Abstract
We discuss the role of comparators in H. pylori treatment trials and why design of an anti-H. pylori therapeutic trial for an infectious disease is fundamentally different from that of common gastrointestinal diseases (eg, the absence of a placebo response, the expectation that cure rates in excess of 95% are routinely possible, and the ability to understand why treatment fails). No comparator is absolutely required other than to 100% success; comparison trials should be limited to comparisons between therapies that reliably achieve 90% or greater success (i.e., good therapies). Comparisons to known low success regimens (i.e., bad therapies) are unethical as is withholding information from the subject regarding current effectiveness of a regimen even if that information would reduce the likelihood that the subject would volunteer. We also discuss how it is possible to predict the outcome of a published but locally untried new regimen. The reason for different outcomes of typical gastrointestinal therapies is shrouded in mystery. In contrast, treatment success for H. pylori should be predictable and treatment failures explainable. For too long expectations and analyses of H. pylori therapy has been confused with what is appropriate for gastrointestinal disease such as constipation or irritable bowel syndrome rather than for infectious diseases such as pneumonia.
Introduction
In this issue of Helicobacter, Calvert et al. present their carefully thought-out views of how to design an H. pylori therapy (1). The authors are among the most experienced in clinical trials of anti-H. pylori therapies as well as in the analysis of trials performed by others. They are also remarkably untainted by big PHARMA. The article is highly recommended as a primer for designing therapeutic anti-H. pylori trials and should also become a valued reference resource. There are however, a few caveats.
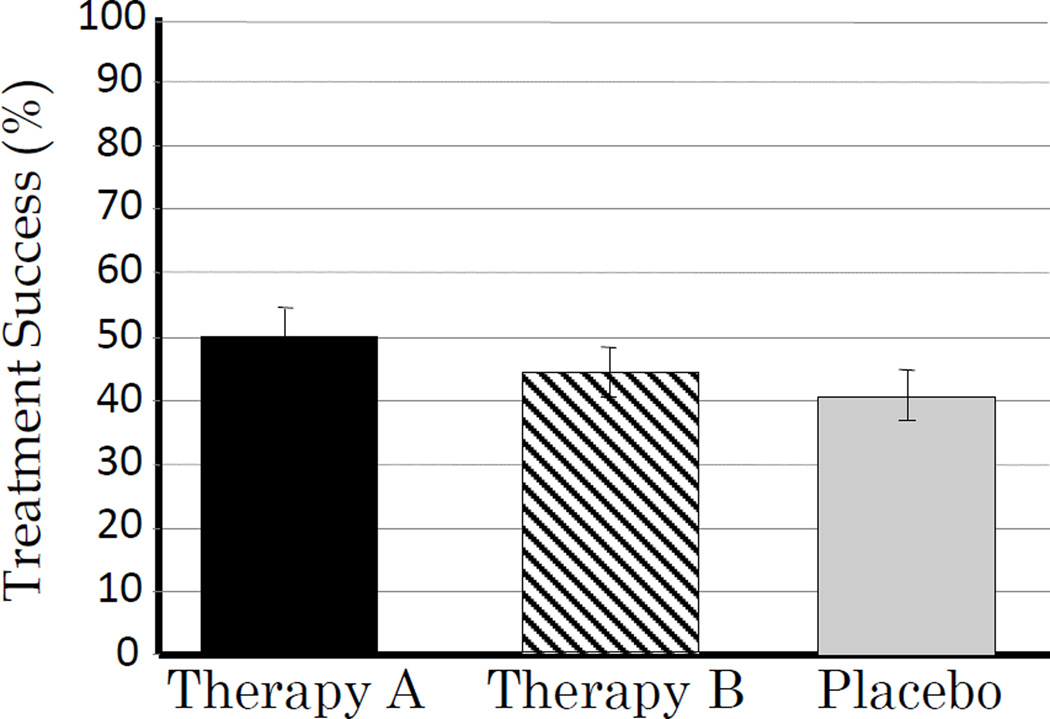
The authors suffer from a mild case of what I call “the course of the gastroenterologist” (also known as, “the compulsion to compare”) (2–4). This need to compare often arises early in gastroenterology training and when unnecessary or inappropriate the urge appears intractable. The diseases seen by gastroenterologists are usually diseases of unknown cause (e,g, functional, “autoimmune” etc) and ones that cannot reliably be cured. Most often we do not fully understand why a particular therapy is effective. We almost never expect our treatment success to approach 100%. Interpretation of studies is further complicated by a considerable placebo response that requires comparisons with placebo to substantiate any claim that the trial actually achieved a positive result (3;4) (Table 1, Figure 1). Even when the comparator is an active agent, a placebo is often required to ensure that the response to the active comparator was also superior to placebo. Not understanding why a regimen is successful, makes it almost impossible to understand why it fails. The degree of response to active drug and placebo often shows variation among trials making meta-analysis a useful tool to assist in identifying differences between therapies and treatment strategies.
Table 1.
Comparison of differences in clinical trials and analyses between typical gastrointestinal diseases and common bacterial infectious diseases
| Disease | ||
|---|---|---|
| GI | Infectious | |
| Result predictable | No | Yes |
| Placebo response | Yes | No |
| Comparator useful | Yes | No* |
| Meta-analyses useful | Yes | Rarely* |
only when comparing highly effective regimens (see text)
Figure 1.
Example of responses to therapy for typical GI disease such as irritable bowel disease, inflammatory bowel disease, constipation where a less than 100% success is expected and a placebo is generally needed.
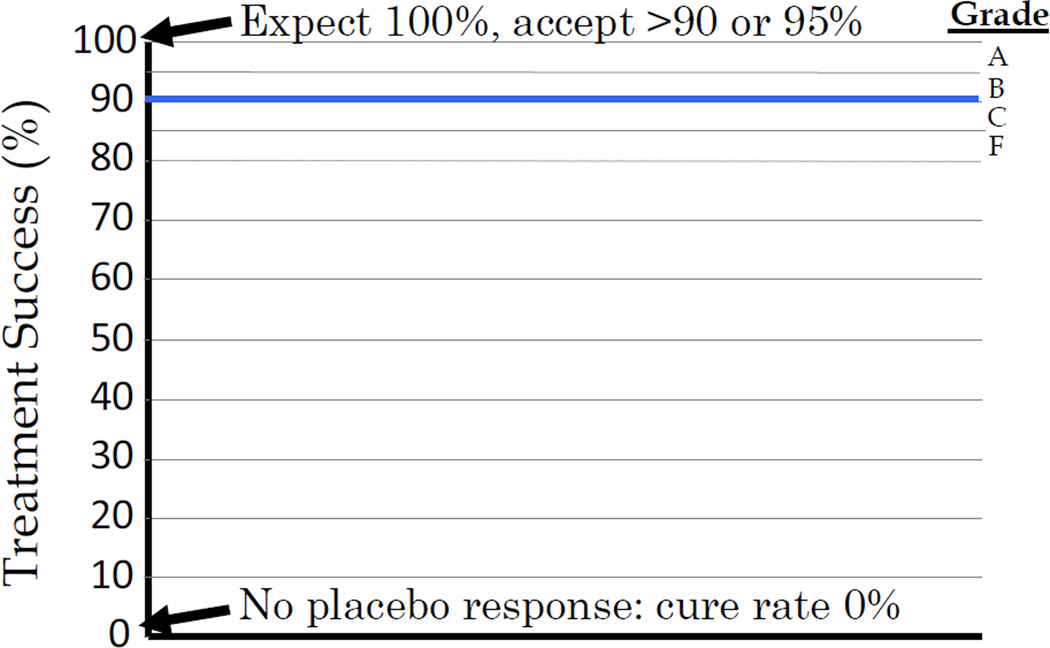
H. pylori infections differ from other problems in gastroenterology primarily because H. pylori is actually an infectious disease that was “captured” by gastroenterology. H. pylori is a common bacterial infections and can be reliably cured using appropriate antimicrobial therapy (i.e., with susceptible organisms one should expect 100% or near 100% treatment success) (3). There is also no placebo response which remarkably changes the requirements for any clinical trial (Table 1, Figure 2). Failure of a H. pylori therapy is also almost always explainable in terms of either antimicrobial resistance or an flawed regimen (e.g., in terms of duration, formulation, etc) (Table 1). Importantly, a regimen that is effective anywhere in the world should be equally effective anywhere else provided that the conditions are the same (pattern of resistance, same drugs and their metabolism). Because the results of an effective anti-H. pylori therapy with susceptible strains should always approach 100% per protocol one can score of the results of a regimen broadly as either good (e.g., reliably provides 90% or greater success, preferably 95% or greater), or bad. Here we define bad as treatment success of less than 90% or less than 85% (if one wishes to include a “gray” zone between 85 and 90%).
Figure 2.
Depiction of the possible outcomes when planning a clinical trial of an anti-H. pylori therapy.
The trial should be planned with stopping points if it becomes clear that it cannot achieve the prespecified criteria for a successful trial (e.g., using ordered categories of success) (5).
Normally, as resistance to common bacterial infections (e.g., E. coli urinary tract infections, pneumococcal pneumonia, gonorrhea, tuberculosis) increases and success declines to unacceptable levels, new regimens are introduced. Few would consider or recommend comparing the new highly successful regimen to a previous “locally best” or “tradition” in which resistance had undermined success (i.e., there would be no need to “prove” that the new regimen was “better” than one that was known to be no longer acceptable locally). However, this seemingly unimaginable scenario occurs often in anti-H. pylori clinical trials. Not only are good and bad anti-H. pylori therapies compared but the results are then subjected to meta-analyses which only prove that what was known to a bad regimen is reliably bad (3). It is unethical to enter subjects into a trial using a known inferior regimen (2). It is also unethical to withhold full information from the subject regarding current effectiveness of a regimen even if that information would reduce the likelihood that anyone would volunteer (i.e., an inferior regimen can never be called the “standard of care” or “approved” in lieu of telling the truth about the actual expected outcome).
If a known bad regimen is not a suitable comparator, what is?
Since 100% success can be achieved, 100% success is a comparator of choice with therapies being judged in terms of how close they come to achieving that level of success. If the best local therapy provides unacceptable low cure rates, it should be abandoned just as was single drug therapy for tuberculosis or low dose penicillin for pneumonia or syphilis. We do not suggest that comparisons between regimens should never be done rather comparisons should be restricted to known good therapies (i.e., in order to identify the best in terms of outcome, cost, convenience, side effects, etc).
Prediction of outcome with published but untried new therapy
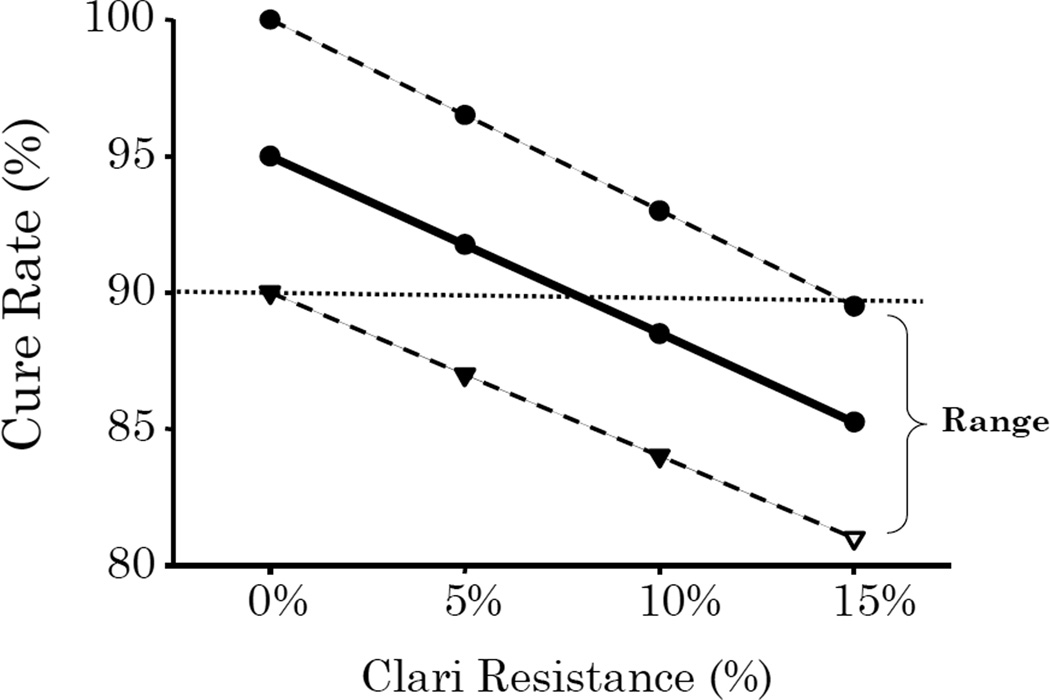
One only needs to know the success rates for a H. pylori regimen and its components, in relation to the presence of resistance and the level of resistance locally to be able to predict the range of possible outcomes. For example, with legacy triple therapy consisting of a PPI, clarithromycin, and amoxicillin, the data needed are: the cure rate for the three drug combination, and each of the two dual therapies (i.e., PPI-clarithromycin and PPI-amoxicillin). Since amoxicillin resistance is extremely rare, one only need to know the rates for the triple therapy and the PPI-amoxicillin dual component (Table 1). In the majority of cases the overall effect is related to the triple component. For example with 20% clarithromycin resistance, the cure with 14 day triple would be the success with susceptible strains plus the success with clarithromycin resistant strains. If the success of triple therapy with susceptible strains was 95%, the calculations would be (per 100 subjects) 100 subjects minus 20 failures = 80 susceptible×the cure rate (95%) = 76 cures + the contribution of the dual therapy @25% or 20×25% or 4 cures for a total treatment success of 80% (from table 1 the range would be 78 to 86%). For a 7 day therapy the corresponding results would be a success rate of 74% (range 72 to 76%). The difference between 7 and 14 day therapy is 6% which also is consistent with data from prior meta-analyses. One can easily calculate the effect of different percentages of clarithromycin resistance (Figure 3) and it becomes clear that on average for a 14 day triple therapy, the success rate will fall below 90% success when the rate of clarithromycin resistance is approximately 8%.
Figure 3.
The range of outcomes in relation to the proportion of the population with clarithromycin resistant infections. The regimen depicted is a cure rate of 95% ± 5% for the three drugs, and 30% for the PPI plus amoxicillin dual therapy given for 14 days.
A similar exercise can be done for any combination regimen (see reference (3) for examples with sequential, concomitant and hybrid therapies). The fact that results with different patterns of resistance have rarely been reported makes the calculations with clarithromycin-containing regimens a bit more complicated but is still clinically useful. That is not to say that new regimens should be introduced without testing in a new population but rather, one would be able to prospectively predict which regimens will be successful and which should not evaluated because they are destined to fail.
Table 2.
Effect of clarithromycin resistance on outcome of triple therapy
| Duration | Regimen Cure rate | (Range) |
|---|---|---|
| 14 day: | PPI-C-A = 95 ± 5% | (90–100%) |
| PPI-A = 30 ± 20% | (10–50%)* | |
| 7 day: | PPI-C-A = 90 ± 5% | (85–95%) |
| – | PPI-A = 10 ± 10% | (0–20%)* |
result with clarithromycin resistant strains.
PPI = proton pump inhibitor, C = Clarithromycin, A = amoxicillin
Acknowledgments
Acknowledgments and potential conflict
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338. which funds the Texas Medical Center Digestive Diseases Center, DK067366 and CA116845. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Dr. Graham is a unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a also a paid consultant for Otsuka Pharmaceuticals regarding diagnostic testing until has received royalties on the Baylor College of Medicine patent covering materials related to 13C-urea breath test. Dr. Dore has nothing to declare.
References
- 1.Calvet X, Gisbert JP, Suarez D. Key points for designing and reporting H. pylori therapeutic trials. A personal view. Helicobacter. 2011 doi: 10.1111/j.1523-5378.2011.00890.x. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8(12):1032–1036. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Rimbara E. Understanding and appreciating sequential therapy for Helicobacter pylori eradication. J Clin Gastroenterol. 2011;45(4):309–313. doi: 10.1097/MCG.0b013e31820ac05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham DY. Efficient identification and evaluation of effective Helicobacter pylori Therapies. Clin Gastroenterol Hepatol. 2009;7(2):145–148. doi: 10.1016/j.cgh.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12(4):275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]