Abstract
C4-dicarboxylic acids appear to be metabolized via the tricarboxylic acid (TCA) cycle in N2-fixing bacteria (bacteroids) within legume nodules. In Sinorhizobium meliloti bacteroids from alfalfa, NAD+-malic enzyme (DME) is required for N2 fixation, and this activity is thought to be required for the anaplerotic synthesis of pyruvate. In contrast, in the pea symbiont Rhizobium leguminosarum, pyruvate synthesis occurs via either DME or a pathway catalyzed by phosphoenolpyruvate carboxykinase (PCK) and pyruvate kinase (PYK). Here we report that dme mutants of the broad-host-range Sinorhizobium sp. strain NGR234 formed nodules whose level of N2 fixation varied from 27 to 83% (plant dry weight) of the wild-type level, depending on the host plant inoculated. NGR234 bacteroids had significant PCK activity, and while single pckA and single dme mutants fixed N2 at reduced rates, a pckA dme double mutant had no N2-fixing activity (Fix−). Thus, NGR234 bacteroids appear to synthesize pyruvate from TCA cycle intermediates via DME or PCK pathways. These NGR234 data, together with other reports, suggested that the completely Fix− phenotype of S. meliloti dme mutants may be specific to the alfalfa-S. meliloti symbiosis. We therefore examined the ME-like genes azc3656 and azc0119 from Azorhizobium caulinodans, as azc3656 mutants were previously shown to form Fix− nodules on the tropical legume Sesbania rostrata. We found that purified AZC3656 protein is an NAD(P)+-malic enzyme whose activity is inhibited by acetyl-coenzyme A (acetyl-CoA) and stimulated by succinate and fumarate. Thus, whereas DME is required for symbiotic N2 fixation in A. caulinodans and S. meliloti, in other rhizobia this activity can be bypassed via another pathway(s).
INTRODUCTION
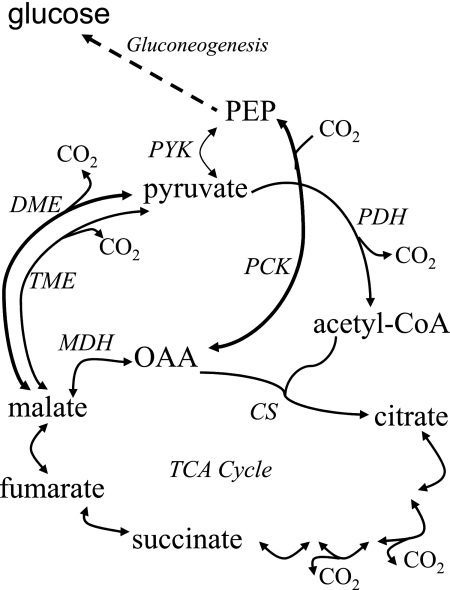
Rhizobia form nodules on leguminous host plants, and the bacteria within these nodules differentiate into N2-fixing bacteroids. N2 fixation requires a high energy input, and the primary carbon and energy sources supplied to bacteroids appear to be malate and succinate (15, 28, 45). To provide energy and reductant for nitrogen fixation, these C4-dicarboxylic acids are metabolized via the tricarboxylic acid (TCA) cycle (13, 18, 24, 32, 33, 40, 44) (Fig. 1). The maintenance of metabolic flux through the TCA cycle during C4-dicarboxylate oxidation requires a pathway for the synthesis of acetyl-coenzyme A (acetyl-CoA) (Fig. 1). In the alfalfa symbiont Sinorhizobium meliloti, acetyl-CoA generation appears to occur via the combined activities of malic enzymes (ME) and pyruvate dehydrogenase (10, 11) (Fig. 1). ME catalyze the decarboxylation of malate to pyruvate and CO2, with the simultaneous reduction of NAD+/NADP+ to NADH/NADPH (51). S. meliloti contains two distinct malic enzymes: DME (EC 1.1.1.39) is an NAD(P)+-dependent ME, and TME (EC 1.1.1.40) is strictly NADP+ dependent (50). DME is considered an important enzyme for regulating C4-dicarboxylic acid metabolism in N2-fixing bacteroids because its activity is strongly inhibited by acetyl-CoA and stimulated by fumarate and succinate. NAD(P)+-ME activity is readily detected in free-living cells and bacteroids of Rhizobium leguminosarum, Bradyrhizobium japonicum, and S. meliloti (6, 7, 10, 11, 22, 30, 31). S. meliloti dme mutants form alfalfa root nodules that fail to fix nitrogen (Fix−), while tme mutants induce wild-type, N2-fixing root nodules (Fix+) (11, 12). Moreover, the levels of symbiotic N2 fixation were reduced in S. meliloti bacteroids with reduced DME activity, whereas increased TME activity failed to rescue the N2 fixation deficiency of dme mutants (10, 34).
Fig 1.
C4-dicarboxylic acid-related metabolism. Abbreviations: DME, NAD(P)+-dependent malic enzyme; TME, NADP+-dependent malic enzyme; PCK, phosphoenolpyruvate carboxykinase; PYK, pyruvate kinase; PDH, pyruvate dehydrogenase; CS, citrate synthase; MDH, malate dehydrogenase; PEP, phosphoenolpyruvate; TCA cycle, tricarboxylic acid cycle.
A second route for the formation of acetyl-CoA is via the enzyme phosphoenolpyruvate carboxykinase (PCK), which catalyzes the decarboxylation of oxaloacetate to phosphoenolpyruvate (Fig. 1). PCK is required for the growth of S. meliloti, R. leguminosarum, and Sinorhizobium sp. NGR234 on C4-dicarboxylic acids (1, 13, 16, 32). However, the symbiotic N2 fixation phenotype of pck mutants varies depending on the plant host. R. leguminosarum pck mutants showed no reduction in the symbiotic N2 fixation phenotype (32), while pckA mutants of S. meliloti showed a reduced N2 fixation efficiency (16). Sinorhizobium sp. NGR234 pck mutants lost 40 to 80% of their N2 fixation efficiency, depending on the plant host (38). PCK activity was detected in R. leguminosarum bacteroids, but no activity was detected in bacteroids of S. meliloti (1, 10, 16, 30, 31, 37, 39).
To gain further insight into the role of malic enzymes in nodules, we isolated dme and dme pckA mutants of the broad-host-range Sinorhizobium strain NGR234. The NGR234 dme mutants were found to form Fix+ nodules with various N2 fixation efficiencies on various host plants (see below). Moreover, while this work was under way, dme mutants of B. japonicum, R. leguminosarum, and Mesorhizobium loti were shown to form Fix+ nodules on soybean, pea, and Lotus, respectively (8, 36, 48). Together, these findings demonstrate that the N2 fixation phenotype of dme mutants is host plant dependent and also suggest that the completely Fix− phenotype of S. meliloti dme mutants is unusual and perhaps unique to the S. meliloti-alfalfa symbiosis. In this respect, two recent reports regarding Azorhizobium caulinodans were of particular note. A. caulinodans forms N2-fixing nodules on Sesbania rostrata, and a large screen for symbiotic mutants of A. caulinodans identified two insertion mutants with mutations in a malic enzyme-like gene (azc3656) that formed Fix− nodules (47). Transcription of a separate malic enzyme-like gene, azc0119, was induced in A. caulinodans bacteroids (49). We therefore characterized the A. caulinodans azc3656- and azc0119-encoded proteins with respect to their malic enzyme activities and showed that azc3656 encodes a DME protein. Thus, whereas NGR234 dme mutants are Fix+ on various host plants, A. caulinodans dme mutants, like S. meliloti dme mutants, have a Fix− phenotype.
MATERIALS AND METHODS
Bacterial strains and culture media.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown at 37°C in Luria-Bertani medium, and Sinorhizobium sp. NGR234 strains were grown at 30°C in tryptone-yeast (TY) medium (5 g liter−1 tryptone, 3 g liter−1 yeast extract, 0.87 g liter−1 CaCl2 · 2H2O) (38). Concentrations of antibiotics (μg/ml) for NGR234 strains were as follows: rifampin, 20; streptomycin (Sm), 50; spectinomycin (Sp), 50; and gentamicin (Gm), 20. Those for E. coli were as follows: ampicillin (Amp), 100; Gm, 10; Sm, 30; Sp, 100; and chloramphenicol (Cm), 10.
Table 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Sinorhizobium strains | ||
| NGR234 | NGR234R rif-1 Nod+ Fix + | 38 |
| NGR234 pckA | NGR234R pckA::Ω Spr | 38 |
| RmP1809 | NGR234R dme-9::Ω Spr | This study |
| RmP1814 | NGR234R dmeΔ14::Ω Spr | This study |
| RmP2190 | RmP1809(pTH1582) | This study |
| RmP2191 | RmP1809(pTH2584) | This study |
| RmP2192 | RmP1814(pTH1582) | This study |
| RmP2193 | RmP1814(pTH2584) | This study |
| RmP2648 | NGR234R pckA::Ω Spr Δdme::kan | This study |
| RmP2651 | NGR234R pckA::Ω Spr Δdme | This study |
| Escherichia coli strains | ||
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | 9 |
| MT616 | MT607(MM294A recA56)(pRK600) | 52 |
| Plasmids | ||
| pBAD/HisA | Expression vector; Apr | Invitrogen |
| pJQ200-mp18 | Suicide vector; Gmr 5% sucroses | 43 |
| pHP45 | Ω Spr Smr Apr | 41 |
| pKD13 | bla FRT ahp FRT PS1 PS4 oriR6K Kmr | 9 |
| pKD46 | bla PBAD gam bet exo pSC101 oriTS Ampr | 9 |
| pRK7813 | Broad-host-range cosmid vector; Mob IncP Tcr | 20 |
| pTH1582 | pJP2 with gusA from pFUS1; Tcr | 52 |
| pTH2415 | pJQ200-mp18; 1,729 bp from NGR234 dme | This study |
| pTH2429 | pTH2415 dme::Ω Spr Smr at 418 bp | This study |
| pTH2457 | pJQ200-mp18; bp −471 to 87 of NGR234 dme | This study |
| pTH2462 | pJQ200-mp18; bp −471 to 87 and 587 to 1186 of NGR234 dme | This study |
| pTH2467 | pTH2462::Ω Spr Smr (between bp 87 and 587) | This study |
| pTH2505 | FLP gene in pRK7813 | Unpublished |
| pTH2530 | pJQ200-mp18; bp −471 to 87 and 1823 to 920 of NGR234 dme | This study |
| pTH2532 | pTH2530 Δdme::FRT Kmr Nmr from pKD13 | This study |
| pTH2584 | pTH1582; sequence from bp −284 and complete NGR234 dme gene | This study |
| pTH2754 | azc3656 with 5′ NheI site and 3′ HindIII site inserted into NheI and HindIII sites of pBAD/HisA | This study |
| pTH2755 | azc0119 with 5′ NheI site and 3′ HindIII site inserted into the NheI and HindIII sites of pBAD/HisA | This study |
Cloning of the dme gene, DNA sequencing, and computer analysis.
The NGR234 dme gene was cloned and sequenced prior to the release of the NGR234 genome sequence (46). Degenerate primers were designed based on highly conserved regions identified through alignment of the dme genes from S. meliloti 1021 and related alphaproteobacteria. The locations of the primers listed below are indicated relative to their 5′ position in the NGR234 dme gene. Two pairs of degenerate primers, FP1 (5′-GTBCTCGGVCTCGGCAATATCGG-3′) (bp 264) plus RP1 (5′-GGATCGGGCCGACATGCAG-3′) (bp 2205) and FP2 (5′-GACCTTCGGCGGCATCAA-3′) (bp 422) plus RP2 (5′-CKWRCGCTTGCCGATGATCTG-3′) (bp 1764), were used to PCR amplify the dme gene region, using genomic DNA of NGR234. The DNA sequences extending to either side of dme were obtained directly from genomic DNA by using primers FP8s (5′-GACATTCTTCACCGACACCTAT-3′) (bp 1814), RP6s (5′-CCGCTCGACTTCAAAGCACTCC-3′) (bp 483), and RP9s (5′-GTCGATTGAGGTTTCGCTTTGTC-3′) (bp 35). Sequencing was performed using a model 3730 DNA analyzer (Mobix) at McMaster University.
Construction and complementation of Sinorhizobium sp. NGR234 dme mutants.
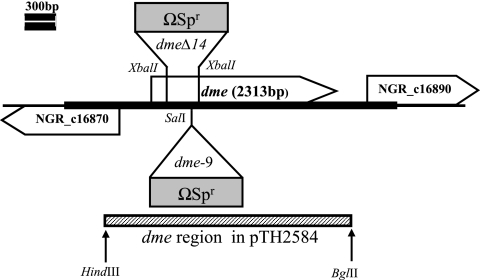
Plasmid pTH2415 was constructed by inserting the NGR234 dme gene region from positions 246 to 1483 (amplified using the primers 5′-ATAAGATCTCGTCGGAAATGAACAGCGTC-3′ and 5′-TATATGCATGGGTTTCGATGATCTGTGGA-3′) into the Gmr plasmid pJQ200-mp18. Plasmid pTH2429, carrying an Ω Spr Smr cassette inserted at the SalI site at position 418 relative to the ATG dme start codon (the dme-9::Ω Spr Smr allele), and pTH2467, in which the dme gene region from bp 88 to 586 was deleted and replaced with the Ω Spr Smr cassette, were transferred from E. coli to NGR234 (Fig. 2). NGR234 dme::Ω Spr Smr homogenates were identified as Rifr Spr Smr Gms colonies. The structure of the dme mutations in genomic DNAs from RmP1809 (dme-9) and RmP1814 (dmeΔ14) was verified by restriction analysis following PCR amplification of the dme region by use of primers from outside the region employed for mutant construction. Plasmid pTH2584 (Tcr), carrying the 2.6-kb NGR234 dme gene region, was used for dme complementation.
Fig 2.
Sinorhizobium sp. NGR234 dme gene region. The dme gene is shown together with relevant restriction sites and the locations of the dmeΔ14::Ω Spr and dme-9::Ω Spr insertion mutations. NGR_c16870 encodes a putative metallophosphoesterase protein, and NGR_c16890 encodes a transcriptional regulator (NgrR). The thick line indicates the 3,328-bp region sequenced in this work, and the lower line indicates the dme region cloned into the complementing plasmid pTH2584.
To construct the NGR234 pckA dme double mutant (strain RmP2648), the dme gene together with its flanking DNA was cloned into the suicide vector PJQ200-mp18 to give pTH2530. The region of dme in pTH2530 was replaced with the FLP recombination target (FRT)-flanked Kmr Nmr cassette from pKD13 by the method of Datsenko and Wanner (9). The resulting plasmid (pTH2532) was transferred into the NGR234 pckA::Ω Spr mutant, and Δdme::kan recombinants (RmP2648) were identified as Rifr Spr Nmr colonies that grew with 5% sucrose. Subsequently, the Kmr Nmr cassette was eliminated upon transfer of the Flp plasmid pTH2505. Deletion of dme and the Kmr Nmr cassette (RmP2651 pckA::Ω Spr Δdme) was verified by PCR, and the absence of the DME protein was verified by Western blot analysis.
Plant assays.
Cajanus cajan cv. Pigeon pea, Lablab purpureus cv. Dolichos Rongai, Leucaena leucocephala (Lain.) de Wit, Macroptilium atropurpureum cv. Aztec Atro, and Vigna unguiculata cv. Red Caloona cowpea seeds were obtained from Queensland Agricultural Seeds Pty. Ltd. (Toowoomba, Queensland, Australia). Prior to germination, these hard-coated seeds were scarified by treatment with concentrated H2SO4, and after rinsing with sterile double-distilled water (ddH2O), the seeds were surface sterilized in 3% (vol/vol) sodium hypochlorite for 10 min. Seeds were washed with sterile ddH2O and germinated on 1.5% (vol/vol) water agar at room temperature for 2 to 4 days, depending on the species.
All plants were grown in Leonard jar assemblies (26) with nitrogen-free sand-vermiculite (1:1 [wt/wt]) and watered with 1× Jensen's medium (19). Seedlings were inoculated with approximately 1 × 108 log-phase cells 2 to 5 days after transplanting. Growth chamber conditions were 16 h of light at 28°C and 8 h of dark at 20°C (2, 42). Plants were grown until the noninoculated controls displayed clear symptoms of nitrogen deficiency (yellowing of leaves and stunted growth). For C. cajan, M. atropurpureum, and V. unguiculata, this occurred after 6 weeks of growth, while L. purpureus and L. leucocephala were grown for 10 weeks.
Shoot dry weight for each pot was determined following incubation of the shoots at 70°C for 2 weeks. Nitrogenase activity was measured by monitoring the reduction of acetylene (10% of total volume) to ethylene by the total root systems from each pot (10, 16). Ethylene was detected using an HP6890 gas chromatograph (GC) system (Agilent Technologies) equipped with a GS-Gaspro column (J&W) with N2 carrier gas. The parameters used were as follows: inlet, 250°C at 100 ml min−1 split flow; column, constant flow at 1.4 ml min−1; oven, 90°C; and flame ionization detector, 260°C, with H2 flow at 40 ml min−1 and airflow at 450 ml min−1. A standard curve was generated using 507 ppm ethylene, and specific activity was calculated as nmol ethylene produced/h/plant (or gram of fresh nodules).
Preparation of bacteroid and free-living cell extracts.
Bacteroids were prepared at 4°C by grinding (with an ice-cold mortar and pestle) ∼3 g of fresh nodules with 10 ml of sterile MMS buffer (40 mM morpholinepropanesulfonic acid [MOPS], 20 mM KOH, 2 mM MgSO4, 0.3 M sucrose, pH 7.0). After filtration through four layers of cheesecloth with MMS, the homogenates were centrifuged at 200 × g for 10 min, and the resulting supernatant was centrifuged at 3,900 × g for 10 min. The pellet was washed twice with MMS, flash frozen in liquid nitrogen, and stored at −80°C.
Free-living cell pellets obtained by centrifugation of 20-ml overnight cultures (optical density at 600 nm [OD600] of around 1.5) were washed twice with 1 ml ice-cold 0.85% (wt/vol) NaCl and resuspended in 500 μl ice-cold sonication buffer (25 mM Tris-Cl, pH 8, 100 mM KCl, 1 mM MgCl2, 10% glycerol, 10 mM 2-mercaptoethanol). Bacteroid cells from ∼3 g of fresh nodules in 1 ml MMS were disrupted using a Cell Disrupter 350 (Branson Ultrasonics Corp.) at an output level of 4 for 10 to 15 cycles per sample at 10 s per cycle. The samples were placed in an ice bath for 3 min after each cycle. The cell lysates were then centrifuged at 4°C and 16,000 × g for 20 min, and the resulting supernatants were aliquoted, flash frozen in liquid nitrogen, and stored at −80°C. Protein concentrations were measured with Coomassie brilliant blue (Bio-Rad) standardized with bovine serum albumin (5).
Enzyme assays, SDS-PAGE, Western blot analysis, and protein purification.
Malic enzyme activity in crude extracts was measured via the l-malate-dependent formation of pyruvate as detected with 2,4-dinitrophenylhydrazine (10, 17). Five-hundred-microliter assay mixtures contained 100 mM Tris-HCl, pH 7.8, 30 mM sodium-l-malate, pH 7.8, 3 mM MnCl2, 5 mM NH4Cl, 0.3 mM NAD+ (for DME) or 0.3 mM NADP+ (for TME), and 5 μl cell extract (∼20 μg total protein). Reaction mixtures were incubated at 30°C for 30 min. A total of 165 μl 0.1% 2,4-dinitrophenylhydrazine in 2 M HCl was added, and following incubation for 10 min at room temperature, 835 μl 2.5 M NaOH was added, reactions were centrifuged, and the absorbance was read at 445 nm. A standard curve was made with pyruvate (0 to 110 nmol). Background activity was measured for the complete reaction mixture without protein. The activity of purified malic enzyme was measured at 340 nm as previously described (50) and as outlined in the figure legends. Malate dehydrogenase (MDH) specific activity was calculated as nmol of NAD reduced/min/mg protein. Two-hundred-microliter assay mixtures contained 100 mM glycine-NaOH, pH 10, 90 mM sodium-l-malate, 2.5 mM NAD+, and 5 μl cell extract (∼10 μg total protein) (10, 14). The PCK specific activity was assayed by monitoring the phosphoenolpyruvate-dependent oxidation of NADH to NAD+ (A340) in an assay system coupled to MDH (3, 10). SDS-PAGE and Western blot analysis with anti-DME and anti-TME antibodies were performed as described previously (25, 35, 50).
The azc0119 and azc3656 genes were PCR amplified from A. caulinodans and cloned into the pBAD/his vector between the His tag and the transcriptional terminator. Following confirmation of the cloned gene sequences, 500-ml E. coli Top10 cultures carrying pTH2754 (azc3656) or pTH2755 (azc0119) were induced for 4 h at 37°C with 1.3 mM l-arabinose. His-tagged AZC3656 and AZC0119 were expressed as soluble proteins, and the proteins were eluted from French press extracts on Ni-nitrilotriacetic acid (Ni-NTA) agarose with increasing concentrations of imidazole. Fractions that carried the pure proteins were pooled, dialyzed, and stored at −80°C in buffer containing 10% glycerol, as previously described (50).
Nucleotide sequence accession number.
The nucleotide sequence of the NGR234 dme region was assigned GenBank/NCBI accession number FJ215683.
RESULTS
Cloning and sequencing of the NGR234 dme gene.
The NGR234 dme gene was PCR amplified, cloned, and sequenced as outlined in Materials and Methods (Fig. 2). The NGR234 and S. meliloti dme genes were 87% identical, and their predicted proteins were 95% identical. A potential ribosome-binding site (5′-AGGGA-3′) and −35 (CTGT) and −10 (AAAT) promoter-like sequences were located at positions similar to those in the S. meliloti dme gene (35). The NGR234 protein contained three conserved domains: the malic enzyme N-terminal domain (pfam00390), the malic enzyme NAD binding domain (pfam03949), and a phosphate acetyl/butaryl transferase-like domain (cl00390) (29). The gene region flanking the NGR234 dme gene (46) was highly syntenic with the S. meliloti dme gene region.
Sinorhizobium sp. NGR234 dme mutants.
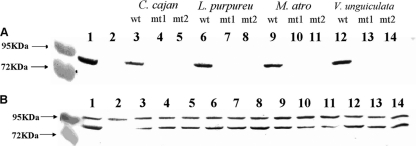
NGR234 dme Ω Spr insertion mutants (dme-9 and dmeΔ14) were constructed, and Western analyses of these mutants, using antibodies raised against the DME and TME proteins from S. meliloti, showed that the dme mutants lacked the DME protein but the TME protein was present at wild-type levels in both mutants (Fig. 3).
Fig 3.
DME and TME proteins in bacteroid extracts from NGR234 strains. Western blots were probed with anti-DME antibody (A) and anti-TME antibody (B). In addition to the TME protein, anti-TME antiserum detects an additional protein band that migrates at 95 kDa and is present in extracts from S. meliloti and NGR234. Each lane was loaded with 25 μg of protein. Lane 1, free-living S. meliloti (wild type); lane 2, free-living S. meliloti dme-3::Tn5 tme-4::Ω Spr. Lanes 3 to 14 show bacteroid extracts from nodules, as follows: lane 3, C. cajan plus NGR234; lane 4, C. cajan plus NGR234 dme-9; lane 5, C. cajan plus NGR234 dmeΔ14; lane 6, L. purpureus plus NGR234; lane 7, L. purpureus plus NGR234 dme-9; lane 8, L. purpureus plus NGR234 dmeΔ14; lane 9, M. atropurpureum plus NGR234; lane 10, M. atropurpureum plus NGR234 dme-9; lane 11, M. atropurpureum plus NGR234 dmeΔ14; lane 12, V. unguiculata plus NGR234; lane 13, V. unguiculata plus NGR234 dme-9; and lane 14, V. unguiculata plus NGR234 dmeΔ14. Data for L. leucocephala are not shown. Prestained protein standards of 95 kDa and 72 kDa are shown in the outer left lane. wt, NGR234; mt1, NGR234 dme-9; mt2, NGR234 dmeΔ14.
Malic enzyme activities in crude extracts of these mutants were assayed using the NAD+- and NADP+-dependent formation of pyruvate from l-malate (Table 2). However, since the 2,4-dinitrophenylhydrazine used to detect pyruvate also reacts with other keto acids, such as oxaloacetate (17), this assay is complicated by the presence of substantial cross-reactivity that is likely to arise from the NAD+-dependent formation of oxaloacetate from l-malate by the enzyme malate dehydrogenase (see below). Nevertheless, the DME activity detected in the two NGR234 dme mutants was approximately 50% of that present in extracts of the wild-type strain (Table 2). TME activities in the dme mutant extracts were generally reduced relative to the wild-type level; this was expected, as the NADP-ME activity of wild-type extracts is catalyzed by both the TME and DME proteins. MDH activity in both of the NGR234 dme mutants was slightly higher than that in the wild-type NGR234 strain. These results are similar to those reported previously for dme mutants of S. meliloti and R. leguminosarum (10, 36).
Table 2.
Enzyme activities of Sinorhizobium sp. NGR234 wild-type and dme mutant bacteroids and free-living cells
| Host planta | Enzyme | Sp actc |
||
|---|---|---|---|---|
| NGR234 wild type | NGR234 dme-9::Ω Spr | NGR234 dmeΔ14::Ω Spr | ||
| Cajanus cajan | DME | 42 ± 1 | 10 ± 1 | 10 ± 2 |
| TME | 26 ± 3 | 27 ± 3 | 20 ± 4 | |
| PCK | 99 ± 9 | 95 ± 4 | 86 ± 2 | |
| MDH | 2,919 ± 157 | 3,394 ± 183 | 3,466 ± 463 | |
| Lablab purpureus | DME | 46 ± 3 | 7 ± 3 | 7 ± 1 |
| TME | 53 ± 4 | 38 ± 3 | 42 ± 5 | |
| PCK | 75 ± 6 | 52 ± 1 | 54 ± 2 | |
| MDH | 2,675 ± 89 | 3,137 ± 94 | 3,223 ± 61 | |
| Leucaena leucocephala | DME | 49 ± 2 | 24 ± 2 | 22 ± 2 |
| TME | 15 ± 2 | 11 ± 3 | 9 ± 2 | |
| PCK | 47 ± 2 | 41 ± 3 | 38 ± 4 | |
| MDH | 1,648 ± 129 | 1,818 ± 109 | 1,577 ± 117 | |
| Macroptilium atropurpureum | DME | 67 ± 4 | 21 ± 4 | 21 ± 1 |
| TME | 45 ± 5 | 39 ± 4 | 33 ± 4 | |
| PCK | 182 ± 10 | 160 ± 1 | 156 ± 3 | |
| MDH | 2,592 ± 74 | 3,344 ± 204 | 3,912 ± 91 | |
| Vigna unguiculata | DME | 74 ± 2 | 23 ± 2 | 21 ± 1 |
| TME | 50 ± 5 | 35 ± 4 | 35 ± 1 | |
| PCK | 113 ± 4 | 100 ± 1 | 96 ± 3 | |
| MDH | 1,736 ± 211 | 1,613 ± 74 | 2,301 ± 193 | |
| Free-living cellsb | DME | 103 ± 13 | 51 ± 3 | 44 ± 7 |
| TME | 80 ± 4 | 67 ± 6 | 61 ± 8 | |
| MDH | 995 ± 50 | 1,395 ± 153 | 1,191 ± 49 | |
Bacteroids were isolated from nodules of 6-week-old Cajanus cajan, 10-week-old Lablab purpureus, 6-week-old Macroptilium atropurpureum, and 6-week-old Vigna unguiculata plants.
Free-living cells of wild-type and dme mutant strains were grown in TY.
Values are means ± standard errors for triplicate samples. For DME (NAD-dependent malic enzyme), specific activity is shown as nanomoles of pyruvate formed/minute/mg protein; for TME (NADP-dependent malic enzyme), specific activity is shown as nanomoles of pyruvate formed/minute/mg protein; for PCK (phosphoenolpyruvate carboxykinase), specific activity is shown as nanomoles of NADH oxidized/minute/mg protein; and for MDH (malate dehydrogenase), specific activity is shown as nanomoles of NADH formed/minute/mg protein. Enzyme activities in S. meliloti bacteroids were measured as 184 ± 7 nmol/min/mg protein for DME, 5,078 ± 109 nmol/min/mg protein for MDH, and 8 ± 2 nmol/min/mg protein for PCK.
Complex symbiotic phenotypes of dme mutants on divergent legume genera.
The symbiotic phenotypes of the NGR234 dme mutants were determined on five host plants, namely, C. cajan, L. purpureus, L. leucocephala, M. atropurpureum, and V. unguiculata (Table 3). The dme mutants formed nodules on all of the plants, but the levels of nodulation and nitrogen fixation varied depending on the host plant (Table 3). The dme mutants formed 20 to 30% fewer nodules than the wild type on C. cajan and V. unguiculata, whereas all strains formed similar numbers of nodules on L. purpureus and M. atropurpureum. The total nodule mass formed by the dme mutants was significantly lower than that of wild-type NGR234 on C. cajan, V. unguiculata, and M. atropurpureum.
Table 3.
Symbiotic phenotypes of NGR234 and NGR234 dme mutants on different host plantsa
| Host (growth period) | Strainb | Shoot dry wt (mg) | Fix%c | AR activity (per plant) (μmol C2H4 produced/h)d | Fix%e | AR activity (per g of nodules) (μmol C2H4 produced/h)d | Fix%f | No. of nodules per plant | Nodule wt (mg) | Nodule wt per plant (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cajanus cajan (6 wk) | Uninoculated control | 97 ± 9a | 0a | 0a | 0a | ND | ND | |||
| Wild type | 542 ± 48b | 100 | 2.5 ± 0.3b | 100 | 7.5 ± 0.9b | 100 | 26 ± 3b | 14 ± 2b | 334 ± 39b | |
| dmeΔ14 mutant | 467 ± 32c | 83 | 1.9 ± 0.2c | 76 | 7.2 ± 0.9b | 96 | 19 ± 2c | 13 ± 1b | 228 ± 17c | |
| dme-9 mutant | 358 ± 24d | 58 | 1.5 ± 0.2d | 60 | 6.9 ± 0.2b | 92 | 18 ± 3c | 12 ± 1b | 213 ± 32c | |
| Lablab purpureus (10 wk) | Uninoculated control | 173 ± 10a | 0a | 0a | 0a | ND | ND | |||
| Wild type | 1,560 ± 124b | 100 | 7.1 ± 1.7b | 100 | 8.4 ± 1.2b | 100 | 19 ± 2b | 43 ± 6b | 831 ± 85b | |
| dmeΔ14 mutant | 1,002 ± 116c | 60 | 4 ± 0.6c | 56 | 4.7 ± 0.8c | 56 | 20 ± 3b | 42 ± 4b | 829 ± 60b | |
| dme-9 mutant | 1,024 ± 108c | 61 | 3.5 ± 0.4c | 49 | 3.9 ± 0.3c | 46 | 21 ± 4b | 44 ± 6b | 890 ± 49b | |
| Leucaena leucocephala (10 wk) | Uninoculated control | 77 ± 7a | 0a | 0 | ND | ND | ND | ND | ||
| Wild type | 285 ± 16b | 100 | 1.4 ± 0.2b | 100 | ND | ND | ND | ND | ND | |
| dmeΔ14 mutant | 226 ± 20c | 72 | 0.96 ± 0.16b | 70 | ND | ND | ND | ND | ND | |
| dme-9 mutant | 240 ± 23c | 78 | 1 ± 0.17b | 75 | ND | ND | ND | ND | ND | |
| Macroptilium atropurpureum (6 wk) | Uninoculated control | 19 ± 1a | 0a | 0a | 0a | 0a | 0a | |||
| Wild type | 362 ± 38b | 100 | 2.6 ± 0.4b | 100 | 9.9 ± 1b | 100 | 21 ± 4b | 13 ± 2b | 268 ± 27b | |
| dmeΔ14 mutant | 222 ± 37c | 59 | 1.3 ± 0.1c | 50 | 6 ± 0.3c | 61 | 23 ± 2b | 9 ± 1c | 204 ± 16c | |
| dme-9 mutant | 218 ± 29c | 58 | 1.2 ± 0.1c | 46 | 5.6 ± 0.2c | 57 | 23 ± 3b | 9 ± 1c | 205 ± 15c | |
| Vigna unguiculata (6 wk) | Uninoculated control | 204 ± 39a | 0a | 0a | 0a | ND | ND | |||
| Wild type | 2,245 ± 166b | 100 | 2.9 ± 0.4b | 100 | 3.6 ± 0.1b | 100 | 83 ± 6b | 10 ± 1b | 825 ± 96b | |
| dmeΔ14 mutant | 790 ± 106c | 29 | 3.3 ± 0.3b | 114 | 5.1 ± 0.3c | 142 | 68 ± 5c | 9.5 ± 0.4b | 641 ± 38c | |
| dme-9 mutant | 758 ± 87c | 27 | 3.4 ± 0.7b | 117 | 5.4 ± 0.4c | 150 | 71 ± 7c | 8.4 ± 0.5c | 595 ± 61c |
Five independent experiments (12 plants per strain per experiment) were performed for each host. Data are means ± SD. Values followed by the same letter do not differ at the 0.05 level of probability, based on one-way analysis of variance. ND, not determined.
The dme-9 and dmeΔ14 mutants were strains RmP1809 and RmP1814, respectively.
Calculated as shoot dry weight per plant: (test − uninoculated)/(wild type − uninoculated).
Nitrogenase activity assayed by acetylene reduction (AR) assay.
Calculated as the amount of acetylene reduced per plant: test/wild type.
Calculated as the amount of acetylene reduction per gram of fresh nodules: test/wild type.
Nitrogen-fixing activity was assessed by measuring plant dry weight and nitrogenase (acetylene reduction) activity (Table 3). Shoot dry weight reflects the cumulative amount of N2 fixed by the plants over the course of the experiment, whereas acetylene reduction measured the ability to fix N2 at the time of the assay. N2 fixation by dme mutants on L. purpureus and M. atropurpureum was 50 to 60% of that for plants inoculated with the wild type, and this appeared to be due mainly to less nitrogenase activity in nodules. We noted that the dme nodules from M. atropurpureum were smaller than those formed by the wild type, whereas the wild-type and mutant nodules on L. purpureus had the same weight (Table 3).
In the case of V. unguiculata plants inoculated with the dme mutants, we observed that greening of the leaves and vigorous growth occurred about 2 weeks later than those for plants inoculated with wild-type bacteria. These observations were reflected in the dry weight values of the plant shoots, which were about 30% of the wild-type level in the case of the dme mutants. However, measurements of nitrogenase activity by V. unguiculata nodules revealed acetylene reduction activities for the dme mutants that were not significantly different from those for wild-type NGR234. To address the lack of congruence between the plant dry weight and nitrogenase activity measurements, the kinetics of root nodule formation by the dme mutants and wild-type NGR234 on V. unguiculata were determined (see Fig. S1 in the supplemental material). These experiments revealed that while dme mutants formed fewer nodules per plant, only a short delay in nodule initiation was apparent. Thus, the dme mutations resulted in a delay in the onset of N2 fixation in V. unguiculata nodules.
Complementation of dme mutants.
To confirm that the Fix phenotype of NGR234 dme mutants resulted from a lack of DME enzyme, the wild-type dme gene and its promoter were cloned into a broad-host-range plasmid (pTH2584) and transferred into the dme mutant strains. The symbiotic phenotype of the resulting strains was determined with M. atropurpureum and V. unguiculata as host plants, and no significant difference was found in the shoot dry weights of plants inoculated with the wild-type strain versus the dme/dme+ merodiploid strains, whereas plants inoculated with the dme mutants showed the same reduced N2 fixation phenotype as that noted in Table 3 (data not shown). We concluded that the symbiotic phenotype of the NGR234 dme mutants resulted from the loss of the DME protein.
Enzyme activities in NGR234 bacteroids.
DME, TME, MDH, and PCK activities were measured in bacteroid extracts of root nodules from C. cajan, L. purpureus, L. leucocephala, M. atropurpureum, and V. unguiculata plants (Table 2). Apparent DME activity detected in dme mutant bacteroids was less than half that of the dme+ parent, whereas both TME and MDH activities in the dme mutant bacteroids were similar to those in wild-type bacteroids. For comparative purposes, we also assayed S. meliloti bacteroid extracts from alfalfa nodules and detected DME (184 ± 7 nmol/minute/mg), MDH (5,078 ± 109 nmol/minute/mg), and PCK (8 ± 2 nmol/minute/mg) activities at levels similar to those reported previously for alfalfa nodules (35). The NGR234 bacteroid enzyme activities revealed several differences from enzyme activity levels reported for S. meliloti bacteroids. DME activities were lower in wild-type NGR234 bacteroids (40 to 70 nmol/minute/mg) than in S. meliloti bacteroids (∼180 nmol/minute/mg). MDH activities detected in S. meliloti bacteroids were about double those measured in wild-type NGR234 bacteroids. Most significantly, PCK activities were high in the NGR234 bacteroids isolated from the different host plants (50 to 180 nmol/minute/mg), while PCK activity was not detected in S. meliloti bacteroids from alfalfa.
Symbiotic phenotype of dme pckA double mutant.
In view of the high PCK activity in NGR234 bacteroids and the recent finding that PCK can function in pyruvate synthesis in pea bacteroids (36), we constructed an NGR234 pckA dme double mutant (RmP2651 pckA::Ω Spr Δdme) and determined its symbiotic phenotype. Acetylene reduction data showed that the NGR234 pckA mutant formed N2-fixing nodules on both L. leucocephala (0.24 ± 0.05 μmol C2H4/plant/hour) and M. atropurpureum (0.37 ± 0.04 μmol C2H4/plant/hour). While the level of N2 fixation on L. leucocephala was less than that previously reported (38), no nitrogenase activity was detected in nodules formed by the NGR234 pckA dme double mutant on L. leucocephala and M. atropurpureum (0.0 μmol C2H4/hour). This result was similar to that reported for R. leguminosarum, where individual dme or pckA mutants fixed N2 but dme pckA double mutants were completely devoid of symbiotic N2-fixing activity (36). Thus, NGR234 bacteroids appear to be capable of using either malic enzyme or phosphoenolpyruvate carboxykinase pathways for the synthesis of pyruvate and acetyl-CoA. We note that wild-type S. meliloti bacteroids isolated from alfalfa nodules have very low PCK activity (16, 39), whereas NGR234 bacteroids isolated from nodules on C. cajan, L. purpureus, L. leucocephala, M. atropurpureum, and V. unguiculata plants had high PCK activities (50 to 180 nmol/min/mg protein) (Table 2). Thus, the PCK activity in NGR234 bacteroids presumably accounts for the symbiotic N2 fixation by NGR234 dme mutants.
The Azorhizobium caulinodans azc3656 gene encodes an NAD(P)+-malic enzyme.
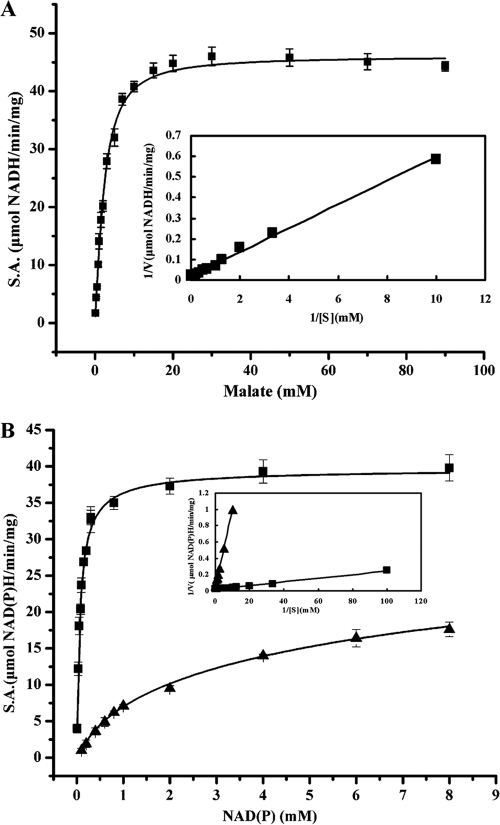
In a symbiotic mutant screen of A. caulinodans ORS571, two insertion mutants with mutations in a malic enzyme-like gene (azc3656) were found to form Fix− nodules (47). In a subsequent study, transcription of a second malic enzyme-like gene (azc0119) was found to be much higher in A. caulinodans bacteroids than in free-living cells (48). The interpretation of these results was hindered by an absence of functional data on either the azc3656 or azc0119 gene product. To examine the malic enzyme-related activities of the proteins encoded by azc3656 and azc0119, these genes were cloned and their encoded proteins were overexpressed and purified as N-terminally His-tagged fusion proteins. Enzyme assays revealed that the AZC3656 protein had an NAD(P)+-malic enzyme activity that was very similar to that of the S. meliloti DME protein (see below). In contrast, the AZC0119 protein had less than 2% of the AZC3656 NAD(P)+-malic enzyme activity over a range of pHs. These data suggest that the AZC0119 protein is not a malic enzyme. AZC3656 showed high NAD+-ME activity at pH 7.8, with Michaelis-Menten-like kinetics, at various concentrations of malate (Fig. 4). The enzyme exhibited only a very limited positive cooperativity with respect to malate, and a Hill coefficient close to 1 (1.17) was calculated from a plot of log[v/(Vmax − v)] versus log[malate] (data not shown). The enzyme had Km values of 101 μM and 2.1 mM for NAD+ and NADP+, respectively, and based on this specificity, AZC3656 belongs to the EC 1.1.1.39 class of malic enzymes.
Fig 4.
AZC3656 protein ME activity in response to various concentrations of l-malate and the cofactors NAD+ and NADP+. (A) ME specific activity (S.A.) in response to l-malate was measured at an NAD+ concentration of 1.5 mM. (B) ME specific activity (S.A.) in response to NAD+ (squares) and NADP+ (triangles) was measured at a concentration of 30 mM l-malate. Insets show Lineweaver-Burk plots (1/V versus 1/S). Data were analyzed using the equation 1/V = 1/Vmax + (Km/Vmax)(1/S) to determine the Km and Vmax with respect to malate and NAD(P)+. Enzyme assay mixtures contained 1.1 μg purified AZC3656, 100 mM Tris-HCl, pH 7.8, 3 mM MnCl2, 5 mM NH4Cl, and 1.5 mM NAD+ in a final volume of 1 ml. Error bars represent standard errors of the means for triplicate samples.
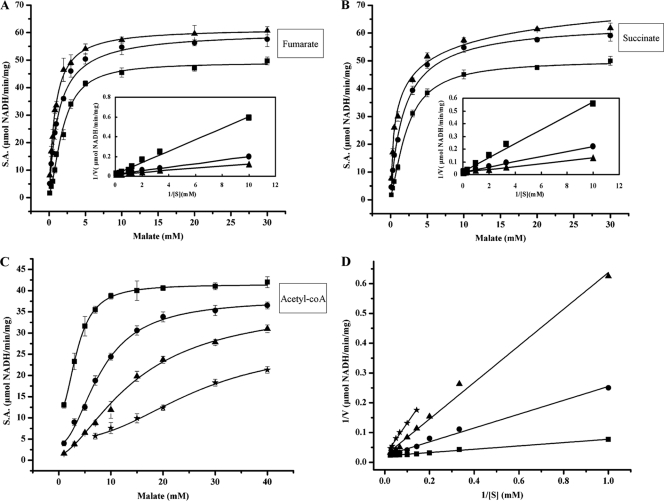
To further investigate the metabolic role of the AZC3656 protein, we tested the metabolites fumarate, succinate, and acetyl-CoA as possible regulators of malic enzyme activity. Fumarate and succinate increased the affinity of AZC3656 for malate, such that the Km for malate dropped from a value of 2.7 mM to 0.59 mM in the presence of 1 mM fumarate and from 2.7 mM to 0.64 mM in the presence of 10 mM succinate (Fig. 5A and B). Acetyl-CoA lowered the affinity of AZC3656 for malate. At 50 μM acetyl-CoA, the Km for malate increased from 2.6 mM to 27.6 mM (Fig. 5C). These effectors had only a modest effect on the Vmax. The allosteric activation of AZC3656 activity by succinate and fumarate coupled with the strong inhibition by acetyl-CoA suggests that AZC3656 plays a role in shunting malate to pyruvate and then to acetyl-CoA (via pyruvate dehydrogenase). Thus, when the TCA cycle flux is limited by insufficient acetyl-CoA, the concentrations of succinate and fumarate accumulate and stimulate AZC3656 to synthesize pyruvate.
Fig 5.
AZC3656 malic enzyme activity is stimulated by fumarate and succinate and inhibited by acetyl-CoA. (A) ME specific activity (S.A.) with 1.5 mM NAD+ and 0 mM (■), 0.1 mM (●), or 1 mM (▲) fumarate. The inset shows a Lineweaver-Burk plot (1/V versus 1/S). (B) ME specific activity (S.A.) with 1.5 mM NAD+ and 0 mM (■), 1 mM (●), or 10 mM (▲) succinate. The inset shows a Lineweaver-Burk plot (1/V versus 1/S). (C) ME specific activity (S.A.) with 1.5 mM NAD+ and 0 μM (■), 25 μM (●), 50 μM (▲), or 100 μM (★) acetyl-CoA. (D) Lineweaver-Burk plot (1/V versus 1/S) of the data in panel C.
To investigate whether A. caulinodans contains a TME-like enzyme, we assayed ME activities in A. caulinodans free-living cells grown in TY medium. NAD+-dependent ME activity in the wild-type strain (201 ± 9 nmol/min/mg protein) was roughly 4-fold that of the activity detected in the two A. caulinodans azc3656 mutants (52 ± 7 and 43 ± 7 nmol/min/mg protein). The NADP+-dependent ME activity (29 ± 3 nmol/min/mg protein) detected in the wild type was much higher than that detected in the azc3656 mutant extracts (3 ± 1 nmol/min/mg protein). The apparent NADP+-ME activity detected in the wild-type extracts was therefore likely to have arisen from the presence of the AZC3656 protein, and the data suggest that A. caulinodans does not contain an NADP+-dependent malic enzyme. This interpretation is entirely consistent with the presence of both NADP+-ME and NAD+-ME in NGR234 wild-type cells and the presence of substantial NADP+-ME activities in NGR234 dme mutant cells (Table 2).
DISCUSSION
Data showing that dme mutants of Sinorhizobium sp. NGR234 form N2-fixing root nodules on diverse host plants (Table 3) clearly suggest that NAD+-ME activity is not absolutely required for symbiotic N2 fixation by NGR234. This conclusion is mitigated by the possibility that, in addition to DME, another NAD+-dependent ME may function in NGR234. However, we failed to detect such an enzyme in the NGR234 genome, where only genes corresponding to the DME (ACP25453) and TME (ACP23834) proteins were detected. The enzyme assay used to detect ME activity in crude extracts relies upon the detection of keto acids (17). Using this assay, the NGR234 dme mutants retained about 50% of the NAD+-dependent ME activity of the wild type. This activity is almost certainly a background activity that results in part from malate dehydrogenase activity. Similar residual activity was also present at similar levels in dme mutants of S. meliloti and R. leguminosarum (10, 36).
The Fix− symbiotic phenotype of the dme pckA double mutant of NGR234 suggests that pyruvate and acetyl-CoA can be generated by either malic enzyme or phosphoenolpyruvate carboxykinase pathways. This result is similar to that obtained for R. leguminosarum, where dme and pckA mutants were Fix+ while dme pckA double mutants were Fix−. We note that in the case of R. leguminosarum, the effect of combining the pckA and dme mutations was more clear-cut, as the individual dme and pckA mutations had little effect on the pea nodule nitrogen fixation phenotype (36). However, in the case of NGR234, the dme and pckA mutations did individually affect symbiotic N2 fixation, and the extent of the symbiotic defects varied with different host plants (Table 3) (38).
PCK activity was readily detected in NGR234 bacteroids from five host plants (50 to 180 nmol/min/mg protein) (Table 2), whereas S. meliloti alfalfa bacteroids had very low PCK activity (8 nmol/min/mg protein) (Table 2) (16, 39). Since the PCK activity in NGR234 bacteroids appeared to be responsible for the Fix+ symbiotic phenotype of the dme mutants, we were interested in determining whether the extent of the symbiotic N2 fixation of the dme mutants (Table 3) was reflected in the PCK activity measured in bacteroids from the different host plants (Table 2). However, no clear correlation between PCK activity and N2 fixation was evident (Tables 2 and 3). This may reflect the complexity of the host-dependent phenotypes of the dme mutants, which include N2 fixation, growth of nodules as reflected by nodule size, and perhaps the initiation of nodule formation. C. cajan and V. unguiculata plants inoculated with dme mutants formed significantly fewer nodules than plants inoculated with the wild type (Table 3). Since DME plays a role in central carbon metabolism, its absence could influence the levels of various metabolic intermediates (Fig. 1). Thus, depending on the carbon sources made available to the bacteria by different host plants, the absence of DME could influence Nod factor synthesis, with possible effects on nodule number.
The A. caulinodans AZC3656 protein is an NAD(P)+-malic enzyme whose kinetic and allosteric properties are remarkably similar to those of the DME protein from S. meliloti (50). Both proteins share similar apparent Kms for l-malate, NAD+, and NADP+, and both activities are stimulated by succinate and fumarate and inhibited by acetyl-CoA (50). Thus, the Fix− phenotype of A. caulinodans azc3656 insertion mutants on Sesbania rostrata (47) appears to be identical to the Fix− phenotype of S. meliloti dme mutants. In view of the proposed alternate pathways for pyruvate synthesis in bacteroids, we postulate that A. caulinodans bacteroids will not show significant PCK activity. We have not found any report where the PCK activity of A. caulinodans bacteroids has been measured. However, data from a comprehensive microarray-transcriptome analysis of A. caulinodans suggest that pckA (azc4063) is transcribed in bacteroids. (Transcript signal data from bacteroids, free-living cells grown in TY, and free-living cells grown with succinate plus lactate were 468 ± 33, 135 ± 37, and 75 ± 9, respectively [49].) Thus, it will be interesting to determine whether A. caulinodans bacteroid extracts have PCK activity, and we hope to perform such measurements in the near future.
Mutations in rhizobia that result in host-dependent symbiotic N2 fixation phenotypes are receiving increasing attention (21, 27). In a comprehensive study of bacteroid transcriptomes and proteomes, Hennecke and colleagues identified “host adaptation” genes in B. japonicum that are expressed differentially in bacteroids from different host plants, i.e., soybean, cowpea, and siratro. Mutations in one such locus that encodes a predicted ABC transporter were shown to cause a host-dependent symbiotic N2 fixation phenotype (21), as were mutations in an RND-type efflux system (BdeAB) in B. japonicum (27). The data presented here and elsewhere (36, 38) demonstrate that dme and pck mutations result in host-dependent N2 fixation phenotypes. These phenotypes presumably reflect differences in the carbon sources that are available to the bacteria and bacteroids in nodules, and the nature of these differences remains to be established.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Sciences and Engineering Council of Canada to T.M.F. and by grants from Genome Canada through the Ontario Genomics Institute and the Ontario Research and Development Challenge Fund.
We are grateful to W. R. Streit for sharing the NGR234 genome sequence data with us prior to its public release.
Footnotes
Published ahead of print 3 February 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Arwas R, McKay IA, Rowney FRP, Dilworth MJ, Glenn AR. 1985. Properties of organic-acid utilization mutants of Rhizobium leguminosarum strain-300. J. Gen. Microbiol. 131:2059–2066 [Google Scholar]
- 2. Bala A, Giller KE. 2001. Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytol. 149:495–507 [DOI] [PubMed] [Google Scholar]
- 3. Blasing OE, Westhoff P, Svensson P. 2000. Evolution of C4 phospho-enolpyruvate carboxylase in Flaveria, a conserved serine residue in the carboxyl-terminal part of the enzyme is a major determinant for C4-specific characteristics. J. Biol. Chem. 275:27917–27923 [DOI] [PubMed] [Google Scholar]
- 4. Reference deleted.
- 5. Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 6. Copeland L, Quinnell RG, Day DA. 1989. Malic enzyme-activity in bacteroids from soybean nodules. J. Gen. Microbiol. 135:2005–2011 [Google Scholar]
- 7. Copeland L, Vella J, Hong ZQ. 1989. Enzymes of carbohydrate metabolism in soybean nodules. Phytochemistry 28:57–61 [Google Scholar]
- 8. Dao TV, et al. 2008. NAD-malic enzyme affects nitrogen fixing activity of Bradyrhizobium japonicum USDA 110 bacteroids in soybean nodules. Microbes Environ. 23:215–220 [DOI] [PubMed] [Google Scholar]
- 9. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Driscoll BT, Finan TM. 1993. NAD(+)-dependent malic enzyme of Rhizobium meliloti is required for symbiotic nitrogen fixation. Mol. Microbiol. 7:865–873 [DOI] [PubMed] [Google Scholar]
- 11. Driscoll BT, Finan TM. 1996. NADP+-dependent malic enzyme of Rhizobium meliloti. J. Bacteriol. 178:2224–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Driscoll BT, Finan TM. 1997. Properties of NAD(+)- and NADP(+)-dependent malic enzymes of Rhizobium (Sinorhizobium) meliloti and differential expression of their genes in nitrogen-fixing bacteroids. Microbiology 143:489–498 [DOI] [PubMed] [Google Scholar]
- 13. Dunn MF. 1998. Tricarboxylic acid cycle and anaplerotic enzymes in rhizobia. FEMS Microbiol. Rev. 22:105–123 [DOI] [PubMed] [Google Scholar]
- 14. Englard S, Siegal L. 1969. Mitochondrial l-malate dehydrogenase of beef heart. Methods Enzymol. 13:99–100 [Google Scholar]
- 15. Finan TM, Wood JM, Jordan DC. 1983. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J. Bacteriol. 154:1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finan TM, McWhinnie E, Driscoll B, Watson RJ. 1991. Complex symbiotic phenotypes result from gluconeogenic mutations in Rhizobium meliloti. Mol. Plant Microbe Interact. 4:386–392 [Google Scholar]
- 17. Friedemann TE, Haugen GE. 1943. Pyruvic acid: the determination of keto acids in blood and urine. J. Biol. Chem. 147:415–442 [Google Scholar]
- 18. Grzemski W, Akowski JP, Kahn ML. 2005. Probing the Sinorhizobium meliloti-alfalfa symbiosis using temperature-sensitive and impaired-function citrate synthase mutants. Mol. Plant Microbe Interact. 18:134–141 [DOI] [PubMed] [Google Scholar]
- 19. Jensen HL. 1942. Nitrogen fixation in leguminous plants. I. General characters of root-nodule bacteria isolated from species of Medicago and Trifolium in Australia. Proc. Linn. Soc. NSW 66:98–108 [Google Scholar]
- 20. Jones JDG, Gutterson N. 1987. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene 61:299–306 [DOI] [PubMed] [Google Scholar]
- 21. Koch M, et al. 2010. Rhizobial adaptation to hosts, a new facet in the legume root-nodule symbiosis. Mol. Plant Microbe Interact. 23:784–790 [DOI] [PubMed] [Google Scholar]
- 22. Kouchi H, Fukai K, Katagiri H, Minamisawa K, Tajima S. 1988. Isolation and enzymological characterization of infected and uninfected cell protoplasts from root-nodules of Glycine-max. Physiol. Plant. 73:327–334 [Google Scholar]
- 23. Reference deleted.
- 24. Kurz WG, LaRue TA. 1977. Citric acid cycle enzymes and nitrogenase in nodules of Pisum sativum. Can. J. Microbiol. 23:1197–2007 [DOI] [PubMed] [Google Scholar]
- 25. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 26. Leonard LT. 1943. A simple assembly for use in the testing of cultures of rhizobia. J. Bacteriol. 45:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindemann A, et al. 2010. Host-specific symbiotic requirement of BdeAB, a RegR-controlled RND-type efflux system in Bradyrhizobium japonicum. FEMS Microbiol. Lett. 312:184–191 [DOI] [PubMed] [Google Scholar]
- 28. Lodwig E, Poole P. 2003. Metabolism of Rhizobium bacteroids. Crit. Rev. Plant Sci. 22:37–78 [Google Scholar]
- 29. Marchler-Bauer A, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKay IA, Dilworth MJ, Lodwig EM, Hosie AH, Glenn AR. 1988. C-4-dicarboxylate metabolism in free-living and bacteroid forms of Rhizobium leguminosarum Mnf3841. J. Gen. Microbiol. 134:1433–1440 [Google Scholar]
- 31. McKay IA, Dilworth MJ, Glenn AR. 1989. Carbon catabolism in continuous cultures and bacteroids of Rhizobium-leguminosarum Mnf-3841. Arch. Microbiol. 152:606–610 [Google Scholar]
- 32. McKay IA, Glenn AR, Dilworth MJ. 1985. Gluconeogenesis in Rhizobium leguminosarum Mnf3841. J. Gen. Microbiol. 131:2067–2073 [Google Scholar]
- 33. Miller RW, McRae DG, Al-Jobore A, Berndt WB. 1988. Respiration supported nitrogenase activity of isolated Rhizobium meliloti bacteroids. J. Cell. Biochem. 38:35–49 [DOI] [PubMed] [Google Scholar]
- 34. Mitsch MJ, Cowie A, Finan TM. 2007. Malic enzyme cofactor and domain requirements for symbiotic N2 fixation by Sinorhizobium meliloti. J. Bacteriol. 189:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitsch MJ, Voegele RT, Cowie A, Osteras M, Finan TM. 1998. Chimeric structure of the NAD(P)+- and NADP+-dependent malic enzymes of Rhizobium (Sinorhizobium) meliloti. J. Biol. Chem. 273:9330–9336 [DOI] [PubMed] [Google Scholar]
- 36. Mulley G, et al. 2010. Pyruvate is synthesized by two pathways in pea bacteroids with different efficiencies for nitrogen fixation. J. Bacteriol. 192:4944–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osteras M, Driscoll BT, Finan TM. 1995. Molecular and expression analysis of the Rhizobium meliloti phosphoenolpyruvate carboxykinase (pckA) gene. J. Bacteriol. 177:1452–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osteras M, Finan TM, Stanley J. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257–269 [DOI] [PubMed] [Google Scholar]
- 39. Osteras M, O'Brien SA, Finan TM. 1997. Genetic analysis of mutations affecting pckA regulation in Rhizobium (Sinorhizobium) meliloti. Genetics 147:1521–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poole P, Allaway D. 2000. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 43:117–163 [DOI] [PubMed] [Google Scholar]
- 41. Prentki P, Krisch HM. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313 [DOI] [PubMed] [Google Scholar]
- 42. Pueppke SG, Broughton WJ. 1999. Rhizobium sp. strain NGR234 and Rhizobium fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant Microbe Interact. 12:293–318 [DOI] [PubMed] [Google Scholar]
- 43. Quandt J, Hynes MF. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15–21 [DOI] [PubMed] [Google Scholar]
- 44. Romanov VI, Hernández-Lucas I, Martínez-Romero E. 1994. Carbon metabolism enzymes of Rhizobium tropici cultures and bacteroids. Appl. Environ. Microbiol. 60:2339–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ronson CW, Lyttleton P, Robertson JG. 1981. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc. Natl. Acad. Sci. U. S. A. 78:4284–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmeisser C, et al. 2009. Rhizobium sp. strain NGR234 possesses a remarkable number of secretion systems. Appl. Environ. Microbiol. 75:4035–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki S, et al. 2007. Rhizobial factors required for stem nodule maturation and maintenance in Sesbania rostrata-Azorhizobium caulinodans ORS571 symbiosis. Appl. Environ. Microbiol. 73:6650–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thapanapongworakul N, et al. 2010. NAD+-malic enzyme affects nitrogenase activity of Mesorhizobium loti bacteroids in Lotus japonicus nodules. Plant Biotechnol. 27:311–316 [Google Scholar]
- 49. Tsukada S, et al. 2009. Comparative genome-wide transcriptional profiling of Azorhizobium caulinodans ORS571 grown under free-living and symbiotic conditions. Appl. Environ. Microbiol. 75:5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voegele RT, Mitsch MJ, Finan TM. 1999. Characterization of two members of a novel malic enzyme class. Biochim. Biophys. Acta 1432:275–285 [DOI] [PubMed] [Google Scholar]
- 51. Wedding RT. 1989. Malic enzymes of higher-plants—characteristics, regulation, and physiological-function. Plant Physiol. 90:367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan ZC, Zaheer R, Finan TM. 2005. Phosphate limitation induces catalase expression in Sinorhizobium meliloti, Pseudomonas aeruginosa and Agrobacterium tumefaciens. Mol. Microbiol. 58:877–894 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.