Abstract
The efficacies of trans-cinnamaldehyde (TC) and eugenol (EG) for reducing Salmonella enterica serovar Enteritidis colonization in broiler chickens were investigated. In three experiments for each compound, 1-day-old chicks (n = 75/experiment) were randomly assigned to five treatment groups (n = 15/treatment group): negative control (-ve S. Enteritidis, -ve TC, or EG), compound control (-ve S. Enteritidis, +ve 0.75% [vol/wt] TC or 1% [vol/wt] EG), positive control (+ve S. Enteritidis, -ve TC, or EG), low-dose treatment (+ve S. Enteritidis, +ve 0.5% TC, or 0.75% EG), and high-dose treatment (+ve S. Enteritidis, +ve 0.75% TC, or 1% EG). On day 0, birds were tested for the presence of any inherent Salmonella (n = 5/experiment). On day 8, birds were inoculated with ∼8.0 log10 CFU S. Enteritidis, and cecal colonization by S. Enteritidis was ascertained (n = 10 chicks/experiment) after 24 h (day 9). Six birds from each treatment group were euthanized on days 7 and 10 after inoculation, and cecal S. Enteritidis numbers were determined. TC at 0.5 or 0.75% and EG at 0.75 or 1% consistently reduced (P < 0.05) S. Enteritidis in the cecum (≥3 log10 CFU/g) after 10 days of infection in all experiments. Feed intake and body weight were not different for TC treatments (P > 0.05); however, EG supplementation led to significantly lower (P < 0.05) body weights. Follow-up in vitro experiments revealed that the subinhibitory concentrations (SICs, the concentrations that did not inhibit Salmonella growth) of TC and EG reduced the motility and invasive abilities of S. Enteritidis and downregulated expression of the motility genes flhC and motA and invasion genes hilA, hilD, and invF. The results suggest that supplementation with TC and EG through feed can reduce S. Enteritidis colonization in chickens.
INTRODUCTION
Salmonella enterica serovar Enteritidis is one of the two most common bacterial agents that cause food-borne illness in the United States (4). Poultry and poultry products are epidemiologically attributed as the critical sources from which humans contract salmonellosis (26, 39). Chickens can harbor S. Enteritidis without showing any obvious clinical signs and disseminate the pathogen to the environment, raising significant public health concerns. Moreover, intestinal colonization of the pathogen in birds leads to carcass contamination during slaughter or contamination of eggs. Despite control measures adopted in reducing the pathogen by preharvest and postharvest approaches, S. Enteritidis is widespread in poultry, leading to the elevated incidence rates of human salmonellosis (4). Recently, the U.S. Centers for Disease Control and Prevention (CDC) reported that food-borne salmonellosis in the last decade has not decreased significantly, and efforts should be targeted for controlling Salmonella (5).
In chickens, S. Enteritidis predominantly colonizes in the cecum. Reducing S. Enteritidis in the intestinal tract of chickens could reduce contamination of poultry products (1). A number of approaches for reducing the colonization by the pathogen in poultry have been explored thus far, but with various degrees of success. These approaches include feeding chickens with competitive exclusion bacteria, bacteriophages, organic acids, oligosaccharides, antibiotics, and vaccines (9, 11, 17, 19, 22, 24, 27, 45, 46). The efficacy of natural antimicrobial compounds for killing pathogenic microorganisms has received recent attention due to the toxicity of synthetic chemicals and concern over emerging antibiotic-resistant strains of bacteria (42). The antimicrobial activities of several plant-derived essential oils have been demonstrated, and a variety of active components in these oils has been identified. Among the various plant compounds, trans-cinnamaldehyde (TC), a major ingredient in cinnamon (Cinnamomum zeylandicum), and eugenol (EG), a component of clove oil (Eugenia caryophillis), possess antibacterial properties against Gram-negative and Gram-positive bacteria (8). Both of these compounds are generally recognized as safe chemicals for use in foods (GRAS) by the U.S. FDA (TC, 21 CFR 182.60 [13]; EG, 21 CFR 582.60 [14]). Previously, we reported that TC was effective in killing S. Enteritidis in chicken drinking water (34). Additionally, we investigated the efficacy of various plant-derived compounds, including TC and EG, for reducing S. Enteritidis in chicken cecal contents in vitro (33). Among the molecules tested, TC and EG were most effective in significantly reducing the pathogen populations. Therefore, the present study was undertaken to determine the efficacies of TC and EG for reducing S. Enteritidis colonization in broiler chickens.
MATERIALS AND METHODS
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut.
Experimental birds.
Day-old commercial, straight-run broiler chicks (Ross × Ross) were procured from Burr Farm Inc., Hampton, CT. They were allocated to floor pens with access ad libitum to nonmedicated feed (Blue Seal Feeds Inc., Londonderry, NH), Salmonella-free water, and arrangements for age-appropriate temperatures and bedding (23) at the Isolation Facility, University of Connecticut.
Experimental design.
Three separate experiments were conducted for each compound. In each experiment, 75-day-old chicks were randomly allocated to five treatment groups (n = 15). In the experiments with TC (99% purity; Sigma-Aldrich, St. Louis, MO), the treatment groups included a negative control (no S. Enteritidis challenge and no supplemental TC), a compound control (no S. Enteritidis challenge but 0.75% [vol/wt] supplemental TC), a positive control (S. Enteritidis challenge but no supplemental TC), a low-dose treatment (S. Enteritidis challenge and 0.5% supplemental TC), and a high-dose treatment (S. Enteritidis challenge and 0.75% supplemental TC). Likewise, in the experiments with EG (99% purity; Sigma-Aldrich), the treatments included a negative control (no S. Enteritidis challenge and no supplemental EG), a compound control (no S. Enteritidis challenge but with 1% [vol/wt] supplemental EG), a positive control (S. Enteritidis challenge and no supplemental EG), a low-dose treatment (S. Enteritidis challenge and 0.75% supplemental EG), and a high-dose treatment (S. Enteritidis challenge with 1% supplemental EG). On day 0, five birds per experimental group were randomly selected and sacrificed to confirm that the birds were initially devoid of any Salmonella. TC or EG was supplemented in the feed for 20 days, starting on day 0. The appropriate volume of each plant compound was measured using a graduated cylinder, added into feed, and mixed thoroughly to obtain the desired concentrations in the feed (0.5 and 0.75% for TC and 0.75 and 1% for EG). On day 8, birds in the positive control, low-dose, and high-dose treatment groups were challenged with S. Enteritidis (8 log10 CFU/bird) by crop gavage. After 24 h (day 9), two birds from each treatment group were sacrificed to determine pathogen colonization in the cecum (n = 10/experiment). After 7 and 10 days of challenge, six birds per treatment group were sacrificed by CO2 asphyxiation, and the cecum with its contents from each bird was collected in 5 ml of sterile phosphate-buffered saline (PBS) for bacteriological analysis.
Bacterial strains and dosing.
A four-strain mixture of S. Enteritidis isolated from chickens (obtained from the Connecticut Veterinary Diagnostic Medical Laboratory, University of Connecticut) was used to colonize the birds, as described previously (32). The isolates were S. Enteritidis 12 (chicken liver, phage type 14b), S. Enteritidis 21 (chicken intestine, phage type 8), S. Enteritidis 28 (chicken ovary, phage type 13a), and S. Enteritidis 31 (chicken gut, phage type 13a). Each strain was preinduced for resistance to 50 μg/ml of nalidixic acid (NA; Sigma-Aldrich, St. Louis, MO) for selective enumeration. One hundred microliters of each nalidixic acid-resistant strain was grown separately in 10 ml tryptic soy broth (TSB; Difco) overnight, transferred into separate conical flasks containing 100 ml TSB with 50 μg/ml NA, and incubated overnight at 37°C with shaking (100 rpm). The cultures were combined and sedimented by centrifugation (3,600 × g, 15 min, 4°C), and the pellet was resuspended in 100 ml of PBS (pH 7.0) and used as the inoculum (∼108 CFU/ml). The bacterial counts in the individual cultures and the four-strain mixture were confirmed by plating 0.1-ml portions of appropriate dilutions on xylose-lysine-desoxycholate agar (XLD; Difco) plates containing NA (XLD-NA) and incubating the plates at 37°C for 24 h.
Cecal S. Enteritidis determination.
S. Enteritidis population numbers in ceca were determined as described previously (32). The ceca with their contents from each bird were weighed and homogenized. Each homogenate was serially diluted (1:10) in PBS, and appropriate dilutions were plated on XLD-NA plates. The plates were incubated for 48 h at 37°C before counting colonies. Representative colonies from XLD-NA plates were confirmed as Salmonella by using the Salmonella rapid detection kit (Microgen Bioproducts Ltd., Camberley, United Kingdom). When colonies were not detected by direct plating, samples were tested for surviving cells by enrichment for 48 h at 37°C in 100 ml selenite-cysteine broth (SCB; Oxoid) (17, 18), followed by streaking on XLD-NA plates. Representative colonies from the plates were confirmed as Salmonella as previously mentioned.
Determination of cecal endogenous bacteria and cecal pH.
Appropriate dilutions of the samples from ceca were plated on duplicate thioglycolate agar plates (TGA; Difco) (15) and incubated at 40°C under 5% CO2 for 24 h. The pH of cecal contents was also recorded for all treatment groups by using a pH meter and direct immersion of the electrode into the samples (33).
Body weight and feed consumption.
The average feed consumption and body weights of birds were also determined for each experiment. Birds were weighed individually at the start and end of each experiment. The average feed consumption per bird was calculated by dividing the total amount of feed consumed per treatment group by the number of birds in the respective treatment group.
Determination of SICs of TC and EG.
The effects of subinhibitory concentrations (SICs) of TC and EG against S. Enteritidis strains were determined as described previously (35). Duplicate 50-ml tubes containing 20 ml Luria-Bertani (LB; Difco) broth or cell culture medium (Dulbecco's modified Eagle medium [DMEM; Gibco, Invitrogen]) supplemented with 10% fetal calf serum (FCS; Gibco, Invitrogen) were separately inoculated with 4 × 106 to 5 × 106 CFU/ml of each S. Enteritidis strain. TC or EG was added with an increment of 1 mg/μl each from 0 to 10 mg/μl to corresponding tubes, and tubes were incubated at 37°C for 24 h. Samples were drawn from each tube, diluted in sterile PBS (pH 7.2), and plated on TSA plates at 0, 2, 4, 6, 8, 10, 12, and 24 h, and the plates were incubated at 37°C for 48 h. The experiment was repeated three times. The highest concentration of TC or EG that did not inhibit the bacterial growth after 24 h of incubation was taken as the SIC for that compound.
Motility assay.
The effects of TC and EG on S. Enteritidis motility were determined according to the modified procedure described in reference 40. Ten microliters (∼106 CFU/ml) of a mid-log-phase S. Enteritidis culture grown in the presence or absence of the SIC of TC (0.01% [vol/vol]) or EG (0.04% [vol/vol]) was inoculated onto the centers of duplicate plates containing LB plus 0.3% agar. The plates were incubated at 37°C for 8 h. The zone of motility (distance the bacteria traversed [in cm] after incubation) was measured. In addition, tubes containing 10 ml of LB broth added with appropriate volumes of TC or EG and bacterial cultures (106 CFU/ml) were also incubated at 37°C for 8 h. The tubes were diluted and plated to determine the number of surviving bacteria.
Cell culture. (i) Intestinal epithelial cell line.
Budgerigar abdominal tumor cells (BATCs), a permanent avian intestinal epithelial cell line, a kind gift of Margie Lee, College of Veterinary Medicine, University of Georgia, Athens, was used for the study. The cell line is a published model for studying Salmonella invasion and pathogenesis in avian species (16, 21, 25). This experiment was carried out to determine if TC and EG reduced S. Enteritidis invasion of avian intestinal epithelial cells. The cells were cultured in DMEM with FCS (16, 21, 25), and after three successful propagations were seeded into wells of 24-well tissue culture plates containing 1 ml DMEM with FCS at 1 × 105 cells/well. The cells were incubated at 37°C with 5% CO2, to a confluence of >95% within 48 h. The viability of cells was confirmed using a trypan blue vital dye exclusion assay (41). Briefly, 50 μl of the diluted BATC suspension prior to each experiment was mixed with 10 μl of trypan blue dye, and 10 μl of the mixture was loaded in the counting chambers of a hemocytometer. After 1 min, the number of nonstained cells was counted under the low-power objective of the microscope.
(ii) Salmonella invasion assay.
S. Enteritidis strains were grown separately in DMEM containing FCS with the SIC of TC (0.01% [vol/vol]) or EG (0.04% EG [vol/vol]) or without compound (control) at 37°C until the mid-log phase. The cultures were sedimented by centrifugation (3,600 × g, 10 min, 4°C), and the pellet was resuspended in DMEM and used as the inoculum. The bacterial counts in the individual cultures were confirmed by plating 0.1-ml portions of appropriate dilutions on TSA plates. A multiplicity of infection (MOI) of ∼50 was used for the study. The tissue culture plates were then centrifuged at 1,000 × g for 3 min at 23°C and incubated for 45 min at 37°C with 5% CO2. Thereafter, the medium was removed from the wells and replaced with DMEM supplemented with 100 μg/ml of gentamicin (Gibco, Invitrogen). The samples were incubated for 1 h to kill all the extracellular bacteria. The wells were then washed with PBS three times, and the intestinal cells were lyzed using 0.1% Triton X-100 (Invitrogen), followed by incubation at 37°C with 5% CO2 for 15 min to release the internalized bacteria. The cell lysates were serially diluted, plated on TSA plates, and incubated at 37°C for 24 h before counting colonies. Duplicate samples of each treatment were included, and the experiment was repeated three times.
Effects of SICs of TC and EG on expression of S. Enteritidis motility and invasion genes. (i) RNA isolation and cDNA synthesis.
Each strain of S. Enteritidis was grown in LB with and without the SIC of TC (0.01% [vol/vol]) or EG (0.04% [vol/vol]) to mid-log phase at 37°C. Three milliliters of bacterial culture was centrifuged at 12,000 × g for 2 min at 4°C, and the resultant pellet was incubated with 1 ml of RNAprotect reagent (Qiagen, Valencia, CA) for 5 min at room temperature. Total RNA was extracted from the control and treated S. Enteritidis cells by using the RNeasy minikit (Qiagen) according to the manufacturer's instructions. Quantitation of RNA was done by measuring the absorbance at 260 and 280 nm (Nanodrop; Bio-Rad). cDNA was synthesized using the SuperScript II reverse transcriptase kit (Invitrogen, Carlsbad, CA).
(ii) RT-qPCR.
The cDNA was used as the template for the amplification of Salmonella motility genes flhC and motA and invasion genes hilA, hilD, and invF. The specific primers for the aforementioned genes and for 16S rRNA (endogenous control) (Table 1) were designed using Primer Express software (Applied Biosystems) based on the Salmonella enterica serovar Enteritidis strain P125109 genome (NCBI reference sequence NC_011294.1). The primers were custom synthesized by Integrated DNA Technologies (Foster City, CA). Real-time quantitative PCR (RT-qPCR) was performed with the ABI Prism 7900 sequence detection system (Applied Biosystems) using the SYBR green assay (Applied Biosystems) under custom thermal cycling conditions. The biological replicates were analyzed in duplicate and normalized against 16S rRNA gene expression. The comparative threshold cycle (CT) method (2−ΔΔCT) was used to assess the relative changes in mRNA expression levels between the control and TC-treated or EG-treated S. Enteritidis cells (7).
Table 1.
Primers used in the study
| Gene | Forward primer | Reverse primer |
|---|---|---|
| hilA | 5′-TTACTGTGCGCTGGCAGAAT-3′ | 5′-TCGCCTTAATCGCATGTTCTT-3′ |
| hilD | 5′-GGCGGTACCCACAGAGAAAG-3′ | 5′-TCGTACAGGAGAACGCCGTT-3′ |
| invF | 5′-ACGCCATAGTCTTCTCCCAGC-3′ | 5′-TCAGTCAACCAGCGGCAAC-3′ |
| flhC | 5′-TTGGCGCTCGTCTACAAATG-3′ | 5′-GACCACGGCTGAGCTGTGTT-3′ |
| motA | 5′-GATTTGCTGGCGTTGCTCTAT-3′ | 5′-CCCCTGCTGACGTGATTTG-3′ |
| 16S | 5′-GTATGCGCCATTGTAGCACG-3′ | 5′-TCATCATGGCCCTTACGACC-3′ |
Statistical analysis.
The number of S. Enteritidis colonies in the cecum was logarithmically transformed (log10 CFU/g) before analysis to achieve homogeneity of variance (10). These data were analyzed using the PROC-MIXED procedure of the SAS statistical analysis software (version 9.2; SAS Institute Inc., Cary, NC). Differences among the means were detected at a P level of ≤0.05 using Fisher's least significance difference (LSD) test. For motility, cell culture, and RT-qPCR assays, the results are provided as mean values and standard errors of the means (SEM). Differences between two independent treatments were analyzed using two-tailed t tests, and a P value of <0.05 was considered statistically significant.
RESULTS
No S. Enteritidis was recovered from the cecal samples of negative or compound control birds (both TC and EG controls) during the entire duration of the study. After 24 h of inoculation, 1 × 107 to 3 × 107 CFU of S. Enteritidis/g of cecal contents was recovered from all the chicks necropsied from the inoculated treatment groups.
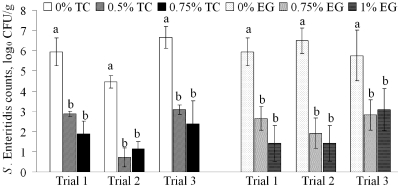
The results for the effects of TC and EG on S. Enteritidis counts in cecal samples are depicted in Fig. 1. The S. Enteritidis counts recovered from the cecal samples of the control birds ranged from 6 to 7 log10 CFU/g after 10 days postinfection (p.i.), with the exception of experiment 2 with TC, where the recovery was ∼4.5 log10 CFU/g (Fig. 1). It was observed that both doses of TC resulted in similar reductions of S. Enteritidis populations in the cecal samples (P < 0.05). The lower dose (0.5% TC) reduced S. Enteritidis by 3.1, 3.7, and 3.6 log10 CFU/g, whereas the higher dose (0.75% TC) resulted in 4, 3.3, and 4.3 log10 CFU/g reductions in experiments 1, 2, and 3, respectively (Fig. 1). Similar results were observed with EG at both concentrations on S. Enteritidis in the cecum (Fig. 1). EG at 0.75 and 1% resulted in 3.3, 4.6, 3.0 and 2.8, 5.1, 2.6 log10 CFU/g reductions of S. Enteritidis, respectively, in experiments 1, 2, and 3 (Fig. 1).
Fig 1.
Effects of TC and EG on S. Enteritidis in cecum and cecal contents on the 10th day postinfection. Error bars represent SEM (n = 6/treatment group/experiment). In each experiment, chicks (except for negative and TC controls) were challenged on day 8 posthatch with a mixture of four S. Enteritidis isolates at 8 log10 CFU/bird. TC was added to the feed prophylactically from day 0 until the completion of the study (20 days). Columns with different letters (a or b) denote significant differences between the treatments (P < 0.05). Negative and compound controls were not included in the statistical analysis, since S. Enteritidis was not recovered from those treatment group birds.
The cecal endogenous bacterial counts and pH did not differ (P > 0.05) between various treatment groups (Table 2). The supplementation with TC at 0.5 or 0.75% did not significantly alter (P > 0.05) the body weights of birds compared to the positive, negative, or compound controls (Table 2). However, all of the EG-supplemented treatment groups had reduced body weights compared to other treatment groups (P < 0.05). The cumulative feed consumption levels of the birds supplemented with 0.75 or 1% EG were also lower than other inoculated treatment groups (P < 0.05) (Table 2). In addition, among the nonchallenged treatment groups, EG lowered cumulative feed consumption compared to the negative and TC controls (P < 0.05).
Table 2.
Effects of TC and EG on cecal pH, cecal endogenous bacteria, body weight, and cumulative feed consumption of 20-day-old chickensa
| Treatment | pHb | Cecal bacteria (log10 CFU/g)b | Avg body wt (g)c | Cumulative feed intake (g)c |
|---|---|---|---|---|
| Negative control | 6.2 ± 0.1 | 8.7 ± 0.2 | 790.5 ± 19.3A | 960.0 ± 5.8A |
| TC control | 6.2 ± 0.1 | 8.6 ± 0.1 | 808.8 ± 26.3A | 952.9 ± 6.0A |
| EG control | 6.2 ± 0.1 | 8.8 ± 0.2 | 582.0 ± 39.7B | 760.3 ± 5.8E |
| Positive control | 6.1 ± 0.1 | 9.4 ± 0.2 | 786.4 ± 20.3A | 896.9 ± 9.1B |
| 0.5% (vol/wt) TC | 6.3 ± 0.1 | 8.5 ± 0.3 | 799.9 ± 36.8A | 885.5 ± 6.2B |
| 0.75% (vol/wt) TC | 6.0 ± 0.1 | 8.9 ± 0.2 | 792.1 ± 40.8A | 883.3 ± 6.0B |
| 0.75% (vol/wt) EG | 6.3 ± 0.1 | 9.3 ± 0.1 | 536.4 ± 26.4B | 821.8 ± 5.9C |
| 1% (vol/wt) EG | 6.3 ± 0.1 | 9.2 ± 0.1 | 530.6 ± 17.2B | 785.5 ± 6.2D |
Values are means ± SEM (n = 18 per treatment group).
Means within the column did not differ significantly (P > 0.05).
Values with different superscript capital letters (A to E) differed significantly from each other within a column (P < 0.05).
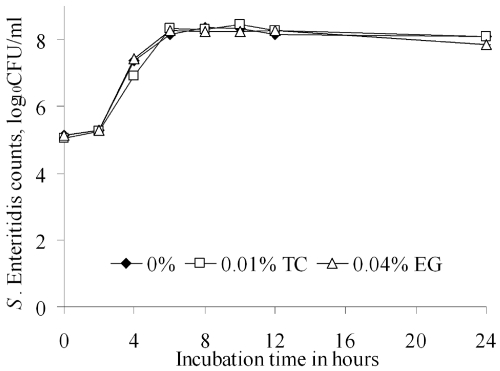
The SICs of TC and EG were 0.01 and 0.04%, respectively (Fig. 2). The average initial S. Enteritidis population in the control and TC- and EG-treated samples was approximately 5.0 log10 CFU/ml. After incubation at 37°C for 24 h, approximately 8.0 log10 CFU/ml of bacteria were recovered from control and treated samples, thereby confirming that the aforementioned concentrations of TC and EG were not inhibitory for S. Enteritidis (Fig. 2).
Fig 2.
Effect of subinhibitory concentrations of TC and EG on S. Enteritidis growth (n = 6/treatment group). The treatment groups did not differ significantly from the controls (P > 0.05). Tubes containing 20 ml LB broth were inoculated with ∼106 CFU/ml of S. Enteritidis. TC or EG was added at increments of 1 mg/μl (from 0 to 10 mg/μl) and incubated at 37°C for 24 h. Samples drawn at various intervals of incubation were diluted and plated on TSA, before enumeration after incubation at 37°C for 24 h. The highest concentration of TC or EG that did not inhibit S. Enteritidis growth after 24 h was taken as the respective SIC.
The effects of the SICs of TC and EG on motility, invasion, and gene expression on the four strains of S. Enteritidis are shown in Table 3. Both plant compounds at their SICs significantly reduced the zone of motility in all strains without reductions in bacterial counts after 8 h of incubation compared to the controls (P < 0.05) (Table 3). The SICs of TC and EG were also found to significantly decrease (P < 0.05) the invasion by S. Enteritidis for BATCs (Table 3). trans-Cinnamaldehyde at 0.01% reduced invasion by 60 to 80%, whereas EG at 0.04% decreased Salmonella invasion by 75 to 85% (P < 0.05). Further, the results of the gene expression studies revealed that TC and EG at their corresponding SICs significantly downregulated expression levels of Salmonella motility and invasion genes (Table 3).
Table 3.
Effects of subinhibitory concentrations of TC and EG on motility, invasion, and virulence gene expression in S. Enteritidis strains used in the studya
| Strain and virulence gene | Motilityb,c (cm) |
% invasionc (CFU/ml) |
Relative fold change in RT-qPCR assayd |
|||||
|---|---|---|---|---|---|---|---|---|
| 0% | 0.01% TC | 0.04% EG | 0% | 0.01% TC | 0.04% EG | 0.01% TC | 0.04% EG | |
| S. Enteritidis 12 | 8.5 ± 0.0 (FL)C | 7.15 ± 0.1B | 5.30 ± 0.0A | 100.00 ± 0.0B (4.16 ± 0.2) | 33.65 ± 17.8A (1.34 ± 0.7) | 17.31 ± 17.3A (0.65 ± 0.7) | ||
| hilA | −1.34 ± 0.2* | −1.90 ± 0.3* | ||||||
| hilD | −1.35 ± 0.2* | −1.98 ± 0.2* | ||||||
| invF | −0.87 ± 0.2NS | −2.52 ± 1.0NS | ||||||
| flhC | −0.81 ± 0.0NS | −1.25 ± 0.0* | ||||||
| motA | −0.94 ± 0.2NS | −1.62 ± 0.1* | ||||||
| S. Enteritidis 21 | 8.5 ± 0.0 (FL)C | 6.20 ± 0.0B | 4.60 ± 0.1A | 100.00 ± 0.0B (4.29 ± 0.2) | 21.48 ± 21.5A (0.89 ± 0.9) | 14.30 ± 14.3A (0.59 ± 0.6) | ||
| hilA | −1.72 ± 0.2* | −1.06 ± 0.1* | ||||||
| hilD | −1.94 ± 0.0* | −1.32 ± 0.0* | ||||||
| invF | −2.14 ± 0.1* | −1.42 ± 0.2* | ||||||
| flhC | −1.99 ± 0.3* | −1.25 ± 0.0* | ||||||
| motA | −1.65 ± 0.1* | −1.18 ± 0.0* | ||||||
| S. Enteritidis 28 | 8.5 ± 0.0 (FL)C | 4.20 ± 0.0A | 4.55 ± 0.1B | 100.00 ± 0.0B (4.10 ± 0.2) | 28.78 ± 18.7A (1.12 ± 0.7) | 27.81 ± 14.4A (1.15 ± 0.6) | ||
| hilA | −1.28 ± 0.2* | −2.10 ± 0.3* | ||||||
| hilD | −1.62 ± 0.1* | −2.14 ± 0.1* | ||||||
| invF | −1.52 ± 0.1* | −2.32 ± 0.0* | ||||||
| flhC | −1.66 ± 0.1* | −1.75 ± 0.1* | ||||||
| motA | −1.45 ± 0.1* | −2.06 ± 0.0* | ||||||
| S. Enteritidis 31 | 8.5 ± 0.0 (FL)B | 3.70 ± 0.0A | 3.80 ± 0.0A | 100.00 ± 0.0B (4.21 ± 0.2) | 43.45 ± 11.1A (1.79 ± 0.4) | 27.68 ± 17.8A (1.12 ± 0.7) | ||
| hilA | −2.42 ± 0.5* | −4.46 ± 0.4* | ||||||
| hilD | −2.36 ± 0.1* | −4.57 ± 0.2* | ||||||
| invF | −2.50 ± 0.1* | −5.52 ± 0.4* | ||||||
| flhC | −2.28 ± 0.1* | −3.81 ± 0.0* | ||||||
| motA | −2.07 ± 0.2* | −4.03 ± 0.2* | ||||||
Values are means ± SEM.
FL, full lawn.
Values with different superscript capital letters (A to C) differ significantly within the row for the indicated experiment (P < 0.05).
The fold change relative to the control.
, TC or EG downregulated expression of the gene compared to the control (P < 0.05). NS, not significant (P > 0.05).
DISCUSSION
Based on our previous observations that TC and EG were effective in significantly reducing S. Enteritidis populations in chicken cecal contents in vitro (33), we investigated the prophylactic efficacies of these molecules as a preharvest treatment for reducing S. Enteritidis populations in chickens. The tested doses of TC (0.5 and 0.75%) and EG (0.75 and 1%) were selected based on our in vitro data (33). The chicken cecum represents the major colonization site for S. Enteritidis (30, 32, 38, 47), thereby allowing further spread of the infection to a healthy uninfected flock. Therefore, the aim of our study was to reduce S. Enteritidis populations in the ceca of chickens.
The results indicated that supplementation with TC and EG consistently decreased S. Enteritidis counts in birds. It was observed that supplementation of both plant molecules in feed significantly reduced the pathogen populations in the cecal samples (Fig. 1). It was also observed that the two concentrations of TC and EG did not significantly differ (P > 0.05) in their efficacies in reducing Salmonella, and both plant compounds were effective after day 10 p.i.
Although supplementation of TC did not significantly alter (P > 0.05) the body weights of chickens, all the EG-supplemented treatment groups of birds had significantly lower body weights than the negative, positive, and TC controls (P < 0.05) (Table 2). The reductions in the body weights in the EG-supplemented treatment groups could have been due to the decreased feed consumption (P < 0.05), as the birds reduced their intake compared to the respective controls (Table 2). However, among the various EG-supplemented treatment groups, the body weights of birds were not different from each other (P > 0.05). Previously, based on the observation that EG supplemented at 850 ppm (0.085%) impaired the absorption of alanine in the rat jejunum (36), Lee and colleagues postulated that EG may impair the normal digestion process (37). While these findings have not been reported in chickens, we believe that the aroma or flavor induced by EG could have reduced the likeability of the feed for the chickens, thereby leading to decreased body weights in EG-supplemented birds.
It was also observed that both TC and EG at the tested concentrations did not cause significant reductions in the total endogenous bacterial populations in the chicken cecum (Table 2). The exact mechanism behind the selective inhibitory effects of TC and EG on Salmonella with no apparent effects on the normal gut flora is not known. However, many previous investigators have reported that plant molecules such as cinnamaldehyde, eugenol, carvacrol, and thymol can cause significant reductions in S. Typhimurium DT104, Escherichia coli O157:H7 (28, 43), pathogenic E. coli, and Clostridium perfringens (28) without any adverse effects on endogenous bacterial populations, including lactobacilli and bifidobacteria (28, 43).
In order to determine if TC and EG produced any effects on major S. Enteritidis colonization factors in chickens, we investigated the effects of SICs of the plant compounds on Salmonella colonization factors, namely, motility and host cell invasion. The SIC of a molecule is the highest concentration below the MIC that does not inhibit bacterial growth (2, 35). However, since the SIC can modify bacterial physico-chemical functions, including that of genes, it is used for studying the effect of antimicrobials on bacterial gene expression and virulence (reviewed in reference 20). Motility plays a critical role in host-bacterium interactions, colonization, and virulence (29). Flagellum-mediated and non-flagellum-mediated motility have been reported to aid in the invasion and colonization by S. Enteritidis (3, 31). The gene motA is associated with the regulation of flagellar assembly (44), and flhC, a transcriptional activator for motility genes, is a global regulator of flagellin production (12). In addition, it was previously demonstrated that S. Enteritidis attaches and invades various host cells prior to establishing an infection process (16, 21, 47). It is also well-established that genes such as hil and inv play major roles in Salmonella invasion (6, 48, 49).
Our experiments revealed that both motility and invasion of avian intestinal epithelial cells were substantially inhibited by TC and EG (P < 0.05) (Table 3). Decreases in these attributes without any concurrent reduction in the bacterial counts led us to hypothesize that these molecules may modulate Salmonella genes responsible for motility and invasion. Therefore, we investigated the effects of TC and EG on the expression of motility and invasion genes, namely, motA, flhC, hilA, hilD, and invF. The results from RT-qPCR experiments revealed that the plant molecules downregulated the virulence genes compared to controls (P < 0.05) (Table 3).
To summarize, supplementation with 0.5 and 0.75% TC or 0.75 and 1% EG was effective in reducing S. Enteritidis populations in 20-day-old commercial broiler chickens. Although both molecules were effective in reducing S. Enteritidis populations in the cecum, TC is more suitable than EG for prophylactic dosing, considering the negative impact of EG on body weight and feed consumption for birds. The results of our in vitro studies indicated that TC and EG at their SICs significantly reduced S. Enteritidis motility and invasion of avian intestinal epithelial cells, and this could be due to the downregulation of the motility genes motA and flhC and invasion genes hilA, hilD, and invF. We conclude that TC could be used as an antimicrobial feed additive to reduce S. Enteritidis colonization in chickens along with standard hygienic practices used on farms.
ACKNOWLEDGMENTS
This work was funded by USDA-NIFA Agriculture and Food Research Initiative grant number 2009-03576, awarded to K. Venkitanarayanan and D. Donoghue.
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that are suitable.
Footnotes
Published ahead of print 10 February 2012
REFERENCES
- 1. Altekruse S, Koehler J, Hickman-Brenner F, Tauxe RV, Ferris KA. 1993. Comparison of Salmonella Enteritidis phage types from egg-associated outbreaks and implicated laying flocks. Epidemiol. Infect. 110:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amalaradjou MAR, Narayanan A, Venkitanarayanan K. 2011. trans-Cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 185:1526–1531 [DOI] [PubMed] [Google Scholar]
- 3. Amy M, et al. 2004. Identification of a new Salmonella enterica serovar Enteritidis locus involved in cell invasion and in the colonization of chicks. Res. Microbiol. 155:543–552 [DOI] [PubMed] [Google Scholar]
- 4. Anonymous 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food: 10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333–337 [PubMed] [Google Scholar]
- 5. Anonymous 2011. Vital signs: incidence and trends of infection with pathogens transmitted through food. Food-borne Diseases Active Surveillance Network, 10 U.S. sites, 1996–2010. MWR Morb. Mortal. Wkly. Rep. 60:749–755 [PubMed] [Google Scholar]
- 6. Bajaj V, Hwang C, Lee CA. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella Typhimurium invasion genes. Mol. Microbiol. 18:715–727 [DOI] [PubMed] [Google Scholar]
- 7. Bookout AL, Mangelsdorf DJ. 2003. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 1:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burt S. 2004. Essential oils: their antibacterial properties and potential applications in food. A review. Int. J. Food Microbiol. 94:223–253 [DOI] [PubMed] [Google Scholar]
- 9. Byrd JA, et al. 2001. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 80:278–283 [DOI] [PubMed] [Google Scholar]
- 10. Byrd JA, et al. 2003. Effect of experimental chlorate product administration in the drinking water on Salmonella Typhimurium contamination of broilers. Poult. Sci. 82:1403–1406 [DOI] [PubMed] [Google Scholar]
- 11. Chadfield MS, Hinton MH. 2004. Effects of furazolidone pretreatment of Salmonella Enteritidis PT4 at sub- and supra-inhibitory concentrations on phagocytosis and intracellular survival in chicken macrophages. Vet. Immunol. Immunopathol. 100:81–97 [DOI] [PubMed] [Google Scholar]
- 12. Chilcott GS, Hughes KT. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Code of Federal Regulations 2011. Title 21, vol 3. Food and drugs. Chapter I. Food and Drug Administration. Subchapter B. Food for human consumption. Part 182. Substances generally recognized as safe. Synthetic flavoring substances and adjuvants. 21 CFR 182.60. [Google Scholar]
- 14. Code of Federal Regulations 2011. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter E. Animal drugs, feeds, and related products. Part 582. Substances generally recognized as safe. Subpart A. Synthetic flavoring substances and adjuvants. 21 CFR 582.60. [Google Scholar]
- 15. Djefal A, Tahtat D, Nacer Khodja A, Saad Bouzid S, Remane N. 2007. Validation and substantiation of 25 kGy as sterilization dose for lyophilized human amnion membrane. Cell Tissue Bank 8:9–12 [DOI] [PubMed] [Google Scholar]
- 16. Dodson SV, Maurer JJ, Holt PS. 1999. Temporal changes in the population genetics of Salmonella pullorum. Avian Dis. 43:685–695 [PubMed] [Google Scholar]
- 17. Fernandez F, Hinton M, Van Gils B. 2002. Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to Salmonella Enteritidis colonization. Avian Pathol. 31:49–58 [DOI] [PubMed] [Google Scholar]
- 18. Filho RLA, da Silva EN, Ribeiro AR, Kondo N, Curi PR. 2000. Use of anaerobic cecal microflora, lactose and acetic acid for the protection of broiler chicks against experimental infection with Salmonella Typhimurium and Salmonella Enteritidis. Braz. J. Microbiol. 31:107–112 [Google Scholar]
- 19. Fiorentin L, Vieira ND, Barioni W., Jr 2005. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 34:258–263 [DOI] [PubMed] [Google Scholar]
- 20. Fonseca AP, Extremina C, Fonseca AF, Souza JC. 2004. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J. Med. Microbiol. 53:903–910 [DOI] [PubMed] [Google Scholar]
- 21. Henderson SE, Bounous DI, Lee MD. 1999. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 67:3580–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heres L, Engel B, Urlings HAP, Wagner JA, van Knapen F. 2004. Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella. Vet. Microbiol. 99:259–267 [DOI] [PubMed] [Google Scholar]
- 23. Hester P, et al. 2010. Poultry, p 102–120 In Federation of Animal Science Societies Writing Committee's guide for the care and use of agricultural animals in research and training, 3rd ed FASS, Champaign, IL [Google Scholar]
- 24. Higgins SE, et al. 2007. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella Enteritidis by abdominal exudate cells. Poult. Sci. 86:2315–2321 [DOI] [PubMed] [Google Scholar]
- 25. Hudson CR, et al. 2000. Genetic relatedness of Salmonella isolates from nondomestic birds in southeastern United States. J. Clin. Microbiol. 38:1860–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Humphrey T, Jorgensen F. 2006. Pathogens on meat and infection in animals: establishing a relationship using Campylobacter and Salmonella as examples. Meat Sci. 74:89–97 [DOI] [PubMed] [Google Scholar]
- 27. Inoue AY, Berchieri A, Jr, Bernardino A, Paiva JB, Sterzo EV. 2008. Passive immunity of progeny from broiler breeders vaccinated with oil-emulsion bacterin against Salmonella Enteritidis. Avian Dis. 52:567–571 [DOI] [PubMed] [Google Scholar]
- 28. Jamroz D, Wiliczkiewicz A, Wertelecki T, Orda J, Skorupińska J. 2005. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 46:485–493 [DOI] [PubMed] [Google Scholar]
- 29. Josenhans C, Suerbaum S. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605–614 [DOI] [PubMed] [Google Scholar]
- 30. Khan MI, Fadl AA, Venkitanarayanan KS. 2003. Reducing colonization of Salmonella Enteritidis in chicken by targeting outer membrane proteins. J. Appl. Microbiol. 95:142–145 [DOI] [PubMed] [Google Scholar]
- 31. Khoramian-Falsafi T, Harayama S, Kutsukake K, Pechere JC. 1990. Effect of motility and chemotaxis on the invasion of Salmonella Typhimurium into HeLa cells. Microb. Pathog. 9:47–53 [DOI] [PubMed] [Google Scholar]
- 32. Kollanoor Johny A, et al. 2009. Prophylactic supplementation of caprylic acid in feed reduces Salmonella Enteritidis colonization in commercial broiler chicks. J. Food Prot. 72:722–727 [PubMed] [Google Scholar]
- 33. Kollanoor Johny A, Darre MJ, Donoghue AM, Donoghue DJ, Venkitanarayanan K. 2010. Antibacterial effect of trans-cinnamaldehyde, eugenol, thymol and carvacrol against Salmonella Enteritidis and Campylobacter jejuni in vitro. J. Appl. Poult. Res. 19:237–244 [Google Scholar]
- 34. Kollanoor Johny A, et al. 2008. Antibacterial effect of trans-cinnamaldehyde on Salmonella Enteritidis and Campylobacter jejuni in chicken drinking water. J. Appl. Poult. Res. 17:490–497 [Google Scholar]
- 35. Kollanoor Johny A, Hoagland TA, Venkitanarayanan K. 2010. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 7:1165–1170 [DOI] [PubMed] [Google Scholar]
- 36. Kreydiyyeh SI, Usta J, Copti R. 2000. Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum. Food Chem. Toxicol. 38:755–762 [DOI] [PubMed] [Google Scholar]
- 37. Lee K-W, Everts H, Kappert HJ, Beynen AC. 2004. Growth performance of broiler chickens fed a carboxymethyl cellulose containing diet with supplemental carvacrol and/or cinnamaldehyde. Int. J. Poult. Sci. 3:619–622 [Google Scholar]
- 38. Li WZ, Watarai S, Kodama H. 2003. Identification of possible chicken intestinal mucosa receptors for SEF21-fimbriated Salmonella enterica serovar Enteritidis. Vet. Microbiol. 91:215–229 [DOI] [PubMed] [Google Scholar]
- 39. Marcus R, et al. 2007. Re-assessment of risk factors for sporadic Salmonella serotype Enteritidis infections: a case-control study in five FoodNet sites, 2002–2003. Epidemiol. Infect. 135:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niu C, Gilbert ES. 2004. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 70:6951–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pazos P, Fortaner S, Prieto P. 2002. Long-term in vitro toxicity models: comparisons between a flow-cell bioreactor, a static-cell bioreactor and static cell cultures. Altern. Lab. Anim. 30:515–523 [DOI] [PubMed] [Google Scholar]
- 42. Salamci E, Kordali S, Kotan R, Cakir A, Kaya Y. 2007. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 35:569–581 [Google Scholar]
- 43. Si W, et al. 2006. In vitro assessment of antimicrobial activity of carvacrol, thymol and cinnamaldehyde towards Salmonella serotype Typhimurium DT104: effects of pig diets and emulsification in hydrocolloids. J. Appl. Microbiol. 101:1282–1291 [DOI] [PubMed] [Google Scholar]
- 44. Soutourina OA, Bertina PN. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27:505–523 [DOI] [PubMed] [Google Scholar]
- 45. Spring P, Wenk C, Dawson KA, Newman KA. 2000. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the caeca of Salmonella-challenged broiler chicks. Poult. Sci. 79:205–211 [DOI] [PubMed] [Google Scholar]
- 46. Stern NJ, Cox NA, Bailey JS, Berrang ME, Musgrove MT. 2001. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 80:156–160 [DOI] [PubMed] [Google Scholar]
- 47. Van Immerseel F, et al. 2004. Medium chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 70:3582–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wallis TS, Galyov EE. 2000. Molecular basis of Salmonella induced enteritis. Mol. Microbiol. 36:997–1005 [DOI] [PubMed] [Google Scholar]
- 49. Zhou D, Galan J. 2001. Salmonella entry into host cells: the work in concept of type III secreted effector proteins. Microbes Infect. 3:1293–1298 [DOI] [PubMed] [Google Scholar]