Abstract
Quantitative real-time PCR assays targeting the groEL gene for the specific enumeration of 12 human fecal Bifidobacterium species were developed. The housekeeping gene groEL (HSP60 in eukaryotes) was used as a discriminative marker for the differentiation of Bifidobacterium adolescentis, B. angulatum, B. animalis, B. bifidum, B. breve, B. catenulatum, B. dentium, B. gallicum, B. longum, B. pseudocatenulatum, B. pseudolongum, and B. thermophilum. The bifidobacterial chromosome contains a single copy of the groEL gene, allowing the determination of the cell number by quantification of the groEL copy number. Real-time PCR assays were validated by comparing fecal samples spiked with known numbers of a given Bifidobacterium species. Independent of the Bifidobacterium species tested, the proportion of groEL copies recovered from fecal samples spiked with 5 to 9 log10 cells/g feces was approximately 50%. The quantification limit was 5 to 6 log10 groEL copies/g feces. The interassay variability was less than 10%, and variability between different DNA extractions was less than 23%. The method developed was applied to fecal samples from healthy adults and full-term breast-fed infants. Bifidobacterial diversity in both adults and infants was low, with mostly ≤3 Bifidobacterium species and B. longum frequently detected. The predominant species in infant and adult fecal samples were B. breve and B. adolescentis, respectively. It was possible to distinguish B. catenulatum and B. pseudocatenulatum. We conclude that the groEL gene is a suitable molecular marker for the specific and accurate quantification of human fecal Bifidobacterium species by real-time PCR.
INTRODUCTION
Microbial colonization of the initially sterile intestine starts with the exposure of the newborn to microbes from the mother and the environment. In this phase, Bifidobacterium species become predominant, representing 60 to 91% of fecal bacteria in breast-fed infants and 28 to 75% in formula-fed infants (17). At 2 years of age, when the complex gut microbiota is fully established, the proportion of fecal bifidobacteria decreases to 1 to 3% (41). Even lower levels or absence of bifidobacteria have been reported for the elderly (3, 15, 19, 38).
The establishment of the intestinal microbiota in early life is a critical phase that affects the maturation of the immune system. Numerous studies show that breast-fed infants have a lower incidence of gastrointestinal infections and atopic diseases than formula-fed infants (20, 46). It has been proposed that the health-promoting effect of breast milk is in part mediated by the intestinal microbiota, which is characterized by low diversity and a high proportion of bifidobacteria (11).
Common Bifidobacterium species of the human intestinal microbiota include Bifidobacterium adolescentis, B. angulatum, B. bifidum, B. breve, B. catenulatum, B. dentium, B. longum, and B. pseudocatenulatum (7). B. gallicum has rarely been detected (6, 7). Recently, B. pseudolongum and B. thermophilum were isolated from human adult and baby feces, respectively (44, 53). These species have previously been considered to be of animal origin (7). B. animalis subsp. lactis is commonly used as probiotic and thus may be detected in human feces.
Bifidobacteria are considered beneficial members of the gut microbiota. The assessment of their diversity and population size in the gastrointestinal tract is therefore important. However, only a few studies have enumerated bifidobacteria to the species level, and the majority of them used cultivation-based techniques (7). The latter are not only laborious but also hampered by partly fastidious growth conditions of bifidobacteria and by the lack of growth medium selectivity (2).
Since the advent of cultivation-independent methods, the 16S rRNA gene has been widely used as a valuable tool for bacterial identification (12). However, the resolution power of the 16S rRNA gene among closely related species is limited. Isolates displaying more than 97% 16S rRNA gene sequence identity are usually considered the same species. Since Bifidobacterium species reveal a relatively high 16S rRNA gene sequence identity (mean, 95% [31, 47]), more discriminative identification markers are needed.
Alternative target genes for the differentiation of Bifidobacterium species include housekeeping genes, such as atpD (49), dnaK (52), groEL (21, 27, 47, 50, 55), groES (50), recA (23, 51), tal (37), tuf (48, 51), and xfp (5, 47, 54). All these genes except groES were demonstrated to have similar or even higher discriminating power for bifidobacteria than the 16S rRNA gene. Unfortunately, only a limited number of sequences of these marker genes are available. The most sequences exist for the groEL gene. The Chaperonin Sequence Database (http://www.cpndb.ca; 18) currently contains more than 13,000 entries for prokaryotes, eukaryotes, and archaea, 121 of which belong to 27 Bifidobacterium species. The groEL gene encodes the chaperonin GroEL (synonyms are Cpn60, GroL, Hsp60, and MopA), which plays an essential role in the handling of cellular stress. For example, it promotes refolding of misfolded polypeptides. Southern blot experiments and sequence analysis of whole genomes have revealed that there is just one groEL copy per genome in bifidobacteria (14, 42, 50, 56). This facilitates quantitative analyses of bifidobacteria.
Here, we report the quantification of Bifidobacterium species in human feces with quantitative real-time PCR (qPCR) using groEL as a discriminative marker. Ninety-seven partial (∼600-bp) or complete (∼1,600-bp) groEL sequences of 12 Bifidobacterium species (Table 1) were used to design species-specific primers. They were applied to SYBR green I chemistry-based qPCR for quantification of Bifidobacterium species. The targeted species include members of the human gut microbiota and B. animalis, some strains of which are used as probiotics.
Table 1.
Bacterial strains used for testing the specificity of the developed primers by qPCR
| Bifidobacterium strain(s)b | qPCR results with primersa: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B_ado-f/B_ado-r | B_ang-f/B_ang-r | B_ani-f/B_ani-r | B_bif-f/B_bif-r | B_bre-f/B_bre-r | B_cat-f/B_cat-r | B_den-f/B_den-r | B_gal-f/B_gal-r | B_lon-f/B_lon-r | B_pcat-f/B_pcat-r | B_plon-f/B_plon-r | B_the-f/B_the-r | |
| B. adolescentisDSM 20083T, DSM 20086 | + | − | − | − | − | − | − | − | − | − | − | − |
| B. angulatumATCC 27535T, DSM 20225 | − | + | − | − | − | − | − | − | − | − | − | − |
| B. animalis DSM 20105 | − | − | + | − | − | − | − | − | − | − | − | − |
| B. animalis subsp. animalis DSM 20104T | − | − | + | − | − | − | − | − | − | − | − | − |
| B. animalis subsp. lactisDSM 10140T | − | − | + | − | − | − | − | − | − | − | − | − |
| B. bifidumDSM 20456T, DSM 20215, DSM 20239 | − | − | − | + | − | − | − | − | − | − | − | − |
| B. breveDSM 20213T, DSM 20091 | − | − | − | −c | + | − | − | − | − | − | − | − |
| B. catenulatumATCC 27539T, DSM 20224 | − | − | − | − | − | + | − | − | − | − | − | − |
| B. dentiumATCC 27534T, ATCC 27678, DSM 20084 | − | − | − | − | − | − | + | − | − | − | − | − |
| B. gallicumDSM 20093T | − | − | − | − | − | − | − | + | − | − | − | − |
| B. longum subsp. longumATCC 15707T | − | − | − | − | − | − | − | − | + | − | − | − |
| B. longum subsp. suis DSM 20211T | − | − | − | − | − | − | − | − | + | − | − | − |
| B. pseudocatenulatumATCC 27919T, DSM 20439 | − | − | − | − | − | − | − | − | − | + | − | − |
| B. pseudolongum subsp. pseudolongumATCC 25526T, DSM 20094 | − | − | − | − | − | − | − | − | − | − | + | − |
| B. pseudolongum subsp. globosum DSM 20092T | − | − | − | − | − | − | − | − | − | − | + | − |
| B. thermophilumDSM 20210T, DSM 20209, DSM 20212 | − | − | − | − | − | − | − | − | − | − | − | + |
In addition to the bifidobacterial strains listed, negative qPCR results were obtained for the following bacterial strains: B. producta DSM 2950T, B. thetaiotaomicron DSM 2079T, B. vulgatus DSM 1447T, Clostridium histolyticum DSM 2158T, Escherichia coli DSM 30083T, Faecalbacterium prausnitzii DSM 17677, Lactobacillus acidophilus DSM 20079T, Lactobacillus gasseri DSM 20243T, Ruminococcus albus DSM 20455T, and Ruminococcus gauvreauii DSM 19829T. +, specific PCR product; −, no PCR product.
The underlined strains served as representative strains for the respective species and were used to generate qPCR standards.
In the case of B. breve DSM 20091, a nonspecific PCR product was observed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains listed in Table 1 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ), Braunschweig, Germany, and the American Type Culture Collection (ATCC), Manassas, VA. All bacteria were cultured anaerobically under a gas phase of N2-CO2 (80:20 [vol/vol]) in 15-ml Hungate tubes containing 10 ml ST medium, except for Faecalibacterium prausnitzii, which was grown on YCFA GSC medium (NCIMB Ltd., Aberdeen, Scotland) (9). The cultures were incubated overnight at 37°C and grown to a density of 9 to 10 log10 cells/ml.
The purity of bacterial cultures was checked by Gram staining and plating on Columbia agar with 5% sheep blood (bioMérieux, Vienna, Austria). The identities of the Bifidobacterium strains underlined in Table 1 (used to generate qPCR standards) were confirmed at the species level by sequencing either the 16S rRNA gene or the groEL gene (Eurofins MWG Operon, Ebersberg, Germany).
Subjects and fecal samples.
Five healthy, full-term, exclusively breast-fed infants (4 males and 1 female) up to 3 months old with birth weights of 2,500 g to 4,500 g were included in the study. The infants had been born by caesarean section, except INF-4, who had been born by vaginal delivery. Infants undergoing antibiotic therapy during the first 14 days of life were excluded. Newborns were enrolled in a clinical study whose protocol was reviewed and approved by an independent ethics committee, and informed written consent was obtained from a legal representative(s). Fecal samples from infants were collected within 30 min after defecation and were kept in an anaerobic jar with a reduced atmosphere created by AnaeroGen (Oxoid, United Kingdom) at 4°C for up to 8 h until they were stored at −20°C. Ten healthy adults from our institute staff (5 males and 5 females), 20 to 40 years old, voluntarily provided fecal samples. They did not have any gastrointestinal disorders during sample collection and did not undergo antibiotic treatment in the 6 months prior to sampling. The fecal samples from the adults were stored at −20°C within 2 h after defecation.
Extraction of DNA from bacterial cultures.
Genomic DNA of Bifidobacterium strains was extracted from 1-ml overnight cultures with the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions for Gram-positive bacteria. Since genomic DNA of B. longum subsp. longum ATCC 15707T was used as a standard for quantification of total bifidobacteria by qPCR, high yields of DNA were required and were obtained with the RTP Invitek Bacteria Mini Kit (Invitrogen, Darmstadt, Germany). The manufacturer's protocol for Gram-positive bacteria was followed with some modifications. After enzymatic cell lysis, an additional mechanical lysis was performed in a 2-ml tube containing 0.75 g of sterile zirconium/silica beads (0.1 mm in diameter; Roth, Karlsruhe, Germany) by running the Fastprep FP120 Instrument (Thermo Electron Corp., Waltham, MA) at speed level 4 for 8 min. Genomic DNA of non-Bifidobacterium strains was extracted with the RTP Invitek Bacteria Mini Kit (Invitrogen, Darmstadt, Germany) following the manufacturer's instructions.
Extraction of bacterial DNA from fecal samples.
Bacterial DNA was extracted from frozen fecal material using the QIAamp DNA stool kit (Qiagen, Hilden, Germany) with a modified protocol for cell lysis. After homogenization of 220 mg feces with 1.2 ml lysis buffer from the kit by vortexing for 2 min in a 2-ml tube containing 0.75 g of sterile zirconium/silica beads (0.1 mm in diameter; Roth, Karlsruhe, Germany), the final suspension was incubated at 95°C for 15 min with continuous shaking (1,400 min−1; Thermomixer 5436; Eppendorf, Hamburg, Germany). The sample was allowed to cool on ice for 2 min. Cells were mechanically lysed by Fastprep treatment for 8 min and 15 s as described above. After cooling on ice for 2 min, coarse particles, cell debris, and the zirconium/silica beads were spun down by centrifugation (20,000 × g; 4°C; 1 min), and the supernatant was transferred to a 2-ml tube. The pellet was mixed with 350 μl lysis buffer from the kit, vortexed for 1 min, and incubated at 95°C for 5 min with continuous shaking as described above. After centrifugation at 20,000 × g and 4°C for 1 min, the supernatants were combined. DNA-damaging substances and PCR inhibitors present in the stool samples were removed by their adsorption to the InhibitEX matrix provided in the kit. The InhibitEX matrix was separated by centrifugation at 20,000 × g for 6 min, and the supernatant was collected and filled up to 1 ml with sterile phosphate-buffered saline (pH 7). DNA was purified with the QIAcube (Qiagen, Hilden, Germany) and eluted from the silica-based membrane with 200 μl ultrapure water.
groEL sequence analysis and design of species-specific primers.
groEL sequences belonging to target Bifidobacterium species (Table 2) and available in GenBank release 185.0 (4) were subjected to a multiple alignment with the program ClustalW2 version 2.1 (24) provided by the European Bioinformatics Institute (http://www.ebi.ac.uk). A region of approximately 600 bp located at positions ∼250 to 840 of the complete groEL gene of ca. 1,600 bp was selected to identify discriminative target sites for the species-specific detection of bifidobacteria. Primer pairs for 12 Bifidobacterium species (Table 3) were manually designed on the basis of 97 partial and complete groEL sequences (Table 2).
Table 2.
groEL sequences used for the design of Bifidobacterium species-specific primers
| Bifidobacterium species | Strain | GenBank accession no. | Size (bp) |
|---|---|---|---|
| B. adolescentis | JCM 1275T | AF210319 | 1,749a |
| ATCC 15703T | AP009256 | 1,617a | |
| B. angulatum | JCM 7096T | AF240568 | 590b |
| B. animalis | B83 | AY166539 | 590b |
| D1 | AY166540 | 590b | |
| III-1 | AY166541 | 590b | |
| III-6 | AY166542 | 590b | |
| subsp. animalis | JCM 1190T | AY004273 | 590b |
| ATCC 25527T | AY488178 | 1,155b | |
| TEEV 1/10 | HQ851043 | 587b | |
| TEEV 3/10 | HQ851044 | 598b | |
| TEEV 1/11 | HQ851047 | 586b | |
| TEEV 4/9 AG | HQ851048 | 608b | |
| subsp. lactis | HN019 | ABOT01000006 | 1,614a |
| 9952 | AF286735 | 590b | |
| DSM 10140T | AY004282 | 1,614a | |
| 9950 | AY004287 | 590b | |
| JB-1 | AY166543 | 590b | |
| NCC 363 | AY488176 | 1,155b | |
| NCC 402 | AY488177 | 1,155b | |
| DSM 27674 | AY488179 | 1,155b | |
| DSM 10140T | AY488180 | 1,155b | |
| ATCC 27536 | AY488181 | 1,155b | |
| NCC 239 | AY488182 | 1,155b | |
| ATCC 27672 | AY488183 | 1,155b | |
| LMG 18906 | AY586539 | 1,614a | |
| AD011 | CP001213 | 1,614a | |
| Bl-04 ATCC SD5219 | CP001515 | 1,614a | |
| DSM 10140T | CP001606 | 1,614a | |
| BB-12 | CP001853 | 1,614a | |
| V9 | CP001892 | 1,614a | |
| CNCM I-2494 | CP002915 | 1,614a | |
| BLC1 | CP003039 | 1,614a | |
| B. bifidum | JCM 1255T | AY004280 | 590b |
| PRL2010 | CP001840 | 1,626a | |
| S17 | CP002220 | 1,626a | |
| B. breve | DSM 20213T | ACCG02000012 | 1626a |
| DSM 20213T | AF240566 | 584b | |
| B. catenulatum | JCM 1194T | AY004272 | 590b |
| JCM 7130 | AY166565 | 590b | |
| B. dentium | ATCC 27679 | AEEQ01000009 | 1,617a |
| JCVIHMP022 | AEHJ01000021 | 1,617a | |
| JCM 1195T | AF240572 | 590b | |
| B. gallicum | DSM 20093T | ABXB03000002 | 1,611a |
| JCM 8224T | AF240575 | 592b | |
| B. longum | N10 | FJ577573 | 552b |
| N103 | FJ577576 | 552b | |
| N81 | FJ577625 | 552b | |
| N86 | FJ577629 | 552b | |
| N88 | FJ577630 | 564b | |
| subsp. infantis | JCM 1210 | AF240576 | 591b |
| JCM 1222T | AF240577 | 591b | |
| ATCC 15697T | AP010889 | 1,626a | |
| 157F | AP010890 | 1,626a | |
| 9901 | AY004286 | 591b | |
| bb52 | AY004288 | 591b | |
| 6w-50 | AY166564 | 590b | |
| D6-9-6 | AY166566 | 590b | |
| D30-3-3 | AY166567 | 590b | |
| D30-3-10 | AY166568 | 590b | |
| D30-3-11 | AY166569 | 590b | |
| L2 | AY166570 | 590b | |
| subsp. longum | ATCC 15697T | CP001095 | 1,626a |
| ATCC 55813 | ACHI01000023 | 1,626a | |
| NCC 2705 | AE014295 | 1,626a | |
| JCM 1217T | AF240578 | 590b | |
| L4 | AY166571 | 590b | |
| TJ | AY166572 | 593b | |
| JCM 7052 | AY166573 | 590b | |
| JCM 7053 | AY166574 | 590b | |
| ATCC 15707T | AY835622 | 552b | |
| DJO10A | CP000605 | 1,626a | |
| JDM 301 | CP002010 | 1,626a | |
| BBMN 68 | CP002286 | 1,626a | |
| KACC 91563 | CP002794 | 1,626a | |
| F8 | FP929034 | 1,626a | |
| R0175 | HM009034 | 1,626a | |
| subsp. suis | JCM 1269T | AY013248 | 591b |
| JCM 7139 | AY166575 | 590b | |
| TEEV 4/9 | HQ851041 | 590b | |
| B. pseudocatenulatum | DSM 20438T | AY004274 | 589b |
| JCM 7040 | AY166552 | 590b | |
| T1-3-15 | AY166553 | 590b | |
| T2-3-4 | AY166554 | 590b | |
| Z2-3 | AY166555 | 590b | |
| B. pseudolongum subsp. globosum | JCM 5820T | AF286736 | 591b |
| 1-23-3 | AY166544 | 590b | |
| 1-25-3 | AY166545 | 590b | |
| 02-2 | AY166546 | 590b | |
| 7#-3-7 | AY166547 | 590b | |
| 7#-10-11 | AY166548 | 590b | |
| 09-25-2 | AY166549 | 590b | |
| Fb9 | AY166550 | 590b | |
| MU8 | AY166551 | 590b | |
| subsp. pseudolongum | JCM 1205T | AF240573 | 591b |
| B. thermophilum | JCM 1207T | AF240567 | 591b |
| RBL67 | DQ340558 | 590b |
Complete coding sequence.
Partial coding sequence.
Table 3.
Bifidobacterium species-specific primers based on the groEL genea
| Target species | Primerb | Sequence (5′ to 3′)c | Primer concn (nM) | Annealing temp (°C) | Amplicon |
|
|---|---|---|---|---|---|---|
| Size (bp) | Melting temp (°C) | |||||
| B. adolescentis | B_ado-f | CTCCGCCGCTGATCCGGAAGTCG | 300 | 75 | 268 | 88.0 |
| B_ado-r | AACCAACTCGGCGATGTGGACGACA | |||||
| B. angulatum | B_ang-f | CTGTCCTCCCAGCAGGACGTGGTC | 300 | 80 | 97 | 85.5 |
| B_ang-r | GCGCTTCGCCGTCAACGTCTTCGG | |||||
| B. animalis | B_ani-f | CACCAATGCGGAAGACCAG | 250 | 64 | 184 | 87.5 |
| B_ani-r | GTTGTTGAGAATCAGCGTGG | |||||
| B. bifidum | B_bif-f | CTCCGCAGCCGACCCCGAGGTT | 300 | 64 | 233 | 88.5 |
| B_bif-r | TGGAAACCTTGCCGGAGGTCAGG | |||||
| B. breve | B_bre-f | GCTCGTCGTTGCCGCCAAGGACGTT | 300 | 72 | 272 | 88.0 |
| B_bre-r | ACAGAATGTACGGATCCTCGAGCACG | |||||
| B. catenulatum | B_cat-f | GGCTATCGTCAAGGAGCTCA | 300 | 64 | 188 | 87.0 |
| B_cat-r | AGTCCAGATCCAAACCGAAAC | |||||
| B. dentium | B_den-f | GGCCCAGTCTTTGGTGCATGAAGGCC | 300 | 75 | 364 | 89.5 |
| B_den-r | GTCTTCGAGCACCGCGGTCTGGTCC | |||||
| B. gallicum | B_gal-f | AGCTCGTCAAGTCCGCCAAGC | 300 | 68 | 188 | 87.5 |
| B_gal-r | CATACCTTCGGTGAACTCGAGG | |||||
| B. longum | B_lon-f | CGGCGTYGTGACCGTTGAAGAC | 250 | 70 | 259 | 88.0 |
| B_lon-r | TGYTTCGCCRTCGACGTCCTCA | |||||
| B. pseudocatenulatum | B_pcat-f | AGCCATCGTCAAGGAGCTTATCGCAG | 250 | 68 | 325 | 87.5 |
| B_pcat-r | CACGACGTCCTGCTGAGAGCTCAC | |||||
| B. pseudolongum | B_plon-f | CRATYGTCAAGGAACTYGTGGCCT | 300 | 66 | 312 | 89.0 |
| B_plon-r | GCTGCGAMGAKACCTTGCCGCT | |||||
| B. thermophilum | B_the-f | ACTGGTCGCTTCCGCCAAGGATG | 300 | 66 | 326 | 88.0 |
| B_the-r | CCARGTCAGCMAGGTGRACGATG | |||||
The target sites are located at positions ∼250 to 840 of the corresponding nucleotide sequence.
f, Forward primer; r, reverse primer.
IUPAC ambiguity codes: M (A or C), K (G or T), R (A or G), Y (C or T).
Quantitative real-time PCR. (i) Standards.
For quantification of the investigated Bifidobacterium species, represented by the strains underlined in Table 1, PCR products containing the target sequence were used as qPCR standards. Therefore, part of the genomic groEL gene was amplified with primers binding to a sequence at least 17 bp up- and downstream of the species-specific primer-binding sites to prevent their degradation during storage. The 50-μl PCR mixture consisted of 1× TopTaq PCR buffer (Qiagen, Hilden, Germany), 200 μM (250 μM for B. pseudocatenulatum) of each deoxynucleoside triphosphate (dNTP) (Invitek, Berlin, Germany), 200 nM each forward and reverse primer (Table 4), 1.25 U of TopTaq DNA polymerase (Qiagen, Hilden, Germany), and 1 μl genomic DNA (30 to 110 ng) of one of the representative strains (Table 1). The reactions were performed in a thermal cycler (Thermo Hybaid MultiBlock System, Ulm, Germany) under the conditions given in Table 4. The amplification products were purified with the High Pure PCR Product Purification Kit (Roche, Mannheim, Germany) following the manufacturer's instructions. The concentration of the amplified DNA (concentrationamplicon [g/liter]) was determined spectrophotometrically at 260 nm (NanoDrop; Peqlab, Erlangen, Germany). The number of PCR products (or partial groEL gene copies) per volume was calculated as follows: (i) concentrationamplicon (g/liter)/molecular massamplicon (g/mol) = concentrationamplicon (mol/liter); (ii) concentrationamplicon (mol/liter) × NA = concentrationamplicon (molecules/liter), where NA is the Avogadro constant (6.022 × 1023 molecules/mol). The molecular massamplicon values are listed in Table 4.
Table 4.
Primers used to generate PCR products containing Bifidobacterium species-specific primer-binding sites (qPCR standards)a
| Target species | Primerb | Sequence (5′ to 3′) | PCR conditions | Amplicon |
|
|---|---|---|---|---|---|
| Size (bp) | Molecular mass (g/mol)c | ||||
| B. adolescentis | B_ado-std-f | GATCAGATCGCTGCCACCGCAAC | 94°C, 3 min; 30 times (94°C, 30 s; 69°C, 30 s; 72°C, 30 s); 72°C, 10 min | 320 | 197,799.00 |
| B_ado-std-r | ATCAGCAGCGGCTTGCCGGTCTT | ||||
| B. angulatum | B_ang-std-f | AGTGCTCGAAGACCCGTACATCCT | 94°C, 3 min; 30 times (94°C, 30 s; 65°C, 30 s; 72°C, 30 s); 72°C, 10 min | 164 | 101,387.60 |
| B_ang-std-r | GGATGTTGTTCAGGATCAGGGTCG | ||||
| B. animalis | B_ani-std-f | GTCACCGTCGAAGACAACAACC | 94°C, 3 min; 30 times (94°C, 30 s, 65°C, 30 s; 72°C, 30 s); 72°C, 10 min | 299 | 184,812.60 |
| B_ani-std-r | CAGGACTTGAAGGTGCCACGG | ||||
| B. bifidum | B_bif-std-f | CAAGGACGTGGAGACCAAG | 94°C, 3 min; 30 times (94°C, 30 s; 65°C, 30 s; 72°C, 30 s); 72°C, 10 min | 391 | 241,687.40 |
| B_bif-std-r | CTTGTTCAGGATGAGGGTCG | ||||
| B. breve | B_bre-std-f | CCACCGAGGTCATCGTC | 94°C, 3 min; 30 times (94°C, 20 s; 58°C, 20 s; 72°C, 20 s); 72°C, 10 min | 322 | 199,032.80 |
| B_bre-std-r | TGCTGGGAGGAGACCTTG | ||||
| B. catenulatum | B_cat-std-f | GTCGTGGTATCGAGAAG | 94°C, 3 min; 30 times (94°C, 30 s; 47°C, 30 s; 72°C, 30 s); 72°C, 10 min | 244 | 150,819.60 |
| B_cat-std-r | TAGCCCTTGTCGAAACG | ||||
| B. dentium | B_den-std-f | CCACTACCGCAACTGTGCTG | 94°C, 3 min; 30 times (94°C, 50 s; 62°C, 50 s; 72°C, 50 s); 72°C, 10 min | 400 | 247,244.00 |
| B_den-std-r | GTCAGGAGGATGTACGGGTC | ||||
| B. gallicum | B_gal-std-f | TGAGAAGGCTTCCGACG | 94°C, 3 min; 30 times (94°C, 30 s; 57°C, 30 s; 72°C, 30 s); 72°C, 10 min | 242 | 149,590.80 |
| B_gal-std-r | GGGAGATGTAGCCCTTG | ||||
| B. longum | B_lon-std-f | CTGAGGCTCTGGACAAGGTCG | 94°C, 3 min; 30 times (94°C, 30 s; 66°C, 30 s; 72°C, 30 s); 72°C, 10 min | 323 | 199,651.20 |
| B_lon-std-r | GGTGCCACGGATGTTGTTCAGG | ||||
| B. pseudocatenulatum | B_pcat-std-f | CGTCGTGGTATCGAGAAGGCTTCCG | 94°C, 3 min; 30 times (94°C, 50 s; 64°C, 50 s; 72°C, 50 s); 72°C, 10 min | 434 | 268,244.60 |
| B_pcat-std-r | AAGGTCGGCAGAGCCTCGCCAT | ||||
| B. pseudolongum | B_plon-std-f | CCTCAAGAACGTTGTGGC | 94°C, 3 min; 30 times (94°C, 30 s; 49°C, 30 s; 72°C, 30 s); 72°C, 10 min | 420 | 259,607.00 |
| B_plon-std-r | CCGGTCTTCATCACGAG | ||||
| B. thermophilum | B_the-std-f | GAAGGCCTGAAGAACGTGGTCG | 94°C, 3 min; 30 times (94°C, 40 s; 65°C, 40 s; 72°C, 40 s); 72°C, 10 min | 449 | 277,522.60 |
| B_the-std-r | TCAGCCACGATCAGCAGCGGA | ||||
The target sites are located at least 17 bp up- and downstream of the Bifidobacterium species-specific primer-binding sites to prevent their degradation during storage.
f, forward primer; r, reverse primer.
Refers to the double-stranded amplicon.
The identities of the generated qPCR standards were confirmed by sequencing (Eurofins MWG Operon, Ebersberg, Germany). For the quantification of total bifidobacteria, genomic DNA from B. longum subsp. longum ATCC 15707T was used as the qPCR standard. After extracting genomic DNA, its concentration was determined spectrophotometrically as described above. Assuming a mean genome size for Bifidobacterium of 2.25 Mb (GenBank [4]), the average mass of a single Bifidobacterium genome would be 2.47 fg: [2.25 Mb × average molecular massbase pair (660 g/mol)]/NA = 2.47 fg/molecule. The number of bifidobacterial genomes per volume was calculated as follows: concentrationgenomic DNA (g/liter)/(2.47 fg/molecule) = concentrationgenomic DNA (molecules/liter). The standards were stored in 10 mM Tris-HCl (pH 8) at −20°C and subjected to 10-fold serial dilutions in ultrapure water prior to each measurement.
(ii) Conditions.
qPCR based on the fluorescent dye SYBR green I was performed with 7500 Fast Real-Time PCR Systems using the 7500 software v2.0.5 (Life Technologies, Darmstadt, Germany). Reactions were measured in triplicate. Each reaction mixture of 25 μl contained 1× QuantiFast SYBR green PCR Master Mix (HotStarTaq Plus DNA polymerase, QuantiFast SYBR green PCR buffer, dNTP mixture, and the ROX passive reference dye; Qiagen, Hilden, Germany), 250 to 300 nM each forward and reverse primer (Table 3), and 1 μl template DNA. Bifidobacterium species were quantified with the following temperature program (Table 3): 95°C for 5 min, 40 cycles at 94°C for 15 s, 64 to 80°C for 15 s, 72°C for 15 s, and 83°C for 15 s. The fluorescence of SYBR green I was measured after each amplification cycle and during the last step at 83°C to get rid of nonspecific fluorescence signals caused by primer dimers or nonspecific minor products (36). Total bifidobacteria were quantified using the 16S rRNA gene-targeting primer pair g-Bifid-F (5′-CTC CTG GAA ACG GGT GG-3′)/g-Bifid-R (5′-GGT GTT CTT CCC GAT ATC TAC A-3′) with the corresponding temperature program as described elsewhere (29). Postamplification melting-curve analysis was performed by slowly increasing the temperature from 68°C to 95°C (increments of 1%, holding for 10 s), while fluorescence was measured continuously. Thus, the specific PCR product was verified based on the specific melting temperature (Table 3) and distinguished from possible nonspecific products. Background fluorescence was calculated during the initial stage of the qPCR corresponding to cycles 3 to 10. The threshold was set within the exponential phase of the amplification plot at 0.01 (single Bifidobacterium species) or 0.05 (total bifidobacteria) relative fluorescence. Threshold cycles (CT) (the PCR cycle numbers at which the fluorescence exceeds the threshold above the calculated background) of less than 11 and more than 33 were excluded from the analysis. The number of groEL copies or bifidobacterial genomes in a fecal sample was calculated using a standard curve, which was generated by plotting the CT values obtained for 10-fold serial dilutions of the qPCR standards as a linear function of the base 10 logarithm of known concentrations. All primers used in this study were commercially synthesized by Eurofins MWG Operon (Ebersberg, Germany).
Spiking experiments.
Fresh fecal samples were collected from two healthy human adults initially tested for the absence of the investigated Bifidobacterium species by the developed qPCR assays. Aliquots of 220 mg fecal material were spiked with 10-fold serial dilutions of known amounts of the representative strain of each Bifidobacterium species (Table 1) ranging from 4 to 9 log10 cells. The concentration of bacterial cells was estimated microscopically by using the Thoma-Zeiss counting chamber (chamber depth, 0.01 mm; small square, 0.0025 mm2). Microscopic counts were determined at least in duplicate. Bacterial DNA from spiked feces was extracted as described above.
Statistics.
Data analysis of the comparison of cell numbers determined with qPCR assays and the Thoma-Zeiss counting chamber was done with GraphPad Prism version 5.0 (San Diego, CA). Significance was tested by one-way analysis of variance (ANOVA) with Bonferroni's posttests. A P value of <0.05 was considered significant.
RESULTS
Specificities of primer pairs.
The properties and specificity of the primer pair for each Bifidobacterium species were checked in silico with OligoAnalyzer 3.1, provided by Integrated DNA Technologies (35), and the Basic Local Alignment Tool (1), provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Each primer pair perfectly matched the groEL sequence of the targeted Bifidobacterium species but had several mismatches with those of the nontargeted Bifidobacterium species investigated in the study (Table 1). In the case of B. thermophilum, the designed primer pairs also matched the groEL of B. thermacidophilum, which, however, is not found in the human intestinal tract (7) and was therefore disregarded. The specificity of each primer pair was experimentally tested by performing qPCR on genomic DNA extracted from 28 Bifidobacterium strains and 10 non-Bifidobacterium strains representing dominant intestinal bacterial species (Table 1). At the appropriate annealing temperature and the chosen primer concentrations, the primer pairs were specific for their respective target species and yielded an amplicon of the expected size (data not shown). Application of the primer pairs to nontargeted bacterial species tested in this study never led to an amplification product. This conclusion was supported by the following observations: CT values obtained for nontarget organisms were close to the CT values of the no-template control, and there was no peak at the melting temperature of the specific amplicon as determined by postamplification melting-curve analysis. However, application of primer pair B_bif-f/B_bif-r to genomic DNA of B. breve DSM 20091 led to a PCR product that, based on the melting-curve analysis, could clearly be identified as nonspecific.
Bifidobacterium species qPCR assays.
For all Bifidobacterium species represented by the strains underlined in Table 1, 10-fold serial dilutions of qPCR standards (PCR products containing the respective partial groEL sequence) were used to generate a standard curve. The standard curves for all Bifidobacterium species were highly linear (R2 > 0.99), at least in the range of 10 to 100,000 groEL copies per PCR, corresponding to 6 to 10 log10 groEL copies/g feces, except for B. angulatum and B. pseudolongum, for which linearity started at 100 groEL copies per PCR. The PCR efficiency (E) was calculated by the slope of the standard curve as follows: E = 10(−1/slope) − 1 (e.g., E = 1, or 100%). For B. adolescentis, B. animalis, B. bifidum, B. breve, B. catenulatum, B. dentium, B. gallicum, and B. pseudocatenulatum, E was at least 90%. For B. angulatum, B. longum, B. pseudolongum, and B. thermophilum, E was somewhat lower but at least 85%.
Reproducibility.
The reproducibility of the qPCR assays was determined for DNA extracts from three adult fecal samples spiked with different cell numbers of the representative strain of each Bifidobacterium species (Table 1) in two replicate runs. The resulting coefficients of variation (CV) based on the groEL copy number were less than 10%, indicating that the developed qPCR assays are precise. The reproducibility between different DNA extracts was determined by extracting DNA three times from adult or infant fecal samples and quantifying B. breve, B. longum, and total bifidobacteria. The CV based on groEL copies varied less than 23%, demonstrating that the DNA extraction method was highly reproducible.
Quantification limit.
To determine the lowest groEL copy number in feces detectable with the developed qPCR assays (quantification limit), adult fecal samples were spiked with the representative strain of each of the Bifidobacterium species under study. The quantification limit in feces was 1 to 10 groEL copies per PCR, corresponding to 5 to 6 log10 groEL copies/g feces (Table 5).
Table 5.
Quantification of Bifidobacterium species in feces of human adults and infants
| Bifidobacterium species | Log10groEL copies/g feces in samplea: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults (n = 10) |
Infants (n = 5) |
|||||||||||||||
| AD-1 | AD-2 | AD-3 | AD-4 | AD-5 | AD-6 | AD-7 | AD-8 | AD-9 | AD-10 | INF-1 (wk 1) | INF-2 (wk 1) | INF-3 (wk 6) | INF-4 (mo 3) | INF-5 |
||
| wk 6 | mo 3 | |||||||||||||||
| B. adolescentis | 9.7 | 9.7 | − | 9.9 | 9.8 | − | 8.9 | 9.3 | 9.3 | − | − | − | − | − | − | − |
| B. angulatum | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| B. animalis | − | 7.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| B. bifidum | 8.2 | 8.6 | − | 9.1 | − | − | − | − | − | 8.6 | − | − | 9.7 | − | − | − |
| B. breve | − | − | − | − | − | − | − | − | − | − | − | 6.2 | 10.4 | 10.1 | − | 8.3 |
| B. catenulatum | − | 8.1 | − | − | 8.1 | − | − | − | − | − | − | − | − | − | − | − |
| B. dentium | − | 6.4 | 6.7 | − | 7.1 | − | − | − | − | − | − | − | 8.8 | − | − | − |
| B. gallicum | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| B. longum | 7.2 | 9.1 | − | 8.9 | 9.2 | 9.3 | 6.6 | 9.1 | − | 9.0 | − | 6.4 | − | 10.1 | − | 10.0 |
| B. pseudocatenulatum | − | − | 9.3 | − | 8.9 | − | − | 8.7 | − | − | − | − | − | − | − | − |
| B. pseudolongum | − | − | 8.0 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| B. thermophilum | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Sum of species | 9.8 | 9.9 | 9.3 | 10.0 | 10.0 | 9.3 | 8.9 | 9.6 | 9.3 | 9.1 | − | 6.6 | 10.5 | 10.4 | − | 10.1 |
| Total bifidobacteriab (log10 bifidobacterial genomes/g feces) | 10.3 | 10.0 | 10.1 | 10.5 | 10.7 | 10.0 | 9.5 | 10.3 | 9.8 | 9.8 | − | 7.6 | 11.1 | 11.0 | 8.1 | 11.1 |
Infants were around 1 week (wk 1), 6 weeks (wk 6), and 3 months (mo 3) old; adults were 20 to 40 years old. −, not quantified (the quantification limits were 5 log10 groEL copies/g feces for B. adolescentis, B. breve, B. catenulatum, B. dentium, B. longum, B. pseudocatenulatum, and B. thermophilum and 6 log10 groEL copies/g feces for B. angulatum, B. animalis, B. bifidum, B. gallicum, and B. pseudolongum).
The total number of bifidobacteria was determined with the 16S rRNA gene-targeting primer pair g-Bifid-F/R by Matsuki et al. (29). The quantification limit was reported to be 6 log10 cells/g feces.
Comparison of cell numbers determined with qPCR assays and the Thoma-Zeiss counting chamber.
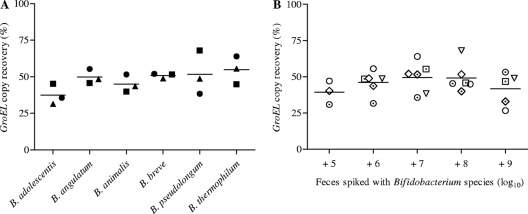
Fecal samples were spiked with cells of six Bifidobacterium species (B. adolescentis, B. angulatum, B. animalis, B. breve, B. pseudolongum, and B. thermophilum). The number of groEL genes measured by the developed qPCR assays was compared with the number of bifidobacteria added to the fecal sample and determined with the Thoma-Zeiss counting chamber prior to spiking. Independent of the Bifidobacterium species, the proportion of groEL copies recovered from fecal samples spiked with 5 to 9 log10 cells/g was 45 to 48% (Fig. 1A and B). These results indicated that the cell numbers of a given Bifidobacterium species present in a fecal sample and determined with the developed qPCR assays were comparable to those obtained by microscopy, suggesting sufficiently effective cell lysis. The recovery of groEL copies was considered in all calculations.
Fig 1.
Comparison of cell numbers determined with qPCR assays and the Thoma-Zeiss counting chamber using human adult feces spiked with Bifidobacterium species cells. (A) Spiking was performed with 6 log10 (▲), 7 log10 (●), and 8 log10 (■) cells of six Bifidobacterium species. (B) Spiking was performed with 5 log10 (n = 3), 6 log10 (n = 6), 7 log10 (n = 6), 8 log10 (n = 6), and 9 log10 (n = 5) cells of B. adolescentis (⊙), B. angulatum (⊡), B. animalis ( ), B. breve (♢), B. pseudolongum (▽), and B. thermophilum (○). The values are means. Significance was estimated by one-way ANOVA with Bonferroni's posttests. Differences of means were not significant. GroEL copy recovery was 48% ± 6% (A) and 45% ± 6% (B) (means ± SD).
), B. breve (♢), B. pseudolongum (▽), and B. thermophilum (○). The values are means. Significance was estimated by one-way ANOVA with Bonferroni's posttests. Differences of means were not significant. GroEL copy recovery was 48% ± 6% (A) and 45% ± 6% (B) (means ± SD).
Presence of PCR inhibitors.
To verify that PCR inhibitors present in feces had been completely removed during DNA extraction, B. longum was quantified with the developed qPCR assay using DNA obtained from four fecal samples (INF-4, INF-5 month 3, AD-5, and AD-6) at dilutions of 0 to −3 log10. Since the cell numbers (groEL copies/g feces) calculated for a given sample were nearly the same for all four dilutions (10.09 ± 0.03 in INF-4, 9.86 ± 0.12 in INF-5 month 3, 9.17 ± 0.02 in AD-5, and 9.28 ± 0.05 in AD-6 [mean ± standard deviation {SD} log10; n = 4]), it was concluded that PCR inhibitors had been completely removed.
Quantification of Bifidobacterium species in fecal samples.
The designed and validated primers were subsequently applied to fecal samples from five healthy, full-term, breast-fed infants and 10 healthy adults (Table 5). Fecal samples from breast-fed infants contained no more than three Bifidobacterium species. B. breve was not detected in adult feces but was found in 4/6 infant samples at up to 10.4 log10 groEL copies/g feces. B. bifidum and B. longum were present at high concentrations (9.7 log10 groEL copies/g feces in INF-3 and 10.1 log10 groEL copies/g feces in INF-4, respectively). B. adolescentis, which is a typical Bifidobacterium species of the adult fecal microbiota, was not detected in infant feces. Most of the adult fecal samples contained between one and three Bifidobacterium species (one species, 2/10; two species, 2/10; three species, 4/10; five species, 1/10; and six species, 1/10). B. adolescentis and B. longum were frequently found in adults (7/10 and 8/10, respectively). Their concentrations were relatively high, with 8.9 to 9.9 log10 groEL copies/g feces for all but two subjects (AD-1 and AD-7), who harbored B. longum at much lower concentrations (7.2 log10 and 6.6 log10 groEL copies/g, respectively). B. pseudocatenulatum, even though detected in just 3/10 samples, also displayed high counts of 8.7 to 9.3 log10 groEL copies/g feces. In adult feces, the concentration of B. bifidum, a predominant Bifidobacterium species in the feces of infants, was in the range of 8.2 to 9.1 log10 groEL copies/g feces. B. catenulatum and B. pseudolongum were found rarely (2/10 and 1/10, respectively) at 8.0 to 8.1 log10 groEL copies/g feces. The lowest numbers, ranging from 6.4 to 7.1 log10 groEL copies/g feces, were measured for B. dentium and B. animalis. The latter was detected only once (AD-2). In adults, total bifidobacteria varied between 9.5 and 10.7 log10 bifidobacterial genomes/g feces. In contrast, 1-week-old infants harbored relatively low levels of total bifidobacteria (7.6 log10 bifidobacterial genomes/g feces in INF-2) or even none (INF-1). However, bifidobacteria were detected in higher numbers in 3-month-old infants than in adults (11.0 log10 bifidobacterial genomes/g feces in INF-4 and 11.1 log10 bifidobacterial genomes/g feces in INF-5). The sum of groEL copies of all targeted Bifidobacterium species was slightly lower than the number obtained with the genus-specific primer pair g-Bifid-F/R targeting the 16S rRNA gene (0.1 to 1.0 log10). The only exception was the sample collected from donor INF-5 at 6 weeks of age, in which none of the investigated Bifidobacterium species could be detected but the concentration of total bifidobacteria was 8.1 log10 bifidobacterial genomes/g feces. B. angulatum, B. gallicum, and B. thermophilum were not detected in any fecal sample from adults or infants.
DISCUSSION
The groEL gene is a powerful phylogenetic marker.
The phylogenetic tree based on fragments of the groEL gene (538 bp, located at positions ∼250 to 840 of the corresponding nucleotide sequence) or larger (∼1,200-bp) or even complete (∼1,600-bp) groEL sequences of Bifidobacterium species (27, 47, 50) was similar to the one based on 16S rRNA gene sequences (31). However, the genetic distances of the groEL sequences were greater than those of the 16S rRNA gene sequences (21, 55). The partial groEL sequence (∼550 bp) identity among different Bifidobacterium species was 80 to 96%, providing considerable discriminative power at the interspecies level (55). The analysis of larger (∼1,200-bp) and complete (∼1,600-bp) groEL sequences even allowed the differentiation of B. animalis and B. longum at the subspecies level (27, 50). The ubiquitous GroEL-encoding gene nevertheless reveals a high degree of sequence conservation, in agreement with the central cellular function of the encoded chaperonin. All in all, the groEL gene fulfills several prerequisites to serve as a reliable alternative phylogenetic marker for Bifidobacterium species.
Advantages of the groEL gene compared to the 16S rRNA gene.
Although the 16S rRNA gene, with a mean sequence identity of 95% (31, 47), allows the discrimination of most Bifidobacterium species, its discriminative power among closely related species is limited. Thus, it is not possible to distinguish B. catenulatum and B. pseudocatenulatum, which share 99.5% sequence identity in their 16S rRNA genes (31, 47). These species have therefore been detected together (16, 29, 33). As demonstrated here, B. catenulatum and B. pseudocatenulatum could easily be distinguished because the two organisms share only 91 to 93% sequence identity in the groEL sequence of ∼550 bp (21, 55). Owing to more than 96% 16S rRNA gene sequence identity, it has not been possible to determine the evolutionary distance between strains belonging to B. breve and B. longum (25). This also applies to B. adolescentis/B. dentium, B. breve/B. pseudolongum, B. catenulatum/B. dentium, and B. longum/B. pseudolongum (25). In contrast, the selected region of the groEL gene used in this study revealed a considerably higher resolution power between these species than the 16S rRNA gene (21, 47, 55).
Limitations.
Initially, phylogenetic analysis of bifidobacteria based on the groEL gene was constrained because the number of available groEL sequences was limited to 73 (21, 55). With increasing interest in the gene as a powerful phylogenetic marker and as a result of the sequencing of bifidobacterial genomes, the database of groEL sequences has continuously grown (18). Although groEL sequences are available for almost all 36 described Bifidobacterium species (http://www.dsmz.de), the majority of these sequences are incomplete; they consist of an approximately 600-bp region located at positions ∼250 to 840 of the gene. For some Bifidobacterium species, only one or two sequences are available (e.g., B. angulatum and B. catenulatum, respectively).
Quantification of Bifidobacterium species in fecal samples.
The colonization of the gastrointestinal tract starts at birth and is characterized by different microbial successions. Within the first days of life, facultative anaerobes, such as coliforms and enterococci, dominate the microbiota. They create a reduced environment, enabling the establishment of strict anaerobes, including bifidobacteria, by days 4 to 7 (26).
In our study, at 1 week after birth, Bifidobacterium species were absent or present at low concentrations. In contrast, at 3 months of age, bifidobacteria had advanced to become one of the dominant population groups in the infant gut. Differences in the patterns and sizes of bifidobacterial populations as reported here have been observed previously (19, 30, 38). Our study revealed low Bifidobacterium diversity and the presence of three or less than three species in a given sample from infants or adults (8/10). This result is similar to a previous study with higher numbers of subjects (30).
High cell counts of B. bifidum, B. breve, and B. longum (including B. longum subsp. infantis) were frequently found in feces from breast-fed infants, suggesting that these three species play a crucial role in gut colonization (16, 30, 39). In accordance with previous studies (15, 37, 38), B. longum was the most common species in human adult feces, followed by B. adolescentis, B. bifidum, and B. pseudocatenulatum. A high incidence of B. longum, B. adolescentis, B. bifidum, and members of the B. catenulatum group was observed in Japanese adults (29, 30). Although we were able to distinguish B. catenulatum and B. pseudocatenulatum in our study, the prevalence of both species together was considerably lower than that reported for these Japanese subjects (40% versus 89% [29] and 92% [30], respectively).
As an inhabitant of the oral cavity (7) B. dentium may pass into the intestine, where it is infrequently detected at low cell numbers (29, 30, 39). In contrast to Matsuki et al. (29, 30), who occasionally detected B. breve, the species was mostly below the quantification limit in the adult samples we analyzed. The donor of sample AD-2 was not aware of having consumed a probiotic food prior to the collection of the sample. However, B. animalis subsp. lactis may be present in normal yogurts that are not explicitly labeled as containing bifidobacteria (3).
None of the few fecal samples tested in our study contained B. angulatum, B. gallicum, or B. thermophilum, all of which have rarely been detected. B. angulatum was occasionally found in infant and adult feces (29, 30), in the latter at relatively low concentrations (∼6.6 log10 cells/g feces [29] and 7.5 to 8.3 log10 CFU/g [3]). B. gallicum was mostly absent (15, 30, 33), except for a baby delivered by caesarean section (6). B. thermophilum, first isolated from baby feces (53), was recently detected in the feces of a breast-fed infant at 6.7 log10 cells/g feces (28). Since B. pseudolongum has so far been associated with animals, it has rarely been investigated in humans. However, in agreement with our study, it was recently found in adults, but not in infants (44).
The numbers of Bifidobacterium species that we detected in adults and infants are essentially in agreement with previous findings (30). Matsuki et al. (30) mostly observed between one and four species in adults (29% harbored three and 31% harbored four species) and up to three species in infants (33% harbored one and 22% harbored three species). However, Matsuki et al. (30) were not able to further discriminate between members of the B. catenulatum group.
The total counts of bifidobacterial genomes determined with the genus-specific primers g-Bifid-F/R targeting the 16S rRNA gene were slightly higher than those resulting from the sum of groEL copies detected with the species-specific primers. It is well known that bifidobacteria possess two to five rrn loci (8, 10, 40). Differences in the 16S rRNA gene copy numbers are adequately considered when the qPCR standard curve uses genomic DNA from a bacterium that represents the predominant species in the sample (34). Taking this into account, B. longum subsp. longum ATCC 15707T, with four 16S rRNA gene copies (13), was used as a standard for quantification of total fecal bifidobacteria in adults and infants. However, Candela et al. (10) have demonstrated that the rrn copies do not differ only at species level. Even different strains of B. adolescentis may harbor between two and five copies (10, 40). The genome size of bifidobacterial species varies between 1.92 and 2.83 Mb (GenBank; corresponding to 2.11 to 3.11 fg/cell). Assuming a mean genome size of 2.25 Mb (2.47 fg/cell) would result in overestimation of the cell number if the predominant Bifidobacterium strain in the sample has a genome size smaller than 2.25 Mb and/or a number of 16S rRNA gene copies higher than four. This would apply to B. adolescentis ATCC 15703T (GenBank accession no. AP009256), B. bifidum PRL2010, and B. bifidum S17 (43, 56). These problems can be avoided by targeting a single-copy gene, such as groEL. However, a multiple alignment of all complete groEL sequences listed in Table 2 did not reveal consensus regions of sufficient length to design genus-specific primers. Quantification limits of qPCR with species-specific primers that target the single-copy groEL gene are comparable with the one targeting the multicopy 16S rRNA gene (Table 5), indicating that the qPCR assays developed in this study are highly sensitive.
During this study, two novel Bifidobacterium species were isolated from human feces, namely, B. stercoris (22) and B. kashiwanohense (32), for which at least partial groEL sequences are available. It is interesting that recent microbiomic analysis suggested the presence of previously undescribed Bifidobacterium species, which, however, have not been identified so far (45).
Conclusion.
This study describes the use of the groEL gene as a target for the identification and quantification of human fecal Bifidobacterium species by qPCR. The method developed allows the distinction between closely related Bifidobacterium species, enabling monitoring of the bifidobacterial population in response to age, antibiotic treatment, pro- and prebiotic intake, or disease.
ACKNOWLEDGMENTS
This study has been part of a cooperation project with Nestec S.A., Nestlé Nutrition, Vevey, Switzerland.
We thank all adults who provided fecal samples.
Footnotes
Published ahead of print 3 February 2012
REFERENCES
- 1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Apajalahti JH, Kettunen A, Nurminen PH, Jatila H, Holben WE. 2003. Selective plating underestimates abundance and shows differential recovery of bifidobacterial species from human feces. Appl. Environ. Microbiol. 69:5731–5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartosch S, Woodmansey EJ, Paterson JC, McMurdo ME, Macfarlane GT. 2005. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 40:28–37 [DOI] [PubMed] [Google Scholar]
- 4. Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2011. GenBank. Nucleic Acids Res. 39:D32–D37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berthoud H, Chavagnat F, Haueter M, Casey MG. 2005. Comparison of partial gene sequences encoding a phosphoketolase for the identification of bifidobacteria. Lebensm. Wiss. Technol. 38:101–105 [Google Scholar]
- 6. Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. 2008. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J. Nutr. 138:1796S–1800S [DOI] [PubMed] [Google Scholar]
- 7. Biavati B, Mattarelli P. 2006. The family Bifidobacteriaceae, p 322–382 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E. (ed), The prokaryotes, 3rd ed, vol 3 Springer, New York, NY [Google Scholar]
- 8. Bourget N, Simonet JM, Decaris B. 1993. Analysis of the genome of the five Bifidobacterium breve strains: plasmid content, pulsed-field gel electrophoresis genome size estimation and rrn loci number. FEMS Microbiol. Lett. 110:11–20 [DOI] [PubMed] [Google Scholar]
- 9. Braune A, Gütschow M, Engst W, Blaut M. 2001. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 67:5558–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Candela M, Vitali B, Matteuzzi D, Brigidi P. 2004. Evaluation of the rrn operon copy number in Bifidobacterium using real-time PCR. Lett. Appl. Microbiol. 38:229–232 [DOI] [PubMed] [Google Scholar]
- 11. Edwards CA, Parrett AM. 2002. Intestinal flora during the first months of life: new perspectives. Br. J. Nutr. 88(Suppl. 1):S11–S18 [DOI] [PubMed] [Google Scholar]
- 12. Fox GE, et al. 1980. The phylogeny of prokaryotes. Science 209:457–463 [DOI] [PubMed] [Google Scholar]
- 13. Fukuda S, et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547 [DOI] [PubMed] [Google Scholar]
- 14. Garrigues C, Johansen E, Pedersen MB. 2010. Complete genome sequence of Bifidobacterium animalis subsp. lactis BB-12, a widely consumed probiotic strain. J. Bacteriol. 192:2467–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gavini F, Cayuela C, Antoine JM, Lecoq CLB, Membré JM, Neut C. 2001. Differences in the distribution of bifidobacterial and enterobacterial species in human faecal microflora of three different (children, adults, elderly) age groups. Microb. Ecol. Health Dis. 13:40–45 [Google Scholar]
- 16. Haarman M, Knol J. 2005. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harmsen HJ, et al. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61–67 [DOI] [PubMed] [Google Scholar]
- 18. Hill JE, Penny SL, Crowell KG, Goh SH, Hemmingsen SM. 2004. cpnDB: a chaperonin sequence database. Genome Res. 14:1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopkins MJ, Sharp R, Macfarlane GT. 2001. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. 1990. Protective effect of breast feeding against infection. Br. Med. J. 300:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jian W, Zhu L, Dong X. 2001. New approach to phylogenetic analysis of the genus Bifidobacterium based on partial HSP60 gene sequences. Int. J. Syst. Evol. Microbiol. 51:1633–1638 [DOI] [PubMed] [Google Scholar]
- 22. Kim MS, Roh SW, Bae JW. 2010. Bifidobacterium stercoris sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 60:2823–2827 [DOI] [PubMed] [Google Scholar]
- 23. Kullen MJ, Brady LJ, O'Sullivan DJ. 1997. Evaluation of using a short region of the recA gene for rapid and sensitive speciation of dominant bifidobacteria in the human large intestine. FEMS Microbiol. Lett. 154:377–383 [DOI] [PubMed] [Google Scholar]
- 24. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 25. Leblond-Bourget N, Philippe H, Mangin I, Decaris B. 1996. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. Int. J. Syst. Bacteriol. 46:102–111 [DOI] [PubMed] [Google Scholar]
- 26. Mackie RI, Sghir A, Gaskins HR. 1999. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69:1035S–1045S [DOI] [PubMed] [Google Scholar]
- 27. Masco L, Ventura M, Zink R, Huys G, Swings J. 2004. Polyphasic taxonomic analysis of Bifidobacterium animalis and Bifidobacterium lactis reveals relatedness at the subspecies level: reclassification of Bifidobacterium animalis as Bifidobacterium animalis subsp. animalis subsp. nov. and Bifidobacterium lactis as Bifidobacterium animalis subsp. lactis subsp. nov. Int. J. Syst. Evol. Microbiol. 54:1137–1143 [DOI] [PubMed] [Google Scholar]
- 28. Mathys S, Lacroix C, Mini R, Meile L. 2008. PCR and real-time PCR primers developed for detection and identification of Bifidobacterium thermophilum in faeces. BMC Microbiol. 8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuki T, et al. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsuki T, Watanabe K, Tanaka R, Fukuda M, Oyaizu H. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyake T, Watanabe K, Watanabe T, Oyaizu H. 1998. Phylogenetic analysis of the genus Bifidobacterium and related genera based on 16S rDNA sequences. Microbiol. Immunol. 42:661–667 [DOI] [PubMed] [Google Scholar]
- 32. Morita H, et al. 2011. Bifidobacterium kashiwanohense sp. nov., isolated from healthy infant faeces. Int. J. Syst. Evol. Microbiol. 61:2610–2615 [DOI] [PubMed] [Google Scholar]
- 33. Mullié C, Odou MF, Singer E, Romond MB, Izard D. 2003. Multiplex PCR using 16S rRNA gene-targeted primers for the identification of bifidobacteria from human origin. FEMS Microbiol. Lett. 222:129–136 [DOI] [PubMed] [Google Scholar]
- 34. Nadkarni MA, Martin FE, Jacques NA, Hunter N. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266 [DOI] [PubMed] [Google Scholar]
- 35. Owczarzy R, et al. 2008. IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 36:W163–W169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfaffl MW. 2001. Development and validation of an externally standardised quantitative insulin-like growth factor-1 RT-PCR using LightCycler SYBR Green I technology, p 281–292 In Meuer S, Wittwer C, Nakagawara K. (ed), Rapid cycle real-time PCR: methods and applications. Springer, Heidelberg, Germany [Google Scholar]
- 37. Requena T, et al. 2002. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 68:2420–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reuter G. 2001. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr. Issues Intest. Microbiol. 2:43–53 [PubMed] [Google Scholar]
- 39. Rinne MM, et al. 2005. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol. Med. Microbiol. 43:59–65 [DOI] [PubMed] [Google Scholar]
- 40. Satokari RM, Vaughan EE, Akkermans AD, Saarela M, de Vos WM. 2001. Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Satokari RM, et al. 2003. Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst. Appl. Microbiol. 26:572–584 [DOI] [PubMed] [Google Scholar]
- 42. Schell MA, et al. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 99:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turroni F, et al. 2010. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. U. S. A. 107:19514–19519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turroni F, et al. 2009. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 75:1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turroni F, et al. 2009. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J. 3:745–751 [DOI] [PubMed] [Google Scholar]
- 46. van Odijk J, et al. 2003. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 58:833–843 [DOI] [PubMed] [Google Scholar]
- 47. Ventura M, et al. 2006. Analysis of bifidobacterial evolution using a multilocus approach. Int. J. Syst. Evol. Microbiol. 56:2783–2792 [DOI] [PubMed] [Google Scholar]
- 48. Ventura M, Canchaya C, Meylan V, Klaenhammer TR, Zink R. 2003. Analysis, characterization, and loci of the tuf genes in Lactobacillus and Bifidobacterium species and their direct application for species identification. Appl. Environ. Microbiol. 69:6908–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ventura M, Canchaya C, van Sinderen D, Fitzgerald GF, Zink R. 2004. Bifidobacterium lactis DSM 10140: identification of the atp (atpBEFHAGDC) operon and analysis of its genetic structure, characteristics, and phylogeny. Appl. Environ. Microbiol. 70:3110–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ventura M, Canchaya C, Zink R, Fitzgerald GF, van Sinderen D. 2004. Characterization of the groEL and groES loci in Bifidobacterium breve UCC 2003: genetic, transcriptional, and phylogenetic analyses. Appl. Environ. Microbiol. 70:6197–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ventura M, Zink R. 2003. Comparative sequence analysis of the tuf and recA genes and restriction fragment length polymorphism of the internal transcribed spacer region sequences supply additional tools for discriminating Bifidobacterium lactis from Bifidobacterium animalis. Appl. Environ. Microbiol. 69:7517–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ventura M, Zink R, Fitzgerald GF, van Sinderen D. 2005. Gene structure and transcriptional organization of the dnaK operon of Bifidobacterium breve UCC 2003 and application of the operon in bifidobacterial tracing. Appl. Environ. Microbiol. 71:487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. von Ah U, et al. 2007. Classification of a moderately oxygen-tolerant isolate from baby faeces as Bifidobacterium thermophilum. BMC Microbiol. 7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yin X, Chambers JR, Barlow K, Park AS, Wheatcroft R. 2005. The gene encoding xylulose-5-phosphate/fructose-6-phosphate phosphoketolase (xfp) is conserved among Bifidobacterium species within a more variable region of the genome and both are useful for strain identification. FEMS Microbiol. Lett. 246:251–257 [DOI] [PubMed] [Google Scholar]
- 55. Zhu L, Li W, Dong X. 2003. Species identification of genus Bifidobacterium based on partial HSP60 gene sequences and proposal of Bifidobacterium thermacidophilum subsp. porcinum subsp. nov. Int. J. Syst. Evol. Microbiol. 53:1619–1623 [DOI] [PubMed] [Google Scholar]
- 56. Zhurina D, et al. 2011. Complete genome sequence of Bifidobacterium bifidum S17. J. Bacteriol. 193:301–302 [DOI] [PMC free article] [PubMed] [Google Scholar]