cells. Blood. 1998;91(6):1966–1976. [PubMed] [Google Scholar]
- 22.Dagher RN, Hiatt K, Traycoff C, Srour EF, Yoder MC. c-Kit and CD38 are expressed by long-term reconstituting hematopoietic cells present in the murine yolk sac. Biol Blood Marrow Transplant. 1998;4(2):69–74. doi: 10.1053/bbmt.1998.v4.pm9763109. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi Y, Zeng H, Ogawa M. CD38 expression by hematopoietic stem cells of newborn and juvenile mice. Leukemia. 2003;17(1):171–174. doi: 10.1038/sj.leu.2402785. [DOI] [PubMed] [Google Scholar]
- 24.Randall TD, Lund FE, Howard MC, Weissman IL. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood. 1996;87(10):4057–4067. [PubMed] [Google Scholar]
- 25.Tajima F, Deguchi T, Laver JH, Zeng H, Ogawa M. Reciprocal expression of CD38 and CD34 by adult murine hematopoietic stem cells. Blood. 2001;97(9):2618–2624. doi: 10.1182/blood.v97.9.2618. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Lin Y, Zhan Y, et al. Murine hematopoietic stem cell characterization and its regulation in BM transplantation. Blood. 2000;96(9):3016–3022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.