Abstract
Dendritic cells play a key role in determining adaptive immunity, and there is growing interest in characterizing and manipulating the interactions between dendritic cells and biomaterial surfaces. Contact with several common biomaterials can induce the maturation of immature dendritic cells, but substrates that reduce dendritic cell maturation are of particular interest within the field of cell-based therapeutics where the goal is to reduce the immune response to cell-laden material carriers. In this study, we use a materials-based strategy to functionalize poly(ethylene glycol) hydrogels with immobilized immunosuppressive factors (TGF-β1 and IL-10) to reduce the maturation of immature dendritic cells. TGF-β1 and IL-10 are commonly employed as soluble factors to program dendritic cells in vitro, and we demonstrate that these proteins retain bioactivity towards dendritic cells when immobilized on hydrogel surfaces. Following stimulation with lipopolysaccharide (LPS) and/or cytokines, a dendritic cell line interacting with the surfaces of immunosuppressive hydrogels expressed reduced markers of maturation, including IL-12 and MHCII. The bioactivity of these immunomodulatory hydrogels was further confirmed with primary bone marrow dendritic cells (BMDCs) isolated from non-obese diabetic (NOD) mice, as quantified by a decrease in activation markers and a significantly reduced capacity to activate T cells. Furthermore, by introducing a second signal to promote BMDC-material interactions combined with the presentation of tolerizing signals, the mulitfunctional PEG hydrogels were found to further increase signaling towards BMDCs, as evidenced by greater reductions in maturation markers.
1. Introduction
Dendritic cells (DCs) bridge the innate and adaptive immune systems and are crucial to initiating and guiding the adaptive immune response. Immature dendritic cells (iDCs) survey the body's periphery in search of foreign and self antigens [1]. Upon antigen uptake, DCs present antigen to lymphocytes, but have the unique capacity to induce either immunity or tolerance to antigen [1, 2]. For example, when iDCs encounter antigen in the presence of stimulatory factors such as “danger signals” or pathogen associated molecular patterns (PAMPs), iDCs undergo maturation into mature DCs and initiate an adaptive immune response [3]. Upon maturation, DCs increase expression of MHC stimulatory molecules, B7 family co-stimulatory molecules (i.e., CD80 and CD86), and inflammatory cytokines, including IL-12, which enable the activation of naïve CD4+ helper T cells to initiate an immune response [1]. Conversely, if iDCs encounter antigen under conditions which prevent full DC maturation, they express an altered pattern of surface proteins, preventing an unfavorable adaptive immune response [2, 4].
Because of their critical importance in dictating the fate of the adaptive immune response, understanding interactions between iDCs and implanted biomaterial surfaces has gained considerable interest in recent years [5]. For example, several commonly-used biomaterials including poly(lactic-co-glycolic acid) (PLGA) and chitosan [6], as well as surface modifications with common extracellular matrix (ECM) proteins [7] and integrins [8], have been identified as regulators that can induce iDC maturation and initiate an adaptive immune response. The induced maturation of iDCs by biomaterials, or adjuvant effect, is desirable for vaccines against infection and tumors where an immune response is favorable [9]. However, in the design of biomaterials for cell delivery, such as the encapsulation of tissues for transplantation, ideal biomaterial cell carriers would prevent the full maturation of iDCs. Babensee and coworkers recently demonstrated that hyaluronic acid and agarose have the capacity to limit immunogenicity compared to surfaces with known adjuvant effect [6, 10], but in general, the investigation of strategies to actively limit iDC maturation via controlled modification of biomaterial surfaces has been limited. In solution, however, iDCs have been cultured in the presence of one or more soluble factors such as transforming growth factor β-1 (TGF-β1) and/or interleukin 10 (IL-10) [11-14]. Resulting DCs have been reported to undergo incomplete maturation upon immune stimulation and can promote T cell anergy or induce regulatory T cell production [2]. For example, Torres-Aguilar et al. recently cultured iDCs with IL-10 and TGF-β1 in the presence of insulin to generate DCs that could induce antigen-specific insulin tolerance in humans [15].
Numerous proteins, peptides and other molecules of interest have been previously incorporated onto biomaterial surfaces and remained bioactive for cell signaling [16, 17]. We have previously investigated PEG-based surfaces for the purposes of immune signaling, and demonstrated that PEG coatings containing immobilized anti-fas are capable of interacting with T cells and inducing apoptosis [18-20]. Notably, Mann et al. tethered TGF-β1 within PEG hydrogels to signal vascular smooth muscle cells and demonstrated that immobilized TGF-β1 maintained bioactivity and increased ECM protein synthesis [21]. Further, it is known that DCs have the capacity to receive biological cues from tethered signaling proteins, as Leclerc et al. immobilized granulocyte-macrophage colony stimulating factor (GM-CSF) upon surfaces to promote the development of iDCs from isolated bone marrow tissue [22]. In the study we describe herein, a general approach to modify biomaterial surfaces with thiolated proteins, specificallyTGF-β1 and/or IL-10, to create immunomodulatory surfaces that signal iDCs and reduce maturation upon stimulation with LPS. A poly(ethylene glycol) (PEG) hydrogel platform, which limits immunogenicity and allows facile modification for incorporation of proteins, was chosen as a basis for tethering anti-inflammatory molecules for iDC signaling. By introducing a second signal that promoted cell-material interactions, along with the immunomodulatory signals, multifunctional PEG hydrogel surfaces could be tailored to suppress iDC maturation to a greater degree than either signal alone.
2.Materials and Methods
2.1. Dendritic cell culture
Initial studies were conducted with the cytokine-dependent, immortalized immature dendritic cell line, JAWSII. The JAWSII dendritic cell line was originally isolated from the bone marrow of p53-/- C57BL/6 mice and has been previously shown to mimic the capacity of primary iDCs to undergo maturation in response to immune stimuli [23-26]. JAWSII cells, an immortalized dendritic cell line of murine bone marrow origin (ATCC, Manassas, VA), were cultured in α-MEM media (Invitrogen, Carlsbad, CA) supplemented with 20% heat inactivated FBS (Invitrogen), 1% penicillin/streptomycin (Invitrogen, and 5 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ). JAWSII were cultured in tissue culture flasks and media was changed weekly. Additionally, primary bone marrow-derived DCs (BMDCs) generated from bone marrow isolated from non-obese diabetic (NOD) mice were evaluated with immunomodulatory hydrogels. Primary bone marrow-derived dendritic cells (BMDCs) were harvested from femurs isolated from NOD mice (4-10 weeks old). The ends of femurs were cut and the marrow was rinsed with 10 ml RPMI media 1640 (Invitrogen) with a 27 gauge syringe needle. Freshly isolated samples were then mixed in an 18 gauge syringe to dissociate clumps and the resulting cell suspension was cultured in media consisting of RPMI 1640 supplemented with 1.5% mouse serum (Invitrogen), 20 ng/ml GM-CSF, and 1% penicillin/streptomycin. BMDCs were seeded onto tissue culture polystyrene (TCPS) in 6-well plates or hydrogels in 96-well plates and 50% fresh media volume was changed daily.
2.2. Thiolation of proteins
To incorporate TGF-β1 and IL-10 as covalent pendant functional groups within hydrogels, proteins were rendered polymerizable via modification by Traut's reagent (Thermo Scientific, Rockford, IL), which conjugates to free amines to create thiols. In brief, proteins were reconstituted in phosphate buffered saline (PBS, pH 7.4, Invitrogen) containing 2 mM EDTA (Sigma) and a 5-fold molar excess Traut's reagent per mol protein. Samples were mixed and reacted at room temperature for 1 hr. Following thiolation, unreacted Traut's reagent was removed via filtration through Zeba™ Spin Desalting Columns (7K MWCO, Thermo Scientific), yielding the final thiolated product of TGF-β1-SH or IL-10-SH. Samples were diluted to a final concentration of 25 μg/ml in PBS with 2 mM EDTA and immediately placed in a -80°C freezer. Prior to use, protein solutions were rapidly thawed and added to pre-polymer solutions in concentrations ranging from 0 to 1 μg/ml for gel formation via photopolymerization.
2.3. PEG hydrogel formation
The synthesis of poly(ethylene glycol) (PEG) diacrylate (PEGDA, 10 kDA) macromolecular monomers from hydroxyl-PEG (Sigma) has been described in detail elsewhere [27]. Briefly, hydroxyl PEG was dissolved in anhydrous toluene by heating to 60°C with mixing. After the dissolved PEG was allowed to cool to room temperature (RT), triethylamine (TEA, 4-fold molar excess per hydroxyl group) and acryloyl chloride were added and reacted overnight at RT with stirring. Next, TEA was removed via filtration through neutral alumina. PEGDA was precipitated in cold diethyl ether and desiccated to dryness overnight. To ensure high levels of purity, PEGDA was dialyzed against deionized water overnight (1 kDa MWCO membrane) with >3 dialysis volume changes.
Pre-polymer solutions were prepared consisting of 10 wt% PEGDA in PBS with 0.05 wt% of the photoinitiator 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure-959, Ciba Specialty Chemicals) and thiolated proteins (0 to 1 μg/ml). For selected studies, poly-L-lysine (PLL, 10 μM, Sigma), laminin (200 μg/ml, BD Biosciences, Bedford, MA) or fibronectin (200 μg/ml, BD Biosciences) were also included in pre-polymer solutions. Pre-polymer solutions were loaded into the tips of 1 ml syringes (30 μl) and polymerized for 10 min under ultraviolet light (6 mW/cm2, centered at 365 nm) for gel formation. Following polymerization, hydrogels were incubated overnight in either PBS or cell culture media (4°C, with orbital shaking) with at least two solution changes to ensure complete removal of residual photoinitiator and untethered proteins. The final, swollen hydrogels were disks with a diameter of ~5.5 mm and height of ~2 mm.
2.4. Measurement of incorporated proteins
TGF-β1 was added to pre-polymer solutions in concentrations ranging from 0 to 1 μg /ml. Following photopolymerization, functionalized hydrogels were rinsed overnight in 3 ml PBS with >2 solution changes to ensure complete removal of non-covalently bound protein. The following day, a modified ELISA was employed to quantify the concentration of protein on the gel surface. Hydrogels were incubated with anti-TGF-β1 monoclonal antibody from mouse (5 μg/ml, Peprotech) in ELISA buffer (sterile filtered PBS with 0.1% BSA and 0.05% TWEEN-20) for 1.5 hrs and then rinsed three times with ELISA buffer. Next, samples were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (100 ng/ml, Jackson ImmunoResearch, West Grove, PA) for 30 min, followed by three rinses with PBS. Samples were then incubated for 60 minutes in ELISA buffer, rinsed three times, and incubated another 15 min in ELISA buffer to ensure complete removal of unbound antibodies. Hydrogels were distributed into individual wells of a 96-well plate containing 70 μl PBS (30 μl hydrogel plus 70 μl PBS resulted in roughly 100 μl total volume / well), and the TMB ELISA substrate (100 μl/well, Thermo Scientific) was added for 20 minutes. Finally, the reaction was quenched via the addition of 100 μl H2SO4 100 μl combined TMB /H2SO4 and the absorbance was measured at 450 nm and compared to that of standards prepared from known quantities of secondary antibody, TMB, and H2SO4.
2.5. PE-25 TGF-β reporter cell assay
The presence of biologically active TGF-β1 on hydrogel surfaces was verified using a PE-25 TGF-β reporter cell line (i.e., mink lung epithelial cells (Mv1Lu or CCL-64)) containing a stably transfected luciferase reporter gene for TGF-β which were generously donated by Dr. Xuedong Liu [28]). PE-25 cells were seeded atop functionalized hydrogels at a density of 1×105 cells/ml for 24 hrs in 200 μl Dulbecco's Modified Eagle Medium (Invitrogen). The following day, the media was gently removed and samples were lysed via the addition of 200 μl passive lysis buffer (Promega, Madison, WI) and incubated for 10 min at 37°C, with shaking, and finally stored at -80°C for >2 hr. Next, the samples were thawed, transferred to microcentrifuge tubes and centrifuged for 10 min (13,000 rpm, 4°C). The lysate was transferred to white, opaque-walled 96-well plate (100 μl per well), and 50 μl luciferase substrate (Promega) was added to each well for 5 min and then luminescence was quantified.
2.6. JAWSII DC studies
JAWSII DCs were seeded into 96-well plates on either empty wells (TCPS) or atop hydrogels at a density of 3.0×105 cell/ml in 200 μl JAWSII cell culture media. Cells were cultured atop blank and functionalized (TGF-β1-SH and/or IL-10-SH) hydrogels or on TCPS with or without the addition of soluble TGF-β1 and/or IL-10-SH for three days. On day 4, DCs were activated via the addition of lipopolysaccharide (LPS, 1 μg/ml, Sigma), with interferon gamma (IFN-γ, 10 ng/ml), tumor necrosis factor alpha (TNF-α, 10 ng/ml) and interleukin 4 (IL-4, 10 ng/ml), each obtained from Peprotech. On day 6, 100 μl of each sample was analyzed for IL-12p70 production via an ELISA kit (eBioscience, San Diego, CA) per the manufacturer's instructions. For flow cytometry analysis, JAWSII were removed from TCPS and hydrogels via incubation in cold PBS (10 min on ice). For flow cytometry analysis, samples were rinsed in PBS + 10% FBS and then incubated for 10 min at 4°C with Fc Block (anti-mouse CD16/CD32, Fcγ III/II Receptor, BD Pharmingen, San Diego, CA). FitC-rat anti-mouse I-A/I-E (clone 2G9, BD Pharmingen) and PE-rat anti-mouse CD86 (clone GL1, BD Pharmingen); antibodies were added and allowed to stain for 60 min at 4°C. Fluorescently labeled antibody isotypes were included as controls. Following staining, samples were rinsed and then analyzed using a CyAn Flow Cytometer and Summit software (Beckman Coulter) and more than 25,000 events were collected per sample.
2.7. BMDC studies
BMDCs were harvested from NOD mice and then seeded in 6 well plates at 5×106 cells/well, in 4 ml BMDC media for four days. Half of the BMDC media was exchanged after 2 days. BMDCs were harvested via cell lifter on day 5 and transferred to either blank or functionalized PEG hydrogels onto TCPS in 96-well plates at a density of 2 × 105 cells/well in 200 μl BMDC media. Cells were cultured for an additional 3 days atop blank or functionalized gels or on TCPS in the presence or absence of soluble TGF-β-SH and/or IL-10. Half of the media volume was exchanged with fresh media each day. On day 8, samples were activated via the addition of LPS (1 μg/ml). On day 8, 100 μl of supernatant per sample was harvested and the IL-12p70 concentration was quantified via ELISA. Cells were lifted for flow cytometry by incubation in PBS with 5 mM Na2EDTA (Sigma) for 20 min at 37°C. Cells were rinsed with 1% BSA in PBS and stained with Fc block (anti-mouse CD16/CD32, Fcγ III/II Receptor, BD Pharmingen) for 10 min on ice. Next, samples were stained with one or more of the following: FitC-anti-MHCII (Clone: RI1B, Ox-6, BD Pharmingen), FITC-anti-CD86 (Clone: GL1, BD Pharmingen), PerCP-anti-CD11b (Clone: M1/70, BD Pharmingen), PE-anti-CD80 (Clone: 16-10A1, BD Pharmingen), APC-Anti-CD11c (Clone: N418, BD Pharmingen), Cells were incubated for 30 min on ice, were rinsed 3 times, and re-suspended for flow cytometry. Samples were analyzed by FACS Caliber.
2.8. T cell activation studies
The diabetogenic CD4 Th1 T cell clone BDC-2.5 was derived from spleen and lymph node cells of a diabetic NOD mouse [29, 30]. T cells were maintained by restimulation with an antigenic membrane fraction from β-cell tumors [31], irradiated NOD spleen cells as antigen-presenting cells (APC), and IL-2 in complete medium (CM). CM is Dulbecco's modified Eagle's minimal essential medium (DMEM) (Life Technologies, Gaithersburg, MD) supplemented with with 44 mM sodium bicarbonate, 0.55 mM L-arginine, 0.27 mM L-asparagine, 1.5 mM L-glutamine, 1 mM sodium pyruvate, 50 □M 2-ME, 10 mM HEPES, and 10% fetal bovine serum. At the end of the two-week cycle, T cells (2 × 104) were cultured with beta cell antigen and BMDCs (1.88×105 cells/ml) as APCs in 96-well plates to assay for T cell responses. Supernatants were harvested 72 hrs later and IFNγ production was measured by ELISA.
2.9. Statistical Analysis
Statistical significance was determined using a two-tailed, unpaired Student's t-test. Differences between datasets were considered statistically significant when the p value was less than 0.05. All results are presented as mean ± standard error of the mean. Each experiment was repeated at least twice.
3. Results
3.1. Bioactivity of thiolated TGF-β1
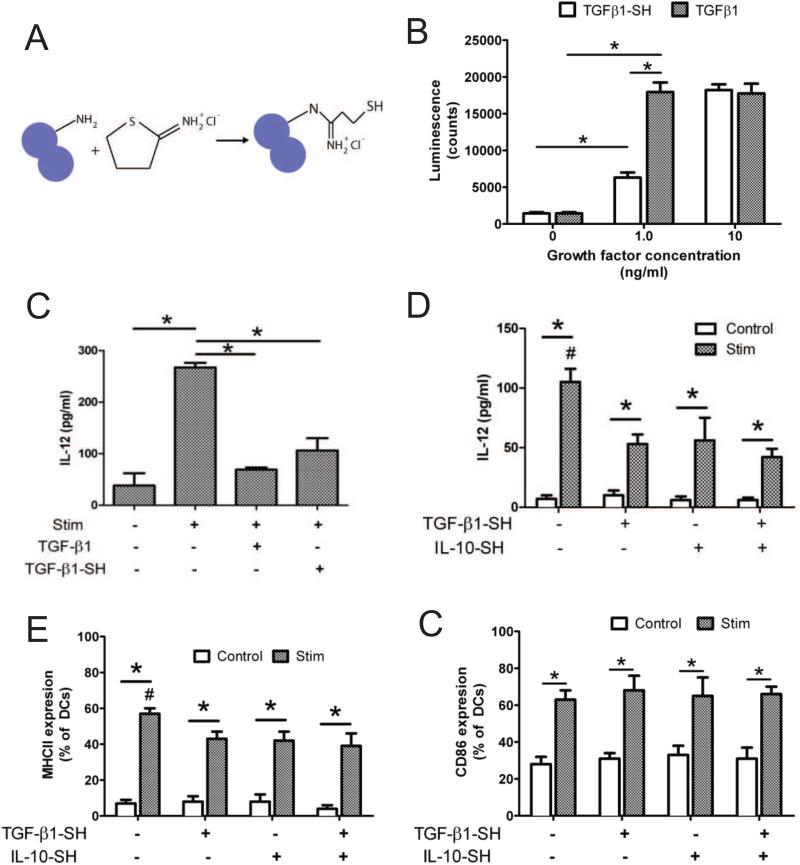
TGF-β1 was converted into a polymerizable form via thiolation with Traut's reagent [32], as schematically illustrated in Fig. 1A. To confirm that TGF-β1-SH remained bioactive, PE-25 (TGF-β reporter) cells were incubated overnight with 0, 1.0 and 10 ng/ml of soluble and unmodified TGF-β1 as compared to TGF-β1-SH. The following day, luciferase activity was quantified as a marker of TGF-β bioactivity. As shown in Fig. 1B, at a concentration of 1.0 ng/ml, a reduction in TGF-β1-SH bioactivity was observed compared to unmodified TGF-β1. At 10 ng/ml, however, both thiolated and unmodified TGF-β1 resulted in high levels of luciferase activity. Bioactivity was also verified via activity with JAWSII DCs. Dendritic cells were incubated with thiolated and unmodified TGF-β1 for 24 hrs (10 ng/ml) and then activated via stimulation with LPS and IFN-γ. As presented in Fig. 1C, both forms of TGF-β1 were able to suppress DC IL-12 production when challenged with LPS, indicating that the bioactivity of TGF-β1-SH was preserved. The same effect was observed with soluble IL-10-SH, shown in Fig. 1D.
Fig 1.
Soluble bioactivity of modified growth factors. (A) Schematic for the thiolation of free amine groups on proteins via Traut's reagent. Circles represent one protein molecule (B) Comparison of bioactivity for thiolated and unmodified TGF-β1 via PE-25 TGF-β reporter assay. (C) IL-12 produced by JAWSII DCs cultured with soluble, thiolated and unmodified TGF-β1. (D) IL-12 produced by JAWSII cultured in the presence of TGF-β1-SH or IL-10-SH following immune stimulation with LPS and cytokines. (E) MHCII and (F) CD86 expression of JAWSII DCs cultured with or without soluble TGF-β1-SH and/or IL-10-SH. * denotes p<0.05 difference. # denotes p<0.05.
The surface expression of MHCII (Fig. 1E) and CD86 (Fig. 1F) was also characterized for JAWSII iDCs cultured with both soluble TGF-β1-SH and/or IL-10-SH. As anticipated, stimulation resulted in a large increase in MHCII expression. However, pretreatment with either TGF-β or IL-10 resulted in DCs with decreased MHCII expression, compared to stimulated controls. Although stimulation also increased the levels of CD86, pre-treatment with immunosuppresants did not affect CD86 expression by JAWSII DCs.
3.2 Formation of immunomodulatory PEG hydrogels
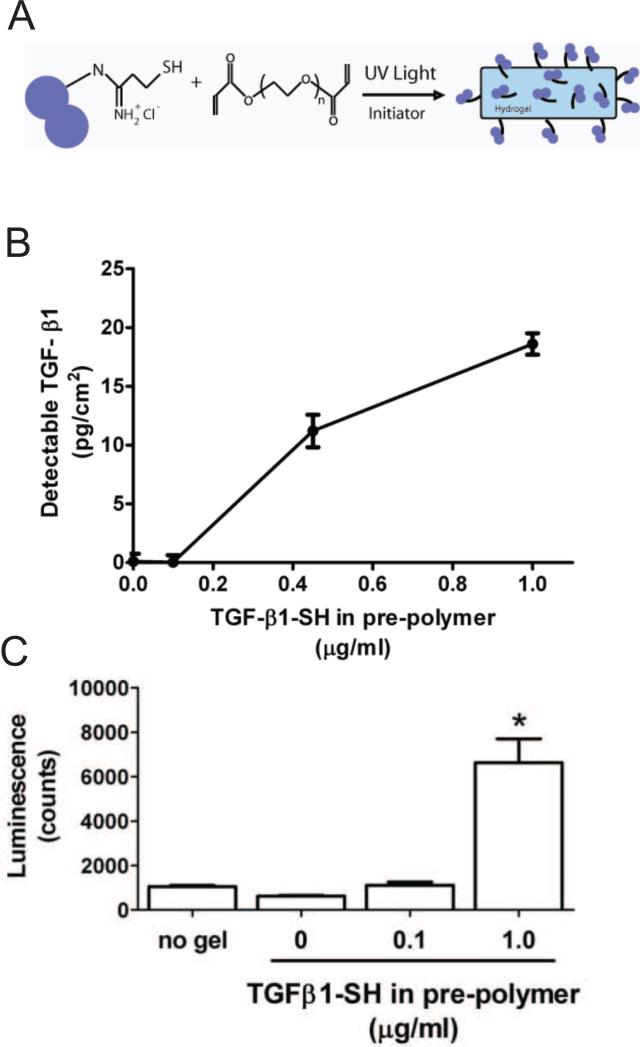
To create functional material systems, PEGDA (10 kDa, 10 wt%) hydrogels were photopolymerized with or without the addition of thiolated proteins, Fig. 2A. Free thiols on proteins were covalently incorporated within hydrogel networks via a mixed-mode, radical-mediated, thiol-acrylate polymerization, as previously described [33]. A modified ELISA was employed to characterize the concentration of TGF-β1 available on the hydrogel surface. As illustrated in Fig. 2B, an increase in the detectable surface concentration of TGF-β1 was observed relative to the concentration of TGF-β1-SH included in the pre-polymer solution. When TGF-β1-SH (1.0 μg/ml) was included in the pre-polymer solution, TGF-β1 (18±2 pg/cm2) was detected on the gel surface. To verify the bioactivity of the TGF-β1 hydrogels, PE-25 TGF-β reporter cells were seeded on gels and allowed to interact for 24 hrs. Luciferase activity was quantified, and as shown in Fig. 2C, significant TGF-β bioactivity was measured on the hydrogel surfaces fabricated with the TGF-β-SH.
Fig 2.
Formation of functionalized PEG hydrogels. (A) Schematic of hydrogel formation in which thiolated proteins are covalently polymerize within PEG hydrogels via thiol-acrylate photopolymerization. (B) Detectable surface density of TGF-β1 on hydrogel surfaces, as detected by modified ELISA. (C) Bioactivity of TGF-β1 incorporated on functionalized hydrogels, as assessed by PE-25 TGF-β reporter cell assay. * denotes p<0.05 difference from all other values.
3.3 JAWSII DCs on immunomodulatory hydrogels
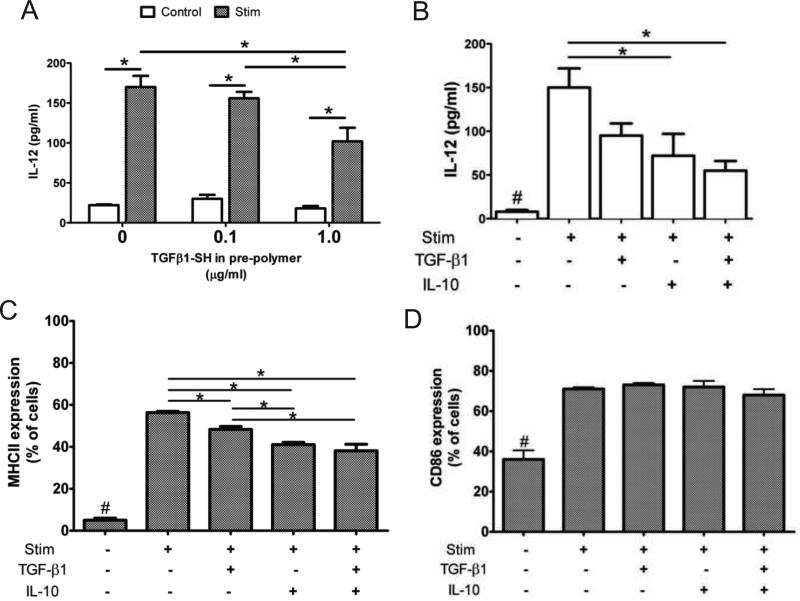
JAWSII iDCs were seeded on hydrogels functionalized with or without the immunosuppressive proteins, TGF-β1 and/or IL-10, and cultured for 3 days. As shown in Fig. 3A, JAWSII iDCs were seeded on hydrogels substrates fabricated with 0, 0.1, and 1.0 μg/ml TGF-β1-SH in the pre-polymer solution. Following stimulation, JAWSII cultured on hydrogels with TGF-β1-SH secreted lower amounts of IL-12, compared to stimulated controls. A marked reduction in IL-12 in response to stimulation was also observed for DCs seeded on hydrogel surfaces containing IL-10, as well as for surfaces duallyfunctionalized with TGF-β1 and IL-10, as shown in Fig. 3B. Stimulated JAWSII DCs on dually-functionalized gels secreted 63% less IL-12 than DCs cultured on unmodified control hydrogels, providing evidence that the immobilized immunosuppressant biological signals remained active and signaled the DCs.
Fig 3.
JAWSII DCs with immunomodulatory hydrogels. (A) IL-12 production of JAWSII DCs stimulated with LPS and cytokines following cell culture atop hydrogels functionalized with various concentrations of TGF-β1. (B) IL-12 production of DCs cultured atop hydrogels polymerized with 1 μg/ml TGF-β1 and / or IL-10. (C) MCHII and (D) CD86 expression of JAWSII DCs cultured atop functionalized hydrogels. * denotes p<0.05 difference. # denotes p<0.05 difference from all other values.
Flow cytometric analysis was performed on JAWSII DCs harvested after seeding on functionalized hydrogels. Increased surface expression of the MHCII and CD86 is commonly observed with these markers of DC maturation, and their expression on JAWSII DCs has been previously observed to correlate with activation state [24]. As illustrated in Fig. 3, JAWSII DCs upregulated MHCII (Fig. 3C) and CD86 (Fig. 3D) expression in response to stimulation. Seeding JAWSII on TGF-β1, IL-10, or dually-functionalized gels, however, significantly decreased the percentage of MHCII+ DCs following stimulation (Fig. 3C). As observed when JAWSII were incubated with soluble immunosuppressants (Fig. 1F), CD86 expression was not affected by hydrogel functionalization (Fig. 3D). In sum, culturing JAWSII with either soluble or immobilized immunosuppressants generated DCs with similar IL-12, MHCII, and CD86 expression, demonstrating the bioactivity of immunomodulatory hydrogels.
3.4 Treating BMDCs with soluble TGF-β1 and IL-10
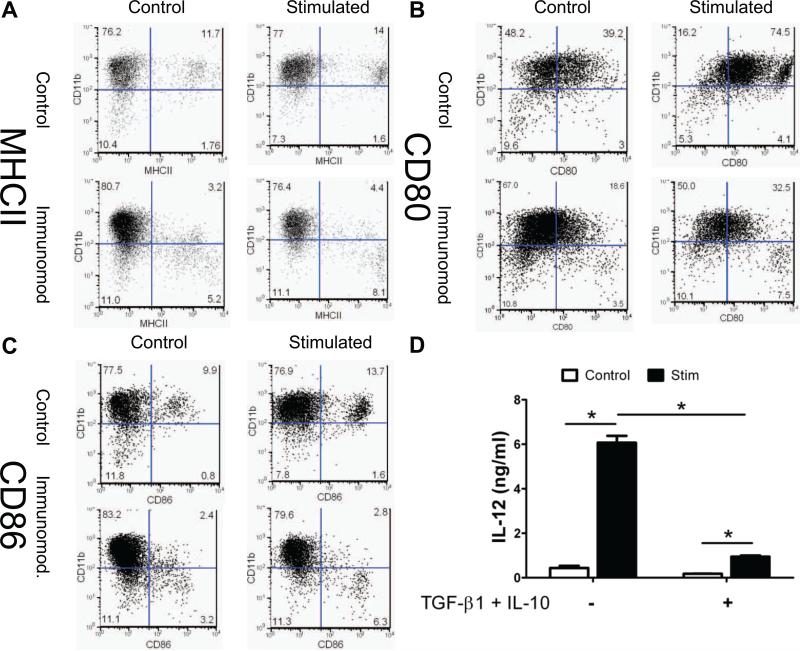
As further evidence of localized immune modulation of DC markers by soluble TGF-β1 and IL-10, we examined primary cells, bone marrow-derived dendritic cells (BMDCs) harvested from NOD mice. BMDCs were first treated with soluble TGF-β1-SH and IL-10-SH; selected samples were stimulated with LPS to induce BMDC maturation, and the media supernatant was assayed for IL-12. Surface levels of MHCII, CD80 and CD86 were assayed via flow cytometry. All samples were co-stained for the dendritic cell marker CD11c, and most of the CD11c+ cells were also CD11b+. Levels of MHCII (Fig. 4A), CD80 (Fig. 4B) and CD86 (Fig. 4C) were reduced in CD11c+ DCs cells by treatment with soluble immunomodulatory factors. Following stimulation with LPS, DCs expressed stimulatory and co-stimulatory markers. The expression of these markers was reduced, however, by pre-treatment with soluble immunosuppressive factors prior to LPS exposure. As shown in Fig 4D, control BMDC cultures treated with LPS secreted markedly higher levels of IL-12. When pre-treated with soluble TGF-β1-SH and IL-10-SH, however, levels of IL-12 were significantly lowered, indicating a less mature phenotype.
Fig 4.
Immunomodulation of primary BMDCs with soluble proteins. (A) CD11b / MHCII, (B) CD11b / CD80, and (C) CD11b/CD86 expression of BMDCs treated with or without soluble immunomodulation (10 ng/ml TGF-β1-SH and IL-10-SH) and with or without stimulation with LPS (1 μg/ml). Numbers are % of cells. Experiment performed by Jing He (D) IL-12 production by BMDCs treated with soluble TGF-β1-SH and IL-10-SH. * denotes p<0.05 difference.
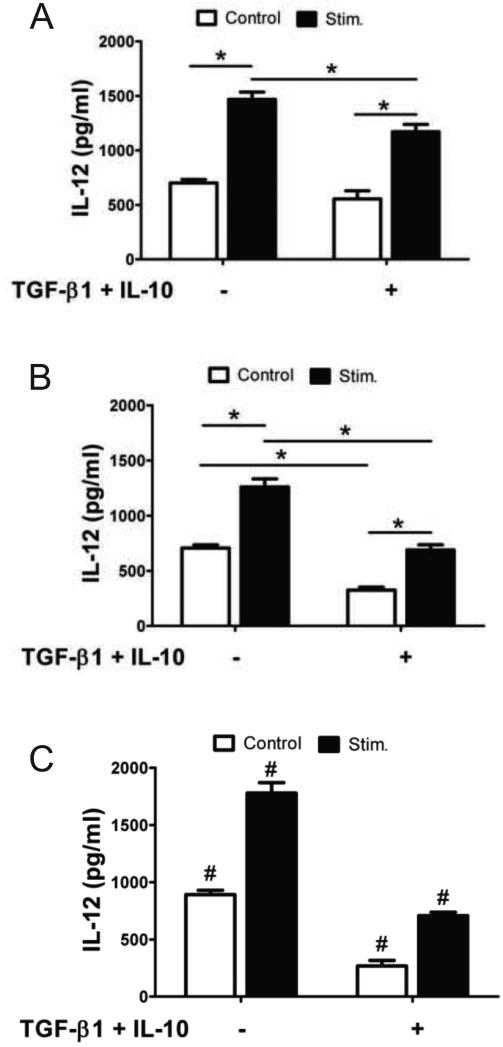
3.5 Culturing BMDCs with immunomodulatory PEG hydrogels
BMDCs were cultured on the surfaces of functionalized and unmodified gels and then stimulated with LPS. IL-12 production was quantified, as shown in Fig. 5A, and, again a significant increase in cytokine production was observed for LPS-stimulated samples. The presence of immunomodulatory factors on the surfaces of the otherwise blank hydrogels, however, only slightly reduced iDC maturation, as evident by a small but significant decrease in IL-12 production with TGF-β1 and IL-10 on the hydrogel surface. We hypothesized that this effect might be due to limited interaction between the immature BMDCs and the PEG surface, as PEG is well documented to resist cell and protein adhesion.
Fig 5.
BMDCs on immunomodulatory hydrogels. IL-12 production by BMDCS cultured on immunomodulatory hydrogels functionalized with (A) unmodified, (B) +PLL, (C) +ECM proteins. * denotes p<0.05 difference, # denotes p<0.05 difference from all values.
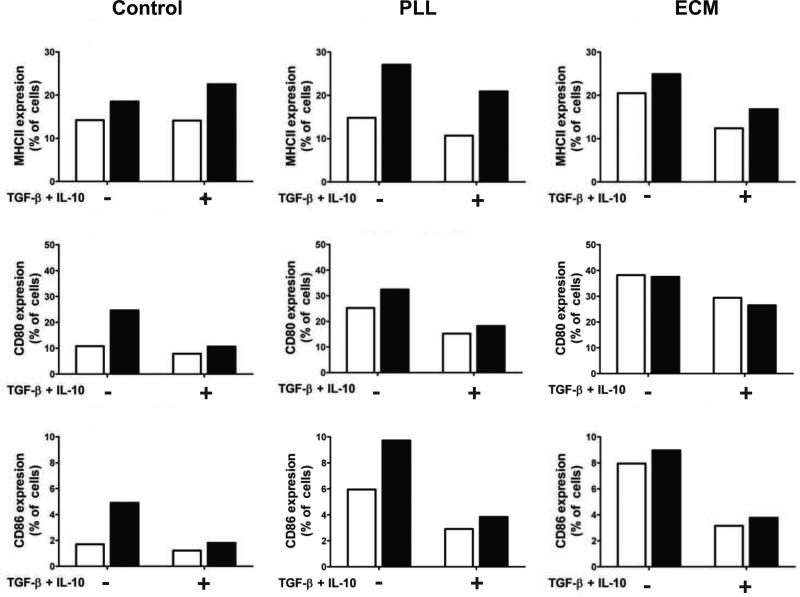
To increase interaction between BMDCs and PEG surfaces, two strategies were explored to promote adhesion. Either poly-L-lysine (PLL) or the extracellular matrix (ECM) proteins, fibronectin and laminin, were incorporated into PEG hydrogels to promote cellular interactions. It is previously known that PLL is cytotoxic at high concentrations, however, when concentrations ≤10 nM were incorporated with PEG hydrogels, no reduction in cellular metabolic activity was observed after 48 hrs of dendritic cell culture atop gels, as shown in Fig. S1. Therefore, 10nM PLL was utilized for subsequent studies. Fibronectin & laminin did not adversely affect cells survival (data not shown). Visually, hydrogel surfaces containing either PLL or ECM proteins appeared to promote iDC/surface interaction, as depicted in Fig. S2. On unmodified PEG hydrogels, BMDCs had a tendency to aggregate, while PLL and ECM hydrogels promoted single-cell attachment to the surface. BMDCs were cultured on PLL and ECM hydrogels functionalized with TGF-β1 and IL-10, and then stimulated with LPS. When seeded on hydrogels co-functionalized with PLL or ECM and immunosuppressive factors, IL-12 measured post-stimulation was decreased by more than 50%, Fig. 5B & 5C. Furthermore, the surface maturation markers, MHCII, CD80 and CD86, were generally reduced prior to and following immune stimulation on immunomodulatory hydrogels, as shown in Fig. 6. The decrease in IL-12 and surface marker expression in these experiments demonstrate that the immunosuppressive hydrogels can result in reduced maturation of BMDC.
Fig 6.
Surface marker expression of BMDCs seeded atop control or immunomodulatory (+TGF-β1 and IL-10) hydrogels, with (black bars) or without (white bars) immune stimulation. Hydrogels were seeded on control hydrogels (first column), or gels co-functionalized with either poly(L-lysine) (PLL, middle column) or hydrogels containing laminin and fibronectin (ECM, right column). The percentage shown represents the fraction of CD11c+ dendritic cells which stained positive for surface markers (MHCII, CD80, or CD86), as compared to isotype controls. Data is shown from 1 experiment but is representative of 3 replicate experiments.
3.6 Co-culture of BMDCs with T cells
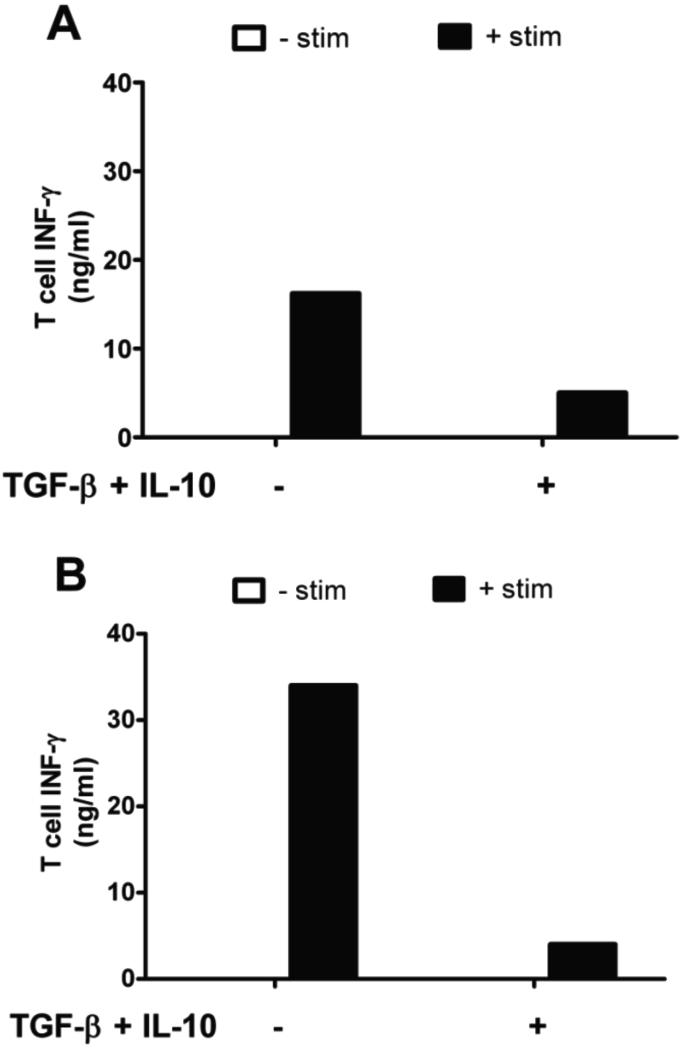
To assess the functional capacity of BMDCs to activate T cells, BMDCs and T cells were co-cultured. As illustrated in Fig. 7A, unstimulated dendritic cells lacked the capacity to activate T cells, evidenced by negligible IFN-γ production. Upon LPS stimulation, however, the BMDCs underwent maturation and were able to activate T cells, as indicated by increased T cell IFN-γ secretion. When BMDCs were pre-treated with soluble TGF-β and IL-10 prior to stimulation, T cell IFN-γ production was significantly decreased, indicating reduced T cell activation by dendritic cells (Fig. 7A). Further, these effects were confirmed when dendritic cells were cultured on hydrogels functionalized with tethered immunosuppressive molecules and PLL (Fig. 7B), providing biological confirmation of the immunosuppressive activity of these functionalized hydrogels on DCs.
Fig 7.
Interferon γ (IFN-γ) production by T cells following culture with BMDCs. BMDCs were cultured on (A) TCPS or (B) PEG hydrogels functionalized with PLL. BMDCs were treated with or without (A) soluble TGF-β1 and IL-10 or (B) covalently immobilized TGF-β1 and IL-10 for two days. Then, + stim samples were treated with LPS overnight to induce DC maturation. Of note, T cells produced undetectable levels of IFN-γ when cultured with unstimulated BMDCs. Data is shown from 1 experiment but is representative of 3 replicate experiments.
4. Discussion
Thiolation of TGF-β1 and IL-10 was achieved via reaction with Traut's reagent [32], which preserved the bioactivity of target proteins and allowed protein to be successfully conjugated to hydrogel networks. Thiolated proteins were added to the pre-polymer mixture and reacted into PEG hydrogels, utilizing a thiol-acrylate mixed-mode photoinitated polymerization. As a consequence, proteins were likely present throughout the hydrogel network as well as on the gel surface. The presence of protein accessible on the hydrogel surface was verified by a modified ELISA, whereby functionalized gels were incubated with a detection antibody. As has been previously reported, antibodies (~150 kDa MW) do not diffuse readily within PEGDA hydrogels on short time scales [34], so protein was detectable only if immobilized and available for binding on the hydrogel surface. Significant surface protein was detectable, quantifiable, and found to be dependent upon the protein concentration added to the pre-polymer solution.
JAWSII DCs were seeded on the surfaces of hydrogels functionalized with TGF-β1 and/or IL-10 to assess bioactivity towards DCs. When cultured on the gels, IL-12 production was suppressed upon stimulation, as was MHCII expression. These molecules were both suppressed to a similar extent by either soluble or immobilized factors, indicating decreased DC maturation and providing evidence that immobilized immunosuppressive factors retain bioactivity for DCs. While the suppression of IL-12 production and MHCII expression was successfully provided by TGF-β1 and/or IL-10, the CD86 co-stimulatory molecule was unaffected. This finding is in contrast to previous observations of decreased co-stimulatory marker expression on stimulated DCs that have been pre-treated with TGF-β1 and IL-10 [2]. Because this finding was consistent for pre-treatment with either soluble or immobilized factors, it is likely that the reduced sensitivity of co-stimulatory expression to immunosuppressive signaling is an inherit property of the JAWSII cell line and not a result of signaling molecule immobilization.
The bioactivity of immunomodulatory hydrogels was further assessed through experiments with primary BMDCs isolated from NOD mice. NOD mice were chosen as the BMDC source in these studies because this animal line is prone to autoimmunity and is a model for Type I diabetes. Type I diabetes has the potential to be cured via cell transplantation, so reducing the immune response to promote graft acceptance has been widely studied within Type I diabetes models. Therefore, we sought to demonstrate the capacity for immunosuppressive hydrogels to reduce NOD mouse dendritic cell activation because this has applications towards graft cytoprotection in a relevant disease model. The activation marker expression profile for BMDCs from NOD mice before and after immune stimulation has been previously characterized [35, 36], and the activation controls reported herein were in good agreement with previous findings [37]. When the BMDCs were treated with soluble, thiolated immunosuppressive proteins, reductions in stimulatory (MHCII), co-stimulatory (CD80 & CD86) and cytokine (IL-12) molecules were observed, in good agreement with previous results [2].
Primary BMDCs were seeded on functionalized hydrogels in order to assess the capacity of the immobilized proteins to signal DCs. Initially, it was observed that PEG hydrogels modified with immunosuppressive factors alone delivered limited signaling to seeded BMDCs, as equivalent IL-12 was produced with our without immunosuppressive factors following stimulation with LPS. We hypothesized that this effect might be due to limited interaction between cells and the PEG surface, as PEG hydrogels have been previously shown to resist cell adhesion. Others have observed that the addition of adhesion ligands to biomaterial surfaces promotes cell attachment and increased cell signaling [38], and we recently reported that the addition of ICAM-1 to PEG surfaces promoted T cell signaling [19]. Therefore, we investigated two methods of introducing adhesion molecules within the PEG hydrogels, either the addition of PLL or the incorporation of the ECM proteins, fibronectin and laminin.
Laminin and fibronectin were incorporated into PEG hydrogels to promote DC adhesion. When seeded upon ECM-hydrogels, BMDCs secreted modestly elevated IL-12 in the absence of LPS stimulation, compared to cells seeded on blank hydrogels. The inherent ability of ECM-functionalized materials to induce the maturation of iDCs has been previously reported [7, 37], so it is not surprising we observed this effect. It was encouraging to note, however, that the introduction of TGF-β1 and IL-10 to the ECM-tethered surfaces significantly reduced the production of IL-12 and expression of MHCII, CD80 and CD86 by BMDCs. The observations that immobilized immunosuppressive proteins have the capacity to suppress ECM-mediated iDC maturation, as well as prevent maturation upon LPS stimulation, provide a striking illustration of the bioactivity of immunomodulatory PEG hydrogels.
Others have incorporated poly-L-lysine (PLL) onto surfaces to promote dendritic cell attachment [39], and it is well known that positively charged groups on PLL result in the rapid binding of serum proteins to material surfaces, enabling cell interactions [40, 41]. Additionally, serum-coated surfaces (e.g., PLL-coated materials) have been used to limitthe adjuvant effect [39, 42], and are therefore a suitable platform for immunomodulation. BMDCs were seeded upon hydrogels functionalized with PLL and TGF-β1 and/or IL-10 and then stimulated. For PLL-hydrogels, an immunosuppressive effect was observed, as DC IL-12 production and surface marker expression on control and immunomodulatory hydrogels were decreased relative to controls. Further, T cell studies confirmed that PLL hydrogels functionalized with immunomodulatory factors significantly reduced dendritic cell maturation. Ultimately, these findings indicated that decreased cell-material interactions were a likely reason for limited signaling on unmodified immunomodulatory gels. Further, the immunosuppressive activity of PLL/immunomodulatory hydrogels towards BMDCs from NOD mice provides evidence that this sort of surface has the potential to interact favorably with DCs from type I diabetes.
5. Conclusion
We report a strategy for the introduction of immunosuppressive proteins, TGF-β1 and IL-10, into PEG hydrogels in a manner that preserves bioactivity towards iDCs. The immunomodulatory hydrogels reported herein reduced dendritic cell activation and possessed bioactivity that approached that of the soluble immunosuppressive proteins when cultured in the presence of the immortalized JAWSII DC cell line or primary BMDCs isolated from NOD mice. This technology suggests strategies for cell delivery applications, such as a coating of cell-laden biomaterials for the treatment of Type I diabetes, where it is desirable to limit the maturation of local dendritic cells and subsequently ameliorate the adaptive immune response.
Supplementary Material
Fig S1. Metabolic activity of JAWSII dendritic cells after 48 hrs atop PEG hydrogels containing 0 to 100 nM PLL. Macromer concentrations of PLL >10 nM had a cytotoxic effect on cells, while concentrations ≤10 nM did not affect cellular metabolic activity, as compared to control JAWSII cells seeded on blank PEG hydrogels. Metabolic activity was quantified via AlamarBlue Assay.
Fig S2. BMDCs on immunomodulatory hydrogels. Brightfield microscopy of BMDCs seeded atop PEG hydrogels that contain (top) no functionalization (middle) poly-L-lysine, and (bottom) the ECM proteins laminin and fibronectin. Scale = 60 μm.
Acknowledgments
The authors wish to acknowledge Dr. Charles Cheung and Dr. Chien-Chi Lin for initial guidance, technical assistance and helpful discussions. Additionally, we would like to thank Kristina Fuerst for her assistance with various experiments, Joshua McCall for his expertise with TGF-β and the reporter cell assay, Huan (Sharon) Wang for her assistance with JAWSII flow cytometry, and Dr. Xuedong Liu for providing PE-25 cells. Finally, we gratefully acknowledge financial support from the National Institute of Health (RO1DK076084), the Howard Hughes Medical Institute, and the Department of Education's Graduate Assistance in Areas of National Need fellowship to P.S.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10(5):399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 4.van Kooten C, Lombardi G, Gelderman KA, Sagoo P, Buckland M, Lechler R, et al. Dendritic Cells as a tool to induce transplantation tolerance: obstacles and opportunities. Transplantation. 2011;91(1):2–7. doi: 10.1097/tp.0b013e31820263b3. [DOI] [PubMed] [Google Scholar]
- 5.Babensee JE. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20(2):101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J Biomed Mater Res. 2005;74(4):503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 7.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Rogers TH, Babensee JE. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials. 2011;32(5):1270–1279. doi: 10.1016/j.biomaterials.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 10.Norton LW, Park J, Babensee JE. Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Control Release. 2010;146(3):341–348. doi: 10.1016/j.jconrel.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 12.Kosiewicz MM, Alard P. Tolerogenic antigen-presenting cells: regulation of the immune response by TGF-beta-treated antigen-presenting cells. Immunol Res. 2004;30(2):155–170. doi: 10.1385/IR:30:2:155. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159(10):4772–4780. [PubMed] [Google Scholar]
- 14.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162(7):3865–3872. [PubMed] [Google Scholar]
- 15.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 184(4):1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20(23-24):2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 17.Hirano Y, Mooney DJ. Peptide and protein presenting materials for tissue engineering. Adv Mater. 2004;16(1):17–25. [Google Scholar]
- 18.Cheung CY, Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconjug Chem. 2006;17(4):1036–1042. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 19.Hume PS, Anseth KS. Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations. Biomaterials. 2010;31(12):3166–3174. doi: 10.1016/j.biomaterials.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume PS, Bowman CN, Anseth KS. Functionalized PEG hydrogels through reactive dip-coating for the formation of immunoactive barriers. Biomaterials. 2011;32(26):6204–6212. doi: 10.1016/j.biomaterials.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22(5):439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 22.Leclerc C, Brose C, Nouze C, Leonard F, Majlessi L, Becker S, et al. Immobilized cytokines as biomaterials for manufacturing immune cell based vaccines. J Biomed Mater Res. 2008;86(4):1033–1040. doi: 10.1002/jbm.a.31751. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Shen C, Rey-Ladino J, Yu H, Brunham RC. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect Immun. 2008;76(6):2392–2401. doi: 10.1128/IAI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen TN, Haase C, Michelsen BK. Treatment of an immortalized APC cell line with both cytokines and LPS ensures effective T-cell activation in vitro. Scand J Immunol. 2002;56(5):492–503. doi: 10.1046/j.1365-3083.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 25.Otsu S, Gotoh K, Yamashiro T, Yamagata J, Shin K, Fujioka T, et al. Transfer of antigen-pulsed dendritic cells induces specific T-Cell proliferation and a therapeutic effect against long-term Helicobacter pylori infection in mice. Infect Immun. 2006;74(2):984–993. doi: 10.1128/IAI.74.2.984-993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinchuk LM, Lee SR, Filipov NM. In vitro atrazine exposure affects the phenotypic and functional maturation of dendritic cells. Toxicol Appl Pharmacol. 2007;223(3):206–217. doi: 10.1016/j.taap.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CC, Anseth KS. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv Funct Mater. 2009;19(14):2325. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke DC, Brown ML, Erickson RA, Shi Y, Liu X. Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics. Mol Cell Bio. 2009;29(9):2443–2455. doi: 10.1128/MCB.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37(10):1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 30.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA. 1989;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergman B, Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994;43(2):197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- 32.Traut RR, Bollen A, Sun TT, Hershey JWB, Sundberg J, Pierce LR. Methyl 4-mercaptobutyrimidate as a cleavable crosslinking reagent and its applications to escherichia-coli 30s ribosome. Biochemistry. 1973;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- 33.Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41(16):6019–6026. [Google Scholar]
- 34.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19(14):1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 35.Langmuir PB, Bridgett MM, Bothwell AL, Crispe IN. Bone marrow abnormalities in the non-obese diabetic mouse. Int Immunol. 1993;5(2):169–177. doi: 10.1093/intimm/5.2.169. [DOI] [PubMed] [Google Scholar]
- 36.Peng R, Bathjat K, Li Y, Clare-Salzler MJ. Defective maturation of myeloid dendritic cell (DC) in NOD mice is controlled by IDD10/17/18. Ann N Y Acad Sci. 2003;1005:184–186. doi: 10.1196/annals.1288.023. [DOI] [PubMed] [Google Scholar]
- 37.Acharya AP, Dolgova NV, Xia CQ, Clare-Salzler MJ, Keselowsky BG. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mouse-derived dendritic cells. Acta biomaterialia. 2010;7(1):180–192. doi: 10.1016/j.actbio.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mant A, Tourniaire G, Diaz-Mochon JJ, Elliott TJ, Williams AP, Bradley M. Polymer microarrays: identification of substrates for phagocytosis assays. Biomaterials. 2006;27(30):5299–5306. doi: 10.1016/j.biomaterials.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 40.Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res. 2000;49(4):435–447. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 41.Wang GX, Deng XY, Tang CJ, Liu LS, Xiao L, Xiang LH, et al. The adhesive properties of endothelial cells on endovascular stent coated by substrates of poly-l-lysine and fibronectin. Artif Cells Blood Substit Immobil Biotech. 2006;34(1):11–25. doi: 10.1080/10731190500428283. [DOI] [PubMed] [Google Scholar]
- 42.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res. 2010;96(1):239–260. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Metabolic activity of JAWSII dendritic cells after 48 hrs atop PEG hydrogels containing 0 to 100 nM PLL. Macromer concentrations of PLL >10 nM had a cytotoxic effect on cells, while concentrations ≤10 nM did not affect cellular metabolic activity, as compared to control JAWSII cells seeded on blank PEG hydrogels. Metabolic activity was quantified via AlamarBlue Assay.
Fig S2. BMDCs on immunomodulatory hydrogels. Brightfield microscopy of BMDCs seeded atop PEG hydrogels that contain (top) no functionalization (middle) poly-L-lysine, and (bottom) the ECM proteins laminin and fibronectin. Scale = 60 μm.