Abstract
Ticks are important disease vectors that can cause considerable economic losses by affecting animal health and productivity, especially in tropical and subtropical regions. In this study, we investigated the prevalence and diversity of bacterial and protozoan tick-borne pathogens in ticks collected from the vegetation and cattle in Nigeria by PCR. The infection rates of questing ticks were 3.1% for Rickettsia species, 0.1% for Coxiella burnetii and 0.4% for Borrelia species. Other pathogens, such as Babesia, Theileria, Anaplasma, and Ehrlichia species, were not detected in ticks from the vegetation. Feeding ticks collected from cattle displayed infection rates of 12.5% for Rickettsia species, 14% for Coxiella burnetii, 5.9% for Anaplasma species, 5.1% for Ehrlichia species, and 2.9% for Theileria mutans. Babesia and Borrelia species were not detected in ticks collected from cattle. Mixed infections were found only in feeding ticks and mainly Rickettsia species and Coxiella burnetii were involved. The diversity of tick-borne pathogens in Nigeria was higher in feeding than in questing ticks, suggesting that cattle serve as reservoirs for at least some of the pathogens studied, in particular C. burnetii. The total estimated herd infection rates of 20.6% for a Rickettsia africae-like species, 27% for Coxiella burnetii, and 8.5% for Anaplasma marginale/centrale suggest that these pathogens may have considerable implications for human and animal health.
INTRODUCTION
Ticks are important disease vectors that can cause considerable economic losses by affecting animal health and productivity, especially in tropical and subtropical regions (28, 32, 37). In Africa, the tick fauna is remarkably diverse, with about 50 endemic tick species that are known to infest domestic animals (38). However, the highest impact on livestock health is caused by species belonging to only three genera, namely, Amblyomma, Hyalomma, and Rhipicephalus (28). Damage is either direct (skin lesions, impairment of animal growth) or indirect, resulting from transmission of a variety of pathogens (32). Major economical impact has been associated with the tick-borne diseases anaplasmosis, heartwater, babesiosis, and theileriosis, all of which are prevalent in Africa (4).
Bovine anaplasmosis is caused by the highly pathogenic species Anaplasma marginale sensu stricto and the naturally attenuated A. marginale subspecies centrale (2, 7). Anaplasma species are commonly detected in cattle, and seroprevalence rates between 4.6% (Kenya) and 98% (South Africa) from different sub-Saharan countries have been reported (4, 18, 26, 31). The causative agents of bovine babesiosis and theileriosis have frequently been detected in blood smears from cattle in Ghana, with prevalences as high as 97% for Theileria mutans, 87% for Theileria velifera, and 61% for Babesia bigemina (4). Tick-borne human ehrlichiosis of varying severity is caused by Ehrlichia chaffeensis and Ehrlichia ewingii (24). Several human-pathogenic tick-borne Rickettsia species have been found in Africa, including Rickettsia conorii conorii, R. conorii caspia, R. africae, R. aeschlimannii, R. massiliae, R. akari, and R. sibirica mongolotimonae (8, 19, 27). Humans are frequently infected with Rickettsia species in Senegal, Burkina Faso, Cameroon, Mali, and the Ivory Coast, where seroprevalence rates from 17 to 36% have been reported (16). Coxiella burnetii causes Q fever in humans, and high serological prevalences have been reported from West African countries (17). Although transmission mainly occurs via contact with infected reservoir hosts (domestic goats, sheep, and cows), C. burnetii can also be transmitted by ticks. The most important borrelial infection of humans in Africa is relapsing fever, transmitted either by lice (louse-borne relapsing fever) or soft ticks (tick-borne relapsing fever [TBRF]). TBRF is caused by at least 16 Borrelia species, of which Borrelia crocidurae seems to be of increasing importance in West Africa (36). In Ghana, 15% of blood smears from cattle were positive for Borrelia species (4).
Pathogens belonging to the genera Anaplasma, Ehrlichia, Coxiella, Rickettsia, Babesia, Theileria, and Borrelia have been detected in ticks from some West African countries. In Mali, Niger, Mauritania, and Cameroon, feeding ticks from cattle were analyzed for Rickettsia species, and prevalence rates ranging from 7.4 to 75% were observed (21, 27). In Cameroon, the prevalence of Ehrlichia species in ticks removed from dogs was found to be 56% for E. chaffeensis and 6% for E. canis (24). However, it is important that these studies on feeding ticks be complemented by pathogen prevalence studies in unfed (questing) ticks collected from vegetation to estimate the risk of infection after tick bites during the next blood meal. So far, throughout West Africa only a single study investigated questing Amblyomma variegatum ticks from Burkina Faso for Ehrlichia ruminantium and reported a prevalence rate of 3.7% (1).
Thus, studies on tick-borne pathogens in ticks are fairly limited in West Africa. Here we present the first comprehensive study on the diversity of bacterial and protozoan tick-borne pathogens in questing and feeding ticks from Nigeria.
MATERIALS AND METHODS
In 2009, questing and feeding ticks were collected in Oyo State in southwestern Nigeria. The field collection sites were located within a 65-km radius of Ibadan, while the collection sites of feeding ticks were located within a 20-km radius. Questing ticks were collected from the vegetation at seven locations (Elepo, Alowo-nle, Fuleni, Orisunbare, Lanlate, Maya, and Igbo-Ora) by cloth dragging and by direct hand-picking from their questing locations. Collection sites included rainforest, derived savannah, shrubs, and herbaceous (mainly graminoid) plant cover and displayed no notable topographical or climatic differences. Feeding ticks were obtained from 11 herds comprising 1 to 13 cattle each at four locations (Moniya, Alakia, Bodija, and Mokola). Collection was performed throughout the year but intensified in the wet season for questing ticks, especially in June and July (67.6%; 473/700). The collection of feeding ticks was most intense from January to March (80.9%; 110/136).
Ticks were stored in 70% ethanol at 4°C until further processing. For molecular analysis, 700 ticks from the vegetation (100 ticks per region) and 136 ticks from 63 cows were randomly selected and morphologically identified to the species level (38). The ticks were washed three times in phosphate-buffered saline, rinsed with distilled water and dried on sterile filter paper before disruption and homogenization. Ticks were crushed individually in 300 μl lysis buffer from an InviMag Tissue DNA minikit (Invitek, Berlin, Germany) using a TissueLyser II (Qiagen, Venlo, Netherlands), and DNA extraction was performed with a KingFisher 96 magnetic particle processor (Thermo Scientific, Waltham, MA) following the manufacturers' instructions.
Detection PCRs were carried out using family-specific primers for members of the Rickettsiaceae and Piroplasmidae and species-specific primers for Coxiella burnetii as described before (references are given in Table 1). The primers for the detection of Anaplasmataceae were modified to be genus specific for Anaplasma and Ehrlichia. The Borrelia spp. primers were adapted to allow amplification of relapsing fever group and Lyme disease spirochetes (Table 1). Primers directed against the flagellar B gene of the Borrelia burgdorferi sensu lato group were used for further characterization of detected Borrelia species (Table 1). The resulting PCR amplicons of the right fragment size were either directly purified (Jet Quick PCR purification spin kit; Genomed, Loehne, Germany) or excised from a 1.5% agarose gel (QIAquick gel extraction kit; Qiagen, Venlo, the Netherlands). Sequencing was performed as described before (30). Neighbor-joining phylogenetic analyses using the Kimura two-parameter model with 1,000 bootstrap replicates and pairwise deletion were performed using MEGA v.4.0.2 software (13). Statistical analyses of differences in the prevalence rates between feeding and questing ticks were performed with Fisher's exact test (in case of a sampling number of <5) or Pearson's goodness of fit chi-square (GFX) test (for P values, see Tables 3 and 4).
Table 1.
Primers and PCR conditions used for the detection of the five pathogen groupsa
| Pathogen | Primer | Orientation | Target gene | Sequence (5′–3′) | Reference | [Primer] (μM) | [MgCl2] (mM) | Annealing temp (°C) | Elongation time (s) |
|---|---|---|---|---|---|---|---|---|---|
| Anaplasmataceae | EHR1 | Forward | 16S rRNA | GAACGAACGCTGGCGGCAAGC | 29 | 0.4 | 2 | 63 | 45 |
| newEHR2b | Reverse | 16S rRNA | CACGCTTTCGCACCTCAGTGTC | 29 | 0.4 | ||||
| EHR3 | Forward | 16S rRNA | TGCRTAGGAATCTRCCTAGTAG | 29 | 0.8 | 2 | 59 | 45 | |
| newEHR2b | Reverse | 16S rRNA | CACGCTTTCGCACCTCAGTGTC | 29 | 0.8 | ||||
| Rickettsiaceae | Rr17k.1p | Forward | 17 kDa | TTTACAAAATTCTAAAAACCAT | 10 | 0.8 | 2 | 55 | 45 |
| Rr17k.539n | Reverse | 17 kDa | TCAATTCACAACTTGCCATT | 10 | 0.8 | ||||
| Rr17k.90p | Forward | 17 kDa | GCTCTTGCAACTTCTATGTT | 10 | 0.8 | 2 | 54 | 45 | |
| Rr17k.539n | Reverse | 17 kDa | TCAATTCACAACTTGCCATT | 10 | 0.8 | ||||
| Piroplasmidae | BJ1 | Forward | 18S rRNA | GTCTTGTAATTGGAATGATGG | 5 | 0.8 | 3 | 61 | 60 |
| BN2 | Reverse | 18S rRNA | TAGTTTATGGTTAGGACTACG | 5 | 0.8 | ||||
| Coxiella | Q5 | Forward | htpB | GCGGGTGATGGTACCACAACA | 35 | 0.4 | 1.5 | 58 | 30 |
| Q3 | Reverse | htpB | GGCAATCACCAATAAGGGCCG | 35 | 0.4 | ||||
| Q6 | Forward | htpB | TTGCTGGAATGAACCCCA | 35 | 0.8 | 2 | 56 | 30 | |
| Q4 | Reverse | htpB | TCAAGCTCCGCACTCATG | 35 | 0.8 | ||||
| Borrelia species | newLDfb | Forward | 16S rRNA | GTAAACGATGCACACTTGGTG | 14 | 0.4 | 2 | 61 | 30 |
| newLDrb | Reverse | 16S rRNA | TCCGRCTTATCACCGGCAGTCT | 14 | 0.4 | ||||
| Outer1 | Forward | flaB | AARGAATTGGCAGTTCAATC | 6 | 0.8 | 2 | 59 | 30 | |
| Outer2 | Reverse | flaB | GCATTTTCWATTTTAGCAAGTGATG | 6 | 0.8 | ||||
| Inner1 | Forward | flaB | ACATATTCAGATGCAGACAGAGGTTCTA | 6 | 0.8 | 2 | 59 | 30 | |
| Inner2 | Reverse | flaB | GAAGGTGCTGTAGCAGGTGCTGGCTGT | 6 | 0.8 |
PCR protocol: 94°C for 3 min; 40 cycles of 94°C for 30 s; specific annealing at 30 s and 72°C for specific elongation; subsequent incubation at 72°C for 10 min.
The primer sequence was modified.
Table 3.
Locations, numbers of questing ticks, and prevalence of pathogensa
| Location | No. of ticks | No. (%) of ticks harboring: |
|||||
|---|---|---|---|---|---|---|---|
| Alphaproteobacterium | R. massiliae | RRG | Borrelia sp. | Unknown bacterium | C. burnetii | ||
| Alowo-nle | 100 | — | 4 (4) | — | — | — | — |
| Igbo Ora | 100 | — | 2 (2) | — | — | — | — |
| Fuleni | 100 | — | 1 (1) | — | — | — | — |
| Maya | 100 | — | 2 (2) | — | 3 (3) | 2 (2) | — |
| Lanlate | 100 | 2 (2) | 8 (8) | — | — | 4 (4) | — |
| Elepo | 100 | — | 2 (2) | 1 (1) | — | — | — |
| Orisunbare | 100 | — | 2 (2) | — | — | 3 (3) | 1 (1) |
| Total | 700 | 2 (0.3) | 21 (3)* | 1 (0.1) | 3 (0.4) | 9 (1.3) | 1 (0.1)* |
RRG, Rickettsia rickettsii group; —, not detected (0%). Asterisks mark rates which differ significantly from those observed in ticks from cattle (P < 0.05).
Table 4.
Mixed infections in ticks feeding on cattle and pathogen species involveda
| Pathogens in mixed infections | No. (%) of: |
|
|---|---|---|
| Ticks feeding on cattle | Potentially infected cattle | |
| RAL + C. burnetii | 4 (2.9) | 4 (6.3) |
| A. marginale/centrale + T. mutans | 2 (1.5) | 2 (3.2) |
| A. marginale/centrale + T. mutans + C. burnetii | 1 (0.7) | 1 (1.6) |
| E. chaffeensis + C. burnetii | 1 (0.7) | 1 (1.6) |
| Ehrlichia sp. + C. burnetii | 1 (0.7) | 1 (1.6) |
| Ehrlichia sp. + RAL | 1 (0.7) | 1 (1.6) |
| Ehrlichia sp. + RAL + C. burnetii | 1 (0.7) | 1 (1.6) |
Ticks from vegetation were not found to harbor multiple pathogens.
Nucleotide sequence accession numbers.
Sequences are available at NCBI under accession numbers JN871727 to JN871848 for Rickettsia species, JN871849 to JN871863 for Coxiella burnetii, JN871864 to JN871869 for Anaplasma species, JN871870 to JN871872 for Borrelia species, JN871873 to JN871879 for Ehrlichia species, and JN871880 to JN871883 for Theileria mutans.
RESULTS
The 836 ticks analyzed comprised four species. The predominant species on cattle were Rhipicephalus (Boophilus) annulatus (37.5%, n = 51) and Amblyomma variegatum (33.8%, n = 46), followed by Hyalomma impeltatum (14.7%, n = 20) and Rhipicephalus evertsi (14%, n = 19). From the vegetation, only R. evertsi (n = 700) was collected. Mainly adult ticks were collected from both environmental sources (males, 45.1%; females, 53.5%; nymphs, 1.4%).
Anaplasmataceae.
Members of the Anaplasmataceae were detected in 11% (15/136) of ticks removed from cattle, with Anaplasma marginale subspecies being the most prevalent (53.3%; 8/15). Both Ehrlichia ewingii and Ehrlichia chaffeensis were detected in a single tick only (Fig. 1A). In five ticks, not clearly identifiable Ehrlichia species with 99% sequence homology to Ehrlichia ewingii (n = 3 ticks) or Ehrlichia ruminantium (n = 2 ticks) were found. All four tick species were found to harbor Anaplasmataceae (R. annulatus: A. marginale subspecies [n = 7] and E. ewingii [n = 1]; H. impeltatum: A. marginale subspecies [n = 1], E. chaffeensis [n = 1], and Ehrlichia sp. [n = 1], A. variegatum: Ehrlichia sp. [n = 1] and R. evertsi: Ehrlichia sp. [n = 3]). In total, 45.5% (5/11) of herds were infected with A. marginale subspecies, 45.5% (5/11) with Ehrlichia sp., 9.1% (1/11) with E. chaffeensis and 9.1% (1/11) with E. ewingii (Table 2). In most cases, only one tick from one cow per herd was infected, except for A. marginale subspecies.
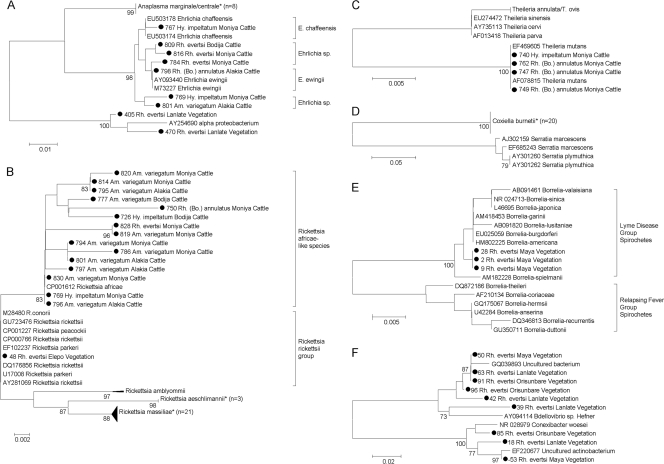
Fig 1.
Neighbor-joining phylogenetic trees based on a 263-nucleotide (nt) fragment of the 16S rRNA gene of Anaplasmataceae (nt 246466 to 246728 of CP001079.1) (A), a 339-nt fragment of the 17-kDa gene of Rickettsiaceae (nt 1194686 to 1195024 of CP000766.2) (B), a 226-nt fragment of the 18S rRNA gene of Piroplasmidae (nt 656 to 881 of HQ184411.1) (C), a 317-nt fragment of the htpB gene of Coxiella (nt 273435–273751 of CP000733.1) (D), a 323-nt fragment of the 16S rRNA gene of Borrelia species (nt 444099 to 443777 of CP002228.1) (E), and a 321-nt fragment of the 16S rRNA gene of Conexibacter woesei (nt 834 to 1151 of NR_028979.1) (F). Nigerian sequences are named with their unique identifier, tick species, geographic location, and biological source. Compressed clusters containing sequences from Nigeria are marked with an asterisk. Only bootstrap values above 70 are shown.
Table 2.
Herds, numbers of cattle and feeding ticks, and prevalence of pathogensa
| Herd | No. of ticks (no./cow) | No. of cattle |
A. marginale/centrale |
E. chaffeensis |
E. ewingii |
Ehrlichia sp. |
R. aeschlimannii |
RAL |
T. mutans |
C. burnetii |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | Inf. ticks (%) | Pot. Inf. cattle (%) | |||
| Alakia | 9 (1–2) | 8 | 1 (11.1) | 1 (12.5) | — | — | 1 (11.1) | 1 (12.5) | 1 (11.1) | 1 (12.5) | — | — | 3 (33.3) | 3 (37.5) | — | — | 2 (22.2) | 2 (25) |
| Aleshinloye | 1 (1) | 1 | — | — | — | — | — | — | — | — | 1 (100) | 1 (100) | — | — | — | — | — | — |
| Bodija 1 | 9 (1–2) | 7 | — | — | — | — | — | — | 1 (11.1) | 1 (14.3) | — | — | — | — | — | — | — | — |
| Bodija 2 | 20 (3–6) | 4 | — | — | — | — | — | — | — | — | — | — | 1 (5) | 1 (25) | — | — | 1 (5) | 1 (25) |
| Bodija 3 | 2 (1) | 2 | — | — | — | — | — | — | — | — | 1 (50) | 1 (50) | 1 (50) | 1 (50) | — | — | 1 (50) | 1 (50) |
| Moniya 1 | 20 (1–3) | 13 | 1 (5) | 1 (7.7) | — | — | — | — | 1 (5) | 1 (7.7) | 1 (5) | 1 (7.7) | 2 (10) | 2 (15.4) | — | — | 1 (5) | 1 (7.7) |
| Moniya 2 | 20 (1–3) | 13 | 1 (5) | 1 (7.7) | — | — | — | — | — | — | — | — | 4 (20) | 3 (23.1) | — | — | 3 (15) | 2 (15.4) |
| Moniya 3 | 45 (1–7) | 11 | 4 (8.9) | 2 (18.2) | 1 (2.2) | 1 (9.1) | — | — | 1 (2.2) | 1 (9.1) | — | — | 2 (4.4) | 2 (18.2) | 4 (8.9) | 3 (27.3) | 8 (17.8) | 8 (72.7) |
| Moniya 4 | 2 (2) | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Moniya 5 | 2 (2) | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Moniya 6 | 6 (3) | 2 | 1 (16.7) | 1 (50) | — | — | — | — | 1 (16.7) | 1 (50) | — | — | 1 (16.7) | 1 (50) | — | — | 3 (50) | 2 (100) |
| Total | 136 (1–7) | 63 | 8 (5.9)* | 6 (9.5) | 1 (0.7) | 1 (1.6) | 1 (0.7) | 1 (1.6) | 5 (3.7)* | 5 (7.9) | 3 (2.2)* | 3 (4.8) | 14 (10.3)* | 13 (20.6) | 4 (2.9)* | 3 (4.8) | 19 (14)* | 17 (27) |
Inf., infected; Pot., potentially; —, not detected (0%). Asterisks mark total prevalence rates in ticks which differ significantly from those observed in ticks from vegetation (P < 0.05).
Ticks from the vegetation were not found to be infected with Anaplasmataceae bacteria. However, two sequences with a highest nucleotide similarity of 99% to an uncultured alphaproteobacterium (GenBank accession number AY254690) were recovered from two questing R. evertsi ticks collected in Lanlate (Fig. 1A; Table 3).
Rickettsiaceae.
Rickettsia species were detected in 12.5% (17/136) of ticks from cattle and in 3.1% (22/700) of ticks from the vegetation. In feeding ticks, a Rickettsia africae-like species (RAL) was predominant (82.4%; 14/17), all sequences of which showed a nucleotide homology of 99 to 100% to those of Rickettsia africae. Rickettsia aeschlimannii was the second most predominant Rickettsia species (17.6%; 3/17). In at least one tick of each species, members of the Rickettsiaceae were detected: A. variegatum carried RAL (n = 10) and R. aeschlimannii (n = 1), R. annulatus carried RAL (n = 1) and R. aeschlimannii (n = 1), R. evertsi carried RAL (n = 1) and R. aeschlimannii (n = 1), and H. impeltatum carried RAL (n = 2). In 63.6% (7/11) of herds, RAL was detected in ticks. In most cases, more than one tick from more than one animal per herd, was positive and the estimated infection rate of cattle in positive herds ranged from 15.4 to 50% (Table 2). R. aeschlimannii was detected in 27.3% (3/11) of herds. Two nymph-stage A. variegatum ticks were infected with Rickettsia species, namely, RAL (8.3%; 1/12) and R. aeschlimannii (8.3%; 1/12).
Rickettsia massiliae was predominantly detected in questing R. evertsi ticks (3%; 21/700) from all locations (Table 3). In 0.1% (1/700) of questing ticks, a Rickettsia species belonging to the Rickettsia rickettsii group was detected (Fig. 1B; Table 3).
Piroplasmidae.
Theileria mutans was the only species of Piroplasmidae detected in ticks from Nigeria (Fig. 1C). It was exclusively detected in feeding ticks, and its prevalence ranged from 0.7% (1/136) in H. impeltatum to 2.2% (3/136) in R. annulatus. The infected ticks originated from three cattle of the same herd in Moniya (Table 2).
Coxiella burnetii.
In 14% (19/136) of feeding ticks, Coxiella burnetii was detected (Fig. 1D). Again, at least one tick of each species was found to harbor C. burnetii (A. variegatum, n = 9; R. annulatus, n = 5; H. impeltatum, n = 2; R. evertsi, n = 3). In total, 63.6% (7/11) of herds were infested with ticks positive for Coxiella and in most cases more than one animal per herd was involved (Table 2). Three A. variegatum nymphs from different cattle of the same herd in Moniya and one from Bodija were found to harbor C. burnetii (33.3%; 4/12). The only C. burnetii-infected questing tick (0.1%; 1/700) was collected in Orisunbare (Table 3).
Borrelia species.
Borrelia species were found only in questing R. evertsi ticks (0.4%; 3/700) collected in Maya (Table 3). Borrelia species identification was not possible with the sequence obtained from the 16S rRNA gene, which showed a nucleotide homology of 99% to members of the Borrelia burgdorferi sensu lato complex (Fig. 1E). Further characterization of the Borrelia species using primers directed against the flagellar gene was also unsuccessful. In addition, 16S rRNA sequences from unknown organisms were detected in nine DNA extracts from questing R. evertsi. Three of these sequences had the highest nucleotide similarity of 94 to 97% to the soil bacterium Conexibacter woesei; the remaining six sequences formed a separate cluster with 83 to 85% nucleotide homology to C. woesei (Fig. 1F).
Mixed infections.
All mixed infections (1.3%; 11/836) were detected in feeding ticks and were predominantly caused by RAL and C. burnetii (36.4%; 4/11) as well as T. mutans and A. marginale subspecies (18.2%; 2/11) (Table 4). Pathogen combinations found only once were E. chaffeensis plus C. burnetii, Ehrlichia sp. plus C. burnetii, and Ehrlichia sp. plus RAL. Two triple infections formed by C. burnetii, T. mutans, and A. marginale subspecies as well as C. burnetii, RAL, and Ehrlichia sp. were found. Tick species in which multiple infections were detected included A. variegatum (n = 5), H. impeltatum (n = 3), R. annulatus (n = 2), and R. evertsi (n = 1). Mixed infections occurred mainly in adult ticks (90.9%), but one nymph-stage A. variegatum tick was found to be coinfected with RAL and C. burnetii.
DISCUSSION
The tick species found in this study are known to commonly infest livestock in West African countries (38). The exclusive finding of R. evertsi from the vegetation can be explained by differences in life cycles and host-seeking behavior of the identified tick species. R. evertsi has a two-host life cycle, in which larval and adult instars display questing behavior (38). A. variegatum and H. impeltatum have a three-host cycle, in which all instars feed on different vertebrate hosts. Larval ticks of both species display questing behavior, whereas the adults are active hunters (38). R. annulatus is a one-host tick species, and only larval instars quest on vegetation. Since the collection of questing larval ticks by cloth dragging can be difficult, it is likely that even though other tick species than R. evertsi may have been present at the field collection sites, they were overlooked due to the limitations of the collection methods.
This is the first comprehensive study on the diversity of bacterial and protozoan tick-borne pathogens in both questing and feeding ticks not only in Nigeria but also in sub-Saharan Africa. All of the investigated pathogens are widespread throughout Africa and represent a threat to both human and animal health (4, 19–25, 27). As expected, the infection rate for most of the pathogens was significantly higher in feeding than in questing ticks (Table 2), suggesting that a number of these pathogens originated from the cow blood ingested before tick collection rather than from transstadially maintained infections acquired during earlier blood meals. Therefore, the detection of pathogens in feeding ticks cannot establish vector competence, whereas infected unfed ticks have at least maintained the pathogen transstadially. Although the latter are more likely to serve as potential vectors of live pathogens, in vitro experiments are required to confirm this. The fact that tick species diversity was much higher in feeding ticks may contribute significantly to the low prevalence rate of pathogens observed in questing ticks. It may well be that R. evertsi is a less suitable vector of pathogens than other tick species, although its vector competence has been reported for Babesia, Theileria, and Anaplasma species (38).
The only study on the prevalence of C. burnetii in ticks from Africa was conducted in Senegal, where 0.7 to 6.8% of feeding ticks from cattle were found to be infected (17). This rate is considerably lower than the 14% of feeding ticks that we found to be infected. Interestingly, C. burnetii was frequently detected in multiple ticks collected from the same cow. In addition, the infection rate of feeding R. evertsi was considerably higher (15.8%; 3/19) than in questing R. evertsi (0.1%; 1/700), and the infection rate of feeding nymph-stage A. variegatum ticks (33.3%; 4/12) was higher than that of adults of the same species (14.7%; 5/34). These observations and the difference between infection rates in questing and feeding ticks (0.1% versus 14%; P < 0.01) may be a reflection of the reservoir status of cattle for C. burnetii. Hence, in Nigeria, C. burnetii seems to represent a considerable risk factor for those in contact with cattle. In Senegal, where the prevalence of C. burnetii in cattle is relatively low (3.6%), seroprevalence rates in humans can be as high as 21.4 to 51% (12, 17), suggesting even higher prevalence rates in Nigeria, where an estimated 27% (17/63) of cattle were infected. Thus, both ticks and cattle must be considered a major source of Q fever and a significant threat to human health in the region.
Eight Anaplasma marginale/centrale-positive ticks were collected, and the estimated prevalence in cattle was 9.5%, which is higher than the prevalence found in bovine blood smears (1.9%) from Nigeria (11). Based on the detection PCR used in the present study, it was not possible to differentiate between the highly pathogenic bovine A. marginale sensu stricto and the naturally attenuated A. marginale subsp. centrale, which is sometimes used as a vaccine (7). As the cattle in this study were not vaccinated, they must have been naturally infected with either one of these subspecies, but the risk of severe disease cannot be estimated.
Theileria mutans, the causative agent of benign bovine theileriosis, was detected in four R. annulatus and H. impeltatum ticks removed from three cows in the same herd in Moniya. This pathogen was not detected in any questing R. evertsi ticks from the seven field locations, suggesting that this tick species may not be a competent vector or that T. mutans has a limited distribution in Nigeria. It seems that in Nigeria, the estimated prevalence rate in cattle (4.8%; 3/63) is comparable to that in bovine blood smears from within the country (3.3%) (11). Interestingly, it is much lower than that in Ghana, where 97% of cow blood smears were found to be positive for T. mutans (4). However, as in that study, all cattle came from an area within a 30-km radius, the result also reflects only a focal prevalence of T. mutans in a few herds.
Interestingly, Babesia species were not detected in any of the ticks analyzed, although reports on the seroprevalence of Babesia bigemina (29.4%) and Babesia bovis (14.1%) in Nigeria exist (3). However, that study was performed in northern Nigeria in the 1980s, suggesting geographical as well as temporal differences in the distribution of Babesia species.
SFG Rickettsia species have been reported in ticks from cattle in Mali (16.2%), Niger (16.3%), and Cameroon (74.7%) (21, 27). We detected R. massiliae and a member of the R. rickettsii group only in questing ticks (P < 0.05). This is compatible with the minor role of vertebrates in the perpetuation and survival of R. massiliae (15). In contrast, RAL and R. aeschlimannii were detected only in feeding ticks, indicating a potential role of cattle as hosts. The estimated prevalence rate of RAL in cattle from the same herd was high (15.4 to 50%), either supporting the suspected role of cattle as a reservoir or reflecting a high transovarial transmission rate of RAL in the local tick population. Further studies are warranted to unambiguously identify the Rickettsia species involved and clarify the life cycle of RAL.
Different Borrelia species have been found in Africa, most of which are transmitted by soft ticks (36). Ticks of the genus Rhipicephalus are known to transmit Borrelia theileri to cattle, causing bovine borreliosis. The 16S rRNA sequences of the Borrelia species detected in this study differed at least in three nucleotide positions from all known Borrelia sequences. These new sequences form a separate cluster within the Borrelia burgdorferi sensu lato group, possibly belonging to a so-far-unknown Borrelia species. Unfortunately, further characterization based on another gene was unsuccessful.
Several sequences from unknown bacteria were obtained in the Anaplasmataceae and Borrelia detection PCRs. As these sequences showed highest similarities to soil bacteria, they were most likely derived from bacteria from the outside rather than the inside of the ticks.
We also report here for the first time mixed infections in feeding ticks from western Africa involving mainly RAL and C. burnetii. Mixed infections involving C. burnetii may originate from subsequent blood meals, cofeeding events, or feeding on coinfected hosts. In mixed infections involving Rickettsia species, transovarial transmission may also play a role (15). Coinfections with multiple pathogens may complicate clinical diagnosis and treatment. In the Northern hemisphere, symptoms of Lyme borreliosis have been reported to be more diverse, intense, and persistent in patients coinfected with either human anaplasmosis or babesiosis (34). As treatment with an additional antimicrobial is necessary for a borreliosis-babesiosis coinfection, misdiagnosis may lead to prolonged illness of patients (34). For other tick-borne human pathogens, including spotted fever group rickettsiae and Coxiella burnetii, the impact of coinfections on the severity of symptoms has not yet been assessed. No such information exists on coinfections in cattle, although coinfections with severe symptoms have been reported in dogs (9, 33).
The diversity of tick-borne pathogens in Nigeria was higher in feeding than in questing ticks, suggesting that cattle serve as reservoirs for at least some of the pathogens studied, in particular Coxiella burnetii. Investigations on the implications for human and animal health as well as on the economic impact of these infections are warranted to determine the cost benefit of vaccination of ruminants against A. marginale marginale, C. burnetii, or ticks. Other preventive measures, such as removal of feeding ticks and the concerted use of acaricides, also need to be assessed.
ACKNOWLEDGMENTS
We thank the staff of the Department of Chemical Pathology and Immunology of the University of Ibadan and others for assisting in the tick collection, as well as livestock traders and abattoir staff for allowing access to cattle.
This work was funded by the Ministry of Cooperation of the Grand-Duchy of Luxembourg and the Centre de Recherche Public-Santé. A. L. Reye was supported by a fellowship from the Bourse Formation Recherche and the Aides à la Formation Recherche of the Fonds National de la Recherche Luxembourg.
Footnotes
Published ahead of print 10 February 2012
REFERENCES
- 1. Adakal H, et al. 2010. Clonal origin of emerging populations of Ehrlichia ruminantium in Burkina Faso. Infect. Genet. Evol. 10:903–912 [DOI] [PubMed] [Google Scholar]
- 2. Adakal H, et al. 2010. Efficiency of inactivated vaccines against heartwater in Burkina Faso: impact of Ehrlichia ruminantium genetic diversity. Vaccine 28:4573–4580 [DOI] [PubMed] [Google Scholar]
- 3. Ajayi SA, Dipeolu OO. 1986. Prevalence of Anaplasma marginale, Babesia bigemina and B. bovis in Nigerian cattle using serological methods. Vet. Parasitol. 22:147–149 [DOI] [PubMed] [Google Scholar]
- 4. Bell-Sakyi L, Koney EB, Dogbey O, Walker AR. 2004. Incidence and prevalence of tick-borne haemoparasites in domestic ruminants in Ghana. Vet. Parasitol. 124:25–42 [DOI] [PubMed] [Google Scholar]
- 5. Casati S, Sager H, Gern L, Piffaretti JC. 2006. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 13:65–70 [PubMed] [Google Scholar]
- 6. Clark K, Hendricks A, Burge D. 2005. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 71:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Wall DT. 2000. Anaplasmosis control and diagnosis in South Africa. Ann. N. Y. Acad. Sci. 916:474–483 [DOI] [PubMed] [Google Scholar]
- 8. Fournier PE, Xeridat B, Raoult D. 2003. Isolation of a Rickettsia related to Astrakhan fever Rickettsia from a patient in Chad. Ann. N. Y. Acad. Sci. 990:152–157 [DOI] [PubMed] [Google Scholar]
- 9. Gal A, et al. 2007. Coinfection with multiple tick-borne and intestinal parasites in a 6-week-old dog. Can. Vet. J. 48:619–622 [PMC free article] [PubMed] [Google Scholar]
- 10. Ishikura M, et al. 2003. Phylogenetic analysis of spotted fever group rickettsiae based on gltA, 17-kDa, and rOmpA genes amplified by nested PCR from ticks in Japan. Microbiol. Immunol. 47:823–832 [DOI] [PubMed] [Google Scholar]
- 11. Kamani J, et al. 2010. Prevalence and significance of haemoparasitic infections of cattle in north-central, Nigeria. Vet. World 3:445–448 [Google Scholar]
- 12. Kamga-Waladjo AR, et al. 2010. Seroprevalence of Neospora caninum antibodies and its consequences for reproductive parameters in dairy cows from Dakar-Senegal, West Africa. Trop. Anim. Health Prod. 42:953–959 [DOI] [PubMed] [Google Scholar]
- 13. Kumar S, Tamura K, Nei M. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150–163 [DOI] [PubMed] [Google Scholar]
- 14. Marconi RT, Garon CF. 1992. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J. Clin. Microbiol. 30:2830–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsumoto K, Ogawa M, Brouqui P, Raoult D, Parola P. 2005. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med. Vet. Entomol. 19:263–270 [DOI] [PubMed] [Google Scholar]
- 16. Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 4:e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mediannikov O, et al. 2010. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 4:e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mtshali MS, et al. 2007. Prevalence and genetic diversity of Anaplasma marginale strains in cattle in South Africa. Zoonoses Public Health 54:23–30 [DOI] [PubMed] [Google Scholar]
- 19. Mura A, et al. 2008. Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans. R. Soc. Trop. Med. Hyg. 102:945–949 [DOI] [PubMed] [Google Scholar]
- 20. Ndi C, Bayemi PH, Ekue FN, Tarounga B. 1991. Preliminary observations on ticks and tick-borne diseases in the north west province of Cameroon. I. Babesiosis and anaplasmosis. Rev. Elev. Med. Vet. Pays Trop. 44:263–265 [PubMed] [Google Scholar]
- 21. Ndip LM, et al. 2004. Detection of Rickettsia africae in patients and ticks along the coastal region of Cameroon. Am. J. Trop. Med. Hyg. 71:363–366 [PubMed] [Google Scholar]
- 22. Ndip LM, Labruna M, Ndip RN, Walker DH, McBride JW. 2009. Molecular and clinical evidence of Ehrlichia chaffeensis infection in Cameroonian patients with undifferentiated febrile illness. Ann. Trop. Med. Parasitol. 103:719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ndip LM, et al. 2005. Ehrlichial infection in Cameroonian canines by Ehrlichia canis and Ehrlichia ewingii. Vet. Microbiol. 111:59–66 [DOI] [PubMed] [Google Scholar]
- 24. Ndip LM, Ndip RN, Esemu SN, Walker DH, McBride JW. 2010. Predominance of Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks from kennel-confined dogs in Limbe, Cameroon. Exp. Appl. Acarol. 50:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ndip LM, et al. 2007. Ehrlichia species in Rhipicephalus sanguineus ticks in Cameroon. Vector Borne Zoonotic Dis. 7:221–227 [DOI] [PubMed] [Google Scholar]
- 26. Okuthe OS, Buyu GE. 2006. Prevalence and incidence of tick-borne diseases in smallholder farming systems in the western-Kenya highlands. Vet. Parasitol. 141:307–312 [DOI] [PubMed] [Google Scholar]
- 27. Parola P, Inokuma H, Camicas JL, Brouqui P, Raoult D. 2001. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7:1014–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajput ZI, Hu SH, Chen WJ, Arijo AG, Xiao CW. 2006. Importance of ticks and their chemical and immunological control in livestock. J. Zhejiang Univ. Sci. B 7:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rar VA, et al. 2005. Tickborne pathogen detection, Western Siberia, Russia. Emerg. Infect. Dis. 11:1708–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reye AL, Hubschen JM, Sausy A, Muller CP. 2010. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl. Environ. Microbiol. 76:2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simuunza M, Weir W, Courcier E, Tait A, Shiels B. 2011. Epidemiological analysis of tick-borne diseases in Zambia. Vet. Parasitol. 175:331–342 [DOI] [PubMed] [Google Scholar]
- 32. Stachurski F. 2000. Invasion of West African cattle by the tick Amblyomma variegatum. Med. Vet. Entomol. 14:391–399 [DOI] [PubMed] [Google Scholar]
- 33. Suksawat J, et al. 2001. Serologic and molecular evidence of coinfection with multiple vector-borne pathogens in dogs from Thailand. J. Vet. Intern. Med. 15:453–462 [DOI] [PubMed] [Google Scholar]
- 34. Swanson SJ, Neitzel D, Reed KD, Belongia EA. 2006. Coinfections acquired from Ixodes ticks. Clin. Microbiol. Rev. 19:708–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. To H, et al. 1996. Q fever pneumonia in children in Japan. J. Clin. Microbiol. 34:647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Dam AP, van Gool T, Wetsteyn JC, Dankert J. 1999. Tick-borne relapsing fever imported from West Africa: diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. J. Clin. Microbiol. 37:2027–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vesco U, et al. 2011. An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Exp. Appl. Acarol. 54:65–83 [DOI] [PubMed] [Google Scholar]
- 38. Walker AR, et al. 2003. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh, Scotland [Google Scholar]