Abstract
Hypertension is a leading cause of cardiovascular, cerebral, and renal disease morbidity and mortality. Here we show that disruption of the Cyp 4a14 gene causes hypertension, which is, like most human hypertension, more severe in males. Male Cyp 4a14 (−/−) mice show increases in plasma androgens, kidney Cyp 4a12 expression, and the formation of prohypertensive 20-hydroxyarachidonate. Castration normalizes the blood pressure of Cyp 4a14 (−/−) mice and minimizes Cyp 4a12 expression and arachidonate ω-hydroxylation. Androgen replacement restores hypertensive phenotype, Cyp 4a12 expression, and 20-hydroxy-arachidonate formation. We conclude that the androgen-mediated regulation of Cyp 4a arachidonate monooxygenases is an important component of the renal mechanisms that control systemic blood pressures. These results provide direct evidence for a role of Cyp 4a isoforms in cardiovascular physiology, establish Cyp 4a14 (−/−) mice as a monogenic model for the study of cause/effect relationships between blood pressure, sex hormones, and P450 ω-hydroxylases, and suggest the human CYP 4A homologues as candidate genes for the analysis of the genetic and molecular basis of human hypertension.
The prevalence, complexity, and multiple medical and socioeconomic consequences of hypertension make it a major health challenge. With few exceptions, the molecular bases of the most common forms of human hypertension have yet to be defined and, despite extensive research, its early diagnosis and clinical management remain challenging and mostly symptomatic. Although environmental factors and coexisting conditions such as hyperlipidemia, diabetes, and obesity play a role in the development and progression of the disease, extensive segregation and linkage analyses indicate that multiple genetic factors contribute to its complex etiology (1–5). Furthermore, gender differences in incidence and severity have suggested the involvement of sex-dependent mechanisms in the pathogenesis of human hypertension (2, 6–9), although the molecular basis of these associations remains undefined. Studies of the genetic causes of the disease have targeted several genes encoding products known to regulate systemic blood pressure, but their roles in the pathogenesis of the most prevalent forms of hypertension have yet to be demonstrated (1, 2, 5). Thus, notwithstanding extensive efforts, the genetic bases of hypertension remain elusive, and a lack of novel candidate genes limits progress in this clinically important area of research.
A role for the CYP 4A arachidonic acid (AA) ω/ω-1 hydroxylases in the pathophysiology of hypertension was first suggested by J. C. McGiff and colleagues in the SHR/WKY rat model of genetically controlled spontaneous hypertension (10). On the basis of: (i) the in vitro ion transport and renovascular effects of 20-hydroxyarachidonic acid (20-HETE) (10–12), (ii) biochemical and temporal correlates of CYP 4A2 expression and activity and SHR hypertension (10–12), and (iii) inhibitor and/or antisense nucleotide studies (10, 13, 14), a prohypertensive role was proposed for 20-HETE, a product of CYP 4A AA-hydroxylases (10). Soon after a role for CYP 4A2 in salt-sensitive hypertension was suggested (11). However, despite extensive studies, direct evidence of a causal link between a genetic defect(s) in CYP 4A isoforms and hypertension is lacking, and thus these proposals have yet to find widespread acceptance. The CYP 4A gene subfamily comprises a group of evolutionarily conserved fatty acid hydroxylases (15–17), whose expression is regulated by physiological and pathophysiological effectors such as lipids, mineralocorticoids, sex hormones, fasting, starvation, insulin, and diabetes (16–20). Cyp 4a10, 4a12, and 4a14 are the murine homologues of rat CYP 4A1, 4A8, and 4A2/4A3, respectively (15, 19). To define the vascular roles of the Cyp 4a monooxygenases, we targeted for disruption the Cyp 4a14 gene and demonstrate that 4a14 (−/−) mice show monogenic hypertension that is both spontaneous and androgen sensitive.
Methods
Cyp 4a14 cDNA Cloning and Expression.
The Cyp 4a14 cDNA (2.5 kb), cloned from a mouse liver library (Stratagene), codes for a protein of 507 amino acids with 90% sequence identity to CYP 4A3 and 4A2 (15). A KpnI-XhoI cDNA fragment (1.9 kb) was subcloned into the pBlueBac IV vector (Invitrogen) and expressed by using a commercial sf9/baculovirus expression system (Invitrogen). Recombinant Cyp 4a14 was purified (15) to a specific content of 6 nmol P450/mg of protein and judged to be ≥70% pure by SDS/PAGE.
Genomic Cloning and Construction of a Targeting Vector.
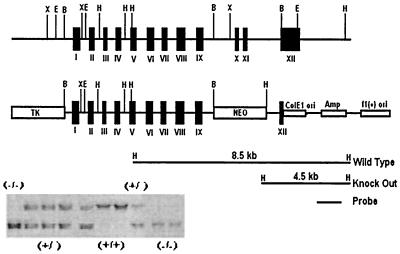
Overlapping genomic clones containing the entire Cyp 4a14 exonic sequences were cloned from a 129/SvJ mouse genomic library (Lambda-FIX II, Stratagene) and partially sequenced. A linearized pNTK targeting vector, in which the sequences coding for the Cyp 4a14 heme-binding peptide (exons 10 and 11) were replaced with a neomycin resistance cassette, resulting in the interruption of in-frame translation at lysine 404 and the insertion of a unique HindIII reporter site for unequivocal genotype analysis (Fig. 1), was electroporated into cultured TL-1 129/SvEv Tac mouse embryonic stem cells (ES) and Cyp 4a14 recombinant ES cells identified by Southern blot analysis. A recombinant ES clone carrying a Cyp 4a14 mutant allele was isolated, expanded, and used for blastocyst implantation and the generation of germline chimeras.
Figure 1.
Strategy used to construct the Cyp 4a14 pNTK targeting vector and for genotype analysis: shown are a partial restriction analysis and exon/intron distribution of the Cyp 4a14 gene and a pNTK targeting vector in which exons 10 and 11 are replaced by a neomycin resistance gene. Included is a Southern analysis of a HindIII digest of tail DNA by using the indicated 1.8-kb DNA probe.
Measurements of Enzyme Activity.
Kidney microsomes were isolated from treated and nontreated Cyp 4a14 (+/+) and (−/−) mice (21), suspended (1–2 mg of protein/ml) in 0.05 M Tris⋅Cl (pH 7.4) containing 0.15 M KCl and 10 mM MgCl2, and incubated with [1-14C]AA or lauric acid (100 μM, 5 μCi/μmol each) and NADPH (1 mM) at 35°C. Reaction products were resolved and quantified as described (21). For antibody inhibition, microsomes were incubated (30 min at 22°C) with rabbit anti-CYP 4A2 or nonimmune serum (0.1–1 mg of protein/ml) before enzymatic analysis. Recombinant Cyp 4a14 (0.1–1 μM) was incubated with [1-14C]-labeled AA or lauric acid in the presence of purified P450 reductase, cytochrome b5, dilauroylphosphatidylcholine, and NADPH (1 mM), as described (15, 21). Urine collections (8–12 h) were incubated at 35°C for 2–3 h with β-glucoronidase (22) (Sigma) (1 mg/ml) and, after purification, the levels of 19- and 20-HETE were quantified by mass spectroscopy (22).
Vascular Physiology Measurements.
The arterial blood pressures of conscious 12- to 14-week-old mice were measured by means of a right carotid artery catheter (300–500 μm OD). After surgery (24–48 h), animals were allowed to become familiar with the environment and, after stabilization, their arterial blood pressures were monitored continuously for at least 30 min by using a pressure transducer. Technical limitations impeded the accurate measurement of blood pressures in animals younger than 8 weeks. For measurements of afferent arteriolar diameter, male kidneys were perfused in vitro with a physiological salt solution supplemented with a mixture of l-amino acids (23), and the juxtamedullary vasculature was monitored continuously by videomicroscopy, as described (23). The relationship between afferent arteriolar diameter and perfusion pressure was determined at 80, 120, and 160 mm Hg. Perfusion pressure changes were followed by a 3-min equilibration before steady-state diameter measurements (23).
Results and Discussion
Disruption of the Cyp 4a14 Gene Causes Spontaneous Hypertension.
Murine germline chimeras carrying a Cyp 4a14 mutant allele were generated as shown in Fig. 1. By mating to wild-type 129/SvJ mice and genetic selection, we generated isogenic homozygous Cyp 4a14 (+/+) and (−/−) mice [from the progeny of an F2 (+/−) × (+/−) cross]. Initial genotype analysis indicated normal offspring patterns after (+/−) × (+/−) crossings. Male and female 4a14 (−/−) mice developed normally and lacked outward symptoms of disease or organ malformation.
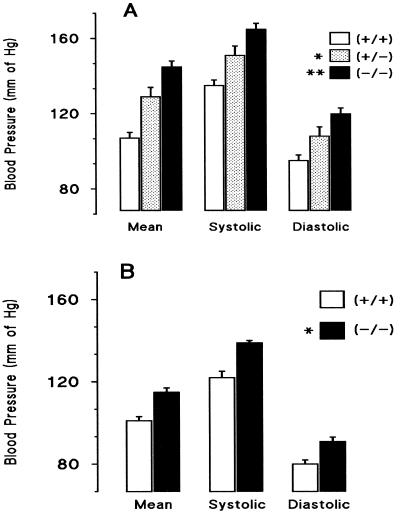
Measurements of systemic blood pressure in sexually mature male 4a14 (−/−) (+/−) and (+/+) mice provided decisive evidence of a physiological role for murine 4a P450s in blood pressure control (Fig. 2A). Compared with wild type, Cyp 4a14 (−/−) mice show significant increases in their mean (MABP), systolic, and diastolic arterial blood pressures (Fig. 2A), whereas 4a14 (+/−) animals show intermediate values (Fig. 2A). The 4a14 (−/−) hypertensive phenotype is spontaneous, i.e., does not require experimental manipulations, and is insensitive to dietary salt [i.e., feeding salt diets containing either 3.0 or 0.03% NaCl (wt/wt) for 4–6 weeks had only minor effects on systemic blood pressure]. Furthermore, 4a14 (+/+) and (−/−) mice showed similar plasma levels of Na+, K+, and aldosterone (not shown).
Figure 2.
Disruption of the Cyp 4a14 gene raises systemic blood pressures in a sexually dimorphic fashion: the blood pressures of conscious adult (10- to 14-week-old) male and female mice were measured by means of a right carotid artery catheter. Shown are averages ± SE calculated from groups of 40 (−/−), 38 (+/+), or 12 (+/−) male mice (Top frame, A) or from groups of 20 (−/−) or 14 (+/+) female mice (Bottom frame, B). [Pressure differentials between Cyp 4a14(+/+) and (−/−) mice were of 38, 30, and 25 mm and of 14, 17, and 11 mm Hg, for mean, systolic, and diastolic blood pressures, and for male and female mice, respectively]. (A) Significantly different from the male wild type: *, Cyp (+/−), P ≤ 0.007; **, Cyp (−/−), P ≤ 1 × 10−5. (B) Significantly different from the female wild type: *, Cyp (−/−), P ≤ 1 × 10−4. No significant pressure differences were observed between female Cyp 4a14 (+/−) and 4a14 (+/+) mice. See Methods for details.
Hypertension in Cyp 4a14 (−/−) Mice Is Sexually Dimorphic.
A large subset of human hypertension is sexually dimorphic, i.e., more severe in males than in females, differences that are minimized after menopause (2, 6–9, 24). Sexual dimorphism is also observed in the hypertensive phenotype of 4a14 (−/−) mice. Blood pressures in female 4a14 (+/+) and (−/−) mice are lower than those of age-matched males, and their pressure differentials are not as pronounced (Fig. 2B). Of interest, disruption of the Cyp 4a14 gene brings the MABPs of knockout females to levels comparable to that of wild-type males [MABPs of 115 ± 2 and 110 ± 4 for Cyp 4a14 (−/−) females and (+/+) males, respectively; n = ≥20; P = 0.3) (Fig. 2)]. A similar sexual dimorphism has been observed in SHR rats, an extensively characterized polygenic model of hypertension (24–27).
These gender differences suggested a role for androgens in the Cyp 4a14 (−/−) phenotype and led us to analyze their plasma levels and role in blood pressure regulation. As shown in Table 1, Cyp 4a14 (−/−) males have plasma testosterone (TST) and 5α-dihydrotestosterone (DHT) levels twice as high as those of (+/+) mice, demonstrating a role for products of this gene in androgen regulation and the existence of a hitherto unrecognized regulatory loop between the fatty acid hydroxylase and mechanisms that control androgen biosynthesis, metabolism, or degradation. Importantly, neither recombinant Cyp 4a14 nor rat CYPs 4A1 or 4A2 catalyzed TST oxidation (not shown), nor was the metabolism of TST by liver microsomes affected by the disruption of the 4a14 gene. Male-specific expression of rat and mouse kidney 4A isoforms and their androgen-dependent regulation have been reported (18, 19).
Table 1.
Plasma androgen levels in Cyp 4a14 (+/+) and (−/−) male mice
| Source of plasma | DHT | TST | |
|---|---|---|---|
| Control | (+/+) | 0.44 ± 0.05 | 1.16 ± 0.1 |
| (−/−) | 1.02 ± 0.1* | 2.06 ± 0.3* | |
| CST/PL | (+/+) | ≤0.05† | ≤0.20† |
| (−/−) | ≤0.05† | ≤0.20† | |
| CST/DHT | (+/+) | 2.64 ± 0.3** | 0.89 ± 0.1 |
| (−/−) | 2.01 ± 0.1** | 0.82 ± 0.1 | |
Fourteen-week-old male Cyp 4a14 (+/+) and (−/−) mice were castrated and, 10 days later, implanted with either placebo (CST/PL) or DHT (CST/DHT)-releasing pellets (21-day pellets, 5 mg DHT/day; Innovative Research of America, Sarasota, FL). Ten days after implantation, plasma samples were analyzed for TST and DHT levels by RIA using commercially available kits. Values (in nanograms/milliliters of plasma) are the mean ± SE of at least 31 different animals for control Cyp 4a14 (+/+) and (−/−) mice and of at least 10 different animals for the rest. Significantly different from control wild type:
P ≤ 0.0009 and P ≤ 0.005 for DHT and TST, respectively. Significantly different from control wild-type and knockout mice, respectively:
P ≤ 2 × 10−4 and P ≤ 1 × 10−3 for castrated DHT-treated (+/+) and (−/−) mice.
, below assay detection limit.
Hypertension in Cyp 4a14 (−/−) Mice Is Androgen-Sensitive.
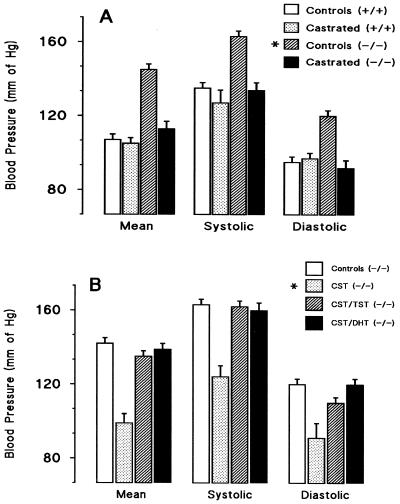
To examine the role of androgens in the Cyp 4a14 (−/−) hypertensive phenotype, we castrated 4a14 (+/+) and (−/−) mice and implanted them with either placebo or TST-releasing pellets. Castration markedly reduced the plasma concentrations of DHT and TST in Cyp 4a14 (+/+) and (−/−) mice (Table 1) and normalized the blood pressures of hypertensive 4a14 (−/−) mice (Fig. 3A). On the other hand, castration had a minor effect on the blood pressures of 4a14 (+/+) mice (Fig. 3A), suggesting that plasma androgen levels must reach a threshold before significant changes in blood pressure can be observed. The administration of DHT or TST to castrated 4a14 (−/−) mice raised the plasma levels of these androgens (Table 1) (1.8 ± 0.04 ng TST/ml; n = 7) and restored the hypertensive phenotype of castrated 4a14 (−/−) mice (Fig. 3B). These androgen-mediated pressure effects were gender and Cyp 4a14 genotype independent, because the administration of DHT also raised the blood pressures of: (i) control and castrated Cyp 4a14 (+/+) mice (MABPs of 140 ± 5 and 137 ± 4 mm of Hg for control and castrated mice, respectively; n = 10) (P ≤ 0.001 and ≤0.0004 for DHT treated vs. nontreated mice, and for castrated and DHT-treated vs. castrated mice, respectively), and (ii) female 4a14 (+/+) or (−/−) mice (MABPs of 133 ± 4 and 132 ± 4 mm of Hg for (−/−) and (+/+) female mice, respectively; n ≥ 9) [P < 0.0003 and 0.0006 for DHT-treated (−/−) and (+/+) mice vs. the respective untreated controls]. Hence, the blood pressures of male and female mice are androgen-sensitive, and male Cyp 4a14 (−/−) hypertension is associated with increases in plasma androgens caused by Cyp 4a14 gene-dependent perturbations in the mechanisms that control the circulating levels of these hormones. A similar androgen sensitivity has been reported in SHR rats (25–28). Castration reduces the MABP of male hypertensive SHR rats by 30 to 40 mm of Hg (26–29) and, as with 4a14 (−/−) mice, the normotensive effects of castration are reversed by TST replacement (25–27). Furthermore, androgen administration equalizes the MABP of hypertensive male and female SHR rats (26, 28). The similarities between a component of the hypertensive phenotypes of SHR rats and of P450 4a14 knockout mice support the proposal that P450 4A isoforms contribute to the full development of high blood pressure in adult SHR rats (10).
Figure 3.
Hypertension in Cyp 4a14 (−/−) mice is androgen-sensitive: (Top frame, A): Groups of Cyp 4a14 (+/+) and (−/−) adult mice were castrated, and 10–12 days later their systemic blood pressures, as well as those of noncastrated knockout and wild-type mice, were determined. (Bottom frame, B): Groups of Cyp 4a14 (−/−) mice were castrated and implanted with either placebo (PL) or TST- or DHT-releasing pellets and their blood pressures, and those of noncastrated knockout controls, determined 9 days later (B). Shown are averages ± SE calculated from groups of 38 4a14 (+/+), 40 4a14 (−/−), 4 castrated 4a14 (+/+), 16 castrated 4a14 (−/−), or from a group of 30 castrated 4a14 (−/−) mice treated with either placebo (8 mice) (CST/PL), TST (14 mice) (CST/TST), or DHT-releasing pellets (8 mice) (CST/DHT). Fig 3A: Significantly different from the MABPs of control (+/+), castrated (+/+), and castrated (−/−) mice: *, P ≤ 1 × 10−5. The MABP of castrated wild-type and knockout mice were not significantly different from that of wild type. (B) Significantly different from the MABP of castrated placebo Cyp (−/−) mice: *, P ≤ 1 × 10−4; **: P ≤ 1 × 10−5. The MABPs of control, castrated, and TST- or DHT-treated Cyp 4a14 (−/−) mice did not differ significantly.
The Expression and Activities of the Kidney Cyp 4a AA Monooxygenases Are Androgen-Sensitive.
To determine whether the Cyp 4a14 (−/−) hypertension was linked to androgen-mediated changes in renal AA metabolism and 20-HETE formation, we characterized microsomal 20-HETE biosynthesis and Cyp 4a expression in the kidneys of control, castrated, and castrated and androgen-treated mice. Significantly, purified recombinant Cyp 4a14 did not metabolize AA even in the presence of cytochrome b5, excess P450 reductase, GSH, EDTA, and/or sodium cholate (15, 30, 31). The enzyme does, however, catalyze lauric acid oxidation (4.0 ± 0.8 nmol product/min/nmol of P450). Thus, Cyp 4a14 is the closest murine 4a family member to rat CYP 4A2, even though it does not metabolize AA. Compared with normotensive Cyp 4a14 (+/+) controls, kidney microsomes from sexually mature hypertensive 4a14 (−/−) male mice metabolize AA to 20-HETE at significantly higher rates (Table 2). In contrast, 4a14 (+/+) and (−/−) females show nearly undetectable renal AA monooxygenase activities (Table 2). Despite these enzymatic differences, mass spectroscopic quantification of urinary 20-HETE (22) showed its concentrations to be low and similar for the 4a14 (+/+) and (−/−) genotypes (0.18 ± 0.04 and 0.24 ± 0.01 ng/ml of urine for wild-type and knockout mice), indicating that, as with most P450 eicosanoids, the urinary levels of 20-HETE may be controlled by degradation and/or metabolism, as opposed to biosynthetic capacity (10–12, 22).
Table 2.
The microsomal Arachidonic acid ω-hydroxylase of mouse kidney microsomes
| Microsomes | ω-Hydroxylase rate | (20-HETE) % of total | |
|---|---|---|---|
| Males | |||
| Control | (+/+) | 38 ± 2 | 86 |
| Control | (−/−) | 85 ± 9* | 84 |
| CST/PL† | (+/+) | <0.2 | — |
| CST/PL† | (−/−) | <0.2 | — |
| CST/DHT | (+/+) | 228 ± 35** | 82 |
| CST/DHT | (−/−) | 225 ± 23 | 82 |
| Females | |||
| Placebo | (+/+) | <0.2 | — |
| Placebo† | (−/−) | <0.2 | — |
| DHT | (+/+) | 140 ± 3 | 76 |
| DHT | (−/−) | 164 ± 3 | 77 |
Fourteen-week-old castrated male and female Cyp 4a14 (+/+) and (−/−) mice were implanted with either placebo (CST/PL) or DHT-releasing pellets (CST/DHT) (Table 1). Ten days later, kidney microsomes were incubated with AA, as described in Methods. Rates, in picomoles of product formed per minute per milligram of microsomal protein, were calculated from the corresponding time courses of product formation. Values are averages ± SE calculated from at least six (males) or three (females) different experiments. Significantly different from control wild type:
, P ≤ 1 × 10−4 and P ≤ 5 × 10−4 for total AA metabolism and 20-HETE formation, respectively. Significantly different from control wild-type and knockout mice, respectively:
, P ≤ 7 × 10−4 and P ≤ 9 × 10−4 for castrated DHT-treated (+/+) and (−/−) mice.
Total reaction rates below detection limits.
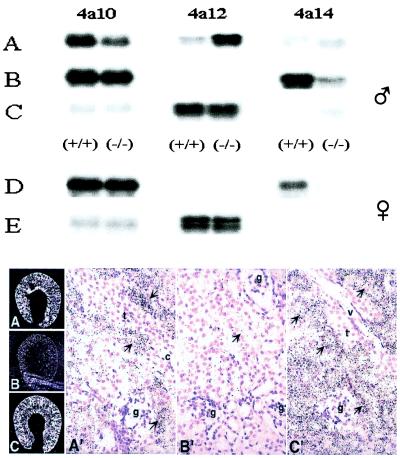
Northern analysis of kidney Cyp 4a isoform expression showed that: (i) Cyp 4a10 is the predominant 4a isoform expressed in the kidneys of wild-type adult males, followed by Cyp 4a12 and low levels of Cyp 4a14 transcripts (Fig. 4). In males, the expression of kidney Cyp 4a14 is variable, age-dependent, and minimized on reaching sexual maturity (not shown). (ii) The female kidney expresses Cyp 4a10 and 4a14 and, as reported (19), lacks detectable Cyp 4a12 transcripts (Fig. 4). (iii) Disruption of the 4a14 gene had little effect on Cyp 4a10 or 4a12 expression by the female kidney (Fig. 4) but causes male-specific up-regulation of the Cyp 4a12 gene and down-regulation of the Cyp 4a10 gene (Fig. 4). Castration drastically decreased renal AA metabolism (Table 2), reduced kidney Cyp 4a12 expression to undetectable levels (Fig. 4), and up-regulated Cyp 4a10 and 4a14 expression (Fig. 4). On the basis of the relative levels of Cyp 4a transcripts and AA monooxygenase activity, kidneys from castrated normotensive 4a14 (+/+) and (−/−) males are similar to those of their corresponding female counterparts (Table 2 and Fig. 4).
Figure 4.
Nucleic acid and in situ hybridization analysis of RNAs present in kidneys of control and DHT-treated adult mice. Top frame: Samples of total RNA (5–10 μg each) from the kidneys of control (A), castrated (B), castrated and DHT-treated (C) males or from control (D) and DHT-treated (E) females were fractionated by agar electrophoresis, transferred to nitrocellulose membranes, and hybridized to 32P-labeled DNA probes (400–500 bp) coding for segments of the 3′-untranslated end of the Cyp 4a10, 4a12, and 4a14 cDNAs. After high-stringency washes, the membranes were exposed to x-ray films for 4, 2, or 21 h for male Cyp 4a10, 4a12, and 4a14, respectively, and 6, 21, or 12 h for female Cyp 4a10, 4a12, and 4a14, respectively. RNA loadings were normalized by using a β-actin cDNA probe. Animal treatment protocols were as in Fig. 2 and Table 2. Long exposures revealed the presence of Cyp 4a14 reactive transcripts in 4a14 (−/−) mice kidneys (for example, lanes A–C). Reverse transcription–PCR amplification, cDNA cloning, and sequence analysis demonstrated that these were truncated mRNAs lacking exons × and XI, transcribed from the disrupted Cyp 4a14 gene. Bottom frame: Dehydrated paraffin sections from the kidneys of control (A and A′), castrated (B and B′) and DHT-treated castrated male mice (C and C′) were hybridized to [35S]-labeled riboprobes encoding 3′-end untranslated segments of the Cyp 4a12 cDNA. After washing, RNase A treatment, and dehydration, the sections were dipped in emulsion (IlfordK5; Knutsford, Cheshire, U.K.), exposed for 4–5 days at 4°C, and developed by using D-19 (Kodak). Slides were counterstained with hematoxylin. Photomicrographs were obtained by using either dark-field (3×) (A–C) or bright-field (100×) (A′, B′, and C′) optics. Thick ascending limbs, collecting ducts, glomeruli, and vessels (v) are indicated by arrows t, c, g, and v, respectively.
Androgen administration to castrated male or female mice minimized Cyp 4a10 and 4a14 expression (Fig. 4) and increased, in a Cyp 4a14 genotype-independent fashion, the kidney expression of Cyp 4a12 and the metabolism of AA to 20-HETE (Fig. 4 and Table 2), indicating that Cyp 4a12 is the isoform responsible for 20-HETE formation. Consistent with this interpretation, an antibody raised against the rat homologue of Cyp 4a14 (CYP 4A2) blocked >90% of the kidney microsomal AA ω-hydroxylase of DHT-treated male or female mice (not shown). The metabolic and regulatory changes shown in Table 2 and Fig. 4 document an androgen-dependent regulation of renal prohypertensive 20-HETE biosynthesis (10, 11). Data in Tables 1 and 2 and Fig. 4 clearly indicate that, whereas androgen administration induces Cyp 4a12-associated hypertension in females, the modest hypertension in the female 4a14 (−/−) mouse is androgen- and Cyp 4a12-independent. The molecular basis of Cyp 4a14 (−/−) female mice hypertension is unknown but presumably results from factors similar to those responsible for lower blood pressures in hypertensive premenopausal women (8, 9, 24).
Synthetic 20-HETE is a powerful renal vasoconstrictor (10, 11, 23, 32), and its documented role in the regulation of glomerular afferent arteriole tone serves as the basis for its proposed prohypertensive roles (10, 11, 23, 32). In situ hybridization of kidney sections from Cyp 4a14 (−/−) mice by using Cyp 4a12 riboprobes demonstrated that hypertensive control and DHT-treated castrated 4a14 (−/−) mice show abundant and selective expression of 4a12 transcripts in the renal cortex (Bottom frame, Fig. 4 A and C). In contrast, only background levels of Cyp 4a12 gene expression are seen in castrated normotensive Cyp 4a14 (−/−) animals (Bottom frame, Figs. 4B and B′, and 5). As shown by increased silver grain density (Fig. 4), expression of the Cyp 4a12 gene in hypertensive control and DHT-treated castrated 4a14 (−/−) mice is mostly restricted to the proximal tubule (Bottom frame, Fig. 4 A′ and C′), whereas thick ascending limbs, collecting ducts, and glomeruli show background expression. The abundance of Cyp 4a12 transcripts in close proximity to the glomeruli microcirculation (Bottom frame, Fig. 4 A and C) shows that 20-HETE biosynthesis is localized in close proximity to the afferent arterioles, a paracrine target for its proposed prohypertensive activity (23, 32, 33).
Increased Renal Vascular Resistance in Cyp 4a14 (−/−) Mice.
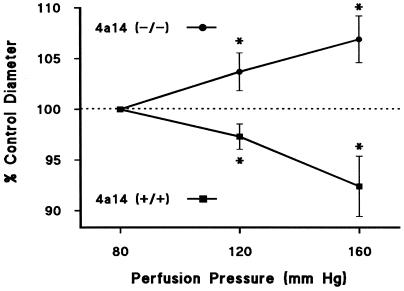
The involvement of an altered renal microvasculature in experimental and human hypertension is well documented (34). Furthermore, elevation in preglomerular vascular resistance may be the determining factor in the decline of renal sodium and water excretion at normotensive pressures and may account for the impaired autoregulatory efficiency frequently observed in chronic hypertension (34). At a perfusion pressure of 80 mm Hg, the kidneys of male Cyp 4a14 (−/−) mice showed a decreased preglomerular vascular diameter [afferent arteriolar diameter: 19 ± 0.5 μm and 17 ± 0.4 μm for Cyp 4a14 (+/+) and 4a14 (−/−) mice, respectively; P ≤ 0.05; n ≥ 5 animals, 10 vessels]. This increase in afferent arteriolar resistance may compromise the excretory ability of the Cyp 4a14 (−/−) kidney and may be responsible for the animal's hypertensive phenotype. As reported for the SHR rat (34), Cyp 4a14 (−/−) males show reduced microvascular autoregulatory efficiency. Thus, whereas in Cyp 4a14 (+/+) mice the afferent arteriolar diameter decreased by 8% when renal perfusion pressure was raised from 80 to 160 mm Hg, under identical conditions, it increased by 7% in Cyp 4a14 (−/−) mice (Fig. 5). Similarly, it has been shown that inhibitors of 20-HETE formation also attenuate the pressure response of rat afferent arterioles (23). These results support the concept that increased renal vascular resistance and impaired autoregulatory capacity contribute to the development of hypertension in 4a14 (−/−) mice and that these changes are associated with an increased biosynthesis of vasoconstrictor 20-HETE (10, 11, 34).
Figure 5.
Impaired afferent arteriolar autoregulatory capacity in male Cyp 4a14 (−/−) mice. Kidneys from adult Cyp 4a14 (+/+) (n = 6 mice; n = 10 vessels) and (−/−) mice (n = 5 mice; n = 10 vessels) were perfused as described in Methods, and the effects of changes in perfusion pressure on the diameter of the afferent arterioles were monitored by videomicroscopy. Values (in percentage of control diameter) are the mean ± SEM. *, significant difference from diameter measured at 80 mm Hg in the same group (P < 0.05). Control afferent arteriole diameters (at 80 mm Hg) were 19 ± 0.5 and 17 ± 0.4 μm for Cyp 4a14 (+/+) and (−/−), respectively. P ≤ 0.05; n = 10 vessels.
In summary, the lack of a functional kidney Cyp 4a14 causes several interrelated metabolic and regulatory effects whose functional manifestations are increased renal vascular resistance, impaired renal hemodynamics, and hypertension. These include increases in: (i) plasma androgens, (ii) Cyp 4a12 gene expression, and (iii) formation of prohypertensive 20-HETE. We postulate that catalytic turnover by Cyp 4a14 generates a yet-to-be-characterized mediator that modulates the levels of circulating androgens. Increased plasma androgen levels induce Cyp 4a12 gene expression and cause attendant increases in proximal tubule 20-HETE biosynthesis, release, and diffusion into the nearby microcirculation. Systemic hypertension results from alterations in nephron hemodynamics, including afferent arteriole autoregulation and renal blood flow (10, 11, 23, 32), caused by increased levels of 20-HETE (10, 11, 32). This interpretation is in agreement with the known renal effects of this eicosanoid (10–12) and provides a molecular/enzymatic description of the relationships between Cyp 4a14 gene function, the regulation of circulating androgens, and renal AA metabolism. These relationships, as well as the Cyp 4a14 (−/−) hypertensive phenotype, suggest new experimental paradigms for future studies of gender differences in human hypertension and the reported prohypertensive effects of male sex hormones (6–9, 24).
A variety of etiologies and the coexistence of additional pathological conditions complicate the study of the molecular basis of human hypertension. Except for a few rare monogenic disorders (1, 2), pathological changes in the regulation of systemic blood pressures are the combined result of abnormalities involving several genes (1, 2, 5). Studies based on functional or pharmacological effects, the characterization of human defective genes, or genetic studies in rat or mouse have identified ≈60 gene products that might contribute to increases or decreases in systemic blood pressure (2). Among these, many of the obvious candidates genes now have been either disrupted and/or overexpressed in mice and, with a few exceptions, their roles confirmed (2). However, their relevance to the pathophysiology of the most prevalent forms of human hypertension remains to be fully established. On the other hand, by focusing on the Cyp 4a14 AA ω/ω-1 hydroxylases, the phenotypic analysis of Cyp 4a14 (−/−) mice demonstrates the crucial role played by Cyp 4a isoforms in renovascular and body physiology and provides a conceptually different and innovative approach to the study of blood pressure regulation.
Finally, the severity and monogenic nature of the Cyp 4a14 (−/−) hypertensive phenotype provide an excellent model to characterize the roles of these factors in the initiation and progress of the disease and make human CYP 4As attractive candidate genes for the study of the molecular basis of hypertension. Importantly, CYP 4A11, the only human 4A isoform so far characterized, is a renal AA ω/ω-1 hydroxylase (29, 31, 35) with a 75% amino acid sequence identity to Cyp 4a12, its closest murine homologue. Inasmuch as epidemiological data suggest associations between gender, sex hormones, and the pathophysiology of human hypertension (1, 2, 6–9), these studies may have important clinical implications. It is now widely accepted that the identification and characterization of genes involved in the pathogenesis of hypertension may advance our understanding of the disease and its causes but, more importantly, may lead to the development of novel and rational targets for diagnosis and clinical intervention.
Acknowledgments
We are grateful to Drs. Tadashi Inagami and Jason Morrow for helpful discussions and suggestions, Shozou Wei and Wendell Nicholson for excellent technical support, and the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK38226) for their generous support. W.J.K. was the recipient of a Career Development Award (Clinical Investigator) from the U.S. Department of Veterans Affairs. D.S.K. was supported in part by Grant AR02002 from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases.
Abbreviations
- AA
arachidonic acid
- HETE
hydroxyarachidonic acid
- MABP
mean arterial blood pressure
- TST
testosterone
- DHT
dihydrotestosterone
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lifton R P. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 2.Garbers D L, Dubois S K. Annu Rev Biochem. 1999;68:127–155. doi: 10.1146/annurev.biochem.68.1.127. [DOI] [PubMed] [Google Scholar]
- 3.Dominiczak AF, Negrin D C, Clark J S, Brosnan J M, McBride M W, Alexander Y. Hypertension. 2000;35:164–172. doi: 10.1161/01.hyp.35.1.164. [DOI] [PubMed] [Google Scholar]
- 4.Pratt R E, Dzau V J. Hypertension. 1999;33:238–247. doi: 10.1161/01.hyp.33.1.238. [DOI] [PubMed] [Google Scholar]
- 5.Halushka M K, Fan J-B, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- 6.Reckelhoff J F, Granger J P. Clin Exp Pharmacol Physiol. 1999;26:127–131. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 7.August P. J Clin Endocrinol Metab. 1999;84:3451–3452. doi: 10.1210/jcem.84.10.6124. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y F. Curr Opin Nephrol Hypertens. 1996;5:181–185. doi: 10.1097/00041552-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Mantzoros C S, Georgiadis E I, Young R, Evagelopoulou C, Khoury S, Hatsilambros N, Sowers J R. Am J Hypertens. 1995;8:606–614. doi: 10.1016/0895-7061(95)00051-P. [DOI] [PubMed] [Google Scholar]
- 10.McGiff J C, Quilley J. Am J Physiol. 1999;277:R607–R623. doi: 10.1152/ajpregu.1999.277.3.R607. [DOI] [PubMed] [Google Scholar]
- 11.Harder D R, Lange A R, Gebremedhin D, Birks E K, Roman R J. J Vasc Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- 12.Capdevila J H, Falck J R, Harris R C. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 13.Su P, Kaushal K M, Kroetz D L. Am J Physiol. 1998;275:R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- 14.Wang M H, Guan H, Nguyen X, Zand B A, Nasjletti A, Laniado-Schwartzman M. Am J Physiol. 1999;276:F246–F253. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- 15.Helvig C, Dishman E, Capdevila J H. Biochemistry. 1998;37:12546–12558. doi: 10.1021/bi981048g. [DOI] [PubMed] [Google Scholar]
- 16.Johnson E F, Palmer C N, Griffin K J, Hsu M H. FASEB J. 1996;10:1241–1248. doi: 10.1096/fasebj.10.11.8836037. [DOI] [PubMed] [Google Scholar]
- 17.Honkakoski P, Negishi M. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundseth S S, Waxman D J. J Biol Chem. 1992;267:3915–3921. [PubMed] [Google Scholar]
- 19.Heng Y M, Kuo S, Jones P S, Savory R, Schultz R M, Tomlinson S R, Gray J B, Bell D R. Biochem J. 1997;325:741–749. doi: 10.1042/bj3250741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroetz D L, Yook P, Costet P, Bianchi P, Pineau T. J Biol Chem. 1998;20:31581–31589. doi: 10.1074/jbc.273.47.31581. [DOI] [PubMed] [Google Scholar]
- 21.Capdevila J H, Falck J R, Dishman E, Karara A. Methods Enzymol. 1990;187:385–394. doi: 10.1016/0076-6879(90)87045-5. [DOI] [PubMed] [Google Scholar]
- 22.Prakash C, Zhang J Y, Falck J R, Chaunhan K, Blair I A. Biochem Biophys Res Commun. 1992;185:728–733. doi: 10.1016/0006-291x(92)91686-k. [DOI] [PubMed] [Google Scholar]
- 23.Imig J D, Falck J R, Inscho EW. Br J Pharmacol. 1999;127:1399–1405. doi: 10.1038/sj.bjp.0702662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips G B, Jing T Y, Laragh J H. J Hum Hypertens. 1997;11:523–526. doi: 10.1038/sj.jhh.1000481. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y F, Meng Q C. Life Sci. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. [DOI] [PubMed] [Google Scholar]
- 26.Turner M E, Johnson M L, Ely D L. Hypertension. 1991;17:1097–1103. doi: 10.1161/01.hyp.17.6.1097. [DOI] [PubMed] [Google Scholar]
- 27.Reckelhoff J F, Zhang H, Srivastava K, Granger J P. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 28.Masabuchi Y, Kumai T, Uematsu A, Komoriyama K, Hirai M. Acta Endocrinol. 1982;101:154–160. doi: 10.1530/acta.0.1010154. [DOI] [PubMed] [Google Scholar]
- 29.Ganten U, Schroder G, Witt M, Zimmermann F, Ganten D, Stock G. J Hypertens. 1989;7:721–726. [PubMed] [Google Scholar]
- 30.Hoch U, Zhang Z, Kroetz D L, Ortiz de Montellano P R. Arch Biochem Biophys. 2000;373:63–71. doi: 10.1006/abbi.1999.1504. [DOI] [PubMed] [Google Scholar]
- 31.Lasker J M, Chen B W, Wolf I, Bloswick B P, Wilson P D, Powel P K. J Biol Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 32.Imig J D, Zou A P, Stec D E, Harder D R, Falck J R, Roman R J. Am J Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 33.Navar L G, Inscho E W, Majid D S A, Imig J D, Harrison-Bernard L M, Mitchell K D. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 34.Cowley AW, Roman R J. J Am Med Assoc. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 35.Imaoka S, Ogawa H, Kimura S, Gonzalez F J. DNA Cell Biol. 1993;12:893–899. doi: 10.1089/dna.1993.12.893. [DOI] [PubMed] [Google Scholar]