Abstract
Several approaches for the inactivation of bacteriophage lambda, including UV germicidal irradiation (UVGI) and the chemical agents Virkon-S, Chloros, Decon-90, and sodium hydroxide (NaOH), were compared. Virkon, NaOH, and UVGI caused a ≥7-log10 reduction in phage titers. This study successfully describes several methods with potential for bacteriophage inactivation in industrial settings.
TEXT
Bacteriophages have been exploited for a range of biotechnological applications (reviewed in reference 11). The use of bacteriophages (especially genetically modified phages) in industry requires rigorous decontamination procedures to prevent cross-contamination within facilities or uncontrolled release into the environment. Previous work has shown that Virkon-S is highly effective for the eradication of several bacteriophage species, including bacteriophage lambda (2), although in this work, the minimum concentration required for inactivation was not established. Similarly, continuous-flow, UV germicidal irradiation (UVGI)-based inactivation methods have been assessed for various bacteria and viruses in water (5–7). Although free-living bacteriophage lambda particles have been found to be susceptible to UV, the methods used to study this are not readily scalable (1). In this work, we explore the effectiveness of several chemical agents and UVGI in the inactivation of bacteriophage lambda in suspension.
Inactivation assays were conducted using bacteriophage λNM1149 (12) carrying a eukaryotic expression cassette encoding Yersinia pestis V antigen (λNM1149-Vsyn) or green fluorescent protein (λNM1149-GFP). Escherichia coli strain LE392 (Promega) was used for the growth and titration of λNM1149-Vsyn. E. coli BDEC-02 (F− λ−, Δrph ΔfhuA ΔhsdS ΔhsdM ΔhsdR ΔmcrB ΔmcrC Δmmr ΔybcN-ybcX Δkil-trkG ΔydfK-ydfO) was used for the growth and titration of λNM1149-GFP. Both E. coli strains encode RecA, so any reactivation of phage damaged by UV radiation would be detected following titration if this was to occur. Growth, purification, and enumeration of bacteriophage particles were conducted as previously described (13). Crude phage lysate was prepared by infection at a multiplicity of infection (MOI) of 1:100 PFU/CFU, followed by overnight growth in L broth. Lysate was treated with chloroform and DNase I/RNase A. Cells and cellular debris were removed by centrifugation at 6,000 × g for 10 min, and the supernatant was recovered (crude phage lysate). Concentrated lysate was prepared by addition of polyethylene glycol (PEG) 8000 (Sigma-Aldrich) to crude phage lysate to 10% (wt/vol). Precipitated phages were recovered by centrifugation and resuspended in SM buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 8 mM MgSO4, 0.01% [wt/vol] gelatin from cold-water fish skin) (concentrated phage lysate). For experiments assessing inactivation of phage on stainless steel surfaces using NaOH, concentrated lysate was prepared using the BDEC-02 host E. coli strain with 3 chloroform extractions, followed by dead-end ultracentrifugation (85,000 × g) and resuspension in SM buffer to the desired concentration (1012 PFU/ml).
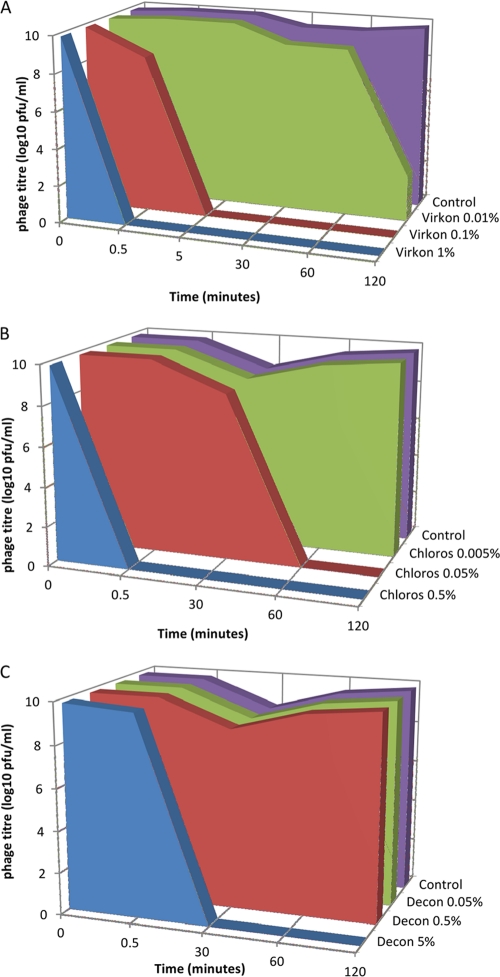
The three chemical agents, Virkon-S (DuPont; contains potassium peroxomonosulfate, sodium dodecylbenzenesulfonate, and sulfamic acid), Decon-90 (Decon Laboratories; a proprietary emulsion of anionic and nonionic surface active agents, stabilizing agents, alkalis, nonphosphate detergent builders, and sequestering agents, all in an aqueous base), and Chloros (Imperial Chemical Industries; provided as an aqueous solution of sodium hypochlorite containing 11% available chlorine), were tested at several concentrations for their effect upon the functionality of bacteriophage lambda in concentrated lysate. Samples were removed for titration immediately following mixing with the chemical agent (∼30 s) and then after 30, 60, and 120 min (Fig. 1). Virkon-S was found to be the most effective agent for inactivating phage at the concentrations tested, potentially due to the multiple mechanisms of bacteriophage inactivation provided by the oxidizing agent, organic acid, and detergent. At the manufacturer's recommended working concentration of 1% (wt/vol), no viable phages were detected after 30 s. The limit of detection was 100 PFU/ml, so this was equivalent to at least a 7-log10-fold reduction in titer. A 0.1% Virkon-S solution caused a 7-log10-fold reduction after 5 min. At 0.01%, a 7-log10-fold reduction was noted following incubation for 2 h. Chloros (0.5% [vol/vol]; 550 parts per million [ppm] of available chlorine) also resulted in at least a 7-log10-fold reduction after 30 s. Both Virkon-S and sodium hypochlorite were previously found to be effective against bacteriophage lambda; however, the concentrations tested in this study were far lower than those previously employed (2). Only the highest concentration of Decon-90 (5% [vol/vol]) showed any effect upon phage functionality. Again, a minimum 7-log10 drop in titer was obtained; however, this effect only occurred following incubation for 30 min. Two-hour time course experiments using Chloros and Decon-90 were carried out once, with titration performed in triplicate. In the case of Virkon, the experiment was repeated a further two times, taking samples only at 0.5, 5, and 30 min.
Fig 1.
Effects of selected disinfectants on phage viability. (A) Virkon. (B) Chloros. (C) Decon. Virkon reduces phage concentrations to the lower limit of detection at 0.1% (one-tenth of the manufacturer's recommended concentration) within 5 min. Chloros gives a 7-log10 reduction in titer within 30 s at the manufacturer's recommended dilution. Decon is apparently the least effective, requiring 30 min at the manufacturer's recommended concentration to produce a 7-log10 reduction in titer. Note: values on charts are set as “zero” for undetectable levels (i.e., no phage recovered from the most-concentrated sample plated). In reality, undetectable signifies a titer of less than 102 PFU/ml.
The use of 0.5 M sodium hydroxide (NaOH) as an agent for decontamination of stainless steel surfaces contaminated with bacteriophages was examined. Aliquots of diluted bacteriophage (50 μl, λNM1149-GFP) were placed on the surface of the steel and spread over a 3-cm by 3-cm area. Aliquots of 150 μl of either 0.5 M NaOH or sterile distilled water were added and spread with a sterile L-shaped spreader. Liquid was absorbed using a sterile viscose swab which was transferred aseptically to a 500-μl aliquot of SM buffer and vortexed for approximately 10 s. Titration of all samples (using BDEC-02 cells) was carried out immediately after swabs were vortexed. Following treatment with NaOH, no plaques resulted from the highest concentration of phage placed on the steel (5.56 × 109 PFU/cm2; i.e., 50 μl at 1 × 1012 PFU/ml spread across a 9-cm2 area). A 400-μl aliquot of the recovered liquid (700 μl) would have been expected to contain 2.86 × 1010 PFU if the NaOH did not affect viability (i.e., the ability of bacteriophage to form plaques on susceptible E. coli strains). The lower limit of reliable detection of phage using the plating assay was approximately 25 PFU per 400 μl (i.e., approximately 5 PFU/cm2; data not shown). Recovery of zero plaques therefore suggests a 9-log10-fold drop in viable titer. The same levels of inactivation were achieved if the phage was allowed to dry on the steel (∼30 min) prior to treatment. Controls treated with sterile distilled water confirmed that approximately 100% of plaques were recovered from the steel, regardless of whether the phages were allowed to dry onto the steel or allowed to remain “wet” (i.e., if phages were present at >5 PFU/cm2, titers obtained were generally within 10% of the expected titer) (data not shown). Published studies detailing the efficacy of sodium hydroxide as a disinfectant are limited. Variability has been noted in the sensitivity of certain human viruses to NaOH treatment, often requiring prolonged incubation and/or heating to result in a multiple-log10-fold reduction in viability (14).
A custom-built UV water treatment apparatus was used for treatment of liquid samples containing bacteriophage. Two flow rates were used: 0.11 liters/s, which equated to a nominal UV dose of 300 mJ/cm2, and 1 liter/s (35 mJ/cm2). The UV lights were left to equilibrate for 15 min before the intensities were measured at 8 points around the tube: 4 points one-quarter of the way along the tube length and 4 points three-quarters of the way along the tube length. The flow rate was altered to achieve a UV dose of either 300 or 30 mJ/cm2. The UV dose was calculated using the following equation: UV dose (mJ/cm2) = [intensity (mW/cm2) × volume exposed to UV (liters)]/[flow rate (liters/s)]. Intensity was calculated using the following equation: intensity = average intensity × correction factor (detector) × correction factor (transmissivity) × number of UV light tubes. The average intensity was calculated from duplicate measurements. A detector correction value of 5 was chosen based on the use of UVP model MS-100, fitted with sensor model EN-125, which was modified to accurately read the high intensity values to be measured. The transmissivity correction factor corrects for the fact that the UV intensity was measured outside the apparatus (i.e., no barrier to the UV), whereas the UV reaching the liquid that passes through the tube must pass through the quartz wall of the flow tube. This correction factor was calculated to equal 0.83. Further correction for the transmissivity of the liquid within the tube was not applied. For phage samples diluted in freshwater or artificial seawater, transmissivity of the liquid was taken to equal 1, as the internal diameter of the flow tube was small (2.5 cm) and this liquid was not turbid. Finally, intensity was multiplied by 4 (intensity was measured from one tube, whereas four were included in the unit design).
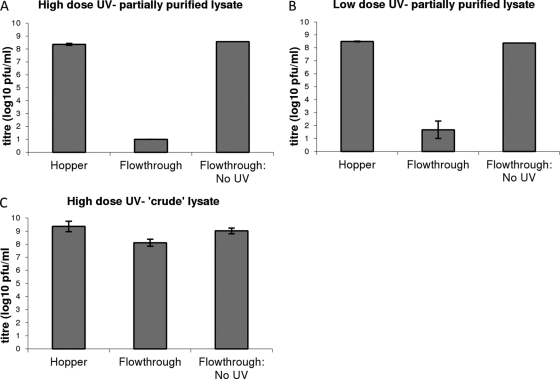
To assess the effect of UV treatment on crude lysate, 1 liter of crude phage lysate was diluted into 4 liters of distilled water to an expected titer of approximately 109 PFU/ml (5 liters total). To assess the effect of UV treatment on partially purified phage lysate, the phage preparation was diluted in distilled H2O or artificial seawater (Instant Ocean; Spectrum Brands Ltd.) to give an expected starting titer of 109 PFU/ml. The 5-liter samples of the liquid containing phages were individually loaded into the hopper, a sample was taken for titration, the valves were opened, and the central 1 liter of run-through was collected at the bottom for titration as the “UV-treated” sample. The UV lights were switched off, and the final 1 liter of the phage lysate was collected as “non-UV-treated” run-through. In fresh water, at the higher UV dose (i.e., a lower flow rate), complete inactivation of all phage (equivalent to a minimum reduction in the titer of at least 7 log10) was observed in all 3 replicate experiments. At a lower UV dose (i.e., a higher flow rate), complete inactivation of all phage (equivalent to a minimum reduction in the titer of at least 7 log10) was observed in 2 out of 3 experiments. In the third experiment, a 5-log10 reduction was observed. Again, no significant reduction in phage titer was observed following a run-through in the absence of UV treatment. Therefore, even at the lower UV dose, a reduction of at least 5 log10 was observed in 3 independent replicates of the experiment (Fig. 2). The titer of the sample in the hopper was slightly lower than the expected 109 PFU/ml (prior to the run-through). However, the titer of the run-through without UV treatment did not differ from the titer in the hopper, showing that loss of phage due to mechanical forces of running the liquid through the device was negligible, i.e., that the UV radiation most likely accounted for any inactivation detected. A 7-log10 reduction was witnessed following treatment of bacteriophage in Instant Ocean with the higher dose of UV (data not shown).
Fig 2.
Effect of treatment of phage lysates with UV. Data shown are the averages of three replicates of the experiment ± the standard error of the mean (SEM). Using high-dose UV (300 mJ/cm2), no viable phages were detected in the flowthrough following UV treatment, meaning that the titer had reduced to below 10 PFU/ml. Without UV treatment, the titer was essentially unchanged in the flowthrough compared to the titer of the liquid in the hopper. Lower-dose UV (35 mJ/cm2) resulted in at least a 5-log10-fold reduction in phage titers following treatment.
Only a modest (1- to 1.6-log10) reduction was witnessed following treatment of crude phage lysate using the UV apparatus. Results of a Student t test (two-tailed, paired per experiment) did not suggest a significant difference in titer between UV-treated and non-UV-treated crude phage lysates (P = 0.135). Therefore, it is unlikely that the device would be applicable to the treatment of crude liquid preparations (e.g., spent fermentor culture), whereas results given above show that the device performs well when used to treat water containing purified phage at a similar titer. UV irradiation has been shown to be effective for the inactivation of several bacteriophages: bacteriophage MS2 has often been used as a model organism for inactivation studies due both to its UV resistance and to the similarity between its inactivation profile and those of enteric viruses, such as poliomyelitis virus (3, 4, 8, 10). UV radiation sensitivities of ϕX174, T4, and T7 bacteriophages have been found to be greater than that of MS2 (4, 9).
In conclusion, the results of this study suggest that the disinfectant Virkon-S is a highly potent agent for inactivation of bacteriophage lambda that is suitable for treatment of waste in small volumes. NaOH (0.5 M) is effective for the decontamination of steel surfaces following contact with phage. UV irradiation is a potential choice for large volumes of liquid containing phage in water with few contaminants other than salts found in seawater.
ACKNOWLEDGMENTS
The UV water treatment device was designed and constructed by Ian Ramsay. We thank Steven Wright of the Moredun Research Institute for technical assistance and advice on the operation of the UV inactivation device.
This work was funded jointly by BigDNA and a grant from Genecom.
Footnotes
Published ahead of print 10 February 2012
REFERENCES
- 1. Fujita H, Endo A, Suzuki K. 1981. Inactivation of bacteriophage lambda by near-ultraviolet irradiation in the presence of chlorpromazine. Photochem. Photobiol. 33:215–222 [DOI] [PubMed] [Google Scholar]
- 2. Halfhide DE, Gannon BW, Hayes CM, Roe JM. 2008. Wide variation in effectiveness of laboratory disinfectants against bacteriophages. Lett. Appl. Microbiol. 47:608–612 [DOI] [PubMed] [Google Scholar]
- 3. Havelaar AH, Meulmans CCE, Pot-Hogeeoom WM, Koster J. 1990. Inactivation of bacteriophage MS2 in wastewater effluent with monochromatic and polychromatic ultraviolet light. Water Res. 24:1387–1393 [Google Scholar]
- 4. Hijnen WA, Beerendonk EF, Medema GJ. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40:3–22 [DOI] [PubMed] [Google Scholar]
- 5. Hill WF, Jr, Hamblet FE, Benton WH. 1969. Inactivation of poliovirus type 1 by the Kelly-Purdy ultraviolet seawater treatment unit. Appl. Microbiol. 17:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill WF, Jr, Hamblet FE, Benton WH, Akin EW. 1970. Ultraviolet devitalization of eight selected enteric viruses in estuarine water. Appl. Microbiol. 19:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly CB. 1961. Disinfection of sea water by ultraviolet radiation. Am. J. Public Health 51:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mamane-Gravetz H, Linden KG, Cabaj A, Sommer R. 2005. Spectral sensitivity of Bacillus subtilis spores and MS2 coliphage for validation testing of ultraviolet reactors for water disinfection. Environ. Sci. Technol. 39:7845–7852 [DOI] [PubMed] [Google Scholar]
- 9. Mamane H, Shemer H, Linden KG. 2007. Inactivation of E. coli, B. subtilis spores, and MS2, T4, and T7 phage using UV/H2O2 advanced oxidation. J. Hazard. Mater. 146:479–486 [DOI] [PubMed] [Google Scholar]
- 10. Meng QS, Gerba CP. 1996. Comparative inactivation of enteric adenoviruses, poliovirus and coliphages by ultraviolet irradiation. Water Res. 30:2668 [Google Scholar]
- 11. Monk AB, Rees CD, Barrow P, Hagens S, Harper DR. 2010. Bacteriophage applications: where are we now? Lett. Appl. Microbiol. 51:363–369 [DOI] [PubMed] [Google Scholar]
- 12. Murray NE. 1983. Phage lambda and molecular cloning, p 395–432 In Hendrix RW, Roberts JW, Stahl FW, Weisberg RA. (ed), Lambda II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 13. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed, p 2.40–2.44 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 14. Sofer G. 2003. Virus inactivation in the 1990s—and into the 21st century. Part 5. Disinfection. BioPharm Int. June 2003:S34–S42 [Google Scholar]