Abstract
The Bacillus cereus sensu lato complex has recently been divided into several phylogenetic groups with clear differences in growth temperature range. However, only a few studies have investigated the actual pathogenic potential of the psychrotolerant strains of the B. cereus group at low temperature, and little information is available concerning gene expression at low temperature. We found that vegetative cells of the psychrotolerant B. weihenstephanensis strain KBAB4 were pathogenic against the model insect Galleria mellonella at 15°C but not at 30°C. A similar temperature-dependent difference also was observed for the supernatant, which was cytotoxic to Vero epithelial cell lines and to murine macrophage J774 cells at 15°C but not at 30°C. We therefore determined the effect of low temperature on the production of various proteins putatively involved in virulence using two-dimensional protein gel electrophoresis, and we showed that the production of the Hbl enterotoxin and of two proteases, NprB and NprP2, was greater at a growth temperature of 15°C than at 30°C. The quantification of the mRNA levels for these virulence genes by real-time quantitative PCR at both temperatures showed that there was also more mRNA present at 15°C than at 30°C. We also found that at 15°C, hbl mRNA levels were maximal in the mid- to late exponential growth phase. In conclusion, we found that the higher virulence of the B. cereus KBAB4 strain at low temperature was accompanied by higher levels of the production of various known PlcR-controlled virulence factors and by a higher transcriptional activity of the corresponding genes.
INTRODUCTION
The Bacillus cereus sensu lato complex, also known as the B. cereus group, is a subdivision of the Bacillus genus that includes B. cereus sensu stricto, B. thuringiensis, B. mycoides, B. pseudomycoides, B. anthracis, B. weihenstephanensis, and the recently proposed new species B. cytotoxicus. These bacteria are routinely isolated from various foods undergoing spoilage (rice, meat, eggs, and dairy products) and are of considerable importance in food microbiology (23, 40, 51). Many strains of the B. cereus sensu lato group are actually opportunistic pathogens that are able to colonize hosts as diverse as insects and mammals (5, 14, 15, 25). They can cause serious gastrointestinal diseases in humans (9, 36, 37, 52), and some strains are also responsible for severe nongastrointestinal infections, such as endophthalmitis and meningitis, particularly in immunocompromised patients and preterm neonates (28, 36, 37, 38).
The food-poisoning strains can cause two types of food-borne illness. The first is characterized by nausea and vomiting with abdominal cramps and has an incubation period of 1 to 6 h (36). This is the emetic form, caused by a small, preformed, cyclic peptide known as the emetic toxin (10, 26). The second type of illness consists principally of abdominal cramps and diarrhea after an incubation period of 8 to 16 h. This is the diarrheal form, which is thought to be mediated by the heat-labile diarrheagenic enterotoxin Nhe and/or the hemolytic enterotoxins Hbl and/or CytK (3, 4, 33, 35, 41). All three enterotoxins are cytotoxic and active against cell membranes, in which they make pores (12, 27). Nhe and Hbl each have three different protein subunits (33, 34) which act together, whereas the third enterotoxin, CytK, is a single-component protein (35). All the strains of the B. cereus sensu lato complex seem to carry genes encoding at least one of the known diarrheal toxins (2, 11, 24), and the expression of these genes is under the control of the PlcR transcriptional regulator, which is activated at the onset of the stationary phase of growth (1). Moreover, the strains of the B. cereus group are also known to synthesize several types of phospholipase C and various hemolysins, collagenases, and proteases (52), which may be implicated in B. cereus pathogenesis (13, 42, 44, 52, 57, 58). These extracellular membrane-active and tissue-degrading proteins constitute a significant proportion of the proteins secreted by B. cereus strains at the start of the stationary growth phase (20). They may, together with one or more of the more specific enterotoxins Hbl, Nhe, and CytK, contribute to the rapid course and severity of the infections caused by this bacterium.
The bacterial species B. weihenstephanensis (31), which includes most of the psychrotolerant strains of the B. cereus sensu lato group, or B. cereus phylogenetic group VI according to the new classification proposed by Guinebretière and collaborators (25), is widespread in nature and can contaminate many raw materials for food production and cold-stored foods (23). Moreover, the spores of these psychrotolerant B. cereus strains are sufficiently heat resistant to survive pasteurization, cooking, and most heat treatments apart from canning, allowing the bacterium subsequently to increase from initially low levels to very high levels at refrigeration temperatures (23, 29). This raises questions about the potential health risk posed by these bacteria when present in refrigerated food products, as both the emetic toxin and the diarrheagenic enterotoxins of food-borne pathogenic B. cereus identified to date are present in psychrotolerant strains (50, 51, 55). In a previous study, we found that psychrotolerant B. weihenstephanensis strains were less virulent in the insect model, G. mellonella, and induced less cytotoxicity in Vero cells at 37°C than B. cereus sensu stricto mesophilic strains (53). However, at 15°C the B. weihenstephanensis strains were as virulent and induced the same cytotoxic activity as B. cereus mesophilic strains. This suggests that virulence in the psychrotolerant B. weihenstephanensis species is, in part, determined by temperature. A reduced stability of the virulence factors of B. weihenstephanensis at the higher temperature could explain this different capacity to induce disease. Alternatively, differential gene expression of the major virulence factors in psychrotolerant strains as an adaptation to a different niche also could explain this temperature-dependent difference. This may have critical implications for the safety of refrigerated foods, and thus there is a need to have a better understanding of the production, both in quality and quantity, of known virulence factors of psychrotolerant B. weihenstephanensis strains at low temperature. The objective of this study is to examine how gene expression and the actual production and stability of active components putatively important for B. weihenstephanensis virulence were affected by temperature.
The complete genome sequence of strain KBAB4 has recently been determined (30). This strain is considered to be representative of the various independently isolated, moderately psychrotolerant B. weihenstephanensis strains (31, 49). Strain KBAB4 contains the two operons encoding the hemolytic (Hbl) and nonhemolytic (Nhe) enterotoxins, but it has no gene for CytK (30). It also carries a second operon encoding all three components of Nhe on a 400-kb plasmid, the first instance of a gene for this toxin being detected on a plasmid. The presence of these potential diarrheic toxin genes, with an additional copy on a plasmid, and the lack of knowledge about the effects of growth temperature on gene expression at low temperature led us to examine the pathogenic potential of the B. weihenstephanensis KBAB4 strain. We determined the effect of temperature on the production of various proteins putatively involved in virulence. We focused on the major known virulence factors, the Nhe and Hbl enterotoxins, and a range of other proteins that are potentially important as pathogenic determinants, such as collagenases, phospholipases, and extracellular proteases. We also analyzed differences in gene expression in strain KBAB4 at different temperatures, with the aim of identifying correlations between temperature, toxin production, and the virulence of strain KBAB4 against G. mellonella, a model insect, and its cytotoxicity to murine macrophages.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The fully sequenced psychrotrophic B. weihenstephanensis strain KBAB4 was used throughout this study. A stock culture of spores from strain KBAB4 was stored in sterile water at 4°C. The spores of strain KBAB4 were reactivated for reisolation by plating on LB agar plates for 16 h at 30°C. For each experiment, a single fresh colony of the microorganism was used to inoculate 100 ml of Luria broth (to give an initial optical density at 600 nm [OD600] of 0.05). Strain KBAB4 was grown at 15 and 30°C in 100-ml cultures in Erlenmeyer flasks with shaking at 175 rpm, and the culture was monitored for various times as indicated in the tables and figures.
Insects and experimental infections in vivo.
Pathogenicity assays were carried out with G. mellonella. This insect was used to assess differences in mortality between 15 and 30°C, because its larvae can survive at both of these temperatures, and its usefulness in comparing the virulence of B. weihenstephanensis strains at low temperature has recently been demonstrated (53). G. mellonella eggs were hatched at 25°C, and the larvae were reared on bee's wax and pollen (Naturalim). Trypsin-activated Cry1C toxin was prepared from the nonsporulating B. thuringiensis strain 407 transformed with pHT1C (45). Crystals were purified on a 67 to 72% sucrose gradient. They were then solubilized in 0.1 M NaCO3 carbonate buffer (pH 10.3), dialyzed against phosphate-buffered saline (pH 8.5), and activated by incubation with trypsin (2% [wt/wt] protein) for 3 h at 37°C. The use of Cry1C toxin in this model is necessary for killing by oral infection, because it helps in the degradation of a structure unique to invertebrates, the peritrophic matrix, which is a non-cell-semipermeable membrane (mainly composed of chitins and proteins) that lines the digestive tract and prevents the invasion of pathogenic microorganisms by forming a natural barrier (54, 60, 61). Cry1C thus helps the bacteria to gain access to the gut surface (C. Nielsen-Leroux, personal communication).
Oral infection tests following force feeding, in the presence of the synergizing toxin Cry1C, were performed with Galleria mellonella last-instar larvae weighing about 250 mg, which were reared at the INRA laboratory by free feeding on pollen and bee's wax at 25°C. The general protocols were described earlier (6). Briefly, last-instar G. mellonella larvae were force fed vegetative cell/Cry1C suspensions containing 3 × 106 to 4 × 106 bacterial cells in mid-exponential growth phase and 3 μg of Cry1C (15, 44) in phosphate-buffered saline (PBS; 10 μl per larva) via a 0.5- by 25-mm ground hypodermic needle (Burckard Manufacturing) and a microinjector (Burckard Scientific). The larvae in the control groups were fed PBS or the Cry1C toxin alone in PBS buffer. Bacterial suspensions used for infection experiments were quantified by plate counting for each experiment to confirm the dose calculated from OD600 measurements before infection. We used 20 larvae for each toxicity assay. Infected larvae were kept at 15 and 30°C in petri dishes (5 larvae per 5-cm petri dish without food), and mortality was recorded each day for the 8 days following infection. All experiments were performed in triplicate. Bacterial growth was monitored simultaneously with additional batches of inoculated larvae. Immediately after inoculation and at 24-h intervals thereafter, we recovered the bacteria from infected live larvae. Two larvae from each batch were surface sterilized by rinsing briefly in 70% ethanol, crushed, and homogenized in 10 ml of PBS with a Polytron homogenizer (Kinematica, Swizerland). Serial dilutions were plated on LB medium.
Assay of the effect of B. cereus supernatants on cell membrane permeability.
Murine macrophage J774 cells were used to assess the toxicity of B. weihenstephanensis KBAB4 supernatants obtained from bacterial cultures grown at different temperatures. The J774 cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin (VWR). The cells were cultured at 37°C in a humid atmosphere containing 5% CO2. The day before use, cells were detached by gentle scraping and used to seed a multiwell plate. For the cytotoxicity assays, J774 macrophages (105 cells/well) were incubated at 37°C for 2 h with or without cell-free supernatants taken at various OD values from the B. weihenstephanensis KBAB4 strain at a concentration of 30% (vol/vol) medium/cell-free supernatant in serum-free medium. We then added trypan blue dye to the cells. Nonpermeabilized (viable) cells remained unstained, whereas permeabilized (killed) cells were stained blue because the dye was able to enter the cytoplasm. At least 300 cells were counted by eye, and cytotoxicity was scored as the percentage of cells stained blue (42, 58, 59). The results presented are the mean values from three independent experiments.
Proteome analysis and the detection and quantification of spots.
The KBAB4 strain was used for proteomic studies. It was grown in 100 ml of LB broth in a 1-liter flask, at 15 or 30°C, on a rotary shaker operating at 175 rpm. Cultures were inoculated to an OD600 of 0.1 with vegetative cells in LB broth, and the culture was stopped when the OD600 was about 5.4. At 30°C, this corresponded to a time point of 2 h after the onset of stationary phase, defined as the breakpoint of the slope of the vegetative-phase growth curve. The bacterial cells were harvested and the culture supernatants were centrifuged at 4,300 × g for 20 min at 4°C (Sigma 3K10 centrifuge). The resulting supernatant was collected and filtered through a membrane (with 0.2-μm pores). The proteins present in the supernatant then were precipitated twice by the deoxycholate-tetrachloroacetic acid method (19, 20). The pellet was washed twice with ethanol-ether (1:1), dried, and stored at −20°C until use. The protein content of the pellet was determined by the Bradford method. Protein pellets were resuspended in sample buffer as previously described (19). The proteins were initially separated by isoelectric focusing (IEF) and then were subjected to SDS-PAGE in a 10 to 15% gradient gel for the second dimension. Gels were silver stained for the detection and quantification of spots or stained with CBB G250 (43) for protein identification. Three independent cultures were grown at each temperature for spot quantification, and a single culture was used to identify the spots. Gel analysis, spot detection, and quantification were carried out as previously described (19).
RNA extraction.
RNA was extracted from strain KBAB4 grown at 15 or 30°C and harvested in mid-exponential growth phase or at the beginning of stationary phase (OD600 = 2.2 and 5.4, respectively). We transferred 2 ml of each culture to an Eppendorf tube and collected the cells by centrifugation at 8,000 × g at 4°C for 30 s (Eppendorf 5804 R centrifuge; Eppendorf, Germany). The supernatant was decanted off, and the pellet was snap-frozen in liquid nitrogen and then stored at −80°C for further processing. Cell pellets were thawed and resuspended in 1 ml of phenol-based RNA extraction buffer (TRI reagent; Applied Biosystems/Ambion Courtaboeuf, France). The resuspended cell pellets were disrupted by bead beating twice for 45 s at 6.5 m/s (Mini 0.1-mm zirconia/silica beads; Biospec Products) with a Fastprep 24 (MP Biomedicals), with the samples kept in glass tubes between the two rounds of beating. The supernatant then was transferred to an Eppendorf tube containing 100 μl BromoChloroPropane (Sigma) and centrifuged at 8,000 × g for 15 min at 4°C. The RNA present in the aqueous phase then was isolated according to the TRI reagent solution protocol (Applied Biosystems/Ambion) for RNA extraction; the RNeasy minikit (Qiagen) was used for RNA precipitation and solubilization, and the Turbo DNA-free kit (Ambion) was used to remove chromosomal DNA contamination as previously described (8).
RNA quality control and cDNA synthesis.
The purity of RNA was assessed by determining the ratio of absorbance at 260 and 280 nm for protein contamination and the ratio of absorbance at 260 and 230 nm for solvent contamination. All RNA samples had an A260/A230 ratio of between 2 and 2.2 and an A260/A280 ratio of between 1.9 and 2.2, indicating a high degree of RNA purity. RNA integrity was further assessed by determining the RNA integrity number (RIN) with the Agilent 6000 Nano kit on a Bioanalyzer 2100 (Stratagene, Agilent Technologies, France). The RIN algorithm provides an estimate of the total RNA ratio (ratio of the area of ribosomal bands to the total area of the electropherogram) and the height of the 16S and 23S peaks (46). The RIN was between 9.7 and 10 for the cultures, indicating RNA samples of very high quality (16). We synthesized cDNA with the AffinityScript quantitative PCR (qPCR) cDNA synthesis kit (Stratagene, Agilent Technologies, France), which was used as recommended by the manufacturer with 1 μg of total RNA and random primers. Quality was checked with the Agilent 6000 Pico kit and an Agilent Bioanalyzer 2100 (Agilent Technologies, France).
Relative quantification of putative virulence gene expression by real-time qPCR.
We performed qPCR on an ABI Prism 7900HT (Applied Biosystems) with SYBR green chemistry. The whole experiment (from strain culture to qPCR) was performed twice, with two technical replicates for each growth condition. The primer pairs used for qPCR were designed with Primer Express software, version 2.0 (Applied Biosystems, Foster City, CA), to amplify specific gene fragments of 79 to 176 bp in size and are listed in Table 1. Primers were purchased from MWG Biotech (France). The efficiency of the primer pairs was validated with a standard curve with four points corresponding to serial dilutions of a cDNA pool (7) spanning from 0.002 to 2 ng cDNA. The regression curves for plcR, hblC, nheAchr, nheBchr, nheAplasmid, plcB, nprP2, nprB, tpi, and rpoB had slopes of −3.4, −3.344, −3.66, −3.63, −3.306, −3.41, −3.43, −3.37, −3.609, and −3.565, respectively, with an r2 of ≥0.99, corresponding to amplification efficiencies of 0.97, 0.99, 0.88, 0.88, 1.00, 0.96, 0.95, 0.97, 0.896, and 0.91, respectively (nheAchr refers to the chromosomal copy of the nheA gene, whereas nheAplasmid refers to an additional copy of the nheA gene present on a plasmid). In the absence of reverse transcription, PCR on RNA samples yielded no significant amplicon, indicating that DNA contamination was negligible. qPCRs and cycling conditions were as recommended by the manufacturer, and amplification was carried out on an ABI Prism 7900 (Applied Biosystems). The final reaction volume for PCR was 20 μl, including 10 μl of SYBR green PCR master mix (Applied Biosystems, Courtaboeuf, France), 300 nM each primer, and 1 ng of cDNA in 5 μl. The thermal profile was 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The specificity of each amplified qPCR product was checked by dissociation curve analysis. All qPCRs were performed in duplicate, and the mean value for the two experiments carried out in duplicate was calculated. Relative expression (or expression ratio) between 15 and 30°C was calculated for each gene by the comparative threshold cycle (ΔΔCT) method using the Relative Expression Software Tool (REST; 2009 V2.0.13; Qiagen). Expression ratios were normalized against two endogenous reference housekeeping genes, rpoB and tpi, whose expression levels did not differ between samples.
Table 1.
Primers used in this studya
| Gene | Gene no. | Size (bp) | Primer name | Primer sequence | Primer locationb |
|---|---|---|---|---|---|
| plcR | BcerKBAB4_5148 | 864 | PlcR_KBAB4 F | CAACTCTCCGATAATGTATGTCATCA | +67 to +93 |
| PlcR_KBAB4 R | CCGCGCCCGATTCA | +124 to +110 | |||
| hblC | BcerKBAB4_2332 | 1,320 | HblC_KBAB4 F | GCTGCTCAAACAGCGGTAG | +718 to +737 |
| HblC_KBAB4 R | CCCTTTTCTGTTGCTTCCTG | +818 to +798 | |||
| nheB | BcerKBAB4_1741 | 1,161 | NheBchr_KBAB4 F | AAGACTTTAATTACAGGGTTATTGGTTACA | +7 to +37 |
| NheBchr_KBAB4 R | TCTGTTTGCCCCTCCTTAGC | +95 to +75 | |||
| nheAchr | BcerKBAB4_1742 | 1,209 | NheAchr_KBAB4 F | TTCATCAGTTGCGACAGCTC | +713 to +733 |
| NheAchr_KBAB4 R | CGATTCCAGCTGTTCCACCAC | +853 to +832 | |||
| nheAplasmid | BcerKBAB4_5600 | 1,155 | NheAplasm KBAB4 F | CAACTGCTCTCGGTGCATAC | +715 to +735 |
| NheAplasm KBAB4 R | CTTTATCTGCTGCTGCGATGTAG | +825 to +802 | |||
| plcB | BcerKBAB4_0591 | 852 | PC-PLC_KBAB4 F | CGAAAGATTGGTTCGTGAAAGC | +698 to +720 |
| PC-PLC_KBAB4 R | AACTTCAGCGCGCCATTTAT | +762 to +742 | |||
| nprB | BcerKBAB4_5149 | 1,761 | NprB_KBAB4 F | TGCTAGCGTCCACGGATT | +1041 to +1059 |
| NprB_KBAB4 R | CCATTTAGCCCGTCACCA | +1157 to +1139 | |||
| nprP2 | BcerKBAB4_2415 | 1,704 | NprP2_KBAB4 F | GTGGCACCATACGGAATAGG | +61 to +81 |
| NprP2_KBAB4 R | TTCACCTGAACGGAATGAGTC | +144 to +123 | |||
| rpoB | BcerKBAB4_0097 | 3,534 | RpoB_KBAB4 F | TCAGTGGTTTCTTGATGAGG | +110 to +130 |
| RpoB_KBAB4 R | CTTTTACACGAAGTGGTGCT | +286 to +266 | |||
| tpi | BcerKBAB4_4929 | 756 | Tpi_KBAB4 F | ACGGCGGTAGCGTAAAAC | +628 to +647 |
| Tpi_KBAB4 R | CCCCAGAAGACCTAAGAACGA | +744 to +723 |
Primer pairs were designed with Primer Express Software v2.0 (Applied Biosystems).
Locations are in reference to the translational start codon of the gene as position +1.
Statistical analysis.
We assessed the significance of the differences in relative expression levels between 15 and 30°C for each gene of interest by a randomized test implemented in REST (2009 V2.0.13; Qiagen). This test was based on the pair-wise fixed-reallocation randomization test (39).
RESULTS
The virulence of strain KBAB4 is higher at low temperature in various toxicity models. (i) Virulence against a model insect, G. mellonella.
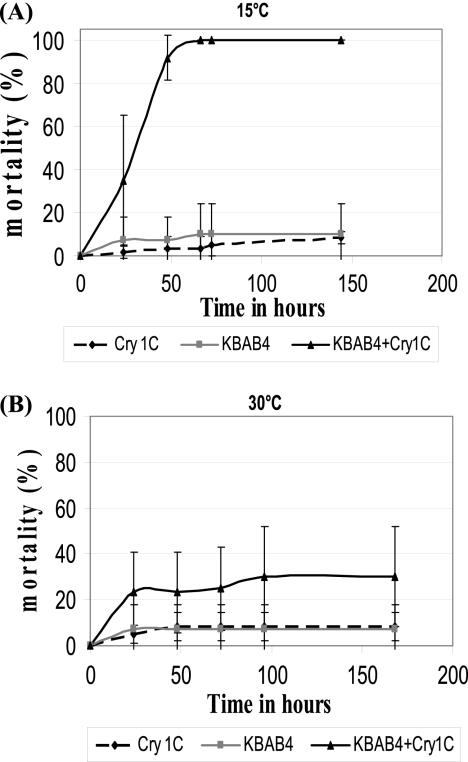
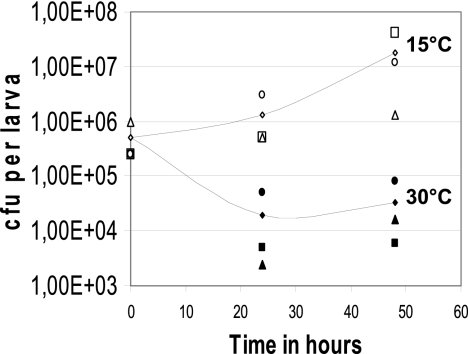
The virulence of strain KBAB4 against Galleria mellonella was investigated by the oral force feeding of groups of 20 fifth-instar G. mellonella larvae with 2 × 106 to 3 × 106 bacterial vegetative cells followed by the incubation of the larvae at two temperatures, 15 and 30°C. The in vivo virulence of strain KBAB4 in G. mellonella larvae significantly differed between 15 and 30°C (Fig. 1): at 15°C, strain KBAB4 caused 100% mortality 72 h after infection but caused only 25% mortality 96 h after infection at 30°C (P < 0.01). We also compared the bacterial growth in the larvae at 15 and 30°C after the insects were force fed about 5 × 105 bacterial vegetative cells. At 15°C the bacterial population increased by about 1 log, to reach a density of 2 × 107 CFU/larva after 48 h. In contrast, at 30°C the bacterial population decreased in size by about 1 log after 24 h and then remained stable at about 3 × 104 CFU/larva throughout the 48-h assay (Fig. 2). This indicates that the lower level of virulence of strain KBAB4 observed in vivo at 30°C is accompanied by a lower capability to persist in the insect environment at this temperature. It also suggests that this lower level of virulence is due at least in part to differences in the survival and/or in the potential of the psychrotolerant strain to colonize the larval intestine at 30°C.
Fig 1.
In vivo virulence of strain KBAB4 against G. mellonella. Last-instar G. mellonella larvae weighing about 250 mg were force-fed Cry1C alone (3 μg), 4 × 106 mid-exponential-growth-phase KBAB4 cells alone, or a mixture of 3 × 106 to 4 × 106 mid-exponential-growth-phase bacteria and 3 μg of Cry1C in PBS. Cry1C alone and KBAB4 alone caused only low levels of mortality. Infected larvae were kept at 30 (A) or 15°C (B) in individual petri dishes, and mortality was recorded each day on the 8 days following infection.
Fig 2.
In vivo bacterial growth monitoring of strain KBAB4 at 15 and 30°C. The bacterial growth of strain KBAB4 in Galleria mellonella last-instar larvae, at 15 and 30°C, after the insects were force fed vegetative cells was monitored. The bacteria were recovered from infected live larvae after inoculation and at 24-h intervals thereafter. White symbols, 15°C; black symbols, 30°C. Different symbols (circles, squares, and triangles) indicate independent experiments. For each independent experiment, two larvae from each batch were surface sterilized by rinsing briefly in 70% ethanol, crushed, and homogenized in 10 ml of PBS. Serial dilutions were plated on LB medium. Values are given as CFU/larva. The curves are the means from three independent experiments.
(ii) Cell membrane permeability assay.
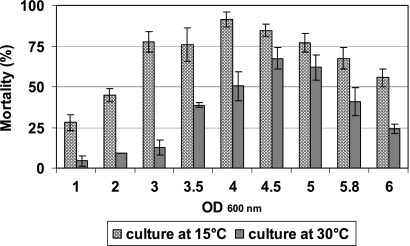
The cytotoxic potential of bacterial cell-free culture supernatants of strain KBAB4, taken at various time points during bacterial growth at 15 and 30°C, was analyzed with J774 murine macrophages by the trypan blue dye exclusion method as previously described (57, 58). Cytotoxicity was recorded 2 h after incubation as the percentage of cells permeable to the blue dye. A similar temperature-dependent difference was seen in the J774 cell culture cytotoxicity assay, in which the supernatants collected during the exponential growth phase at 15°C were highly toxic, whereas those collected at 30°C displayed low levels of cytotoxicity. At both temperatures, supernatant cytotoxicity progressively increased during the growth cycle (Fig. 3), peaking at the start of the stationary phase, together with the expression of PlcR-controlled genes (19, 21). Interestingly, the cytotoxicity of the supernatants of cultures grown at 15°C was moderate (50% at an OD600 of 2) during midexponential growth and high (75% at an OD600 of 3) during the late exponential phase. In contrast, at 30°C no such cytotoxic activity was observed during the exponential growth phase (less than 15% at an OD600 of 1, 2, or 3) (Fig. 3). During early stationary phase (OD600 of 4 or 4.5), the cytotoxicity of the supernatant also was significantly higher at 15°C than that at 30°C (90 versus 65%). Overall, the supernatant of strain KBAB4 was significantly less toxic for cultures grown at 30°C than for cultures grown at 15°C.
Fig 3.
Effect of strain KBAB4 supernatant on cell membrane permeability. J774 murine macrophages (1 × 105) cultured in RPMI medium were incubated with bacterial cell-free supernatants of strain KBAB4 collected at various points in the growth cycle (OD600) at 15 and 30°C. After 2 h of incubation, cell mortality was evaluated by trypan blue dye exclusion. At least 300 cells were counted visually, and the percentage of blue cells was determined for the assessment of cytotoxicity. Results are mean values from three independent experiments. For each OD600 value, the cytotoxicity was significantly higher when the culture was grown at 15°C (P < 0.05). Noninfected cells or cells incubated with water gave less than 5% mortality.
Major extracellular PlcR-controlled virulence factors display differential production at 15 and 30°C.
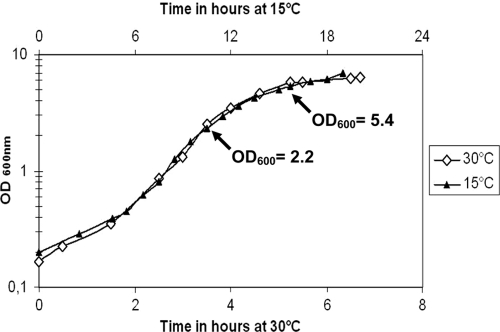
Another powerful approach to display the bacterial proteins expressed under particular conditions and for studying the relative production levels is the use of two-dimensional protein gel electrophoresis (2DE). We first checked the growth kinetics at both temperatures and found that the generation time was three times longer for cultures at 15°C than for cultures at 30°C (Fig. 4). Thus, for accurate comparison at both temperatures, the supernatants were collected when the strain had reached identical bacterial densities rather than after a given time of culture. The extracellular proteomes of strain KBAB4 grown in LB medium at either 15 or 30°C were harvested at an OD600 of 5.4. Indeed, it is known that the key virulence factors, such as toxins, phospholipases, and degradative enzymes, thought to be involved in the pathogenic properties of the members of the B. cereus group are produced during early stationary phase (1, 19, 21). This optical density was reached 2 h after the onset of the stationary phase for cultures grown at 30°C and about 7 to 8 h after the onset of the stationary phase when the KBAB4 strain was grown at 15°C. The qualitative analysis by 2DE of the extracellular proteome of three independent cultures of strain KBAB4 sampled at the same bacterial density indicated similar levels of extracellular virulence factor during early stationary phase for the three replicates under standardized conditions at 15 and 30°C. Some of the large extracellular protein spots were similar at both temperatures, whereas others were specifically affected (Table 2). The PlcR-dependent proteins produced in similar amounts in all experimental conditions included the collagenase ColB and the phospholipase PC-PLC. The two lytic factors of the Nhe complex, NheA and NheB, were also found to be present in similar amounts at both temperatures (Table 2). The NheA component of the Nhe enterotoxin also was detected by Western blotting, at both 15 and 30°C, using monoclonal antibodies (data not shown). Several differences also were found between the supernatants of cultures grown at 15 and 30°C. The protein spot identified as the lytic component of the Hbl enterotoxin complex (HblC) was consistently more intense at 15°C than at 30°C (Table 2). The L2 component of Hbl was also detected at both 15 and 30°C with the antibody-based Oxoid kit but with positive reactions obtained at greater dilutions when the culture was grown at 15°C (data not shown). Moreover, the spots corresponding to two PlcR-controlled proteases, NprB and NprP2, were found to be specifically present at 15°C but not at 30°C. These findings suggest that temperature affects the production of various known extracellular virulence factors.
Fig 4.
Growth curves of B. weihenstephanensis strain KBAB4 in LB medium at 15 and 30°C. Arrows indicate the OD600 at which the cultures or supernatants were harvested, before and after the onset of stationary phase, for qPCR. The onset of stationary phase was defined as the breakpoint in the slope of the vegetative-phase growth curve. The time interval between seeding and the onset of the stationary phase was about 4.5 h at 15°C and 14 h at 30°C.
Table 2.
Comparative analysis of the levels of the major PlcR-controlled virulence proteins identified on the 2DE gelsa
| Protein | Function | Gene no. | Production atb: |
Localization | |
|---|---|---|---|---|---|
| 30°C | 15°C | ||||
| ColB | Collagenase | Bcer KBAB4_0468 | 1.90 ± 0.15 | 1.92 ± 0.07 | Extracellular |
| NheB | Nonhemolytic enterotoxin lytic component L2 | Bcer KBAB4_1741 | 2.06 ± 0.50 | 2.34 ± 1.51 | Extracellular |
| NheA | Nonhemolytic enterotoxin lytic component L1 | Bcer KBAB4_1742 | 5.35 ± 0.73 | 4.46 ± 0.45 | Extracellular |
| PC-PLC | Phospholipase C | Bcer KBAB4_0591 | 3.84 ± 0.42 | 4.10 ± 1.17 | Extracellular |
| HblL2 | Hemolysin BL lytic component L2 | Bcer KBAB4_2332 | 0.66 ± 0.15 | 3.19 ± 1.04 | Extracellular |
| NprP2 | Bacillolysin | Bcer KBAB4_2415 | Absent | 1.75 ± 0.22 | Extracellular |
| NprB | Bacillolysin | Bcer KBAB4_5149 | Absent | 0.85 ± 0.13 | Extracellular |
The culture supernatants from three independent cultures grown at 15 or 30°C were harvested in early stationary phase at an OD600 of 5.4 either 2 h after entry into the stationary phase at 30°C or 6 h after the onset of the stationary phase at 15°C. Twenty μg of protein was loaded onto IEF strips in a linear range (pH 4 to 7), and the supernatants were separated by 2DE as described in Materials and Methods.
The spots were quantified after normalization, and spot volumes (pixel intensity × area) are expressed as a percentage of the total volume of the spots on the gel as described in Gohar et al. (20). Data are averages ± standard errors of the means. Significant differences between samples grown at 15 and 30°C are indicated in boldface.
The expression of various virulence genes in strain KBAB4 is temperature dependent.
We investigated whether the higher levels of these virulence factors at 15°C were the result of transcriptional regulation by quantifying the amounts of mRNA for these proteins by real-time qPCR. The qPCR experiments were performed on reverse-transcribed RNA from cultures grown at 15 and 30°C. The CT value was used for the relative quantification of gene expression between temperatures. In the qPCR experiment, CT was correlated with the sensitivity of the PCR experiment and inversely proportional to the original relative expression level of the gene of interest. The expression ratio for each selected gene was assessed after normalization with two housekeeping genes, tpi and rpoB, which were used for standardization, because these two housekeeping genes were stably expressed regardless of the growth temperature (Table 3) and because their expression was in the same range as that of our genes of interest. In addition, as gene expression levels may also vary during infection and have different levels in different growth phases (i.e., stationary versus exponential), the KBAB4 strain was sampled during both the exponential and stationary phases (at an OD600 of 2.2 and 5.4, respectively) after growth in LB medium at 15 and 30°C (Fig. 4) to target the specific gene transcripts produced at these two points in the growth cycle. Table 3 shows the relative expression values calculated from two independent experiments performed in duplicate for each condition and for each gene. Clear differences in expression profile were observed for plcR, nprP2, nprB, hblC, and nheAplasmid at 15 and 30°C (P < 0.005) during both vegetative growth and early stationary phase (Table 3). The expression level of plcR was significantly higher at 15°C than at 30°C by a mean factor of about 25 (P < 0.01) during exponential growth (OD600 of 2.2) and of 148 (P < 0.023) during stationary phase (OD600 of 5.4). The levels of expression of the two protease-encoding genes, nprP2 and nprB, were also significantly higher, differing between the two temperatures by factors of 350 and 440, respectively, during exponential growth (P < 0.005) and by a factor of >1,000 (P < 0.019 and P < 0.012) during stationary phase. The level of expression of hblC was 1,600 times higher (P < 0.003) at 15°C than at 30°C during exponential growth but only 13 times higher (P < 0.012) after the culture entered stationary phase. The additional copy of the nhe gene present on a plasmid (nheAplasmid) also was more strongly expressed at 15°C than at 30°C by a mean factor of 38 (P < 0.003) during exponential growth. This gene was also more strongly expressed at 15°C during early stationary phase but only by a mean factor of 8.5 (P < 0.021). Thus, the expression of various virulence factors is induced in strain KBAB4 during growth at low temperature. In contrast, the other major PlcR-controlled genes analyzed during this study were stably expressed regardless of growth temperature. The expression of the nheAchr and plcB genes was higher by a factor of 3.6 and 3.4, respectively, at 15°C than at 30°C (Table 3), but this difference was significant only during exponential growth (P < 0.005), with no such effect being observed during stationary phase (P > 0.07 and P > 0.744, respectively), and the expression of nheBchr did not differ significantly between the two time points in the growth cycle tested (P > 0.083 and P > 0.425, respectively).
Table 3.
Effects of temperature on the relative expression levels of various virulence genesa
| Gene | Growth by phase |
|||
|---|---|---|---|---|
| Exponential |
Stationary |
|||
| Expression ratio | P value | Expression ratio | P value | |
| plcR | 25 | 0.010 | 148 | 0.023 |
| hblC | 1603 | 0.009 | 13 | 0.014 |
| nheBchr | 1.6 | 0.083 | 0.6 | 0.425 |
| nheAchr | 3.6 | 0.005 | 3.3 | 0.070 |
| nheAplasmid | 38 | 0.002 | 8.5 | 0.024 |
| plcB | 3.4 | < 0.001 | 0.9 | 0.744 |
| nprB | 442 | 0.007 | 1066 | 0.015 |
| nprP2 | 358 | < 0.001 | 1003 | 0.026 |
| rpoB | 1.1 | 0.9 | ||
| tpi | 0.9 | 1.1 | ||
The data shown are the relative expression levels obtained by qPCR in samples from cultures grown at 15 versus 30°C during the exponential growth phase or stationary phase along with two endogenous control genes. Values are given as relative expression ratios and as the means from four samples from two separate biologically independent experiments. The P value obtained with REST is the probability of the alternative hypothesis that the difference between the sample and control groups is due only to chance.
DISCUSSION
It has often been suggested that the virulence of the B. cereus group is multifactorial and dependent on the extracellular release of toxins and degradative enzymes targeting the host (1, 2, 18, 21, 52). It has also been suggested that the level of expression of the genes encoding these virulence factors accounts for most of the pathogenic potential of a given strain (17, 38, 56). In this study, we have investigated various aspects of the biological activity and production of major putative secreted virulence factors in the psychrotolerant B. weihenstephanensis strain KBAB4 as a function of growth temperature. Vegetative cells of strain KBAB4 were pathogenic against G. mellonella at 15°C but not at 30°C (Fig. 1). A similar temperature-dependent difference was seen in the J774 murine macrophage cell cytotoxicity assay, and this effect was much stronger during the exponential growth phase (Fig. 3). These observations are in agreement with our earlier findings that growth temperature affects the virulence of the psychrotolerant members of the B. cereus group (53). This differential cytotoxicity pattern cannot be attributed to the production of emetic toxins at low temperature, as strain KBAB4 was negative for the presence of the ces genes that are responsible for the synthesis of cereulide, and the KBAB4 strain does not produce the HlyII hemolysin (gene frameshift), which has been shown to be responsible for macrophage death in other B. cereus strains (58). In the case of strain KBAB4, these differences in pathogenicity and toxicity were accompanied by differences in the amounts of Hbl enterotoxin and of two other extracellular putative virulence factors, the extracellular proteases NprP2 and NprB. These data suggest a role for the Hbl enterotoxin complex and for the two extracellular proteases, NprP2 and NprB, in the higher levels of virulence and cytotoxicity observed at 15°C. The Hbl enterotoxin, in particular, has already been described as being involved in cytotoxicity to epithelial cells (3, 22, 34, 52) and could be responsible for the higher levels of cytotoxicity observed at 15°C. It is also possible that all three components of the toxin are not produced to adequate levels at 30°C because of a lower stability of one of the proteins of the complex or a higher sensitivity to proteases at 30°C. It is also notable that NprP2 and NprB are orthologs of the Staphylococcus aureus zinc metalloprotease aureolysin. Aureolysin production by S. aureus contributes to its resistance to the innate immune system by the cleavage of the human antimicrobial peptide LL-37 (47). In insects, bacteria belonging to group B. cereus are also exposed to high concentrations of antimicrobial peptides, major effectors of the innate immune response that are essential for the elimination of pathogens (32). Therefore, the lower level of infectiousness of strain KBAB4 observed in vivo at 30°C (Fig. 2) may well be related to the absence of the NprP2 and NprB proteases at this temperature (Table 2), resulting in a lessened ability to overcome host defenses. However, the virulence of strain KBAB4 also depends on host immunological conditions, and some of the differences in pathogenicity observed could be due in part to host-specific responses to growth temperature rather than bacterium-specific responses.
PlcR-controlled virulence factors are known to accumulate only during stationary phase, when high bacterial densities are reached (1, 21, 48), but these findings were obtained exclusively for bacterial cultures grown between 30 and 37°C and for mesophilic strains with an optimum growth temperature of 30 to 37°C. To our knowledge, no data are available for the strains of the B. cereus group adapted to growth at lower temperatures, such as strain KBAB4. We found high mRNA levels for various PlcR-controlled genes during the mid-exponential growth phase when the KBAB4 strain was grown at 15°C, indicating that these genes also were expressed during exponential phase. Our results also indicate that, at 15°C, PlcR is expressed during late exponential growth, and that its level of expression is also significantly higher at 15°C than at 30°C after the culture entered stationary phase. Taken together, our results suggest that temperature somehow exerts an effect on the regulation of plcR and that a higher expression of PlcR is required to ensure the correct adaptation of the bacterium to the temperatures encountered in the environment. Indeed, in the environment, these bacteria are continuously subjected to temperature variations, and temperature is probably an important parameter that can affect gene expression and modify their physiology. However, in our conditions, only the amounts of HblC, NprP2, and NprB increased at 15°C, whereas the amounts of ColB, NheA, and/or NHeB and PC-PLC did not differ significantly between 15 and 30°C (Table 2). Therefore, the higher expression of PlcR at 15°C did not result in a higher expression of these PlcR-controlled genes. It is possible that, at 15°C, hblC, nprP2, and nprB are efficiently transcribed only above a threshold level of PlcR, whereas the other plcR-controlled genes, whose expression is not strongly affected by temperature, have a lower threshold. However, we cannot exclude that other regulatory mechanisms that are not active when the bacterium is grown at 30°C are directly responsible for the higher expression of hblC, nprP2, and nprB at 15°C. Whether other PlcR-controlled virulence genes are also differently regulated by PlcR at low temperature or in different environmental or growth conditions, and how this may have an effect on the virulence, certainly deserves further investigation.
The higher levels of mRNA transcripts for plcR, hblC, nprP2, and nprB at 15°C (Table 3) may result from higher levels of transcription, from greater mRNA stability at the lower temperature used in this study, or both. The approach employed in this study cannot distinguish between increased transcription initiation and mRNA stabilization by low temperature. However, a >1,600-fold difference for hblC, a >350-fold difference for nprP2, and a 440-fold difference for nprB suggests the induction of the expression of these virulence factor genes during exponential growth in response to temperature. Moreover, the two housekeeping genes used for standardization in this study, as well as the nheB, nheA, and plcB genes, were stably expressed (e.g., mRNAs found in similar amounts) regardless of growth temperature. This strongly argues that, in strain KBAB4, the higher levels of plcR, hblC, nprP2, and nprB mRNA transcripts at 15°C result principally from higher levels of transcriptional activity rather than from temperature-dependent differences in mRNA stability.
In conclusion, this study shows that virulence gene expression in the psychrotolerant KBAB4 strain of the B. cereus group seems to be determined, in part, by temperature. We also found that the higher virulence and cytotoxicity at 15°C of the B. cereus KBAB4 strain or supernatant was accompanied by higher levels of the production of Hbl, NprP2, and NprB and by a higher transcriptional activity of the corresponding genes. Therefore, the production of these virulence factors seems to be important for the survival and colonization of the bacteria in larval intestine at 15°C. Another finding of our work is that these virulence genes were expressed during the exponential growth phase, and this demonstrates that the transcription of various PlcR-controlled genes can occur during the exponential growth phase. Taken together, our findings suggest that, in nature, B. weihenstephanensis is a pathogen of invertebrates, such as soil insects, with whom it may share the same environmental habitat and thermal niches, rather than a pathogen of warm-blooded mammals. Therefore, the most psychrotolerant strains of the B. cereus group may be less capable of causing diarrhea in warm-blooded hosts, such as mammals, than the mesophilic strains of the group.
ACKNOWLEDGMENTS
This work was supported by grants from the Institut National de la Recherche Agronomique (INRA) and the Agence Nationale de la Recherche (ANR) (France) under the ANR-05-PNRA-013 B. cereus contract.
We thank Didier Lereclus, in whose laboratory this work was conducted. We also thank Christophe Buisson for assistance and advice for insect rearing and bioassays and Christina Nielsen-Leroux and Didier Lereclus for helpful discussions.
Footnotes
Published ahead of print 3 February 2012
REFERENCES
- 1. Agaisse H, Gominet M, Oaøkstad Kolstø AB, Lereclus D. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043–1053 [DOI] [PubMed] [Google Scholar]
- 2. Anderson Borge GI, Skeie M, Sorhaug T, Langsrud T, Granum PE. 2001. Growth and toxin profiles of Bacillus cereus isolated from different food sources. Int. J. Food Microbiol. 69:237–246 [DOI] [PubMed] [Google Scholar]
- 3. Beecher DJ, Schoeni JL, Wong ACL. 1995. Enterotoxin activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beecher DJ, Wong AC. 1997. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 272:233–239 [DOI] [PubMed] [Google Scholar]
- 5. Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23:382–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouillaut L, et al. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bustin S, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 8. Cadot C, et al. 2010. InhA1, NprA, and HlyII as candidates for markers to differentiate pathogenic from nonpathogenic Bacillus cereus strains. J. Clin. Microbiol. 48:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dierick K, et al. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43:4277–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehling-Schulz M, et al. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197 [DOI] [PubMed] [Google Scholar]
- 11. Ehling-Schulz M, et al. 2006. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 260:232–240 [DOI] [PubMed] [Google Scholar]
- 12. Fagerlund A, Lindbäck T, Storset AK, Granum PE, Hardy SP. 2008. Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 154:693–704 (Erratum, 154:1554.) [DOI] [PubMed] [Google Scholar]
- 13. Fedhila S, Nel P, Lereclus D. 2002. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184:3296–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fedhila S, Daou N, Lereclus D, Nielsen-LeRoux C. 2006. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol. Microbiol. 62:339–355 [DOI] [PubMed] [Google Scholar]
- 15. Fedhila S, et al. 2010. Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella. J. Invertebr. Pathol. 103:24–29 [DOI] [PubMed] [Google Scholar]
- 16. Fleige S, Pfaffl MW. 2006. RNA integrity and the effect on real time qRT-PCR performance. Mol. Aspects Med. 27:126–139 [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Arribas ML, Kramer JM. 1990. The effect of glucose, starch, and pH on growth, enterotoxin and haemolysin production by strains of Bacillus cereus associated with food poisoning and non-gastrointestinal infection. Int. J. Food Microbiol. 11:21–33 [DOI] [PubMed] [Google Scholar]
- 18. Gilbert RJ, Kramer JM. 1984. Bacillus cereus enterotoxins: present status. Biochem. Soc. Trans. 12:198–200 [DOI] [PubMed] [Google Scholar]
- 19. Gilois N, et al. 2007. Growth-related variations in the Bacillus cereus secretome. Proteomics 7:1719–1728 [DOI] [PubMed] [Google Scholar]
- 20. Gohar M, et al. 2002. Two dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784–791 [DOI] [PubMed] [Google Scholar]
- 21. Gohar M, et al. 2008. The PlcR virulence regulon of Bacillus cereus. PLoS One 3:e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Granum PE, Lund T. 1997. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 157:223–228 [DOI] [PubMed] [Google Scholar]
- 23. Guinebretière M-H, et al. 2001. Identification of bacteria in pasteurized zucchini purées stored at different temperatures and comparison with those found in other pasteurized vegetable purées. Appl. Environ. Microbiol. 67:4520–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guinebretière M-H, Broussolle V, Nguyen-The C. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40:3053–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guinebretière M-H, et al. 2008. Ecological diversification in the Bacillus cereus group. Environ. Microbiol. 10:851–865 [DOI] [PubMed] [Google Scholar]
- 26. Häggblom MM, Apetroaie C, Andersson MA, Salkinoja-Salonen MS. 2002. Quantitative analysis of cereulide, the emetic toxin of Bacillus cereus, produced under various conditions. Appl. Environ. Microbiol. 68:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hardy SP, Lund T, Granum PE. 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197:47–51 [DOI] [PubMed] [Google Scholar]
- 28. Hilliard NJ, Schelonka RL, Waites KB. 2003. Bacillus cereus bacteremia in a preterm neonate. J. Clin. Microbiol. 41:3441–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson KM, Nelson CL, Busta FF. 1984. Influence of heating and cooling rates on Bacillus cereus spore survival and growth in a broth medium and in rice. J. Food Sci. 49:34–39 [Google Scholar]
- 30. Lapidus A, et al. 2008. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Biol. Interact. 171:236–249 [DOI] [PubMed] [Google Scholar]
- 31. Lechner S, et al. 1998. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 48:1373–1382 [DOI] [PubMed] [Google Scholar]
- 32. Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25:697–743 [DOI] [PubMed] [Google Scholar]
- 33. Lund T, Granum PE. 1996. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 141:151–156 [DOI] [PubMed] [Google Scholar]
- 34. Lund T, Granum PE. 1997. Comparison of biological effect of the two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 143:3329–3336 [DOI] [PubMed] [Google Scholar]
- 35. Lund T, De Buyser ML, Granum PE. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254–261 [DOI] [PubMed] [Google Scholar]
- 36. Mahler H, et al. 1997. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336:1142–1148 [DOI] [PubMed] [Google Scholar]
- 37. Miller JM, et al. 1997. Fulminating bacteremia and pneumonia due to Bacillus cereus. J. Clin. Microbiol. 35:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moravek M, et al. 2006. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 257:293–298 [DOI] [PubMed] [Google Scholar]
- 39. Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pirttijärvi TS, Andersson MA, Scoging AC, Salkinoja-Salonen MS. 1999. Evaluation of methods for recognising strains of the Bacillus cereus group with food poisoning potential among industrial and environmental contaminants. Syst. Appl. Microbiol. 22:133–144 [DOI] [PubMed] [Google Scholar]
- 41. Prüss BM, Dietrich R, Nibler B, Märtlbauer E, Scherer S. 1999. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl. Environ. Microbiol. 65:5436–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramarao N, Lereclus D. 2005. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell Microbiol. 7:1357–1364 [DOI] [PubMed] [Google Scholar]
- 43. Reisner AH, Nemes P, Bucholtz C. 1975. The use of Coomassie brilliant blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal. Biochem. 64:509–516 [DOI] [PubMed] [Google Scholar]
- 44. Salamitou S, et al. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825–2832 [DOI] [PubMed] [Google Scholar]
- 45. Sanchis V, Agaisse H, Chaufaux J, Lereclus D. 1996. Construction of new insecticidal Bacillus thuringiensis recombinant strains by using the sporulation non-dependent expression system of cryIIIA and a site specific recombination vector. J. Biotechnol. 48:81–96 [DOI] [PubMed] [Google Scholar]
- 46. Schroeder A, et al. 2006. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sieprawska-Lupa M, et al. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 48:4673–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slamti L, Lereclus D. 2002. A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 21:4550–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sorokin A, et al. 2006. Multiple locus sequence typing analysis of Bacillus cereus and Bacillus thuringiensis reveals separate clustering and a distinct population structure of psychrotrophic strains. Appl. Environ. Microbiol. 72:1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stenfors LP, Mayr R, Scherer S, Granum PE. 2002. Pathogenic potential of fifty Bacillus weihenstephanensis strains. FEMS Microbiol. Lett. 215:47–51 [DOI] [PubMed] [Google Scholar]
- 51. Stenfors Arnesen LP, O'Sullivan H, Granum PE. 2007. Food poisoning potential of Bacillus cereus strains from Norwegian dairies. Int. J. Food Microbiol. 116:292–296 [DOI] [PubMed] [Google Scholar]
- 52. Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 53. Stenfors Arnesen L, Granum PE, Buisson C, Bohlin J, Nielsen-LeRoux C. 2011. Using an insect model to assess correlation between temperature and virulence in Bacillus weihenstephanensis and Bacillus cereus. FEMS Microbiol. Lett. 317:196–202 [DOI] [PubMed] [Google Scholar]
- 54. Terra WR. 2001. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 47:47–61 [DOI] [PubMed] [Google Scholar]
- 55. Thorsen L, et al. 2006. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 72:5118–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thorsen L, Budde BB, Henrichsen L, Martinussen T, Jakobsen M. 2009. Cereulide formation by Bacillus weihenstephanensis and mesophilic emetic Bacillus cereus at temperature abuse depends on pre-incubation conditions. Int. J. Food Microbiol. 134:133–139 [DOI] [PubMed] [Google Scholar]
- 57. Tran SL, Guillemet E, Gohar M, Lereclus D, Ramarao N. 2010. CwpFM (EntFM) is a Bacillus cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence. J. Bacteriol. 192:2638–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tran SL, et al. 2011. Hemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell. Microbiol. 13:92–108 [DOI] [PubMed] [Google Scholar]
- 59. Tran S-L, Puhar A, Ngo-Camus M, Ramarao N. 2011. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PLoS One 6:e22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang P, Granados RR. 2001. Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch. Insect. Biochem. Physiol. 47:110–118 [DOI] [PubMed] [Google Scholar]
- 61. Walters LL, Irons KP, Guzman H, Tesh RB. 1993. Formation and composition of the peritrophic membrane in the sand fly, Phlebotomus perniciosus (Diptera: Psychodidae). J. Med. Entomol. 30:179–198 [DOI] [PubMed] [Google Scholar]