Summary
Mast cells are essential in allergic responses and beyond. White adipose tissue from obese humans contains large numbers of mast cells. Serum mast cell tryptase levels are also significantly higher in obese subjects than in lean subjects, suggesting a role of these inflammatory cells in obesity and diabetes. Two types of mast cell-deficient mice, along with corresponding wild-type control mice, were fed a Western diet to induce obesity and diabetes. We also used two anti-allergy drugs, cromolyn and ketotifen (Zaditor), to treat wild-type mice during intake of a Western diet or after the onset of obesity and diabetes, to examine the possible prevention or reversal of these conditions. Mast cell deficiency or pharmacological stabilization reduced body weight gain and improved glucose and insulin sensitivities. These common, side effect-free drugs also reduced pre-established obesity and diabetes without noticeable toxicity. Mechanistic studies suggest that mast cells participate in these metabolic disorders by affecting energy expenditure, protease expression, angiogenesis, apoptosis, and preadipocyte differentiation. These observations open a new era of basic research regarding mast cells, and offer hope to patients suffering from these metabolic disorders.
Keywords: mast cell, obesity, diabetes, cromolyn, ketotifen (Zaditor)
Mast cells are important inflammatory cells that participate in immune responses during allergic reactions in airways, skin, and the gastrointestinal tract – such as asthma, allergic rhinitis, atopic dermatitis, and food allergies [1,2]. Recent discoveries, however, suggest that these cells also participate in other inflammatory diseases, such as cancers, inflammatory bowel disease, metabolic bone disease, renal injury, arthritis, atherosclerosis, abdominal aortic aneurysms, obesity, and diabetes [3–10]. After activation, mast cells degranulate and release histamine, proteases, cytokines, chemokines, growth factors, arachidonic acid metabolites, and reactive oxygen and nitrogen species [11]. Using these inflammatory mediators, mast cells influence neighbouring cell and tissue pathobiology. For example, mast cells release interleukin (IL)-6 and interferon (IFN)-γ to stimulate vascular smooth muscle cell (SMC) and endothelial cell expression of cysteinyl cathepsins [8] to degrade extracellular matrix (ECM) proteins; to promote angiogenesis; to induce vascular SMC, endothelial cell, and macrophage apoptosis; and to activate T lymphocytes [12]. Mast cells also release their unique serine proteases – chymase, tryptase, and carboxypeptidase A. Like cysteinyl cathepsins, these proteases promote vascular SMC and endothelial cell apoptosis, ECM protein degradation, leukocyte migration, and activation of zymogens and latent cytokines [12].
Obesity and diabetes are chronic inflammatory diseases. Along with providing structural support and serving as an energy reservoir, adipose tissues cooperate with other endocrine organs to regulate energy metabolism, blood pressure, coagulation, and immune responses [13]. Adipocytes and preadipocytes are the main components by volume in adipose tissue. Recent analysis of the adipose tissue stromal vascular fraction (SVF) revealed the presence of inflammatory cells in adipose tissues, including macrophages [14–16], regulatory T cells [17], CD8+ T cells [18], CD4+ T cells [19], and natural killer T cells [20]. Adipocytes and inflammatory cells in the SVF secrete hormones, cytokines, and chemokines, such as adiponectin, leptin, resistin, visfatin, tumour necrosis factor (TNF)-α, IL6, IL1, and monocyte chemotactic protein (MCP)-1, as part of the adipose tissue inflammatory mechanism [21]. Adipose tissue mast cells initially seemed to correlate with fat cell numbers in subcutaneous and epididymal adipose tissues from lean mice, and to increase in number after mice became obese [22,23]. It was thought that mast cells increased the uptake of chylomicron triglyceride in the fat depot [22]. But recent epidemiologic surveys have shown that mast cells or allergic responses might associate with obesity and diabetes. For example, asthma is more prevalent in obese children and adults [24–27], and obesity worsens asthmatic conditions [28–30]. One study shows that overweight and obesity are common among allergic women (76%) [31], suggesting that obesity and allergic conditions are linked – although the exact nature of that link remains unclear [32]. Diabetes also associates with allergic responses. Insulin resistance associates significantly with an increased incidence of wheezing and asthma-like symptoms [33]. Increased insulin contributes to enhanced airway SMC contractility, thereby leading to increased bronchial hyperresponsiveness [34]. Diet-induced obese (DIO) mice and genetically generated obese mice (ob/ob or db/db mice) are diabetic and exhibit increased baseline airway hyperresponsiveness [35–37]. Exogenous administration of the insulin-sensitizing adipokine adiponectin attenuates allergen-induced airway hyperreactivity and inflammation in mice [38], whereas adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodelling in chronic asthma in mice [39].
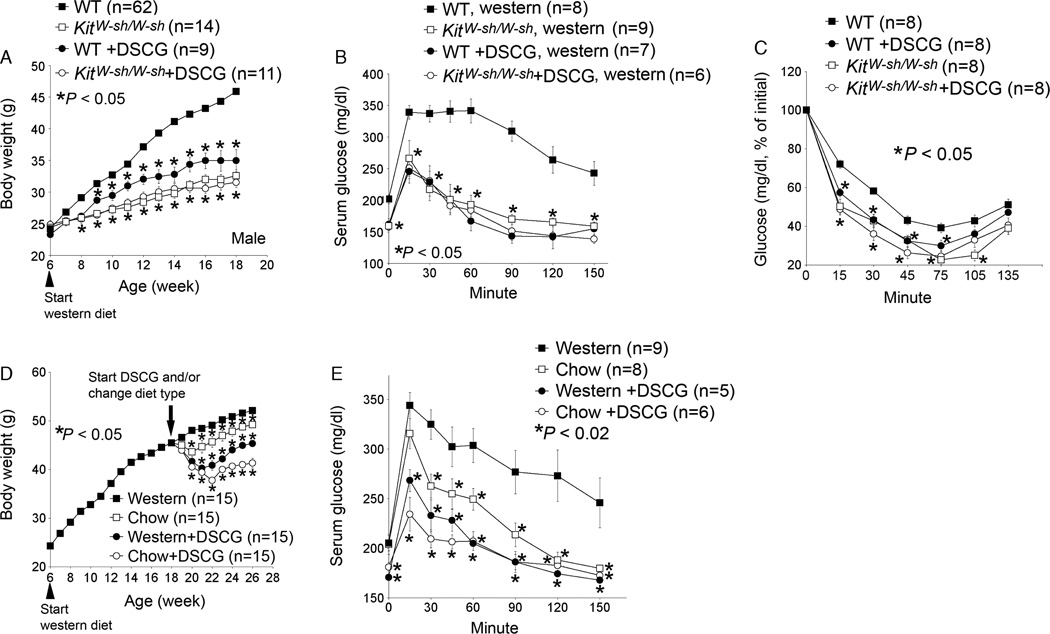
We recently demonstrated that mast cells participated directly in obesity and diabetes. In humans and mice, white adipose tissue (WAT) from obese subjects contained significantly higher numbers of mast cells than did WAT from lean subjects. Mast cell tryptase levels were significantly higher in serum from obese patients than in serum from lean subjects. Using mast cell-deficient KitW−sh/W−sh mice and the mast cell stabilizer disodium cromoglycate (DSCG or cromolyn), we demonstrated that the absence or inactivation of mast cells significantly reduced body weight gain (Figure 1A), glucose tolerance (Figure 1B), and insulin tolerance (Figure 1C). We obtained the same results when mast cell-deficient KitW/Wv mice or the mast cell stabilizer ketotifen (Zaditor) were used [10]. Two strains of mast cell-deficient mice and two mast cell stabilizers proved the same concept – that mast cells are essential to diet-induced obesity and associated type 2 diabetes. KitW−sh/W−sh mice and KitW/Wv mice showed significantly reduced body weight gain and reduced glucose and insulin tolerance, and cromolyn and ketotifen had similar effects. Furthermore, we demonstrated that these over-the-counter (OTC) drugs reversed pre-established DIO and diabetes. After 3 months on a Western diet, mice developed both DIO and glucose tolerance, but they lost significant body weight and had significantly improved glucose intolerance after being switched to a regular chow diet. The administration of cromolyn (Figure 1D and E) or ketotifen with a Western diet or a regular chow diet, however, yielded much greater reductions in body weight gain and glucose tolerance compared with the diet change alone, suggesting a role of mast cell inactivation in reversing obesity and diabetes. Combination of diet change and mast cell stabilizer administration (cromolyn (Figure 1D and E) or ketotifen) showed the best control of diabetes and obesity. These mice had body weight gain and glucose tolerance similar to those at the same age that had never been fed a Western diet. Therefore, the two OTC drugs not only reduced body weight gain and improved glucose and insulin tolerance in mice, but also reversed pre-established DIO and diabetes. Mast cells contributed to DIO and diabetes mechanistically by changing energy expenditure, adipose tissue microvessel growth, adipocyte apoptosis, and preadipocyte differentiation. Both KitW−sh/W−sh mice and those receiving cromolyn consumed similar amount of food and water to those of wild-type control mice, but showed significantly increased oxygen consumption, carbon dioxide production, and brown fat uncoupling protein 1 expression. Decreased body weight gain in KitW−sh/W−sh mice or cromolyn-treated mice was mainly due to the loss of fat mass. Lean mass percentage over total body weight in KitW−sh/W−sh mice or those receiving cromolyn was significantly increased, compared with that from wild-type control mice [10]. Although the precise roles of mast cell IL6 and IFN-γ in obesity and diabetes remain incompletely understood, the absence of these inflammatory cytokines in mast cells reduced body weight gain, glucose tolerance, and serum levels of leptin, insulin, and glucose.
Figure 1.
Absence or stabilization of mast cells reduces body weight gain and glucose and insulin tolerance. Wild-type (WT) or mast cell-deficient KitW−sh/W−sh mice at 6 weeks of age received disodium cromoglycate (DSCG) intraperitoneally while consuming a Western diet for 3 months. Body weight was monitored weekly (A), and glucose tolerance assay (B) and insulin tolerance assay (C) were performed at the end of Western diet consumption. In an independent group, 6-week-old WT mice consumed a Western diet for 3 months to develop obesity and diabetes. Mice were then separated into four groups and given different treatments (remaining on a Western diet with or without DSCG, and switched to a regular chow diet with or without DSCG) for an additional 2 months. Body weight was monitored weekly (D), and glucose tolerance assay (E) was performed at the end of all treatments. The number of mice for each treatment is shown in parentheses. *p < 0.05 was considered statistically significant, nonparametric Mann–Whitney U test. Modified from Ref. [8]
Although mast cells appear important to the pathogenesis of obesity and diabetes in mice, several critical issues remain. For example, WAT contains more than just mast cells. Adipose tissue SVF contains macrophages and several kinds of T cells, although different T cells have different pro-inflammatory or anti-inflammatory functions. In addition, we should determine whether our observed reversal of DIO and diabetes by OTC mast cell stabilizers in mice could be translated fully or partially to humans. We recently showed that mast cells, macrophages, and neutrophils all release TNF-α, IL6, and IFN-γ to activate endothelial cells. When mast cells from Tnf−/−, Il6−/−, and Ifng−/− mice were used to treat endothelial cells, the expression of adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, P-selectin, and E-selectin) from endothelial cells was reduced significantly, compared with endothelial cells exposed to wild-type mast cells [40]. Leukocyte migration and adhesion were increased on endothelial cell monolayer after treatment with degranulated mast cell supernatant, and endothelial cell pathological activities were significantly reduced when mast cell supernatants from Tnf−/−, Il6−/−, and Ifng−/− mice were used. Macrophages and neutrophils from Tnf−/−, Il6−/−, and Ifng−/− mice yielded similar results to those of mast cells [40]. Different blood leukocytes therefore may release similar sets of inflammatory cytokines to mediate their own adhesion to the endothelium. Based on our current understanding, however, both mast cells and CD8+ T cells are responsible for monocyte and macrophage recruitment to WAT. In DIO mice, depletion of CD8+ T cells with anti-CD8 antibody significantly reduced F4/80+ CD11c+ CD206− pro-inflammatory classically activated M1 macrophages in SVF, but did not affect F4/80+ CD11c− CD206+ alternatively activated anti-inflammatory M2 macrophages [18], suggesting a role of CD8+ T cells in M1 macrophage recruitment in adipose tissues. In contrast, the numbers of F4/80+ CD11c+ CD206− M1 cells in SVF from DIO mice lacking CD8+ T cells were similar to those in SVF from lean mice. Reconstitution of CD8+ T cells increased the F4/80+ CD11c+ CD206− M1 cells in SVF from DIO to the level of those in wild-type DIO mice [18]. In mast cell-deficient KitW−sh/W−sh mice, we detected low Mac3+ macrophages in WAT. Similarly, wild-type DIO mice treated with cromolyn or ketotifen [10] also showed reduced macrophage accumulation without affecting mast cell content in WAT. These observations suggest that both CD8+ T cells and mast cells drive the recruitment of macrophages in WAT, but more investigations are merited to further understand the mechanisms of these cell–cell interactions during the development of obesity at the molecular level.
Obesity and diabetes are not only public health issues, but also social problems in the United States and worldwide. Whether cromolyn, ketotifen, or other untested anti-allergy drugs could be used in patients with obesity and/or diabetes and whether their effects are limited only to experimental models are thus areas of great interest. Both cromolyn and ketotifen have been on the market for asthmatic or allergic patients for nearly 30 years. However, the molecular mechanisms, by which they inactivate mast cells, still has not been fully understood and there is no record whether these drugs demonstrate similar effects in controlling obesity and diabetes to what we observed in animal models. It is possible that asthmatic and allergic patients are not always obese or diabetic and they may stop using these anti-allergy drugs after the symptoms are relieved. Unnecessary chronic treatment, lack of systemic record, and unnecessary obese or diabetic among asthmatic or allergic patients make it inconclusive whether these mast cell stabilizers are effective in treating human metabolic diseases. Although formal clinical trials are not available currently, patient with type 2 diabetes showed improved serum glucose and hemoglobin A1c levels after a few months of treatment with oral cromolyn (Shi, unpublished data). In a small clinical trial of 35 asthmatic patients between 15 and 65 years of age, 3 months of inhaled cromolyn reduced body weight by 1.4 ± 0.8 kg [41]. Cromolyn also affects eosinophils and neutrophils [42], and has limited effect on intestinal mucosal mast cells in rodents [43] and humans [44]. Nevertheless, it still has been used widely as a mast cell stabilizer among allergy patients [45]. Both cromolyn and ketotifen are used commonly to treat pediatric allergic symptoms. Tranilast, another common mast cell stabilizer, is used in bronchial asthma, atopic dermatitis, and allergic conjunctivitis. The anti-angiogenic activity of tranilast recently was shown via inhibition of mast cell chymase and TGF-β [46]. Omalizumab, a humanized monoclonal antibody approved by the U.S. Food and Drug Administration (FDA), binds to free IgE for the treatment of allergic diseases and also is used as a mast cell stabilizer [47]. IgE activates mast cells by binding on its receptor FcεRI, and triggering the Syk-JNK-MAPK signalling cascade [48]. The Syk kinase therefore plays a central role in FcεRI signalling; interruption of this signalling pathway may impair mast cell activation. R112 is the first Syk inhibitor in clinical studies [49]. All of these drugs may have the same effects as cromolyn in controlling obesity and/or diabetes, but development and focus in this field are still limited to allergy and associated symptoms. Mast cell proteases are also possible targets for the development of medications for metabolic diseases. We recently showed that mast cell chymase [50] and tryptase [51] are important in regulating vascular cell apoptosis and microvessel growth in cardiovascular disease in mice. Mechanistically, these mast cell proteases caused vascular cell death and promoted angiogenesis by degrading ECM proteins (e.g. fibronectin, vitronectin, and nidogen) and vascular endothelial cadherin [52], and by producing angiotensin-II [53,54]. We also found that the absence of tryptase or chymase led to reduced expression and activities of cysteinyl cathepsins and matrix metalloproteinases [50,51], which have been implicated in obesity and diabetes [55–58]. Tryptase inhibitors (e.g. APC 2059) have been used to treat asthma and ulcerative colitis [59]. The heparin antagonists protamine and Polybrene® also inhibit tryptase activity by dissociating active tetrameric tryptase into inactive monomers [60]. Several oral chymase inhibitors are also available [61]; these protease inhibitors may have therapeutic applications in patients with metabolic diseases. We hope that in the near future, human trials will emerge to examine the efficacy of mast cell stabilizers and mast cell protease inhibitors in obesity, diabetes, and associated metabolic complications.
Acknowledgements
The authors thank Ms. Sara Karwacki for editorial assistance. This work is supported by National Institutes of Health (NIH) grants HL60942, HL81090, HL88547 (G. P. S), and by an American Heart Association Established Investigator Award, 0840118N (G. P. S).
Footnotes
Conflict of interest
None declared.
References
- 1.Mathias CB, Freyschmidt EJ, Caplan B, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182(4):2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34(11):1660–1664. doi: 10.1111/j.1365-2222.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- 4.Chichlowski M, Westwood GS, Abraham SN, Hale LP. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS One. 2010;5(8):e12220. doi: 10.1371/journal.pone.0012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner RT, Iwaniec UT, Marley K, Sibonga JD. The role of mast cells in parathyroid bone disease. J Bone Miner Res. 2010;25(7):1637–1649. doi: 10.1002/jbmr.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack M, Rosenkranz AR. Basophils and mast cells in renal injury. Kidney Int. 2009;76(11):1142–1147. doi: 10.1038/ki.2009.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13(6):719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Sukhova GK, Yang M, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117(11):3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci. 2011;68(6):965–975. doi: 10.1007/s00018-010-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Shi GP. Cysteinyl cathepsins and mast cell proteases in the pathogenesis and therapeutics of cardiovascular diseases. Pharmacol Ther. 2011;131(3):338–350. doi: 10.1016/j.pharmthera.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184(4):285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 14.Villena JA, Cousin B, Pénicaud L, Casteilla L. Adipose tissues display differential phagocytic and microbicidal activities depending on their localization. Int J Obes Relat Metab Disord. 2001;25(9):1275–1280. doi: 10.1038/sj.ijo.0801680. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 19.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30(2):193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 22.Hellman B, Larsson S, Westman S. Mast cell content and fatty acid metabolism in the epididymal fat pad of obese mice. Acta Physiol Scand. 1963;58:255–262. doi: 10.1111/j.1748-1716.1963.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 23.Hellman B. Studies in obese-hyperglycemic mice. Ann N Y Acad Sci. 1965;131(1):541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- 24.Beuther DA, Sutherland ER. Over-weight, obesity, and incident asthma: a meta analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910. [DOI] [PubMed] [Google Scholar]
- 26.Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008;121(5):1075–1084. doi: 10.1016/j.jaci.2008.03.005. quiz 1085–1086. [DOI] [PubMed] [Google Scholar]
- 27.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122(3):507–511. e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 30.Saint-Pierre P, Bourdin A, Chanez P, Daures JP, Godard P. Are overweight asthmatics more difficult to control? Allergy. 2006;61(1):79–84. doi: 10.1111/j.1398-9995.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferencz V, Meszaros S, Csupor E, et al. Increased bone fracture prevalence in postmenopausal women suffering from pollen-allergy. Osteoporos Int. 2006;17(3):484–491. doi: 10.1007/s00198-005-0011-z. [DOI] [PubMed] [Google Scholar]
- 32.Schaub B, von Mutius E. Obesity and asthma, what are the links? Curr Opin Allergy Clin Immunol. 2005;5(2):185–193. doi: 10.1097/01.all.0000162313.64308.b5. [DOI] [PubMed] [Google Scholar]
- 33.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39(5):700–707. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 34.Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hyper-contractile airway smooth muscle phenotype. Eur J Pharmacol. 2003;481(1):125–131. doi: 10.1016/j.ejphar.2003.08.081. [DOI] [PubMed] [Google Scholar]
- 35.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104(6):1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 36.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95(3):938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- 37.Lu FL, Johnston RA, Flynt L, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 38.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118(2):389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Medoff BD, Okamoto Y, Leyton P, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41(4):397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Alcaide P, Liu L, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6(1):e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke CW, May CS. A comparison of the efficacy of ketotifen (HC 20–511) with sodium cromoglycate (SCG) in skin test positive asthma. Br J Clin Pharmacol. 1980;10(5):473–476. doi: 10.1111/j.1365-2125.1980.tb01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moqbel R, Walsh GM, Macdonald AJ, Kay B. Effect of disodium cromoglycate on activation of human eosinophils and neutrophils following reversed (anti-IgE) anaphylaxis. Clin Allergy. 1986;16(1):73–83. doi: 10.1111/j.1365-2222.1986.tb01956.x. [DOI] [PubMed] [Google Scholar]
- 43.Pearce FL, Befus AD, Gauldie J, Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982;128(6):2481–2486. [PubMed] [Google Scholar]
- 44.Befus AD, Dyck N, Goodacre R, Bienenstock J. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol. 1987;138(8):2604–2610. [PubMed] [Google Scholar]
- 45.Patala Patalano F, Ruggieri F. Sodium cromoglycate: a review. Eur Respir J Suppl. 1989;6:556s–560s. [PubMed] [Google Scholar]
- 46.Jones SE, Gilbert RE, Kelly DJ. Tranilast reduces mesenteric vascular collagen deposition and chymase-positive mast cells in experimental diabetes. J Diabetes Complicat. 2004;18(5):309–315. doi: 10.1016/j.jdiacomp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Djukanović R, Wilson SJ, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170(6):583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 48.Novak N, Bieber T, Peng WM. The immunoglobulin E-Toll-like receptor network. Int Arch Allergy Immunol. 2010;151(1):1–7. doi: 10.1159/000232565. [DOI] [PubMed] [Google Scholar]
- 49.Meltzer EO, Berkowitz RB, Gross-bard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005;115(4):791–796. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120(11):973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Sun J, Lindholt JS, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108(11):1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17(7):611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 53.Blair RJ, Meng H, Marchese MJ, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99(11):2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muramatsu M, Katada J, Hayashi I, Majima M. Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem. 2000;275(8):5545–5552. doi: 10.1074/jbc.275.8.5545. [DOI] [PubMed] [Google Scholar]
- 55.Yang M, Zhang Y, Pan J, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9(8):970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 56.Yang M, Sun J, Zhang T, et al. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. 2008;28(12):2202–2208. doi: 10.1161/ATVBAHA.108.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Hul M, Lijnen HR. A functional role of gelatinase A in the development of nutritionally induced obesity in mice. J Thromb Haemost. 2008;6(7):1198–1206. doi: 10.1111/j.1538-7836.2008.02988.x. [DOI] [PubMed] [Google Scholar]
- 58.Chun TH, Inoue M, Morisaki H, et al. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59(10):2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tremaine WJ, Brzezinski A, Katz JA, et al. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Aliment Pharmacol Ther. 2002;16(3):407–413. doi: 10.1046/j.1365-2036.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- 60.Hallgren J, Estrada S, Karlson U, Alving K, Pejler G. Heparin antagonists are potent inhibitors of mast cell tryptase. Biochemistry. 2001;40(24):7342–7349. doi: 10.1021/bi001988c. [DOI] [PubMed] [Google Scholar]
- 61.Doggrell SA, Wanstall JC. Vascular chymase: pathophysiological role and therapeutic potential of inhibition. Cardiovasc Res. 2004;61(4):653–662. doi: 10.1016/j.cardiores.2003.11.029. [DOI] [PubMed] [Google Scholar]