Abstract
Enterococci are used to evaluate recreational-water quality and health risks in marine environments. In addition to their occurrence in feces of warm blooded animals, they are also common epiphytes. We investigated the contribution of plankton- or particle-associated enterococci in estuarine and coastal water. Seven water and size-fractionated plankton samples were collected monthly between April 2008 and January 2009 in the tidal reaches of the Skidaway River (Georgia, USA). Each size fraction, along with filtered (<30 μm) and bulk estuarine water, was processed according to U.S. Environmental Protection Agency method 1600. Presumptive enterococci were selected and species were identified using carbon substrate utilization patterns. The highest average densities occurred within the 30-, 63-, 105-, and 150-μm size fractions, which also represented the majority (>99%) of the particles within the sampled water. Particle-associated enterococci accounted for as little as 1% of enterococci in bulk water in April to as much as 95% in July. Enterococcus faecalis was the most commonly isolated species from both water and plankton and represented 31% (16/51) and 35% (6/17) of the identified Enterococcus species from water and plankton, respectively. Enterococcus casseliflavus represented 29% of the selected isolates from plankton and 16% from water. Both E. faecalis and E. casseliflavus were able to survive and grow in plankton suspensions significantly longer than in artificial seawater. Enterococcus spp. may be highly concentrated in plankton and associated particles, especially during summer and fall months. These findings could have implications for the effectiveness of enterococci as an indicator of coastal water quality, especially in particle-rich environments.
INTRODUCTION
Enterococci are Gram-positive bacteria common in the feces of warm-blooded animals, including humans. In 1986, the U.S. Environmental Protection Agency (EPA) recommended using enterococci in place of fecal coliform bacteria as the preferred indicator of fecal pollution and health risk in marine water (29). The recommendation was based on epidemiological studies showing that culturable enterococci were positively correlated with reports of highly credible gastroenteritis at beaches throughout the United States (29). Enterococci also survive longer in marine environments than fecal coliform bacteria, making the group easier to detect, and they are more closely associated with human fecal matter than animal feces (6, 32).
Although enterococci are more useful than other historically used indicators in marine environments, there are limitations to using the group to assess fecal pollution and health risk. EPA recreational-water quality indicator recommendations were based on a limited set of studies performed in the 1970s and 1980s (6). Additionally, these studies focused only on beaches impacted by sewage treatment plants (6), excluding beaches affected by non-point-source pollution, such as septic tanks and urban and agricultural runoff. More recent epidemiological studies continue to support the relationship between enterococcal density and risk of gastrointestinal illness (33), but these were also focused on sewage-impacted waters. Enterococci also have a range of sources other than humans, including livestock and domestic and wild birds (7, 14). Additionally, they have been found in association with soil, plants, zooplankton, and algae (23, 25, 28, 34). Even Enterococcus faecalis, the species present in the highest concentration in human feces (22), has been found to adhere to zooplankton and to persist in the environment for extended periods of time (24, 25). Due to the potential for introduction by sources other than human feces and the persistence of enterococci in the environment, high levels may not indicate continuous addition of human fecal wastes in an area and therefore may present confounding information for regulators charged with assessing water quality.
Under the federal Beaches Environmental Assessment and Coastal Health (BEACH) Act of 2000 (Public Law 106-284), every state with marine or Great Lakes beaches was required to adopt enterococcal standards for recreational-water quality criteria by 2004. Furthermore, the BEACH Act also called for the continued evaluation and improvement of fecal indicator standards for recreational marine waters through scientific research. In the 2007 Scientific Experts Report, scientists specifically recognized the importance of an understanding of the ecology of fecal indicator bacteria when water quality criteria are being evaluated (31).
The overall goal of this research was to investigate the distribution, persistence, and possible growth of the fecal indicator bacteria enterococci in the plankton community and detrital particles of coastal Georgia waters. The following hypotheses were tested: (i) enterococci are enriched in estuarine plankton or particles relative to the water column, (ii) enterococci are associated with specific groups of plankton or sizes of particles, (iii) enterococcal species distribution varies between free-living forms found in the water column and forms found associated with plankton and/or particles, and (iv) specific environmental conditions such as temperature and plankton or particle concentration will affect the ability of Enterococcus species to grow or persist.
MATERIALS AND METHODS
Sample collection.
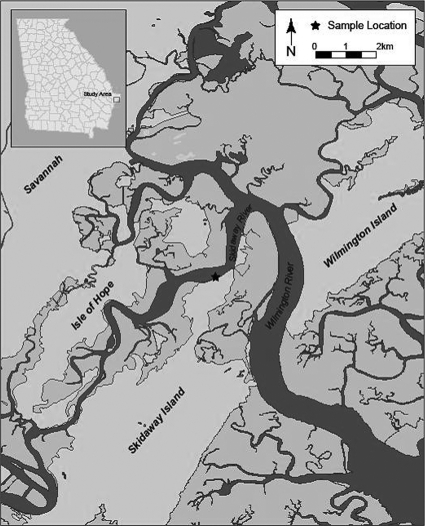
Samples were collected seven times between April 2008 and January 2009 at a tidally influenced fixed station on Skidaway Island (GA) along the Intracoastal Waterway (Fig. 1). Surface water temperature, pH, salinity, and conductivity were measured with a YSI model 556 meter (YSI, Inc., Yellow Springs, OH). Rainfall data were obtained for Skidaway Island (www.georgiaweather.net) for the day, week, and month prior to each sampling date. Tidal stage (www.tidesandcurrents.noaa.gov) for each sampling event was recorded. At each collection, 10 sample fractions were obtained, including two water samples and eight plankton fractions. Water samples were collected in 1-liter sterile polypropylene bottles as grab samples <0.5 m below the surface of the water. Bulk water was split, with one subsample being filtered through a 30-μm mesh net to provide a final filtered sample; both unfiltered “bulk” water and the 30-μm-filtered sample were used in analyses. An ISCO 3700 sampler water pump (ISCO, Inc., Lincoln, NE) was used to deliver water, collected at a depth of <0.5 m, through a series of mesh nets fixed in PVC housings. The pumping rate was determined at the start of each sample set. Mesh nets were arranged in descending order (500, 335, 250, 200, 150, 105, 63, and 30 μm), and samples were filtered sequentially. Water pumping time was recorded and converted to volume of water pumped. Plankton and particles in each net were collected by rinsing with phosphate-buffered saline (PBS) into individual sterile glass beakers to a final volume of 200 ml. Samples were transported immediately to the laboratory for bacterial processing.
Fig 1.
Water and plankton samples were collected between April 2008 and January 2009 at a fixed station on Skidaway Island (GA) along the Intracoastal Waterway (★).
Bacterial analysis.
Each concentrated plankton sample was subdivided into four 50-ml aliquots. Prior to membrane filtration, each concentrated plankton sample was homogenized (Pro 200 homogenizer; ProScientific Inc., Oxford, CT) for 2 min. Homogenization was used to ensure that any internalized bacteria (e.g., within zooplankton) would be detected, following the standard protocols used for assaying plankton for Vibrio spp. (for example, see reference 11). Homogenized plankton samples and filtered and bulk water were filtered in duplicate onto 47-mm-diameter, 0.45-μm-pore-size mixed cellulose membranes according to EPA method 1600 (30) as soon as possible following homogenization. Membranes were placed on mEI (membrane enterococcus indoxyl-β-d-glucoside) agar and incubated for 24 ± 2 h at 41°C. All colonies with a blue halo were considered enterococci, and final counts were recorded as CFU 100 ml−1 of concentrated plankton.
The contribution of each plankton fraction (and all fractions collectively) to the enterococcal load in a bulk water sample was determined. Briefly, the concentration factor for each fraction was determined by dividing the total volume pumped by 200 ml (the concentrated amount). The amount of enterococci (CFU) per 100 ml of concentrated material (designated EP below) was divided by this concentration factor to provide the equivalent amount of CFU 100 ml−1 in the total pumped volume (this value was used to compare enterococcal levels between fractions). To obtain the contribution of each fraction to the total amount of enterococci observed in a bulk water sample (as a percentage), this number was then divided by the enterococcal CFU 100 ml−1 measured in bulk water (designated EB), and the result was multiplied by 100: [(EP + concentration factor)/EB] × 100.
Bacterial species determination.
Following incubation on mEI, up to 10 presumptive enterococci colonies were picked from each membrane filter with a sterile toothpick (e.g., up to 20 isolates per sample, based on duplicate plates), representing a minimum of 10% of the colonies per sample. Selected colonies were reisolated three times on mEI or mE agar. In general, final isolation (third round) was completed on mE to minimize costs associated with repeated use of mEI agar (mEI agar uses the mE agar base but adds indoxyl-ß-d-glucoside, which significantly increases the per-sample cost); at this step, the level of specificity afforded with mE was sufficient to ensure no mixed colonies. Final isolates were grown overnight on Biolog universal growth medium with 5% sheep's blood and inoculated into Biolog GP2 MicroPlates (Biolog, Inc., Hayward, CA) for phenotypic identification based on carbon substrate utilization patterns. Isolates with similarity index value ratings greater than 0.5 were included for analysis (26). Biolog GP2 MicroPlates correctly identify common enterococcal species with 73% overall accuracy and 100, 90, 64, 100, and 100% accuracy for E. casseliflavus, E. faecalis, E. faecium, E. gallinarum, and E. mundtii, respectively (20). All identified isolates were cultured overnight in brain heart infusion broth mixed with 20% glycerol (final concentration) and stored in cryovials at −80°C.
Plankton identification.
A 25-ml aliquot of each plankton sample (particle sizes of 30 to 63, 63 to 105, 105 to 150, 150 to 200, 200 to 250, 250 to 335 and 335 to 500 μm) was fixed (4% [vol/vol] formalin, final concentration) and stored at 5°C. Samples were preserved for long-term storage in 70% ethanol (vol/vol) and stored at room temperature. A stereoscope (Olympus SZX9; Olympus America Inc., Center Valley, PA) was used to determine the actual concentration of plankton and detritus (including plant matter, fecal pellets, and pieces of plankton and exuviae) in each sample. For the larger size fractions (200 to 250, 250 to 335, and 335 to 500 μm), the entire 25-ml volume was counted. For the smaller size fractions (30 to 63, 63 to 105, 105 to 150, and 150 to 200 μm), a Hensen-Stemple pipette (Wildco Wildlife Supply Company, Buffalo, NY) was used to obtain a countable subsample (100 to 400 organisms). Briefly, samples were poured or pipetted onto square petri dishes divided by 5-mm grids (Fisher Scientific Inc., Pittsburg, PA) and immobilized with 3 to 5 drops of Protoslo (Carolina Biological Supply Company, Burlington, NC). The concentration of plankton or detritus (no. of particles/ml−1) was determined after categorizing particles into general taxonomical groups or a detritus group. Taxonomical groups consisted of diatoms, cyanobacteria, dinoflagellates, ciliates, cnidarians, rotifers, cladocerans, ostracods, polychaetes, bivalve nauplii, copepods, copepod nauplii, crab zoea, amphipods, annelids, and sessilia. Unless otherwise noted, the word “plankton” refers to all particles greater than 30 μm, which may include detritus.
Microcosm. (i) Plankton collection.
Plankton for use in laboratory microcosm studies was collected in the Intracoastal Waterway in Brunswick, GA, in December 2008 during three 10-min tows by boat performed using 63-μm and 200-μm mesh nets. The contents of each tow (all sizes) were combined into one sterile 2-liter bottle and allowed to settle. As with previous field collections, particles were dominated by detritus. Water was pipetted from the top of the plankton slurry, and the wet weight of the settled plankton was used to create a 1% (wt/vol) plankton suspension in artificial seawater (ASW) (Instant Ocean; Aquarium Solutions, Mentor, OH) and a 5% (wt/vol) plankton suspension in ASW with a salinity of 30. All plankton samples were stored at 5°C.
(ii) Bacterial cultures.
Environmental strains of E. faecalis (representing a likely fecal source) and E. casseliflavus (representing a likely epiphytic source) were obtained during this study. Each strain was identified using the Biolog system and secondarily confirmed by sequencing of the full 16S rRNA gene (Macrogen, Rockville, MD). Sequences were searched using BLASTn in GenBank (1). A series of eight microcosms were used to compare the persistence and/or growth of the two species within 1% plankton suspensions, 5% plankton suspensions, and artificial seawater at 10 and 30°C.
Overnight cultures of E. faecalis and E. casseliflavus were grown in 5 ml brain heart infusion broth at 35°C. Cultures were centrifuged at 2,450 × g for 10 min at 4°C. Cells were washed by resuspension in 5 ml 1× PBS and vortexed, centrifuged again, and resuspended in a final volume of 5 ml 1× PBS. Cells were inoculated into each microcosm for an estimated final concentration of 103 to 104 CFU ml−1.
(iii) Plankton preparation.
A 60-ml sample of each of two prepared plankton suspensions, 1% and 5% (used as operationally defined “moderate” and “high” plankton/particle suspensions, respectively), was pipetted into each of six 200-ml flasks and placed in a boiling water bath for 2 min to heat-kill bacteria present in the natural plankton. This heat treatment was selected following preliminary testing, including no treatment and boiling for 2, 5, and 10 min. Two minutes of boiling produced the best results with the lowest heat exposure required to reduce background growth of bacteria on mE agar and to result in no observable difference in particle counts and characteristics. Flasks were covered, immediately placed in a refrigerator, and allowed to cool overnight to 5°C. The following day, each of three replicate 1% and 5% plankton flasks was inoculated with one of two test Enterococcus species (E. faecalis or E. casseliflavus). The remaining flasks (three each of 1% and 5% plankton) were not inoculated and served as negative plankton controls. Triplicate flasks with 60 ml ASW were also inoculated with each species, and triplicate flasks each containing 60 ml of uninoculated ASW served as negative seawater controls. After inoculation, the contents of each flask were kept in suspension by mixing on a stir plate, and 5.5-ml aliquots were pipetted into 10 15-ml sterile polypropylene tubes. Tubes were placed on an end-over-end rotator (Rugged Rotator, model 099A RD4512; Glas-Col, Terre Haute, IN) and held at 10 or 30°C in the dark. These temperatures represented the typical minimum and maximum temperatures found during this and previous studies in coastal Georgia (27). Tubes were removed at 0, 4, 8, 12, 24, and 48 h and at days 4, 7, 11, 16, and 22. Once removed, the entire contents of each tube were homogenized, diluted with 1× PBS (as needed), and immediately spread plated onto mE agar in duplicate. For microcosm experiments, mE agar was used rather than mEI agar. Preliminary work showed that following heat treatment mE was sufficiently selective to accurately detect the seeded Enterococcus populations, allowing a substantial reduction in cost. To evaluate longer persistence, the tubes with homogenized contents from day 22 were placed back on the end-over-end rotator, and additional aliquots were taken for spread plating on days 28, 35, 42, and 65. Because the microcosm environment was different between days 0 to 22 (intact plankton or particles) and days 22 to 65 (homogenized plankton or particles), these results are presented separately. After 42 ± 6 h incubation at 41°C, colonies (brick red) were counted and recorded as CFU ml−1. For each time point, change from initial conditions was defined as counts at time n divided by counts at time zero (Tn/T0), where a Tn/T0 value of >1 represented growth and a value of <1 represented die-off.
Statistical analysis.
All enterococcal counts were log transformed to approximate a normal distribution. Normality was tested with the Shapiro-Wilk normality test (α = 0.05). Log values were used in all statistical tests, and means are reported as geometric means in the text. Associations between environmental characteristics (temperature, salinity, dissolved oxygen concentration, and rainfall) and enterococcal counts (CFU 100 ml−1) in plankton fractions and bulk water were analyzed using the Pearson correlation coefficient (r). When counts were below the limit of detection, a value of zero was used in all statistical calculations. In all cases, significance was declared at a P value of ≤0.05.
For microcosm experiments, time points when maximum growth was reached for each flask (highest Tn/T0 value) were determined. Analysis of variance (ANOVA) was used to determine if this maximum density was significantly different among the treatments. Tukey's post hoc test was used to evaluate pairwise differences. Significance was declared at a P value of ≤0.05.
RESULTS
Enterococcal density in environmental samples.
Enterococcal densities in bulk water ranged from 4 CFU 100 ml−1 in early July to 938 CFU 100 ml−1 in late April (mean, 71 CFU 100 ml−1). Enterococcal counts from bulk water exceeded the EPA's single-sample maximum (104 CFU 100 ml−1) in April, June, and November. In filtered water, enterococcal densities ranged from 3 CFU 100 ml−1 in June to 102 CFU 100 ml−1 in April (mean, 20 CFU 100 ml−1); however, over the course of the study, there was not a significant difference between mean enterococcal densities in bulk and filtered water.
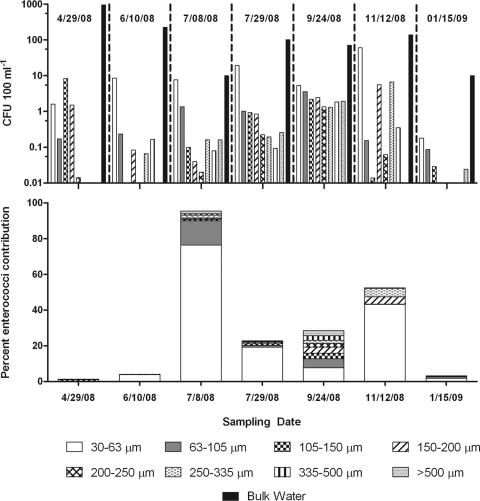
Among the plankton fractions, enterococci were detected in all months only in the two smallest fractions (30 to 63 and 63 to 105 μm). The abundance of enterococci represented in the bulk (unconcentrated) 30- to 63-μm fraction ranged from <1 CFU 100 ml−1 (January) to 60 CFU 100 ml−1 (November), with a mean of 15 CFU 100 ml−1) (Fig. 2). Within the 63- to 105-μm fraction, enterococcal levels were ∼0.9 CFU enterococci 100 ml−1, ranging from <0.1 CFU 100 ml−1 in January to 3.5 CFU 100 ml−1 in September. The abundance of enterococci in the 105- to 150-μm fraction averaged 1.6 CFU 100 ml−1, with the largest abundance being in April (8.2 CFU 100 ml−1) and levels falling below the detection limit (<0.01 CFU 100 ml−1) in June. The abundance of enterococci in the 150- to 200-μm fraction averaged 0.7 CFU 100 ml−1, with the largest abundance being in November (5.5 CFU 100 ml−1) and levels falling below the detection limit in January. The abundance of enterococci in the 200- to 250-, 250- to 335-, 335- to 500, and >500-μm fractions averaged 0.2, 0.3, 1.3, and 0.5 CFU 100 ml−1, respectively, with enterococcal recovery below the detection limit for all of these plankton fractions in January. Additionally, enterococcal density was below the detection limit for the 200- to 250- and >500-μm plankton fractions in June and the 250- to 335-, 335- to 500-, and >500-μm plankton fractions in April (Fig. 2). While there was considerable variability between sample dates, mean levels in the 30- to 63-μm fraction were significantly greater than those in the 200- to 250- and >500-μm fractions (n = 7; P = 0.03).
Fig 2.
(Top) Mean enterococcal densities in plankton fractions and bulk water (n = 2 for each date or fraction). Enterococcal levels in each plankton fraction were calculated by dividing the abundance in the concentrated fraction by the overall concentration factor for each size fraction to provide the equivalent enterococcal load in bulk water. (Bottom) Contribution of enterococci associated with each plankton fraction to the bulk water sample. Bars are additive for all plankton, and the relative contribution of each fraction is shown.
The percent contribution of enterococci in each plankton fraction to bulk water was determined (Fig. 2). Enterococci from all plankton fractions combined contributed as much as 95% of the enterococci in bulk water in early July and as little as 1.2% in April. The 30- to 63-μm plankton fraction contributed the most enterococci to bulk water (mean, 22%) on all dates except in April. In April, the 105- to 150-μm plankton fraction contributed the most enterococci (4%); this fraction provided an average 1% contribution across the sampling dates. The 63- to 105-μm plankton fraction provided an average 3% contribution across the sampling dates. The 150- to 220-, 200- to 250-, 250- to 335-, 335- to 500-, and >500-μm fractions each averaged less than 1.5% of the enterococci contribution to bulk water. In September, the contributions of all fractions were remarkably similar, with each fraction contributing between 2 and 7.6% of enterococcal density in bulk water.
Physical and chemical parameters measured over the sampling period are included in Table 1. Enterococcal levels among the sampled fractions correlated with environmental parameters or rainfall on few occasions, which may have been due to low power provided by the small number of samples from each fraction. However, there was a significant positive correlation (r = 0.76; P = 0.05) between enterococcal concentration in the >500-μm plankton fraction and salinity. Furthermore, in the 250- to 335- and 335- to 500-μm plankton fractions, enterococcal concentrations were significantly negatively correlated with rainfall in the week prior to sampling (r = −0.77 and r = −0.084; P = 0.04 and 0.02, respectively).
Table 1.
Physiochemical parameters at time of sample collection
| Sampling date | Temperature (°C) | Salinity | Dissolved oxygen concn (mg ml−1) | pH | Rainfall (mm)a |
|---|---|---|---|---|---|
| 29 Apr 2008 | 22.1 | 24.3 | 7.1 | 7.7 | 0.91 |
| 10 June 2008 | 29.7 | 29.2 | 4.4 | 7.6 | 0.13 |
| 8 July 2008 | 28.4 | 30.6 | 3.4 | 7.6 | 0.64 |
| 29 July 2008 | 29.7 | 30.5 | 3.7 | 7.7 | 0.99 |
| 24 Sept 2008 | 22.0 | 30.6 | 5.9 | 7.9 | 0.08 |
| 12 Nov 2008 | 16.2 | 27.0 | 8.5 | 7.9 | 0.00 |
| 15 Jan 2009 | 11.6 | 28.2 | 7.6 | 7.6 | 1.12 |
In 7 days prior to sampling.
Enterococcus species distribution.
A total of 502 well-isolated colonies presenting the characteristic blue halo on mEI agar were selected and taken through at least three rounds of isolation. Seventy-four of the 502 isolates were eventually taken to pure culture and identified using the Biolog system. Abundant growth of nontarget bacteria, including Staphylococcus species, on the mEI agar plates made proper isolation of enterococcal colonies from plankton samples difficult, even after multiple rounds of subculture. Of the 74 pure-culture isolates, 68 (91.9%) were identified as Enterococcus species; 1 (1.4%) was identified only to the Enterococcus genus level. Five (6.8%) were identified as non-Enterococcus genera. Among the isolates that could be identified to species level, the most common species isolated from both water and plankton (all fractions combined) was E. faecalis (16/51 [31%] and 16/17 [35%], respectively). Eight of 51 (16%) of water isolates and 5 of 17 (29%) plankton isolates were identified as E. casseliflavus. Sixteen of 51 (31%) water isolates and 4 of 17 (24%) plankton isolates were identified as E. mundtii. E. faecium was found less frequently than these species but with similar percentages between water and plankton samples (Table 2). Enterococcus gallinarum and E. flavescens isolates were identified in water but not plankton.
Table 2.
Summary of Enterococcus sp. distribution among water and plankton samples
| Rank | Water |
Plankton |
||
|---|---|---|---|---|
| Organism | % of total (n = 51) | Organism | % of total (n = 17) | |
| 1 | E. faecalis | 31 | E. faecalis | 35 |
| 2 | E. mundtii | 31 | E. casseliflavus | 29 |
| 3 | E. casseliflavus | 16 | E. mundtii | 24 |
| 4 | E. faecium | 10 | E. faecium | 12 |
| 5 | E. flavescens | 8 | E. flavescens | 0 |
| 6 | E. gallinarum | 4 | E. gallinarum | 0 |
Description of plankton/particle fractions.
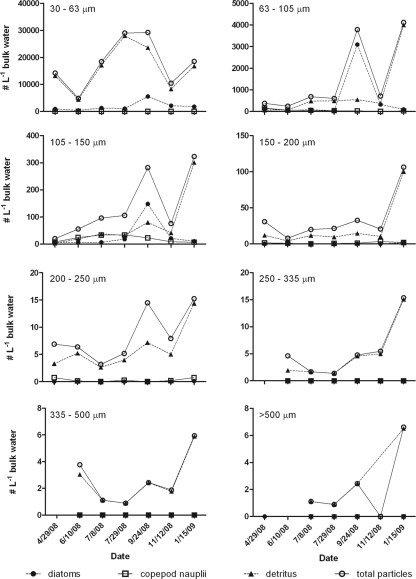
Detritus particles, diatoms, and copepod nauplii made up ∼93% of all particles observed throughout the study. Eighty-five percent of all plankton fraction particles were classified as detritus, which was found in all fractions ranging from an average of 42% of particles in the 105- to 150-μm fraction to 99% of particles in the >500-μm fraction; however, there were very few total particles in this large size class (Fig. 3). Of the total particles, 13.5% were classified as diatoms (found in all four plankton fractions with particles of <200 μm), and 1% were copepod nauplii (found in the 63- to 105-, 105- to 150-, and 150- to 200-μm fractions). All other plankton categories combined made up less than 1% of the total plankton particles. Over 99% of all plankton particles were from the 30- to 63-, 63- to 105-, and 105- to 150-μm fractions, with the highest particle load being consistently in the 30- to 63-μm size range and with the load decreasing with larger size fractions. Across the study period, the greatest diversity of plankton was noted in September (Fig. 3), coincident with the highest diatom levels of the study period. The highest detritus and total particle levels were generally noted in January (Fig. 3).
Fig 3.
Abundance of plankton (diatoms and copepod nauplii), detritus, and total particles among each of the eight sampled size fractions for each sample date.
Microcosms.
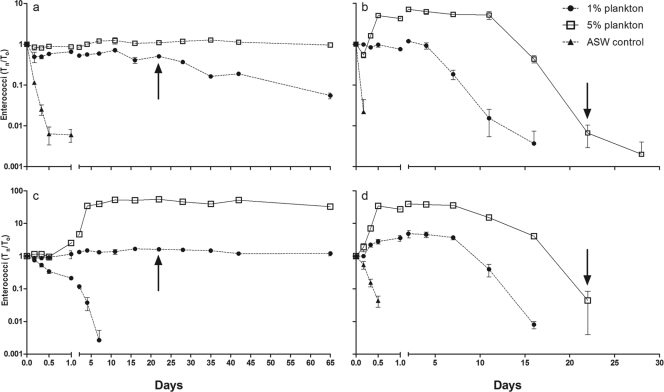
At 10°C, there were notable differences between E. faecalis and E. casseliflavus among the seawater and plankton treatments (Fig. 4). In both cases, bacterial concentrations declined in ASW alone; however, E. casseliflavus continued to form colonies for 7 days, while E. faecalis was unculturable by 24 h. Persistence was greatly enhanced for both species by the presence of plankton. In both 1% and 5% suspensions, both species showed viable (culturable) populations through day 65. In the 1% plankton suspension, E. casseliflavus showed a nearly static population density, with a Tn/T0 of 1.62 at day 22 (in a suspension of intact plankton and particles), and changed little through day 65 (Tn/T0 of 1.21 at day 65) despite being in a suspension of homogenized plankton and particles beginning at day 22. The maximum growth was reached at day 16, with a Tn/T0 of 1.67. E. faecalis showed greater decay than E. casseliflavus, with a Tn/T0 that was 0.51 on day 22 and declined to 0.06 on day 65 (in homogenized plankton beginning at day 22), and no growth (Tn/T0 always ≤1) during the course of the experiment. In the 5% plankton suspension, E. casseliflavus showed logarithmic growth early in the treatment, resulting in a maximum Tn/T0 of 58.09 on day 11 and persisting near this level through day 65. E. faecalis also showed growth, although less dramatic, reaching a Tn/T0 of 1.24 (day 11) but also persisting near this level throughout the experiment. All noninoculated controls (1% plankton, 5% plankton, and ASW) remained negative throughout the experiment.
Fig 4.
Growth and decay trends for enterococci in seawater and plankton microcosms. (a) E. faecalis at 10°C; (b) E. faecalis at 30°C; (c) E. casseliflavus at 10°C; (d) E. casseliflavus at 30°C. Arrows indicate day 22, when the contents of all microcosm containers were homogenized.
Persistence at 30°C was less than that observed for 10°C in all experimental treatments. For both species, populations declined rapidly in ASW, with E. faecalis losing culturability by 4 h and E. casseliflavus by 12 h. In the 1% plankton suspension, E. casseliflavus reached a maximum growth of 4.49 (Tn/T0) at day 2 but lost culturability by day 16. For E. faecalis, growth was observed, albeit moderate (Tn/T0 = 1.19), but culturable cells persisted to day 16. In the 5% plankton fraction, E. casseliflavus grew to a maximum Tn/T0 of 39.09 at day 2 and persisted to day 22, after which it could no longer be cultured. Enterococcus faecalis also grew in the 5% suspension, reaching 7.19 (Tn/T0) at day 2 and persisting to day 28. Controls remained negative throughout the experiment, with the exception of the 5% plankton suspension, in which 1 CFU was noted at the 4-h time point (negative at all other times).
At both 10°C and 30°C, a significant difference was observed between the maximum growth levels achieved by E. faecalis and E. casseliflavus in the 5% plankton treatment. E. casseliflavus Tn/T0 levels were significantly greater than those of E. faecalis at both temperatures (P < 0.0001). E. casseliflavus growth was also significantly greater in 5% plankton than 1% plankton at both temperatures (P < 0.0001).
DISCUSSION
This study compared the density of the fecal indicator bacteria enterococci in water and size-fractionated plankton samples from an estuary in Georgia over the course of 7 months. We found that plankton-associated enterococci contributed up to 95% of the culturable enterococci in bulk water samples and in vitro studies indicated that plankton may serve as a reservoir for growth and persistence of this fecal indicator. Enterococci have previously been found in association with specific types of plankton such as copepods, green algae, and seaweed (2, 24, 25, 34). In this study, we compared the contribution of groups of plankton, separated by size, to enterococcal densities. The density of enterococci greatly varied between plankton fractions and between sampling dates; however, the highest densities were found consistently in the 30- to 63-, 63- to 105-, and 105- to 150-μm plankton fractions. These plankton fractions also represented the majority (>99.%) of the plankton particles in bulk water, which was comprised of an average of 85% plant detritus, 13.5% diatoms and 1% copepod nauplii. Contrary to what was observed in this study, a coastal marine study in Italy found enterococcal densities in greater numbers in larger plankton (>200 μm and mostly zooplankton) than smaller plankton (<64 μm and mostly phytoplankton) (18). The large tidal range and particle load in the estuary where our study was conducted could explain the differences observed between our study and a coastal study performed 50 m from shore (18).
Data suggest that, unlike levels of some Vibrio spp., which tend to attach to specific types of organisms (10, 12), levels of enterococci may be more related to the general particle load present in the environment. In areas of higher particle concentrations, such as within an estuary with high levels of tidal mixing, particle-associated bacterial counts increase relative to counts of free-living bacteria (3). This is consistent with results found here, where the smaller plankton fractions (mostly plant detritus) made up the majority of particles collected and therefore provided more opportunity for bacterial association. Enterococci, acting as detritivores, may be able to colonize the organic material in the marine environment and persist. Enterococci may not only find necessary nutrients in plankton but also find shelter from sunlight inactivation (13).
While we hypothesized that the recognized epiphytic Enterococcus species would be more common among the plankton fractions, we found that species distribution was actually similar between plankton and water. Enterococcus faecalis represented the majority of the isolates identified in both categories. Enterococcus faecalis is commonly isolated from the intestines of warm-blooded animals, including humans (7, 22), and has been found in large concentrations (>30% of enterococci population) in estuarine areas along the Georgia coast (19). E. casseliflavus and E. mundtii were also identified frequently among both plankton and water isolates. Unlike E. faecalis, E. casseliflavus and E. mundtii are considered epiphytic species found commonly on plant matter and in soil but not often isolated from the intestines of animals (7, 15). Our results are consistent with a study in Orange County, CA, that found 31% of presumptive Enterococcus isolates from ocean samples to be E. casseliflavus and 24% of isolates were E. faecalis (21). In Greece, the majority of marine recreational-water enterococcal isolates were human-fecal-matter-associated: E. faecium (11/94 18%) and E. faecalis (10/94 17%) (8). These studies demonstrate regional variation in the composition of the bacterial community. They also illustrate the fact that nonhuman strains are a common component of the bulk enterococcal densities and species level differences in growth or persistence in coastal environments may impact the utility of this group as a fecal indicator.
Enterococcal survival in the environment depends upon several biotic and abiotic factors, including temperature, sunlight, and the presence of organic matter, among others. Our microcosm results show persistence and growth of both E. casseliflavus and E. faecalis at 10°C and 30°C in the presence of plankton; however, at 10°C, both species persisted at near-inoculum levels (∼103 CFU ml−1) for more than 2 months, whereas culturability was lost by 28 days at 30°C. Although enterococci can survive and even grow at higher temperatures with sufficient nutrients and organic matter, our findings in artificial seawater (without plankton) for both E. faecalis and E. casseliflavus were consistent with previous studies on the survival of total enterococci from natural sewage in aquatic environments, with survival being reduced to only several hours at 30°C compared to that at lower temperatures (4, 16, 17).
With the availability of nutrients and protection, as may be found in algae or plankton, enterococci may be able not only to survive but also to multiply in marine environments. In our study, which was restricted to culturable enterococci, growth was enhanced by the presence of plankton compared to artificial seawater. Relative to seawater, both 1% and 5% plankton concentrations resulted in significant growth and enhanced persistence of both E. casseliflavus and E. faecalis. Similarly, indigenous enterococci demonstrated 100-fold growth for the first 18 h in Cladophora leachate, filtered supernatant from a centrifuged suspension of Cladophora (a green alga), and lake water at 35°C (5). The enterococcal concentration remained above or at the starting concentration for the 168-hour study duration (5). Moreover, in situ evidence also supports the potential for enterococci to grow in marine environments. Anderson et al. (2) found that seaweed contained significantly greater concentrations of enterococci at recreational beaches in New Zealand and suggested that seaweed may support a rapid expansion in the environment. Further analysis of the isolates from the seaweed and water demonstrated the presence of clonal enterococci populations in seaweed but their absence in water samples (2). Results from the present study confirm these results but also indicate the potential for nonclonal growth with similar presence of Enterococcus species in water and in plankton and/or particles. While E. casseliflavus, a plant-associated species, grew to significantly higher densities than E. faecalis, this fecal-matter-associated species still exhibited measurable growth in association with plankton, especially with increasing plankton concentrations and temperature. These results are also consistent with our field survey, which showed an increased contribution of plankton-associated enterococci during the warmest months of the year, with 95% of the enterococci in bulk water attributed to plankton in early July.
In addition to growth in association with plankton, these organic-matter-rich particulates could also protect enterococci from damage by UV irradiance, which has been cited as an important driver for enterococcal survival in the environment (13). Estuarine waters with high turbidity due to suspended solids provide protection from sunlight inactivation. In microcosm experiments testing enterococcal survival in estuarine water at 15°C, enterococci persisted longer in high-turbidity waters than low-turbidity waters in both irradiated and dark experiments (13).
While this and other studies suggest the importance of plankton and particulate organic matter on enterococci growth and persistence (2, 5, 13), there is evidence that plankton may also control enterococci abundance (9). Although not accounted for in the size fractions studied here (which were all >30 μm), protozoan predators (i.e., flagellates measuring <20 μm) have been shown to graze on enterococci in controlled laboratory studies (9). Furthermore, once introduced into the environment, enterococci may be temporarily sequestered in sediments, where they remain in high concentrations but are not accounted for in routine water monitoring during calm conditions (19). Storm events could resuspend these populations, leading to artificially high levels (19). Together, these studies suggest that enterococci, once introduced into the natural aquatic environment, are subject to multiple factors which may result in net growth or loss, independent of the human activities that these bacteria are taken to represent, such as loading from sewage discharge or other wastewater disposal.
This study contributes to the knowledge of the ecology of enterococci within estuarine plankton communities, which can aide in the evaluation of these bacteria as water quality indicators. Our investigation demonstrates the potential for culturable enterococci to associate and proliferate within the plankton community along Georgia's coast, suggesting that abundance may be decoupled from pollution sources. In estuaries, which are typically high-particle-load environments, counts of particle-associated bacteria increase relative to those of free-living bacteria (3). By providing nutrients and protection from sunlight, particles such as plankton are potential reservoirs for enterococci. Whereas enterococci may serve as a reliable indicator of water quality in some regions of the world, in particle-rich marine environments, enterococcal density analysis in water alone may provide misleading information for regulators assessing water quality.
ACKNOWLEDGMENTS
This work was supported by grants from the NOAA Oceans and Human Health Initiative (NA04OAR460020 and S0867882 [Georgia Oceans and Health Initiative Graduate Training Consortium]).
Marc Frischer and Aaron Peck at the Skidaway Institute of Oceanography and Dominic Gualdognoli at the Georgia Division of Natural Resources provided valuable field and lab support.
Footnotes
Published ahead of print 10 February 2012
REFERENCES
- 1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Anderson SA, Turner SJ, Lewis GD. 1997. Enterococci in the New Zealand environment: implications for water quality monitoring. Water Sci. Technol. 35:325–331 [Google Scholar]
- 3. Bidle KD, Fletcher M. 1995. Comparison of free-living and particle associated bacterial communities in the Chesapeake Bay by stable low-molecular-weight RNA analysis. Appl. Environ. Microbiol. 61:944–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bordalo AA, Onrassami R, Dechsakulwatana C. 2002. Survival of faecal indicator bacteria in tropical estuarine waters (Bangpakong River, Thailand). J. Appl. Microbiol. 93:864–871 [DOI] [PubMed] [Google Scholar]
- 5. Byappanahalli MN, Shively DA, Nevers MB, Sadowsky MJ, Whitman RL. 2003. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 46:203–211 [DOI] [PubMed] [Google Scholar]
- 6. Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. 1983. A marine recreational water quality criterion consistent with indicator concepts and risk analysis. J. Water Pollut. Cont. Fed. 55:1306–1314 [Google Scholar]
- 7. Devriese LA, Van De Kerckhove A, Kilpper-Bälz R, Schleifer KH. 1987. Characterization and identification of Enterococcus species isolated from the intestines of animals. Int. J. Syst. Bacteriol. 37:257–259 [Google Scholar]
- 8. Grammenou P, Spiliopoulou I, Sazakli E, Papapetropoulou M. 2006. PFGE analysis of enterococci isolates from recreational and drinking water in Greece. J. Water Health 4:263–269 [PubMed] [Google Scholar]
- 9. Hartke A, Lemarinier S, Pichereau V, Auffray Y. 2002. Survival of Enterococcus faecalis in seawater experiments is limited in the presence of bacterivorous zooflagellates. Curr. Microbiol. 44:329–335 [DOI] [PubMed] [Google Scholar]
- 10. Huq A, et al. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huq A, Grim C, Colwell RR, Nair B. 2006. Detection, isolation and identification of Vibrio cholerae from the environment. Curr. Protoc. Microbiol. 2:6A.5.1–6A.5.38 [DOI] [PubMed] [Google Scholar]
- 12. Kaneko T, Colwell RR. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl. Environ. Microbiol. 29:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kay D, et al. 2005. Decay of intestinal enterococci concentrations in high-energy estuarine and coastal waters: towards real-time T90 values for modeling faecal indicators in recreational waters. Water Res. 39:655–667 [DOI] [PubMed] [Google Scholar]
- 14. Kuntz RL, Hartel PG, Rodgers K, Segars WI. 2004. Presence of Enterococcus faecalis in broiler litter and wild bird feces for bacterial source tracking. Water Res. 38:3551–3557 [DOI] [PubMed] [Google Scholar]
- 15. Leclerc H, Devriese LA, Mossel DAA. 1996. Taxonomical changes in intestinal (faecal) enterococci and streptococci: consequences on their use as indicators of faecal contamination in drinking water. J. Appl. Bacteriol. 81:459–466 [DOI] [PubMed] [Google Scholar]
- 16. Lessard EJ, Sieburth JM. 1983. Survival of natural sewage populations of enteric bacteria in diffusion and batch chambers in the marine environment. Appl. Environ. Microbiol. 45:950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lleó MM, Bonato B, Benedetti D, Canepari P. 2005. Survival of enterococcal species in aquatic environments. FEMS Microbiol. Ecol. 54:189–196 [DOI] [PubMed] [Google Scholar]
- 18. Maugeri TL, Carbone M, Fera MT, Irrera GP, Gugliandolo C. 2004. Distribution of potentially pathogenic bacteria as free living and plankton associated in a marine coastal zone. J. Appl. Microbiol. 97:354–361 [DOI] [PubMed] [Google Scholar]
- 19. McDonald JL, et al. 2006. Identifying sources of fecal contamination inexpensively with targeted sampling and bacterial source tracking. J. Environ. Qual. 35:889–897 [DOI] [PubMed] [Google Scholar]
- 20. Moore DF, et al. 2006. Comparison of 16S rRNA sequencing with conventional and commercial phenotypic techniques for identification of enterococci from the marine environment. J. Appl. Microbiol. 100:1272–1281 [DOI] [PubMed] [Google Scholar]
- 21. Moore DF, Guzman JA, McGee C. 2008. Species distribution and antimicrobial resistance of enterococci isolated from surface and ocean water. J. Appl. Microbiol. 105:1017–1025 [DOI] [PubMed] [Google Scholar]
- 22. Noble CJ. 1978. Carriage of group D streptococci in the human bowel. J. Clin. Pathol. 31:1182–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roll BM, Fujioka RS. 1997. Sources of faecal indicator bacteria in a brackish, tropical stream and their impact on recreational water quality. Water Sci. Technol. 35:179–186 [Google Scholar]
- 24. Signoretto C, et al. 2004. Adhesion of Enterococcus faecalis in the nonculturable state to plankton is the main mechanism responsible for persistence of the bacterium in both lake and seawater. Appl. Environ. Microbiol. 70:6892–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Signoretto C, Burlacchini G, Pruzzo C, Canepari P. 2005. Persistence of Enterococcus faecalis in aquatic environments via surface interactions with copepods. Appl. Environ. Microbiol. 71:2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solit R. 2001. MicroLog system, release 4.2 user guide. Biolog, Inc., Hayward, CA [Google Scholar]
- 27. Turner JW, Good B, Cole D, Lipp EK. 2009. Environmental factors affect the status of plankton as a reservoir for Vibrio species. ISME J. 3:1082–1092 [DOI] [PubMed] [Google Scholar]
- 28. Ulrich A, Müller T. 1998. Heterogeneity of plant associated streptococci as characterized by phenotypic features and restriction analysis of PCR amplified 16S rDNA. J. Appl. Microbiol. 84:293–303 [DOI] [PubMed] [Google Scholar]
- 29. U.S. Environmental Protection Agency 1986. Ambient water quality criteria for bacteria—1986. EPA-440/5-84/002. U.S. Environmental Protection Agency, Office of Water, Washington, DC [Google Scholar]
- 30. U.S. Environmental Protection Agency 2002. Method 1600: enterococci in water by membrane filtration using membrane-enterococcus indoxyl-β-d-glucoside agar (mEI). EPA 821-R-02-022. U.S. Environmental Protection Agency, Office of Water, Washington, DC [Google Scholar]
- 31. U.S. Environmental Protection Agency 2007. Recreational water quality criteria. EPA 823-R-07-006. U.S. Environmental Protection Agency, Office of Water, Washington, DC [Google Scholar]
- 32. Vasconcelos GJ, Swartz RG. 1976. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl. Environ. Microbiol. 31:913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wade TJ, et al. 2010. Rapidly measured indicators or recreational water quality and swimming-associated illness at marine beaches: a prospective cohort study. Environ. Health 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whitman RL, Shively DA, Pawlik H, Nevers MB, Byappanahalli MN. 2003. Occurrence of Escherichia coli and enterococci in Cladophora (Chlorophyta) in nearshore water and beach of Lake Michigan. Appl. Environ. Microbiol. 69:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]