Abstract
Conceptual metaphor theory suggests that knowledge is structured around metaphorical mappings derived from physical experience. Segregated processing of object properties in sensory cortex allows testing of the hypothesis that metaphor processing recruits activity in domain-specific sensory cortex. Using functional magnetic resonance imaging (fMRI) we show that texture-selective somatosensory cortex in the parietal operculum is activated when processing sentences containing textural metaphors, compared to literal sentences matched for meaning. This finding supports the idea that comprehension of metaphors is perceptually grounded.
Keywords: fMRI, parietal operculum, grounded cognition, tactile
1. INTRODUCTION
Some accounts of cognition propose that knowledge is represented in abstract codes, distinct from the sensory modalities through which the knowledge was acquired, and that cognitive processes involve computations on these amodal representations (Fodor, 1975; Pylyshyn, 2007). Theories of grounded cognition reject this notion, proposing instead that knowledge is represented in modal systems derived from perception and that cognition depends on perceptual simulations (Barsalou, 2008).
Conceptual metaphor theory is one approach to grounded cognition which suggests that knowledge is structured by metaphorical mappings from sensory experience (Lakoff & Johnson, 2003). It is argued that this is true even for abstract concepts like time, for which metaphorical mappings can be made from experience of more concrete domains, such as space (Boroditsky, 2000; Casasanto & Boroditsky, 2008); for example, we speak of falling ‘behind’ schedule or looking ‘forward’ to an event. Conceptual metaphor theory is supported by a number of behavioral studies (e.g. Ackerman, Nocera & Bargh, 2010; Boot & Pecher, 2010; Gibbs & Matlock, 2008; Oullet, Santiago, Israeli & Gabay, 2010: Wilkowski, Meier, Robinson, Carter & Feltman, 2009). For example, the experiences of light/heavy, rough/smooth, or hard/soft objects influenced subsequent judgments of importance, difficulty of social interaction, or flexibility in negotiation, respectively (Ackerman et al., 2010); and anger primes led to overestimation of actual room temperature (Wilkowski et al., 2009). These studies suggest that metaphorical expressions such as ‘weighty matters’, ‘coarse language’, ‘unbending attitude’, and ‘hot-headed’ have a perceptual basis. By contrast, others suggest that conventional metaphors, such as the time/space mappings noted above, are merely overlearned idiomatic associations and that lexicalization of such metaphors results in separate stored meanings (Keysar & Bly, 1999; Keysar, Shen, Glucksberg & Horton, 2000). On this account, there is no need to invoke associative mapping to understand that having a ‘rough’ day means having a ‘bad’ day, since the metaphor is in such common usage that the meaning of the word ‘rough’ is stored as both ‘abrasive’ and ‘unpleasant’.
These two approaches to metaphor comprehension make very different predictions about the neural basis of metaphor processing. If lexicalized metaphors do not require access to the corresponding concrete concept, then activity should be largely limited to classical language areas, whereas conceptual metaphor theory predicts activity in sensory areas involved in processing the domain from which the metaphor is derived. Previous studies showed increased activity in language areas for both conventional (Eviatar & Just, 2006; Lee & Dapretto, 2006) and novel (Rapp, Leube, Erb, Grodd & Kircher, 2004) metaphors. However, since the metaphors in these studies were drawn from multiple domains, the locus of sensory activity might have varied across trials, accounting for the failure to observe consistent sensory processing.
More recent studies have begun to address whether there is a sensorimotor basis for metaphor comprehension by restricting the metaphors employed to a single domain (Chen, Widick & Chatterjee, 2008; Raposo, Moss, Stamatakis & Tyler, 2009; Boulenger, Hauk & Pulvermüller, 2009; Desai, Binder, Conant, Mano & Seidenberg, 2011). These studies have concentrated on action verbs, particularly in relation to the idea of embodiment. However, the picture that emerges from this work is not entirely consistent. Raposo et al. (2009) found activity in motor and premotor cortex for literal, but not metaphorical, usages of arm- or leg-related verbs. Conversely, Boulenger et al. (2009) found somatotopic activation for arm- and leg-related verbs in both literal and metaphorical usages. Aziz-Zadeh and Damasio (2008), in a critique of studies reporting somatotopic recruitment of motor regions by action verbs, suggested that alternative organizing principles such as action goals, rather than somatotopy, may govern the simulations presumed to underlie motor cortical recruitment. Recently, Desai et al. (2011) reported activity for both metaphorical and literal action sentences in the left anterior inferior parietal lobule and the cerebellum: this activity was interpreted as motor-related, although these regions also have non-motor functions. Interestingly, activity in primary and supplementary motor cortices and in the superior temporal sulcus (implicated in biological motion perception) was inversely related to familiarity of both metaphorical and literal usages (Desai et al., 2011). Metaphorical use of motion verbs recruited a part of the middle temporal gyrus, close to the visual motion area MT (Chen et al., 2008) but, since this study did not include a functional localizer, the precise relationship between regions processing motion perception and motion metaphors remains unclear.
We reasoned that the segregated processing of object properties in sensory cortex offers alternative approaches to testing conceptual metaphor theory. In the present study, we restricted metaphors to the single domain of texture, which is commonly perceived both haptically and visually. We previously demonstrated texture-selective regions in somatosensory cortex of the parietal operculum for haptic stimuli and in early visual cortex for both haptic and visual stimuli (Stilla & Sathian, 2008; Sathian, Lacey, Stilla, Gibson, Deshpande, et al., 2011). Here, we used rapid event-related fMRI to compare processing of sentences containing familiar, textural metaphors against literal sentences with similar meanings and sentence structures. We present preliminary evidence for the hypothesis that processing textural metaphors activates texture-selective somatosensory areas defined on independent functional localizer scans.
2. RESULTS
Mean response time (±SEM) for metaphorical sentences (.84s ± .12s) was longer than for literal sentences (.63s ± .07s), 2-tailed t6 = −3.25, p = .02). A contrast of the metaphorical and literal conditions showed that the textural metaphors activated somatosensory cortex in the parietal operculum, in bilateral OP1 and left OP3, which were previously shown to be texture-selective during haptic perception in a different group of subjects (Stilla & Sathian, 2008), and also cortex around the right inferior precentral sulcus (Table 1).
Table 1.
Regions active during the textural metaphor (TM) task. x,y,z: Talairach coordinates for the center of gravity of activations.
| Region | x | y | z |
|---|---|---|---|
| Left OP1 | −51 | −23 | 19 |
| Left OP3 | −43 | −12 | 19 |
| Right OP1 | 58 | −26 | 22 |
| Right inferior precentral sulcus | 35 | −6 | 32 |
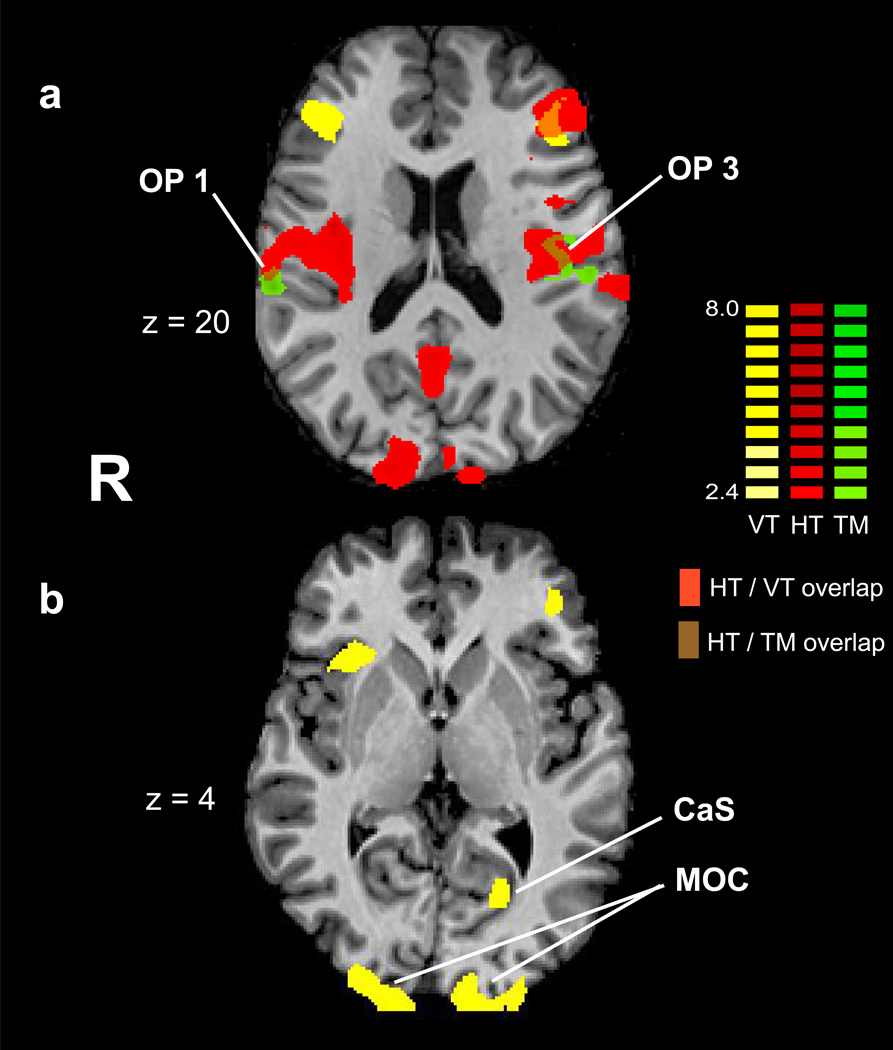
To verify haptic texture-selectivity at the parietal opercular foci in the participants of the present study, we overlaid the activations during processing of textural metaphors onto the functionally localized texture-selective activations from the same group of individuals during haptic perception (Gibson et al., 2008). This confirmed that the parietal opercular regions shown here to be active during listening to textural metaphors were within regions of haptic texture-selectivity (Figure 1). However, there was no metaphor-specific activity within visually or multisensory texture-selective regions of early visual (medial occipital) cortex.
Figure 1.
Textural metaphor and texture perception activations. Axial slices showing (a) textural metaphor activations (green) overlaid on the same individuals’ haptic (red) and visual (yellow) texture-selective regions. Overlap zones between these activations (brown) are seen only in the parietal operculum (right OP1 and left OP3 are shown; the overlap in left OP1 was on a more superior axial slice that is not illustrated). Textural metaphor activations do not overlap with other haptic, visual, or multisensory (orange) texture-selective regions in either frontal cortex (bilateral inferior frontal sulcus and gyrus) or (b) visual cortex (MOC: medial occipital cortex; CaS: calcarine sulcus). VT: visual texture; HT: haptic texture; TM: textural metaphor. Talairach z plane shown for each slice; color scale: t-scales for the contrasts.
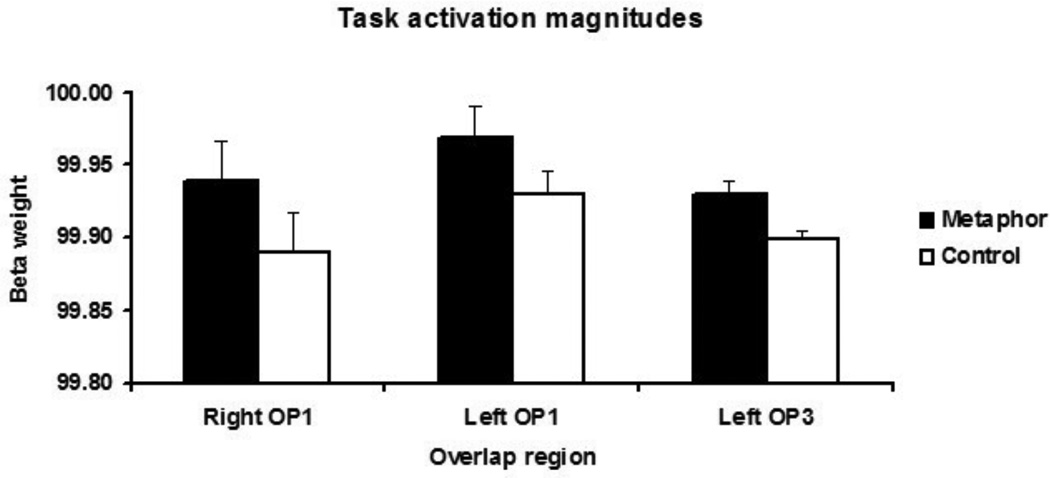
For further analysis, we created regions of interest (ROIs) centered on the parietal opercular overlap zones. The activation magnitudes in these overlap zones, shown in Figure 2, were significantly greater in the metaphorical than the literal condition (as expected based on the contrasts used). Although the metaphorical and control sentences differed in word frequency and imageability, activation magnitudes (z-transformed betas) in the parietal opercular ROIs were uncorrelated with either word frequency or imageability for both metaphorical and control literal sentences (r values ranged from −.2 to .2, p values ranged from .2 to .9). We therefore conclude that the parietal opercular activations observed during texture metaphor comprehension were not likely to result from differences in word frequency or imageability between the metaphorical and literal sentences that we used.
Figure 2.
Activation magnitudes in the textural metaphor/ haptic texture-selective overlap zones for the textural metaphor and literal control conditions. Error bars: SEM.
Because of the relatively small sample size, we verified the robustness of the parietal opercular activations during texture metaphor processing by calculating effect sizes and 95% confidence intervals (95% CI) for the activation magnitudes, as indexed by the baseline-referenced z-transformed beta weights for the parietal opercular ROIs in each condition (see Lacey, Hagtvedt, Patrick, Anderson, Stilla et al., 2011). Cohen’s d was .65 (95% CI .61–.69) for left OP1, 1.63 (95% CI 1.61–1.64) for left OP3, and .74 (95% CI .69–.79) for right OP1. Thus there were medium-to-large effects, with very narrow confidence intervals, that were clearly distinct from zero. An important concern with small samples is that they can be associated with wide confidence intervals that make effect size estimates difficult to interpret. Here, we can be reassured that the parietal opercular activations in response to texture metaphors are robust because the effect sizes are associated with narrow confidence intervals that are far from zero.
Whereas activations on the metaphor > literal contrast were limited to the parietal operculum and inferior precentral sulcus, the reverse contrast revealed a widely distributed set of areas (Supplementary Table 2), the majority of which showed baseline-referenced deactivations to the metaphorical sentences. Neither contrast showed differential activation in classical language areas, suggesting that linguistic processing in these areas was equivalent across sentence type.
3. DISCUSSION
Our findings provide the first clear evidence for activity in functionally localized, domain-specific sensory cortical areas during processing of metaphors. Using metaphors drawn from the single domain of texture and contrasting these with literal sentences matched for meaning and sentence structure, we observed activation in somatosensory texture-selective areas, but did not find differential activation either in language areas or in visual or bisensory texture-selective areas. Although texture can also be perceived in the auditory modality (e.g., Guest, Catmur, Lloyd & Spence, 2002), selective activation was not found in auditory cortical regions. Texture is particularly salient to touch (Klatzky, Lederman & Reed, 1987), which tends to dominate vision in texture perception (Guest and Spence, 2003) and exceeds vision at discriminating fine textures (Heller, 1989). In addition, the texture words used were predominantly related to touch (see, for example, Stadtlander & Murdoch, 2000; Lynott & Connell, 2009) and many metaphors were likely more easily interpreted by reference to touch than vision. For example, the phrases ‘a rough day’ or ‘a slimy person’ have negative connotations because they refer to attributes that may be particularly unpleasant to touch. Thus, it appears that metaphor processing selectively activated sensory areas in the modality from which the metaphors primarily derived their meaning. Additional research might manipulate the emotional valence of metaphors by reference to different modalities in order to examine this further.
We did not find differences in classical language areas between the activity evoked by metaphorical and literal sentences. This does not rule out a role for classical language areas in metaphor processing. However, it is noteworthy that previous studies reporting activity in such areas during metaphor processing did not use controls as tightly matched for meaning and sentence structure as we did. For instance, the literal and metaphorical sentences used by Eviatar and Just (2006) were not matched either for meaning or sentence structure; Lee and Dapretto (2006) used sets of single words in which the target word was used in either a literal or metaphorical sense; and Rapp et al. (2004) matched sentence structure but not meaning. Further work is necessary to address the potential role of classical language areas in metaphor processing.
The right inferior precentral sulcus was specifically active during processing of textural metaphors, but did not display perceptual texture-selectivity. Although its role here is unclear, nearby foci are involved in visuo-somatic body-centered spatial coding (Galati, Committeri, Sanes & Pizzamiglio, 2001) and body ownership (Ehrsson, Holmes & Passingham, 2005), suggesting that this region could perhaps mediate a more general grounding of metaphors or language in somatic representations. Interestingly, this area is active when observing sign language but not when hearing spoken language (Pa, Wilson, Pickell, Bellugi & Hickok, 2008).
Although the metaphorical and literal sentences differed in word frequency and imageability, there was no correlation between these variables and activation magnitudes in the parietal opercular ROIs. It seems unlikely, therefore, that the activation observed during processing of texture metaphors was influenced by these factors. Further, the metaphor > literal contrast did not show activation in visual cortical areas that are known to be active during visual imagery (e.g., Ganis, Thompson & Kosslyn, 2004). This suggests that the difference in imageability between conditions was not sufficient to elicit differential activity due to visual imagery. However, more work is required to unequivocally exclude the contribution of factors such as word frequency and imageability to results such as those reported here.
The two sentence types used in the present study differed in both metaphoricity and use of a specific sensory domain. Thus, it is difficult to estimate the contribution of each of these factors to the observed results. One way to dissociate these factors is to use literal sentences referring to specific sensory domains in addition to metaphorical sentences; we are currently pursuing this approach. Analogously, Desai et al. (2011) showed that both literal and metaphorical action sentences activate the left anterior inferior parietal lobule which is involved in action planning.
We did not attempt to manipulate the degree of conventionality, or lexicalization, of the metaphorical sentences, selecting only familiar metaphors (as borne out by the high familiarity ratings with low variability, see 4.2 below) and avoiding novel metaphors. A strong version of conceptual metaphor theory would predict sensory cortex to be activated regardless of familiarity, whereas a weak version would predict such activation only for novel metaphors, or activation correlating with the degree of metaphor novelty because the underlying simulations become less critical as metaphors become more familiar (Bowdle & Gentner, 2005). In keeping with this latter idea, Desai et al. (2011) showed that activation in motor regions was inversely related to familiarity of action metaphor sentences. Tremblay and Small (2011) also argue against a strong simulation-based account of language comprehension, at least for action-related language, on the basis that there are different patterns of activity in ventral premotor cortex for observation of and processing sentences about actions; however, this disparity could have arisen because the actions were not matched across these conditions. Our present results, which are in a sensory rather than action domain, tend to favor a strong conceptual metaphor account but replication in other metaphoric domains and explicit testing of novel domain-specific metaphors are required in future work.
The present findings, together with numerous behavioral studies (e.g. Ackerman et al., 2010; Boot & Pecher, 2010; Gibbs & Matlock, 2008; Oullet et al., 2010: Wilkowski et al., 2009), are consistent with the conceptual metaphor theory of grounded cognition (Lakoff & Johnson, 2003), but not with accounts of lexicalized metaphors in which these are simply learned linguistic conventions that do not require access to root concepts (Keysar & Bly, 1999; Keysar et al., 2000). Earlier behavioral research on spoken word recognition has shown that representations of multiple candidate words with the same onset are transiently activated before the complete word is disambiguated (e.g., Marslen-Wilson & Zwitserlood, 1989; Yee & Sedivy, 2006). These transient activations extend to fMRI-observable, perceptually based, semantic representations in the specific case of motion-related words in a newly-learned artificial lexicon (Revill, Aslin, Tanenhaus & Bavelier, 2008). At present, it is unclear whether such transient activations occurring during processing of natural language can be recorded using fMRI, since such activations are presumably even more transient than those occurring during processing newly learned words. However, although our results are consistent with conceptual metaphor theory, ascertaining whether the metaphor-specific activations found in the present study are actually necessary for comprehending conventional metaphors requires other approaches. One approach we are currently pursuing is to use transcranial magnetic stimulation over domain-specific sensory cortex to disrupt metaphor processing. Another approach would be to test patients with lesions confined to specific sensory cortical regions to see whether they exhibit deficits in comprehending metaphors referencing the corresponding domains.
Previous neuroimaging studies using action verbs to examine the role of embodiment in metaphor processing have yielded inconsistent results, perhaps because the underlying simulations might be organized in different ways, either somatotopically or according to action goals (Aziz-Zadeh & Damasio, 2008). Here, we took advantage of the segregated sensory processing of different object properties and the fact that texture perception is commonly achieved haptically or visually. By restricting metaphors to the single domain of texture and employing visual and haptic texture localizers, we were able to show that the simulations underlying such metaphors are likely somatosensory, since we did not find metaphor-related activation in either visual or bisensory texture-selective cortex. In conclusion, although based on a relatively small sample, the present study provides a preliminary proof of concept for conceptual metaphor theory.
4. METHODS
4.1 Participants
Seven people took part in the study (2 males, 5 females; mean age 20 years, 8 months). Due to the nature of the task, we excluded participants for whom American English was a second/non-native language. All were right-handed based on the validated subset of the Edinburgh handedness inventory (Raczkowski, Kalat & Nebes, 1974). All were people for whom we had localized visual, haptic and bisensory texture-selective areas in a separate fMRI study of texture perception (Gibson, Stilla & Sathian, 2008). At that time, the present study had not been planned and the average delay between participating in the two studies was 4.6 months. Participants were not aware of the connection between the two studies and were naïve as to the aim of the current experiment; no mention was made of the use of metaphors, textural words or imagery. Informed consent was obtained and all procedures were approved by the Emory University Institutional Review Board.
4.2 Materials
We paired 54 sentences containing conventional texture metaphors (‘She had a rough day’) with control sentences containing an equivalent literal meaning (‘She had a bad day’), by replacing the texture metaphor with non-metaphorical words. For 45 sentence-pairs this entailed replacement of a single word; for the other 9 sentences, a short phrase containing the texture metaphor was replaced. The sentences used are listed in Supplementary Table 1. The metaphorical and control sentences were matched as a group for the average number of syllables per sentence (t106 = −.25, p = .8). The metaphorical texture words used differed in frequency (Kucera & Francis, 1967) (t106 = −2.32, p = .02) and imageability (Toglia & Battig, 1978; Gilhooly & Logie, 1980) (t106 = 6.26, p <.001) from the corresponding words used in the literal control sentences. Pilot testing (n = 12) showed 94.2% agreement that the meanings of the metaphorical sentences and their literal counterparts were similar. Pilot testing in the same group also provided imagery ratings, using the same methods as Toglia & Battig (1978), for a small number of words lacking published ratings. A separate group (n = 10) rated familiarity of the metaphors on a 1–7 scale, where 1 = very unfamiliar and 7 = very familiar. The mean rating was 5.86 (standard deviation .96); a one-sample t-test showed that this was significantly higher than the mid-point of the scale (t53=18.13, p< .001). We tested against the mid-point as this represents a point at which the metaphors are not clearly familiar or unfamiliar; it therefore serves as a suitable reference point to give meaning to the observed ratings (Lacey et al., 2011). The sentences were recorded in Adobe Audition (Adobe Systems Inc., San Jose, California) and edited to last 2s. The speaker recited the sentences in a neutral tone with minimum inflection. Acoustic analyses of the sound files, carried out using PRAAT (Boersma & Weenink, 2008), showed that the metaphorical and literal sentences were matched for duration (t106 = −.02, p = .98), pitch (t106 = −1.57, p = .12), amplitude (t106 = .46, p = .64), and speech rate (syllables per second) (t106 = −.62, p = .53).
4.3 Functional imaging
Participants lay supine in the scanner, with foam blocks positioned around the head to minimize movement. A mirror angled over the head coil enabled participants to see a centrally placed fixation cross projected on a screen placed in the rear magnet aperture. Participants were instructed to keep their eyes open and fix their gaze on the cross. They were instructed to listen to the sentences and press a response button with the left index finger as soon as they understood the sentence, using a fiberoptic response box. The sentences were presented through headphones that attenuated external sounds by 20 dB to muffle scanner noise. Each participant completed 2 runs in a single scan session, in counterbalanced order across participants. Each run consisted of 54 trials of 4s duration, with jittered inter-trial intervals of 4, 6, 8, and 10s in a quasi-exponential distribution (Ollinger, Corbetta & Shulman, 2001). In each trial, a sentence was presented for 2s, followed by a 2s response period. Half the trials contained metaphors, while the other half were literal controls. The two trial types were interleaved in pseudorandom orders so that a metaphorical sentence and its literal counterpart were never presented together. There were six 10s baseline periods of fixation without stimulation, one at the beginning and end of each run and four at pseudorandom intervals during each run. The stimuli were presented, and responses recorded, using Presentation software (Neurobehavioral Systems Inc., Albany, California).
4.4 MR scanning
MR scans were performed on a 3 Tesla Siemens Trio whole body scanner (Siemens Medical Solutions, Malvern, PA), using a twelve-channel matrix headcoil. T2*-weighted functional images were acquired using a single-shot gradient-recalled echoplanar imaging (EPI) sequence for blood oxygenation level-dependent (BOLD) contrast. These functional scans acquired 29 horizontal slices of 4mm thickness using the following parameters: repetition time (TR) 2000ms, echo time (TE) 30ms, field of view (FOV) 220mm, flip angle (FA) 90°, in-plane resolution 3.4×3.4mm, and in-plane matrix 64×64. High-resolution 3D anatomic images were acquired using an MPRAGE sequence (TR 2300ms, TE 3.9ms, inversion time 1100ms, FA 8°) comprising 176 sagittal slices of 1mm thickness (FOV 256mm, in-plane resolution 1×1mm, in-plane matrix 256×256). Once magnetic stabilization was achieved in each run, the scanner triggered the computer running the Presentation software so that the sequence of experimental trials was synchronized with scan acquisition.
2.5 Image processing and analysis
Image processing and analysis was performed using BrainVoyager QX v1.6.3 (Brain Innovation, Maastricht, Netherlands). Each subject's functional runs were real-time motion-corrected utilizing Siemens 3D-PACE (prospective acquisition motion correction). Functional images were preprocessed utilizing sinc interpolation for slice scan time correction, trilinear-sinc interpolation for intra-session alignment of functional volumes, and high-pass temporal filtering to 3 cycles per run to remove slow drifts in the data. Anatomic 3D images were processed, co-registered with the functional data, and transformed into Talairach space (Talairach & Tournoux, 1988). Activations were localized with respect to 3D cortical anatomy with the help of an MRI atlas (Duvernoy, 1999). For group analysis, the transformed data were spatially smoothed with an isotropic Gaussian kernel (full-width half-maximum 4mm). Runs were percent signal change normalized. Statistical analysis of group data used random-effects, general linear models (GLM) followed by pairwise contrasts (metaphor > literal and vice versa). Correction for multiple comparisons (corrected p<0.05) was achieved by imposing a threshold for the volume of clusters comprising contiguous voxels that passed a voxel-wise threshold of p<0.05, using a 3D extension (implemented in BrainVoyager QX) of the 2D Monte Carlo simulation procedure described by Forman et al. (1995). Corrections were performed within a mask comprising all voxels active in either task relative to baseline.
Highlights.
Texture metaphors activate texture-selective somatosensory cortex
Consistent with theory that metaphor comprehension is perceptually grounded
Supplementary Material
Acknowledgments
Support to KS from the National Eye Institute, National Science Foundation and the Veterans Administration is gratefully acknowledged. We thank Amy Anderson for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman JM, Nocera CC, Bargh JA. Incidental haptic sensations influence social judgments and decisions. Science. 2010;328:1712–1715. doi: 10.1126/science.1189993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Damasio A. Embodied semantics for actions: Findings from functional brain imaging. Journal of Physiology-Paris. 2008;102:35–39. doi: 10.1016/j.jphysparis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. PRAAT: doing phonetics by computer. (v5.0.41) [computer program] 2008 Retrieved 23 November 2008 from http://www.praat.org/
- Boot I, Pecher D. Similarity is closeness: metaphorical mapping in a conceptual task. Quarterly Journal of Experimental Psychology. 2010;63:942–954. doi: 10.1080/17470210903134351. [DOI] [PubMed] [Google Scholar]
- Boroditsky L. Metaphoric structuring: understanding time through spatial metaphors. Cognition. 2000;75:1–28. doi: 10.1016/s0010-0277(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Hauk O, Pulvermüller F. Grasping ideas with the motor system: semantic somatotopy in idiom comprehension. Cerebral Cortex. 2009;19:1905–1914. doi: 10.1093/cercor/bhn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdle B, Gentner D. The career of metaphor. Psychological Review. 2005;112:193–216. doi: 10.1037/0033-295X.112.1.193. [DOI] [PubMed] [Google Scholar]
- Casasanto D, Boroditsky L. Time in the mind: using space to think about time. Cognition. 2008;106:579–593. doi: 10.1016/j.cognition.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chen E, Widick P, Chatterjee A. Functional-anatomical organization of predicate metaphor processing. Brain & Language. 2008;107:194–202. doi: 10.1016/j.bandl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RH, Binder JR, Conant LL, Mano QR, Seidenberg MS. The neural career of sensory-motor metaphors. Journal of Cognitive Neuroscience. 2011;23:2376–2386. doi: 10.1162/jocn.2010.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain. Surface, Blood Supply and Three-dimensional Sectional Anatomy. New York: Springer; 1999. [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. Journal of Neuroscience. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eviatar Z, Just MA. Brain correlates of discourse processing: an fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia. 2006;44:2348–2359. doi: 10.1016/j.neuropsychologia.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J. The Language of Thought. Cambridge MA, USA: Harvard University Press; 1975. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Galati G, Committeri G, Sanes JN, Pizzamiglio L. Spatial coding of visual and somatic sensory information in body-centred coordinates. European Journal of Neuroscience. 2001;14:737–746. doi: 10.1046/j.0953-816x.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cognitive Brain Research. 2004;20:226–241. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gibbs RW, Matlock T. Metaphor, imagination, and simulation. In: Gibbs RW, editor. The Cambridge Handbook of Metaphor and Thought. New York: Cambridge University Press; 2008. pp. 161–176. (2008). [Google Scholar]
- Gibson G, Stilla R, Sathian K. Segregated visuo-haptic processing of texture and location. Melbourne: Abstract, Human Brain Mapping; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooly J, Logie RH. Age-of-acquisition, imagery, concreteness, familiarity, and ambiguity measures for 1,944 words. Behavior Research Methods & Instrumentation. 1980;12:395–427. [Google Scholar]
- Guest S, Catmur C, Lloyd D, Spence C. Audiotactile interactions in roughness perception. Experimental Brain Research. 2002;146:161–171. doi: 10.1007/s00221-002-1164-z. [DOI] [PubMed] [Google Scholar]
- Guest S, Spence C. What role does multisensory integration play in the visuotactile perception of texture? International Journal of Psychophysiology. 2003;50:63–80. doi: 10.1016/s0167-8760(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Heller MA. Texture perception in sighted and blind observers. Perception & Psychophysics. 1989;45:49–54. doi: 10.3758/bf03208032. [DOI] [PubMed] [Google Scholar]
- Keysar B, Bly BM. Swimming against the current: Do idioms reflect conceptual structure? Journal of Pragmatics. 1999;31:1559–1578. [Google Scholar]
- Keysar B, Shen Y, Glucksberg S, Horton WS. Conventional language: how metaphorical is it? Journal of Memory and Language. 2000;43:576–593. [Google Scholar]
- Klatzky RL, Lederman S, Reed C. There’s more to touch than meets the eye: the salience of object attributes for haptics with and without vision. Journal of Experimental Psychology: General. 1987;116:356–369. [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-day American English. Providence RI, USA: Brown University Press; 1967. [Google Scholar]
- Lacey S, Hagtvedt H, Patrick VM, Anderson A, Stilla R, Deshpande G, Hu X, Sato JR, Reddy S, Sathian K. Art for reward’s sake: visual art recruits the ventral striatum. NeuroImage. 2011;55:420–433. doi: 10.1016/j.neuroimage.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoff G, Johnson M. Metaphors We Live By. Chicago IL, USA: The University of Chicago Press; 2003. [Google Scholar]
- Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. NeuroImage. 2006;29:536–544. doi: 10.1016/j.neuroimage.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lynott D, Connell L. Modality exclusivity norms for 423 object properties. Behavior Research Methods. 2009;41:558–564. doi: 10.3758/BRM.41.2.558. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson W, Zwitserlood P. Accessing spoken words: the importance of word onsets. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:576–585. [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Santiago J, Israeli Z, Gabay S. Is the future the right time? Experimental Psychology. 2010;57:308–314. doi: 10.1027/1618-3169/a000036. [DOI] [PubMed] [Google Scholar]
- Pa J, Wilson SM, Pickell H, Bellugi U, Hickok G. Neural organization of linguistic short-term memory is sensory modality-dependent: evidence from signed and spoken language. Journal of Cognitive Neuroscience. 2008;20:2198–2210. doi: 10.1162/jocn.2008.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn Z. Things and Places: How the Mind Connects With the World. Cambridge MA, USA: MIT Press; 2007. [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss ME, Stamatakis EA, Tyler LK. Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia. 2009;47:388–396. doi: 10.1016/j.neuropsychologia.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TTJ. Neural correlates of metaphor processing. Cognitive Brain Research. 2004;20:395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Revill KP, Aslin RN, Tanenhaus MK, Bavelier D. Neural correlates of partial lexical activation. Proceedings of The National Academy of Sciences USA. 2008;105:13111–13115. doi: 10.1073/pnas.0807054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Lacey S, Stilla R, Gibson GO, Deshpande G, Hu X, LaConte S, Glielmi C. Dual pathways for haptic and visual perception of spatial and texture information. NeuroImage. 2011;57:462–475. doi: 10.1016/j.neuroimage.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtlander LM, Murdoch LD. Frequency of occurrence and rankings for touch-related adjectives. Behavior Research Methods, Instruments & Computers. 2000;32:579–587. doi: 10.3758/bf03200831. [DOI] [PubMed] [Google Scholar]
- Stilla R, Sathian K. Selective visuo-haptic processing of shape and texture. Human Brain Mapping. 2008;29:1123–1138. doi: 10.1002/hbm.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Brain. New York, USA: Thieme Medical Publishers; 1988. [Google Scholar]
- Toglia MP, Battig WF. Handbook of Semantic Word Norms. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1978. [Google Scholar]
- Tremblay P, Small SL. From language comprehension to action understanding and back again. Cerebral Cortex. 2011;21:1166–1177. doi: 10.1093/cercor/bhq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkowski BM, Meier BP, Robinson MD, Carter MS, Feltman R. “Hot-headed” is more than an expression: the embodied representation of anger in terms of heat. Emotion. 2009;9:464–477. doi: 10.1037/a0015764. [DOI] [PubMed] [Google Scholar]
- Yee E, Sedivy JC. Eye movements to pictures reveal transient semantic activation during spoken word recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:1–14. doi: 10.1037/0278-7393.32.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.