Abstract
Quantification of the abundance of Vibrio parahaemolyticus in water and oysters from Rhode Island showed the presence of environmental strains and low levels of potentially pathogenic strains when water temperatures were ≥18°C, with peak levels in late July to early August. A higher abundance of the trh gene than of the tdh gene was observed.
TEXT
Vibrio parahaemolyticus is a Gram-negative, halophilic bacterium occurring naturally in estuarine and coastal marine waters that is able to cause illness in humans (12, 14, 18, 19). Filter feeders, such as oysters, are capable of acquiring V. parahaemolyticus from the water column and concentrating it in their tissues (10). Because of the potential for human illness through consumption of raw or undercooked shellfish with V. parahaemolyticus, these bacteria pose a threat to shellfish aquaculture and fisheries through loss of revenue due to concerns about seafood safety and negative publicity in cases of outbreaks. Illnesses due to V. parahaemolyticus from consuming shellfish in Rhode Island and the Northeast United States are rare (3). Because of a strong association between V. parahaemolyticus levels and temperature (5, 7, 18, 19), there is an increased awareness of the danger posed by V. parahaemolyticus due to a rise in marine and estuarine water temperatures. In Narragansett Bay (Rhode Island), spring-summer sea surface temperatures have warmed by 1.6°C from 1959 to 2005 and are generally over 15.0°C from late May through mid-October (1). Warmer water temperatures could potentially extend the season in which V. parahaemolyticus could be a concern.
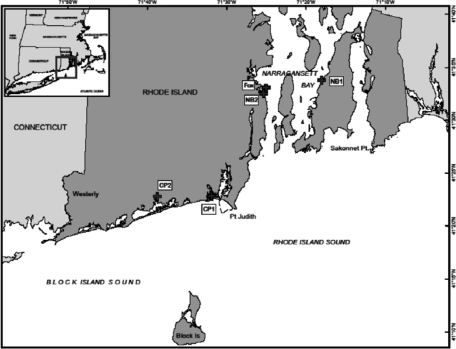
The total and relative abundances of environmental and potentially pathogenic V. parahaemolyticus bacteria in Rhode Island coastal waters have not been determined. A 1985 study showed the presence of V. parahaemolyticus in Narragansett Bay, but no pathogenic strains were detected (19). The purpose of this study was to determine the seasonal abundance of environmental and pathogenic V. parahaemolyticus in Rhode Island. Water (1 liter) and oyster (n = 10 to 15) samples were collected from two farms (NB1, 41°33.678′N, 71°18.431′W; NB2, 41°32.583′N, 71°25.351′W) in Narragansett Bay and two farms (CP1, 41°22.883′N, 71°31.954′W; CP2, 41°22.804′N, 71°33.720′W) in coastal salt ponds (Fig. 1). Water samples were also collected from a site near Fox Island (41°34.2′N, 71°23.4′W) within Narragansett Bay. A 3-tube most-probable-number (MPN) method combined with quantitative PCR detection of the tlh, tdh, and trh genes was used for detection of V. parahaemolyticus (16, 20). After incubation of the MPN tubes, a 1-ml portion from each enrichment tube in the serial dilution series was transferred to cryotubes and stored at −80°C. Bacterial DNA was extracted by boiling, and quantitative PCR (qPCR) was used to score the tubes as positive or negative for tlh, trh, and tdh. Vibrio parahaemolyticus densities (+1 to account for 0 values) were log10 transformed for statistical analysis using one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way ANOVA on ranks and the appropriate post hoc tests (IBM SPSS Statistics 19; IBM, Somers, NY; Sigmastat 3.1; Systat, Chicago, IL).
Fig 1.
Partial map of Rhode Island showing the locations of the sampling sites in Narragansett Bay (NB1, NB2, and Fox Island) and coastal salt ponds (CP1 and CP2). (Map courtesy of Christopher Damon, adapted from the Rhode Island Geographic Information System [RIGIS; copyright 2011].)
Vibrio parahaemolyticus densities in oysters and water.
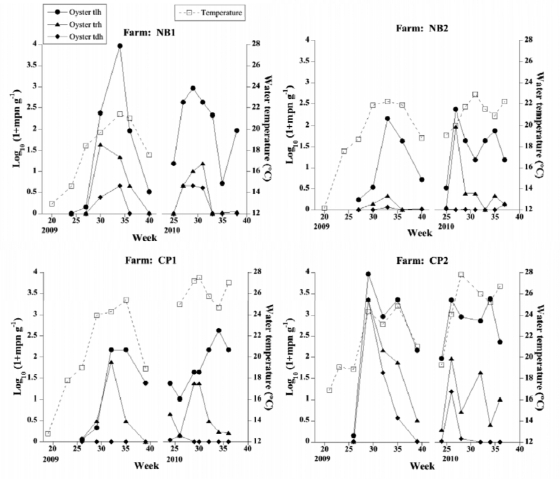
V. parahaemolyticus was detected in Rhode Island oyster and water samples for approximately 15 weeks during the summers of 2009 and 2010 (Fig. 2 and 3). For most locations, densities of V. parahaemolyticus increased rapidly in a period of 2 to 3 weeks after water temperatures reached approximately 18°C, starting in early July for 2009 or mid-June for 2010. This study confirmed July to August as the period of highest risk for V. parahaemolyticus. This is similar to the case with the Chesapeake Bay, where V. parahaemolyticus first peaked in June and July in the water column after water temperature rose to 19°C (13, 18). The maximum level of total V. parahaemolyticus seen in oysters in this study (9 × 103 MPN g−1 oyster tissue) corresponds to a very low risk of gastroenteritis from oysters (10). Levels of total V. parahaemolyticus bacteria detected in Rhode Island oysters are comparable to levels observed in shellfish from other U.S. and European coastal areas (<104 MPN g−1) (2, 5, 6, 11, 15, 16, 17, 18, 20).
Fig 2.
Temporal variation in temperature (right axis, squares, broken line) and levels of V. parahaemolyticus tlh (circles, solid line), trh (triangles, solid line), and tdh (diamonds, solid line) (left axis) in oysters collected from four Rhode Island farms during the summers of 2009 and 2010.
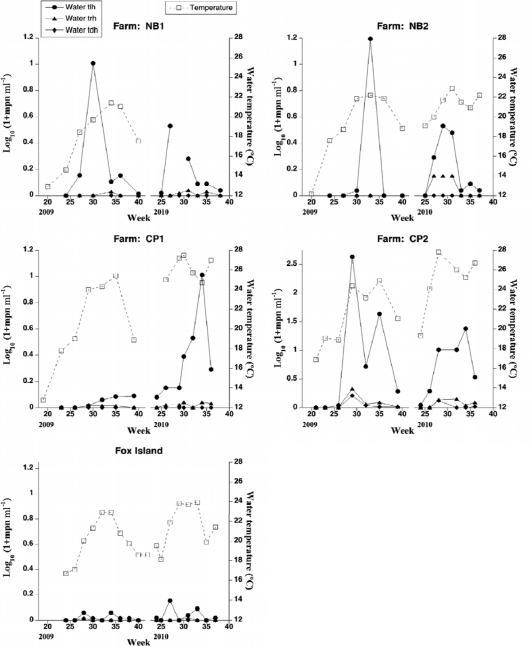
Fig 3.
Temporal variation in temperature (right axis, squares, broken line) and levels of V. parahaemolyticus tlh (circles, solid line), trh (triangles, solid line), and tdh (diamonds, solid line) (left axis) in water collected from four Rhode Island farms and Fox Island station during the summers of 2009 and 2010.
Densities of V. parahaemolyticus in Narragansett Bay water samples in 2009 and 2010 (≤15 MPN ml−1) are comparable to the maximum densities found in a 1985 study (4.95 CFU ml−1 [19]) (Fig. 3). Levels of V. parahaemolyticus in water collected from oyster farms were in general slightly higher than levels at the nonfarm reference site at Fox Island. Maximum densities of tlh in water were significantly higher in 2009 than in 2010 (Kruskal-Wallis one-way ANOVA; P = 0.049). The summer of 2009 was a colder summer with more precipitation, possibly explaining some of the differences between these 2 years.
Abundance of pathogenic V. parahaemolyticus relative to total V. parahaemolyticus.
For 21 of 48 (43.8%) oyster samples, only the trh gene was detected (tdh was nondetectable [Table 1]), showing a predominance of trh+ strains in Rhode Island. The percentage of pathogenic V. parahaemolyticus relative to total V. parahaemolyticus, calculated by dividing trh and tdh values by that for tlh, varied greatly both spatially and temporally for both years, and changes occurred in a matter of weeks (Table 1 and Fig. 2 and 3). Studies of other U.S. coastal waters (Gulf Coast and Pacific) typically show that most samples contain either a majority of tdh+ strains lacking trh or of tdh+ trh+ strains (6, 16, 20). Only one other study of U.S. waters (Chesapeake Bay) has shown a higher prevalence of trh+ over tdh+ (18). However, studies from other countries, such as Norway, India, and France, have also shown a higher relative prevalence of trh+ (4, 8, 9). The relative percentage of pathogenic V. parahaemolyticus in oysters in Rhode Island (2.5 to 31.9% on average) (Table 1) is higher than what has been reported for other coastal locations in the United States (0.3 to 3.2%) (10). However, recent studies have shown that the percentage of pathogenic V. parahaemolyticus can be high (16).
Table 1.
Percentages of pathogenic V. parahaemolyticus relative to total V. parahaemolyticus in water and oyster samples from Fox Island, Narragansett Bay, and two farms in the coastal ponds for 2009 and 2010 combined
| Farma | % trh or tdh prevalence [avg ± SD (range)] in: |
|||
|---|---|---|---|---|
| Oysters |
Water |
|||
| trh | tdh | trh | tdh | |
| NB1 | 2.5 ± 5.0 (0–17.8) | 0.2 ± 0.3 (0–0.8) | 6.2 ± 11.8 (0–31.8) | 0 (0) |
| NB2 | 7.2 ± 11.2 (0–39.8) | 0.4 ± 0.8 (0–2.4) | 10.5 ± 16.7 (0–45.8) | 0 (0) |
| CP1 | 31.9 ± 39.2 (0–100) | 0.4 ± 1.3 (0–4.6) | 15.7 ± 29.0 (0–100) | 0 (0) |
| CP2 | 6.3 ± 4.7 (0.1–24.8) | 3.5 ± 7.6 (0–24.8) | 2.4 ± 3.0 (0–4.6) | 1 ± 1.5 (0–3.8) |
| Fox Island | NTb | NT | 7 ± 14.4 (0–38.9) | 2.7 ± 8.1 (0–24.3) |
| Total | 12.1 ± 23.4 (0–100) | 1.1 ± 3.9 (0–24.8) | 8.2 ± 17.1 (0–100) | 0.7 ± 3.4 (0–24.3) |
NB1 and NB2 are farms in Narragansett Bay; CP1 and CP2 are farms in coastal ponds.
NT, not tested.
Conclusions.
This study provides a range of V. parahaemolyticus densities found in Rhode Island oysters and water samples. We detected a higher abundance of the trh gene than of the tdh gene. It is unknown if the levels of pathogenic V. parahaemolyticus measured in this study by qPCR were due to the presence of one or a few trh+ strains lacking tdh in relatively higher abundance or to the accumulation of low levels of multiple tdh+ trh+ strains and of trh+ strains lacking tdh. This study provides a baseline for further studies of the ecology of V. parahaemolyticus in coastal waters in a temperate estuary in the Northwest Atlantic.
ACKNOWLEDGMENTS
Funding for this project was provided by USDA AFRI Special Research Grant NIFA 2008-03240 to D. A. Bengtson, M. Gómez-Chiarri, and M. A. Rice. Equipment (Stratagene MX3005 and Nanodrop 8000) used for this study was available at the Rhode Island Genomics and Sequencing Center, supported in part by the National Science Foundation under an EPSCoR Grant (no. 0554548).
We thank the East Coast Shellfish Growers Association for their support and for providing oyster samples. We thank L. Maranda, B. D. Jenkins, P. S. Cohen, and C. A. Oviatt from the University of Rhode Island and S. Jones and C. A. Whistler from the University of New Hampshire for their helpful comments on the manuscript. Also, we thank Katherine Markey, Tyler Ferrara, and Paul Torbett for help with summer field and lab work and Christopher Damon from URI's Environmental Data Center for the map in Fig. 1.
Footnotes
Published ahead of print 3 February 2012
REFERENCES
- 1. Collie JS, Wood AD, Jeffries HP. 2008. Long-term shifts in the species composition of a coastal fish community. Can. J. Fish. Aquat. Sci. 65:1352–1365 [Google Scholar]
- 2. Cook DW, Bowers JC, DePaola A. 2002. Density of total and pathogenic (tdh+) Vibrio parahaemolyticus in Atlantic and Gulf Coast molluscan shellfish at harvest. J. Food Prot. 65:1873–1880 [DOI] [PubMed] [Google Scholar]
- 3. Daniels NA, et al. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 181:1661–1666 [DOI] [PubMed] [Google Scholar]
- 4. Deepanjali A, Kumar S, Karunasagar I, Karunasagar I. 2005. Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl. Environ. Microbiol. 71:3575–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DePaola A, Hopkins LH, Peeler JT, Wentz B, McPhearson RM. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Appl. Environ. Microbiol. 56:2299–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DePaola A, Kaysner CA, Bowers J, Cook DW. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl. Environ. Microbiol. 66:4649–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DePaola A, Nordstrom JL, Bowers JC, Wells JG, Cook DW. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deter J, et al. 2010. Ecology of pathogenic and non-pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll a. Environ. Microbiol. 12:929–937 [DOI] [PubMed] [Google Scholar]
- 9. Ellingsen AB, Jorgensen H, Wagley S, Monshaugen M, Rorvik LM. 2008. Genetic diversity among Norwegian Vibrio parahaemolyticus. J. Appl. Microbiol. 105:2195–2202 [DOI] [PubMed] [Google Scholar]
- 10. FDA 2005. Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters. FDA, Washington, DC [Google Scholar]
- 11. Hervio-Heath D, et al. 2002. Occurrence of pathogenic vibrios in coastal areas of France. J. Appl. Microbiol. 92:1123–1135 [DOI] [PubMed] [Google Scholar]
- 12. Jones SH, Summer-Brason B. 1998. Incidence and detection of pathogenic Vibrio sp. in a northern New England estuary, USA. J. Shellfish Res. 17:1665–1669 [Google Scholar]
- 13. Kaneko T, Colwell RR. 1978. The annual cycle of Vibrio parahaemolyticus in Chesapeake Bay. Microb. Ecol. 4:135–155 [DOI] [PubMed] [Google Scholar]
- 14. Levin RE. 2006. Vibrio parahaemolyticus, a notably lethal human pathogen derived from seafood: a review of its pathogenicity, characteristics, subspecies characterization, and molecular methods of detection. Food Biotechnol. 20:93–128 [Google Scholar]
- 15. Martinez-Urtaza J, et al. 2008. Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the Rias of Galicia, Spain. Appl. Environ. Microbiol. 74:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordstrom JL, Vickery MCL, Blackstone GM, Murray SL, DePaola A. 2007. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic V. parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73:5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oberbeckmann S, Wichels A, Wiltshire KH, Gerdts G. 2011. Occurrence of Vibrio parahaemolyticus and Vibrio alginolyticus in the German Bight over a seasonal cycle. Antonie Van Leeuwenhoek 100:291–307 [DOI] [PubMed] [Google Scholar]
- 18. Parveen S, et al. 2008. Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. Int. J. Food Microbiol. 128:354–361 [DOI] [PubMed] [Google Scholar]
- 19. Watkins WD, Cabelli VJ. 1985. Effect of fecal pollution on Vibrio parahaemolyticus densities in an estuarine environment. Appl. Environ. Microbiol. 49:1307–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zimmerman AM, et al. 2007. Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Appl. Environ. Microbiol. 73:7589–7596 [DOI] [PMC free article] [PubMed] [Google Scholar]