Abstract
Mast cells are well known for their role in allergic and anaphylactic reactions, as well as their involvement in acquired and innate immunity. Increasing evidence now implicates mast cells in inflammatory diseases where they are activated by non-allergic triggers, such as neuropeptides and cytokines, often exerting synergistic effects as in the case of IL-33. Mast cells can also release pro-inflammatory mediators selectively without degranulation. In particular, IL-1 induces selective release of IL-6, while corticotropin-releasing hormone secreted under stress induces the release of vascular endothelial growth factor. Many inflammatory diseases involve mast cells in cross-talk with T cells, such as atopic dermatitis, psoriasis and multiple sclerosis, which all worsen by stress. How mast cell differential responses are regulated is still unresolved. Preliminary evidence suggests that mitochondrial function and dynamics control mast cell degranulation, but not selective release. Recent findings also indicate that mast cells have immunomodulatory properties. Understanding selective release of mediators could explain how mast cells participate in numerous diverse biologic processes, and how they exert both immunostimulatory and immunosuppressive actions. Unraveling selective mast cell secretion could also help develop unique mast cell inhibitors with novel therapeutic applications.
Keywords: Brain, inflammation, mast cells, mitochondria, multiple sclerosis, psoriasis, skin, stress, selective release, vascular permeability
Introduction
Mast cells derive from distinct precursors in the bone marrow or other hematopoietic tissues [1,2]. They mature under the influence of local tissue microenvironmental conditions, through various cytokines such as stem cell factor (SCF) [2,3]. SCF enhances mast cell degranulation and cytokine production through cross-linking of their high affinity surface receptors for IgE (FcεRI), even though it does not induce degranulation on its own [4-7]. Other molecules that promote mast cell maturation include nerve growth factor (NGF) [8], which acts via tyrosine kinase receptors (TrkA, B, C), different from the c-kit activated by SCF [9]. Neurotrophin-3 was also shown to promote maturation of both fetal mouse skin mast cells [10] and human intestinal mast cells [11]. Moreover, human mast cells express mRNA and protein for the Trk ligands NGF, brain-derived neurotrophic factor (BDNF) and neurotrophin-3 [9], suggesting autocrine actions. However, unlike NGF, which stimulates mast cell degranulation [12], neurotrophins do not. Mast cell chemoattractants include SCF, monocyte chemoattractant protein-1 (MCP-1) and the “regulated upon activation, normal T cell expressed and secreted” (RANTES) [13]. SP is also a potent chemoattractant for human basophils [14]. Depending on their location, stage of maturation or species [15], mast cells express different types and levels of surface antigens and receptors, some of which are involved in activation and others in cell recognition (Table 1) [16].
Table 1. Mast cell receptors and their agonists*.
| Adenosine receptors A2A, A2B, A3 |
Adenosine |
|---|---|
| β2-Adrenoreceptor | Adrenaline |
| C3αreceptor | C3α |
| C5α receptor | C5α |
| Cannabinoid CB2 receptor | 2-Arachidonoyl-glycerol, anandamide |
| CD47 (=integrin-associated protein, IAP) | Integrins |
| CD200 receptor | CD200 (0X2) |
| Cd300α receptor | Eosinophil granule proteins |
| Chemokine receptors CXCR1-4, CX3 CR1, CCR1,3-5 | Chemokines |
| CRHR-1, CRHR-2 | Corticotropin releasing hormone |
| Estrogen receptors (A,B) | Estrogens |
| FcαR (CD89) | IgA |
| FcεRI | IgE |
| FcγRI | IgG |
| FcγRIIA | IgG |
| FcγRIIB | IgG |
| FcγRIII | IgG |
| GPR34 | Lysophosphatidylserine |
| GPR92 | Lysophosphatidic acid |
| Histamine receptors H1, H2, H3, H4 |
Histamine |
| 5-HT1A | Serotonin |
| Kit receptor tyrosine kinase (CD17) | Stem cell factor |
| LPA1, LPA3 | Lysophosphatidic acid |
| Leptin receptor | Leptin |
| Leukotriene receptors 1 and 2 | Leukotrienes |
| MRGX2 | Mastoparan, somatostatin, SP |
| Myeloid-associated Ig-like receptor 1 | ? |
| Neurokinin receptors NK1R, NK2R, NK3R, VPAC2 |
CGRP, Hemokinin-A, SP, VIP |
| Neurotensin receptor | Neurotensin |
| Neurotrophin receptors TrkA TrkB TrkC |
NGF BDNF Neurotrophin 3 |
| Nicotinic acetylcholine receptor | Acetylcholine |
| 0X40 | 0X40-ligand |
| Protease activated receptors 1-4 | Serine proteases (e.g. trypsin, tryptase) |
| Peripheral benzodiazepine receptor | ? |
| Progesterone receptor | Progesterone |
| Prostaglandin E receptors EP2, EP3, EP4 |
Prostaglandin E |
| Purinoreceptors P2Y1, P2Y12, P2Y13, P2Y2, P2Y11 |
ADP ATP, UTP ATP |
| Sphingosine-1-phosphate S1P1, S1P2, S1P5 |
S1P |
| Toll-like receptors 1-9 | Bacterial and viral products |
| Urokinase receptor | Urokina5s2e |
| Vitamin D receptor | Vitamin D |
There are differences in the expression of cell surface receptors between human and rodent mast cells.
In addition to IgE and antigen [5], immunoglobulin free light chains [17,18], anaphylatoxins, hormones and neuropeptides [19,20] can trigger mast cell secretion [21-23] (Table 2). The latter include substance (SP) [24], hemokinin [25], neurotensin (NT) [26], NGF [12,27] which is released under stress [28], and pituitary adenylate cyclase activating polypeptide (PACAP) [29,30]. Skin mast cells are located close to sensory nerve endings and can be triggered by neuropeptides [21,31], such as NT [26], NGF [12], SP [32], and PACAP [30] (Fig. 1), which can be released from dermal neurons. In fact, skin mast cells contain SP [33], while cultured mouse and human mast cells contain and secrete NGF [34]. Thymic stromal lymphopoietin (TSLP), released in response to inflammation, pathogens and trauma [35], also activates mast cells, but only in the presence of interleukin-1 (IL-1) and tumor necrosis factor (TNF) [35,36]. A number of additional immune and infectious triggers (e.g. stimulants of Toll-like receptors, TLR) can lead to selective release of mast cell mediators (See under “Selective release” below).
Table 2. Mast Cell Mediators*.
| Mediators | Main Pathophysiologic Effects |
|---|---|
| Prestored | |
| Biogenic Amines | |
| Histamine | Vasodilation, angiogenesis, mitogenesis, pain |
| 5-Hydroxytryptamine (5-HT, serotonin) | Vasoconstriction, pain |
| Chemokines | |
| IL-8(CXCL8), MCP-1(CCL2), MCP-3(CCL7), | Chemoattraction and tissue infiltration of leukocytes |
| MCP-4, RANTES (CCL5), Eotaxin (CCL11) | |
| Enzymes | |
| Arylsulfatases | Lipid/proteoglycan hydrolysis |
| Carboxypeptidase A | Peptide processing |
| Chymase | Tissue damage, pain, angiotensin II synthesis |
| Kinogenases | Synthesis of vasodilatory kinins, pain |
| Phospholipases | Arachidonic acid generation |
| Tryptase | Tissue damage, activation of PAR, inflammation, pain |
| Matrix metalloproteinases | Tissue damage, modification of cytokines/chemokines |
| Peptides | |
| Angiogenin | Neovascularization |
| Corticotropin-releasing hormone | Inflammation, vasodilation |
| Endorphins | Analgesia |
| Endothelin | Sepsis |
| Kinins (bradykinin) | Inflammation, pain, vasodilation |
| Leptin | Food intake regulator |
| Renin | Angiotensin synthesis |
| Somatostatin | Anti-inflammatory (?) |
| Substance P | Inflammation, pain |
| Urocortin | Inflammation, vasodilation |
| VEGF | Neovascularization, vasodilation |
| Vasoactive intestinal peptide | Vasodilation, mast cell activation |
| Proteoglycans | |
| Chondroitin sulfate | Cartilage synthesis, anti-inflammatory |
| Heparin | Angiogenesis, nerve growth factor stabilization |
| Hyaluronic acid | Connective tissue, nerve growth factor stabilization |
| De novo synthesized | |
| Cytokines | |
| Interleukins (IL)-1,2,3,4,5,6,8,9,10,13,16,18 | Inflammation, leukocyte migration, pain |
| IFN- α , IFN-β, IFN-γ; MIF; TGFβ; TNF-α, | Inflammation, leukocyte proliferation/activation |
| MIP-1α, MCP-1 | |
| Growth Factors | |
| SCF, GM-CSF, β-FGF, neurotrophin 3, NGF, | Growth of a variety of cells |
| PDGF, TGFβ, VEGF | |
| Nitric oxide | Vasodilation |
| Phospholipid metabolites Leukotriene B4 | Leukocyte chemotaxis |
| Leukotriene C4 | Vasoconstriction, pain |
| Platelet activating factor | Platelet activation, vasodilation |
| Prostaglandin D2 | Bronchonstriction, pain |
There are differences in the expression of mediators between human and rodent mast cells.
β-FGF, β-fibroblast growth factor; GM-CSF, granulocyte monocyte-colony stimulating factor; IFNγ, interferon-γ; MCP, monocyte chemoattractant protein; MIF, macrophage inflammatory factor; MIP, macrophage inflammatory protein; NGF, nerve growth factor; PDGF, platelet-derived growth factor; SCF, stem cell factor; TGFβ, transforming growth factor β ; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
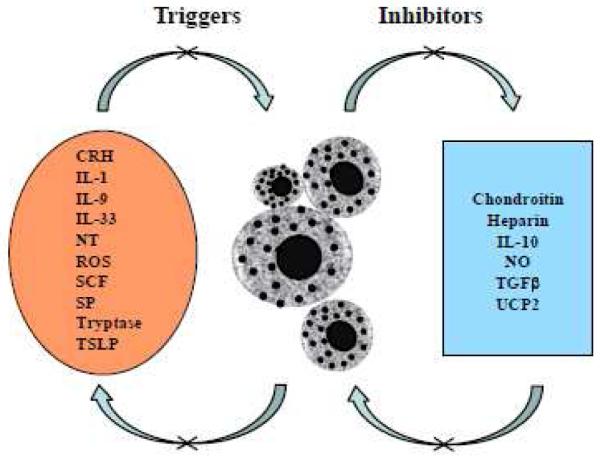
Figure 1.
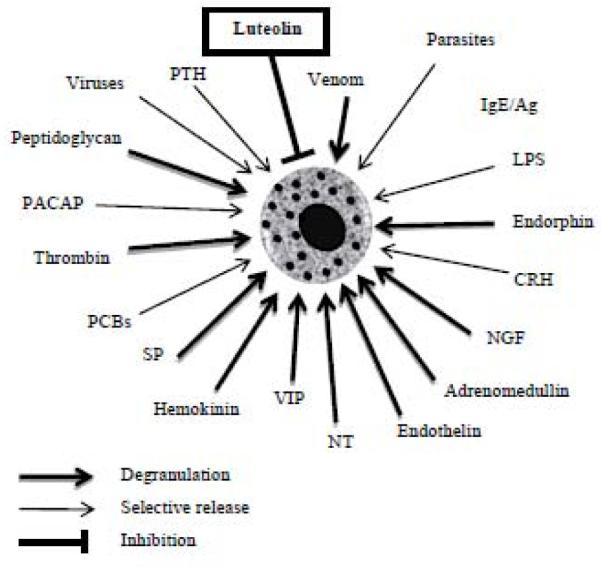
Schematic representation of physiological and environmental mast cell triggers, and the inhibitory effect of certain flavonoids, such as luteolin. Many of these triggers stimulate selective release of mediators such as IL-6, TNF or VEGF without degranulation.
CRH, corticotropin releasing hormone; LPS, lipopolysaccharide; NT, neurotensin; PACAP, pituitary adenylate cyclase activating polypeptide; PCBs, polychlorinated biphenols; PTH, parathyroid hormone; SP, substance P; VIP, vasoactive intestinal peptide.
Once activated, mast cells secrete numerous vasoactive and pro-inflammatory mediators [37-42]. These include pre-formed molecules such as histamine, serotonin, TNF, kinins and proteases stored in secretory granules. Leukotrienes (LT), prostaglandins and platelet activated factor (PAF) are synthesized during mast cell activation from arachidonic acid liberated by the action of phospholipases. In addition, a number of cytokines (e.g. IL-1, 2, 5, 6, 8, 9, 13, TNF) and vascular endothelial growth factor (VEGF) [43] are synthesized de novo and released several hours after stimulation (Table 2). VEGF is also released from normal human cultured mast cells selectively in response to corticotropin-releasing hormone (CRH) [44].
CRH is secreted from the hypothalamus under stress and regulates the hypothalamic-pituitary-axis (HPA) axis [45] through specific receptors [46]. These include CRHR-1 [47] and CRHR-2 [48], the latter being subdivided in CRHR-2α and CRHR-2β [49]. All CRHR are activated by urocortin (Ucn), a peptide with about 50% structural similarity to CRH [50]. Ucn II [51] and Ucn III [52] are potent selective CRHR-2 agonists. CRH can also be secreted from immune cells [53] and mast cells [54]. CRH and related peptides released locally under stress may regulate mast cell function [55], and the brain-skin connection [56]. It was recently reported that CRH stimulates generation of mast cells from human hair follicle precursors [57].
Mature mast cells vary considerably in their cytokine [58] and proteolytic enzyme content, but their phenotypic expression is not fixed [59,60]. Mast cells in the presence of SCF produce predominantly pro-inflammatory cytokines, whereas when used together with SCF and IL-4, they produce mostly Th2 cytokines [61]. For instance, human umbilical cord-derived mast cells (hCBMCs) primed with IL-4 or IL-5 before stimulation with IgE released more TNF, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF), compared to hCBMCs maintained in SCF alone. In contrast, IL-4 enhanced SCF-dependent mast cell proliferation and shifted IgE-stimulated response to Th2 cytokines such as IL-3, IL-5 and IL-13, but not IL-6 [62].
Mast cells play an important role in innate or acquired immunity [63], bacterial infections [64-66], as well as in autoimmunity [67]. Mast cells are also important for maturation of Th17 cells and are recognized as key cells in autoimmune disorders [68]. For instance, mast cells in the presence of IL-6 and transforming growth factor β (TGFβ) are necessary for the production of Th17 cells [69], while TNF and vasoactive intestinal peptide (VIP) drive IL-6-independent Th17 cell maturation [69-71]. A number of immune molecules also contribute to mast cell activation. Addition of complement fragment 3a (C3a) led to increased degranulation of human mast cells stimulated by aggregated IgG [72]. Immunoglobulin-free light chains elicited immediate hypersensitivity-like reactions [18,73], with subsequent T cell-mediated immune responses. The antibacterial peptides, human B-defensins, can activate mast cells and induce degranulation [74]. In fact, mast cells interact with T cells [75,76] and superactivate them through TNF, as shown with mouse [77,78] and human [79,80] mast cells. It was recently shown that T cells release “microparticles” that stimulate human mast cell degranulation and IL-8 release [81]. Mast cells, in turn, secrete heparin “microparticles” that contain and deliver TNF to lymph nodes [82].
Mast cells, specifically a subset highly expressing both FcεRI and MHC II [83], can function as antigen presenting cells [84-86]. Basophils can also act as Th2-inducing antigen-presenting cells [87,88]. Basophils promote Th2 responses [89,90] and co-operate with dendritic cells for optimal Th2 responses [91]. Moreover, basophil activation by “autoreactive IgE” induces their “homing” to lymph nodes, where they promote Th2 cell differentiation and production of auto-reactive antibodies that contribute to lupus nephritis [92]. Interestingly, mast cells can act both as positive and negative modulators of immunity [93]. In addition, mast cells can coordinate the adaptive immune response by directing migration of dendritic and T cells to lymph nodes and secreting T cell-polarizing cytokines [94]. Such regulatory activities of mast cells may stem from selective release of immunomodulatory molecules that could have both autocrine and paracrine actions (Fig. 2).
Figure 2.
Schematic representation of mast cell autocrine triggers and modulators. Numerous molecules secreted by mast cells can have autocrine actions, either activating or inhibiting mast cells.
CRH, corticotropin-releasing hormone; IL, interleukin; NT, neurotensin; NO, nitic oxide; ROS, reactive oxygen species; SCF, stem cell factor; SP, substance P; TGFβ, transforming growth factor β; TSLP, thymic stromal lymphopoietin; UCP2, uncoupling protein 2.
Mast cells also have the unusual ability to be triggered by certain molecules and then either activate them or degrade them. For instance, mast cells can act on precursor protein molecules and generate active peptides [95], such as histamine-releasing peptides [96] and NT, [97] from plasma. However, mast cells can also degrade NT [98] and limit its biologic effects [99]. Mast cells can also synthesize endothelin [100], but also release proteases that degrade endothelin [64]. Finally, mast cells can be activated by snake toxins [101,102], but also degrade them [103]. Whether these actions will prove useful or detrimental obviously depends on the ability of mast cells to secrete specific mediators selectively in a well-regulated fashion.
Inflammatory processes and the role of selective release
Increasing evidence indicates that mast cells are critical for the pathogenesis of inflammatory diseases [19,20], such as arthritis [104], atopic dermatitis, psoriasis [105,106], and multiple sclerosis [107] (Fig. 3). Gene array analysis of human mast cells activated by IgE showed overexpression of numerous, mostly inflammation-related genes [108]. Proteases released from mast cells could act on plasma albumin to generate histamine-releasing peptides [96,109] that would further propagate mast cell activation and inflammation. Proteases could also stimulate protease-activated receptors (PAR) inducing microleakage and widespread inflammation [110,111]. However, unlike allergic reactions, mast cells are rarely seen to degranulate during inflammatory processes. The only way to explain mast cell involvement in non-allergic processes would be through “differential” or “selective” secretion of mediators without degranulation [112].
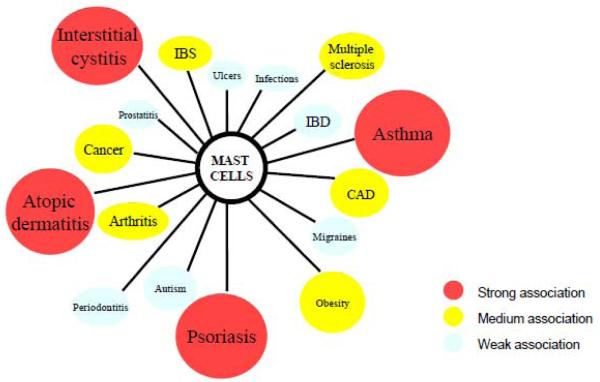
Figure 3.
Mast cell involvement in inflammatory diseases. Increasing evidence indicates that mast cells are involved in many diseases. Colors indicate the strength of the association (red = strongest, white = weakest).
CAD, coronary artery disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
This ability could occur through different mechanisms: (A) mast cells can secrete the content of individual granules [113]; (B) mast cells can secrete some granular contents through a process associated with ultrastructural alterations of their electron dense granular core indicative of secretion, but without evidence of degranulation [114], a process that has been termed “activation” [115], “intragranular activation” [116] or “piecemeal” degranulation [117] (Table 3, Fig. 4); (C) mast cells can undergo selective release of specific mediators such as serotonin without histamine [118]. Selective release of serotonin occured through sequestration from secretory granules inside vesicles containing high affinity serotonin-binding proteins from which it was released [119]. A somewhat similar process was reported for eosinophils where it was shown that eotaxin stimulation induced movement of preformed IL-4 from granules into secretory vesicles from which it was released [120]. Human mast cells stimulated by IL-1 selectively released IL-6 without degranulation through vesicles (40-80 nm) much smaller than the secretory granules (800-1000 nm) [121]. Selective release of eicosanoids has also been shown [122-124].
Table 3. Selective release of mast cell mediators.
| Stimuli | MC type |
Mediators released |
Mediators NOT released |
Pathophysiological importance |
References |
|---|---|---|---|---|---|
| ENDOGENOUS | |||||
| CD8 ligands | RPMC | TNF, NO | H | T cell interaction | [279] |
| CRH | hCBMC | VEGF | H, tryptase, IL-8 | Inflammation | [25] |
| Endothelin-1 -3 | RMMC | TNF, IL-12↑ | IL-4, IL-10, IL-134* | Th1 immunity | [280] |
| IL-1 | hCBMC | IL-6, IL-8, TNF | H, tryptase | Inflammation | [92] |
| IL-1β | RPMC | NO | PAF, H | Inflammation | [281] |
| IL-12 | P815 | IL-13 | Host defence against bacteria |
[282] | |
| IL-12 | RPMC | IFN-y | H | Th1 immunity | [283] |
| LTC4/LTD4 | IL-4- primed hCBMC |
TNF, MIP-1α, IL-5 |
H | Non-IgE mediated inflammation |
[284] |
| Monomeric IgE | BMMC | IL-6 | H, LTC4 | Mast cell survival | [285] |
| PGE2 | RPMC | IL-6 | H, TNF | Cytoprotection | [286] |
| SCF | BMMC | IL-6 | H, LTC4, TNF | Mast cell development | [83] |
| SDF | hCBMC | IL-8 | H, GM-CSF, IFN-γ, IL-1β |
Endothelial transmigration | [88] |
| Thrombin | BMMC | IL-6 | Serotonin, TNF | Anticlotting | [287] |
| Urocortin | hCBMC | IL-6 | H, tryptase, IL-8, VEGF |
Inflammation | [288] |
| EXOGENOUS/PHARMACOLOGICAL | |||||
| Amitriptyline | RPMC | Serotonin | HA | Headaches | [73] |
| Cholera Toxin | RPMC | IL-6 | HA, TNF | Inflammation | [289] |
| Clostridium difficile Toxin A |
RPMC | TNF | HA | GI tract inflammation | [290] |
| CpG DNA | BMMC | TNF, IL-6 | HA, IL-4, IL-12, GM-CSF, IFN | Host response to bacteria | [291] |
| H. pylori VacA Toxin |
BMMC | IL-6, IL-8, TNF | HA | Gastric injury | [102] |
| LPS (TLR-4) | RPMC | IL-6 | HA | Bacterial infection | [81] |
| PMA | BMMC | VPF/VEGF | 5HT | Angiogenesis | [292] |
| S.a.peptidoglycan (TLR-2) |
hCBMC | HA, IL-1p, RANTES, LTC4 |
IL-6 | Exacerbation of asthma by bact. infection |
[98] |
| Suboptimal FcεRI stimulation |
BMMC | MCP-1, HA low | IL-10, HA | Chemokines ⪡Cytokines /HA |
[120] |
| Viruses (TLR- 3,5,9) |
FSMC | TNF, IL-6 | HA | Recruitment of other immune cells |
[103] |
BMMC, bone marrow mast cells; CRH, corticotropin-releasing hormone; FSMC, fetal skin-derived cultured mast cells; GM-CSF, granulocyte monocyte-colony stimulating factor; H, histamine; HA, hexosaminidase; hCBMC, human cord blood-derived mast cells; IFN, interferon; LT, leukotriene; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NO, nitric oxide; PAF, platelet activating factor; PMA, phorbol myristate acetate; PG, prostaglandin; RMMC, rat mucosal mast cells ; RPMC, rat peritoneal mast cells; SCF, stem cell factor; SDF, stromal cell-derived factor; TLR, toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
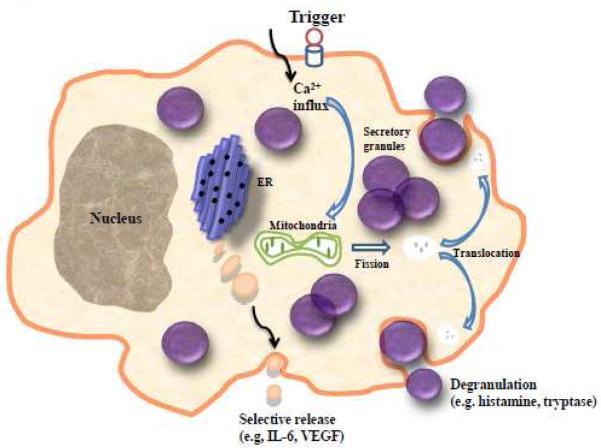
Figure 4.
Schematic representation showing mast cell degranulation as compared to selective mediator release. During selective release, vesicles much smaller than secretory granules transport mediators to the cell surface for exocytosis.
ER, endoplasmic reticulum; VEGF, vascular endothelial growth factor.
Selective release of IL-6 was reported in response to bacterial lipopolysaccharide (LPS), in the presence of the phosphatidylinositol 3-kinase (PI3-K) inhibitor wortmannin, or triggered by SCF [125-127]. CRH induced selective VEGF release [128], and PGE2 also induced release of VEGF [129] and MCP-1 without degranulation [130]. Yet, PGE2 inhibited FcεRI-induced histamine release from human lung mast cells [131]. Stromal cell-derived factor-1 alpha (SF-1α) selectively produced IL-8 from human mast cells without degranulation as well [132]. Activation of human cultured mast cells by CD30 ligands led to release of the chemokines IL-8 and MCP-1 without histamine and without degranulation [133]. IL-33 induced IL-13 release independent of IgE stimulation [134].
TLR are critical in innate and acquired immunity [135,136]. TLR activation on mast cells leads to release of different cytokines [137]. For instance, rodent mast cell TLR-4 activation by LPS induces TNF release without degranulation. TLR-4 is also activated by extra domain A of fibronectin to release several cytokines, including TNF, in the same way as LPS [138]. Furthermore, LPS induces secretion of IL-5, IL-10 and IL-13, but not GM-CSF, IL-1 or LTC4. [139,140]. In contrast, staphylococcal peptidoglycan induces degranulation and histamine release through TLR-2 [139,141]. TLR-2 and TLR-4 activation has a synergistic action with antigen in enhancing cytokine production from rodent mast cells [142]. Elsewhere, it was shown that TLR-2 activation produces IL-4, IL-6 and IL-13, but not IL-1, while LPS produces TNF, IL-1, IL-6 and IL-13, but not IL-4 or IL-5, again without degranulation [143].
TLR 3, 7 & 9 activation by poly-oligodeoxynucleotide and CpG induces release of TNF and IL-6 without degranulation from fetal rat skin-derived mast cells [144]. Human mast cells produce IL-6 through viral TLR-9 activation [145], while they produce interferon (IFN) following TLR-3 activation by double-stranded RNA [146].
Regulation of mast cell activation
FcεRI-induced mast cell degranulation involves calcium-dependent exocytosis, and SNAP-23 phosphorylation [147], but granule translocation to the surface is calcium-independent [148]. Mast cell activation by different triggers apparently engages different downstream pathways. FcεRI aggregation induces PI3K, ERK, JNK, NF-κB and PKC activation, although the PKCε isozyme may be redundant [149,150]. PI3K inhibition by the “phosphatase and tensin homologue deleted on chromosome ten” (PTEN) or PTEN knockdowns induce constitutive cytokine production, without degranulation, that involves phosphorylation of AKT, p38/MAPK and JNK [151]. Secretion in response to compound 48/80 requires PLC, tyrosine kinase, p38/MAPK and PKC [152]. In contrast, IL-1 stimulation of selective IL-6 release is extracellular calcium-independent and involves p38/MAPK, but only PKCθ isozyme activation [153]. CRH-induced selective VEGF release from mast cells is also extracellular calcium-independent, and involves only PKA and p38/MAPK activation [128].
Degranulation in response to FcεRI-aggregation was severely impaired in IL-2-inducible T cell kinase −/− mice [154]. FcεRI-induced mast cell activation in rat basophil leukemia (RBL) cells was inhibited by the Syk-tyrosine kinase inhibitor Piceatannol [155]. Suboptimal antigen challenge of human mast cells led to FcεRI-unresponsiveness that correlated with reduced Syk levels [156], apparently through actin assembly that blocked degranulation [157]. However, low antigen still permitted MCP-1 release, suggesting yet another mechanism of differential release [158].
The Src family kinase Lyn is a negative regulator of allergic mast cell activation, but Lyn −/− mice had increased FcεRI expression, circulating histamine and eosinophilia [159]. Fyn deficient mast cells could not generate IL-6, TNF or MCP-1 during FcεRI aggregation, but IL-13 production was intact, suggesting divergent regulatory pathways [160].
Adaptor complexes such as B cell lymphoma 10-mucosal-associated lymphoid tissue 1 (Bcl10-Malt1) permit FcεRI-dependent IL-6 and TNF release without degranulation [161]. Mice deficient in either Bcl10 or MALT1 proteins did not produce TNF or IL-6 upon FcεRI signaling: yet, degranulation and LT secretion was normal [162]. Neutralization of the inhibitory receptor IRp60 (CD300a) in human cord blood mast cells in mice led to increased mediator release [163]. In contrast, engagement of the myeloid cell inhibitory receptor CD200 in human mast cells inhibited FcεRI-induced activation [164]. Mast cells also express the inhibitory receptors CD300 and Siglec-8, as well as the death receptor TRAIL [165]. Two peptides derived from the complement components C3a, C3a+ and C3a9 inhibited FcεRI-induced degranulation and TNF release [166].
There appear to be some innate inhibitors of mast cell secretion (Fig 2). Chondroitin sulfate and heparin, the major constituents of mast cell granules, inhibit human mast cell secretion [167]. Nitric oxide (NO) blocks FcεRI-induced cytokine secretion through inhibition of Jun [168]. In contrast IL-10 appears to have divergent effects depending on the mast cell type and stimulus [169]. The natural chymase inhibitors alpha 1-antitrypsin and secretory leukocyte protease inhibitor (SLPI) inhibit histamine release from human cells [170].
Recent evidence indicates that mitochondria are involved in the regulation of mast cell degranulation (Fig. 4). Mitochondrial uncoupling protein 2 (UCP2) inhibited mast cell activation [171]. Moreover, our recent results indicate that mast cell degranulation requires mitochondrial translocation to the cell surface [172] (Fig. 5). Inhibition or downregulation of Dynamin Related Protein 1 (Drp1), a cytoplasmic protein responsible for mitochondrial fission and translocation, blocks mast cell degranulation [173]. The involvement of mitochondria in mast cell regulation may also explain the ability of certain flavonoids [174] to inhibit mast cell degranulation [175], since quercetin was shown to accumulate in mitochondria [176].
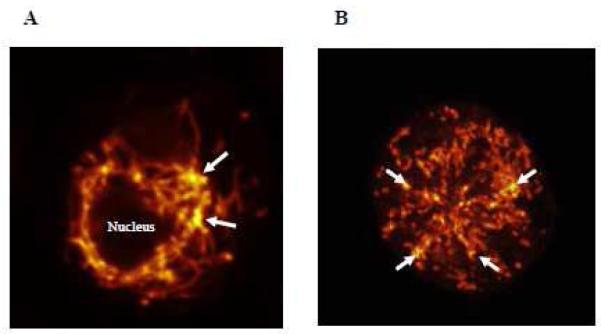
Figure 5.
Two human cultured LAD2 mast cells, showing distribution of mitochondria stained with MitoTracker and photographed using Confocal microscopy; (A) control in which mitochondria form a “net” around the nucleus and (B) after stimulation with SP (2 M for 30 min at 37°C) in which mitochondria are distributed throughout the cell. (Magnification: x 1000). Arrows point to the areas with the highest concentration of MitoTracker (yellow color); thus the highest aggregation of mitochondria.
Atopic dermatitis and psoriasis
Skin mast cells may have important functions as “sensors” of environmental and emotional stress [56], possibly due to direct activation by CRH secreted under stress, and related peptides [55]. Mast cell-related atopic dermatitis (AD) and psoriasis, are triggered or exacerbated by stress through mast cell activation [177,178]. Mast cell activation in AD may also be induced by cytokines, such as TSLP. We recently reported increased serum levels and skin gene expression of TSLP in AD patients as compared to controls [179], in agreement with previous studies [180,181].
Computer-induced stress enhanced allergen specific responses with concomitant increase in plasma SP levels in patients with AD [182]. Similar findings with increased plasma levels of SP, VIP and NGF, along with a switch to a Th2 cytokine pattern, was reported in patients with AD playing video games [183]. Skin has its own equivalent of the HPA axis [184,185]. CRH and CRHR mRNA is expressed in human and rodent skin [186,187] and CRH can be secreted from dorsal root ganglia and from sympathetic ganglia [188,189]. CRH administration in humans causes peripheral vasodilation and flushing reminiscent of mast cell activation [190]. Moreover, intradermal administration of CRH and Ucn activates skin mast cells and increases vascular permeability in rodents [191] and humans [192,193], through activation of CRHR-1 [56]. CRHR-1 expression was increased in chronic urticaria [194]. Acute stress released CRH in the skin and increased local vascular permeability [195]. Acute stress also exacerbated skin delayed hypersensitivity reactions [196], and chronic contact dermatitis in rats, an effect that involved significantly increased mast cells in the dermis, and was dependent on CRHR-1 [197]. Acute restraint stress induced rat skin vascular permeability [198], which was inhibited by a CRH receptor antagonist, and was absent in mast cell deficient mice [191,199].
Psoriasis is also triggered or exacerbated by acute stress [105,200-202]. We showed that psoriasis is associated with increased serum CRH and decreased lesional skin CRHR-1 gene expression possibly due to downregulation [203]. Psoriasis is characterized by keratinocyte proliferation and inflammation, as well as mast cell accumulation and activation [106,204]. Mast cells are increased in lesional psoriatic skin [105,106]. Neuropeptides [205], especially SP [206], are involved in the pathogenesis of psoriasis. In particular, SP reactive fibers are localized close to mast cells [105,207]. SP can stimulate mast cells [208,209] and contributes to inflammation [210,211]. SP-positive nerve fibers are more dense in psoriatic lesions and have an increased number of mast cell contacts compared to normal skin [207,212,213]. SP-positive nerve fibers and mast cell contacts are also increased by acute stress in mice [214], leading to dermal mast cell degranulation [201,208,215]. Keratinocytes also express neurokinin (NK) 2 receptors and can be stimulated by SP [216], to release IL-1 [217]. Keratinocyte proliferation is accelerated by PAF, which can be secreted from mast cells [218], and stimulates human mast cells [219].
Psoriasis is associated with chronic inflammation and it often co-exists with inflammatory arthritis [220], in which IL-33 was recently implicated [221]. IL-33 is one of the newest members of the IL-1 family of inflammatory cytokines [222], and can mediate IgE-induced anaphylaxis in mice [223]. IL-33 also induces release of IL-6 from mouse bone marrow-derived cultured mast cells [224], and IL-8 from hCBMCs [225]. We showed that IL-33 augments SP-stimulated VEGF release from human mast cells and IL-33 gene expression is increased in lesional skin from patients with psoriasis [226]. Mast cells may, therefore, be involved in the pathogenesis of psoriasis and other inflammatory skin diseases.
Multiple sclerosis
Functional mast cell-neuron interactions occur in the brain [227,228] and could mediate neuroinflammation [20]. In the brain, mast cells are found in the leptomeninges [228,229], the choroid plexus, thalamus and hypothalamus, especially the median eminence [230,231], where most of histamine derives from mast cells [232-235]. We had proposed that mast cells can act as “the immune gate to the brain” [107], and we later showed that mast cells regulate BBB permeability [236,237]. BBB breakdown [238] precedes any pathological or clinical signs of MS [239-241], as shown by MRI-gadolinium studies and trans-BBB leakage of albumin [242]. Mast cells have been implicated in multiple sclerosis (MS), a demyelinating condition involving brain and MS plaque infiltration [243] by lymphocytes and activated mast cells [244,245]. Gene array analysis of MS plaques showed overexpression of genes for FcεRI, the histamine-1 (H1) receptor and tryptase, all of which are associated with mast cells [246,247]. A recent paper reported that experimental allergic encephalomyelitis (EAE) development depends on H1 receptor activation [248]. Mast cells are located close to the cerebral microvasculature and do not express FcεRI protein under normal conditions [249]. This is not surprising as the brain is not known to develop allergic reactions since IgE does not cross the blood-brain-barrier (BBB). Brain mast cells also do not normally express their surface growth factor (c-kit) receptor [250], but do so during EAE [251]. We first showed that mast cells migrate into the brain from the meninges, and it was later shown that they can also enter the CNS from blood [252]. Mast cell-derived products can enter neurons, a process termed “transgranulation”, indicating a novel form of brain-immune system communication [253]. We further hypothesized that perivascular brain mast cells could come in contact with circulating T cells and not only allow them to enter the BBB, but also activate them [80]. TNF can be released from rat brain mast cells [254], and is involved in both brain inflammation [255,256] and increased vascular permeability [257]. Mast cell tryptase is elevated in the CSF of MS patients [258] and can activate peripheral mononuclear cells to secrete TNF and IL-6 [259], as well as stimulate PAR that can lead to microvascular leakage and widespread inflammation [260]. It was recently reported that meningeal mast cells promote T cell infiltration in the CNS by disrupting BBB integrity through TNF [261]. However, this paper did not include any of earlier publications discussed above and did not consider the possibility that lack of TNF may eventually worsen EAE [262]. The above findings imply that mast cells may be able to secrete both prestored and de novo synthesized TNF [263,264] with different biological actions.
The role of CD4+ T cells is well-documented in MS, but this CD4-Th1 model has recently been questioned [265], because increasing evidence also implicates Th2 processes typically associated with allergic reactions [266,267]. Some studies reported the inability of mast cell deficient mice to fully develop EAE, but suggested that reduced T cell activation may also be involved [268,269]. Mast cell contact with activated T cells leads to secretion of matrix metalloproteinase (MMP)-9 and IL-6 from human mast cells [270]. Moreover, mast cells can promote IgE-dependent and T cell-independent proliferation and activation through TNF release [77], [78]. We showed that mast cells superstimulate activated T cells, an action which is further increased when mast cells are activated by myelin basic protein (MBP) and is partially dependent on TNF [79,80]. MBP could induce homogeneic mast cell activation and brain demyelination [271]. Moreover, virally-induced encephalomyelitis could not develop in W/Wv mast cell deficient mice, and EAE was attenuated and delayed in these mice [272].
Mast cell-derived mediators can increase BBB permeability [273]. Selective release of IL-6 could have profound effects on brain function [274] and could activate the HPA axis [275]. Selective release of VEGF, an isoform of which is particularly vasodilatory [43,276], could lead to BBB disruption [277]. Mast cells are localized close to CRH-positive neurons in the median eminence [278] and express functional CRH receptors [44]. Activation of hypothalamic mast cells can stimulate the HPA axis [279-281], through histamine, which regulates the hypothalamus, and can also increase hypothalamic CRH mRNA expression [282]. Moreover, human mast cells can synthesize and secrete large amounts of CRH [283], as well as IL-1 and IL-6 which are independent activators of the HPA axis [284].
The effect of stress and CRH on mast cell activation and BBB permeability may help explain some of the clinical findings in MS patients. Acute stress worsens the symptoms of MS, and the appearance of new MRI lesions has been repeatedly shown to be precipitated by psychological stress [285-288]. In one study in Denmark, parents who had unexpectedly lost a young child had a significantly increased risk of MS, compared to other bereaved parents [289]. Meta-analysis of 14 prospective studies showed a significantly increased risk of MS exacerbations after stressful events [290]. A review of the effect of stress on MS proposed that it may be due to glucocorticoid-insensitive immune cells [291]. Another study argued that stress could not affect MS because the function of peripheral blood leukocytes in MS patients was apparently unaffected by stress [292]. However, such findings may not be relevant as stress may predominantly affect mast cells and T cells, but not peripheral leukocytes. Release of CRH and cytokines outside the brain may be more relevant instead. For instance, examination-stress dramatically increased serum TNF levels in medical student volunteers [293], and restraint stress induced mast cell-dependent increase in mouse serum IL-6 [294]. Rat brain mast cells were activated by acute stress, and led to CSF elevation of rat mast cell protease I [278], the equivalent of tryptase in humans. These effects were abolished by polyclonal antiserum to CRH and by the CRHR-1 antagonist Antalarmin [228,278]. A short period of restraint [295] or maternal deprivation stress [296] increased the severity of EAE. Acute restraint stress also shortened the time required for the development of EAE in mice [295]. Moreover, EAE was characterized by decreased clinical disability and brain infiltration by immune cells in CRH −/− mice as compared to normal controls [297]. Restraint stress was also reported to increase mortality rates and lead to higher CNS viral load during Theiler’s virus infection [298]. Stressed mice had increased inflammatory spinal cord lesions and developed autoimmune antibodies to MBP [299]. Mast cell activation was shown to occur in response to isolation stress [300], restraint stress [278], subordination stress [301], and during courtship following isolation of male doves [302].
Mast cells could, therefore, participate in the pathogenesis of MS in many different ways: they could (A) be stimulated to release cytokines/chemokines selectively inducing T cell/macrophage recruitment and activation; (B) present myelin antigens to T cells; (C) disrupt the BBB and permit entry of active T cells that are sensitized to MBP; (D) damage myelin and release fragments that could stimulate secretion of tryptase, which may in turn enhance demyelination and induce further inflammation through stimulation of PAR. As a result mast cells were considered as a possible therapeutic target for MS [303]. It is of interest that flavonoids [174] known to inhibit mast cell secretion [175] have also been shown to inhibit macrophage myelin phagocytosis [304], and EAE [305,306]. The flavone luteolin, which is structurally related to quercetin, was also a strong inhibitor of human autoimmune T cells [307]. Quercetin and luteolin also inhibit IL-6 release from microglia [308] and induce an anti-inflammatory phenotype [309]. Luteolin is neuroprotective [309] and is closely related to dihydroxyflavone recently shown to mimic the action of BDNF [310]. We showed that luteolin can inhibit mast cell activation and mast cell-dependent superstimulation of activated T cells with or without stimulation by MBP [80]. Luteolin can also inhibit activation of peripheral lymphocytes from MS patients [311], and it was, therefore, proposed as adjuvant therapy for MS [312].
Conclusion
Mast cells clearly participate in the induction and/or propagation of certain inflammatory diseases, through selective release of mediators. The pharmacologic inhibition of this process would, therefore, have clear therapeutic potential. Luteolin formulations, alone or together with drugs that can selectively inhibit the release of pro-inflammatory mediators hold promise for the treatment of skin and brain inflammatory diseases.
Research highlights.
Mast cells release pro-inflammatory mediators selectively without degranulation
Mast cells are activated by CRH released under stress
Neuropeptide mast cell triggers have synergistic action with cytokines, like IL-33
Unique flavonoid combinations can effectively block mast cell secretion
Mast cells may serve as new therapeutic targets for psoriasis and multiple sclerosis
Acknowledgments
Aspects of our work discussed here were supported in part by US National Institutes of Health (NIH) grants: AR47652, NS71361, NS55681 and NS66205 to TCT. Konstantinos-Dionysios Alysandratos and Asimenia Angelidou are recipients of postgraduate scholarships from the Hellenic State Scholarships Foundation (Athens, Greece). Bodi Zhang is partially supported by a graduate fellowship from Galenica, SA (Athens, Greece).
Abbreviations
- AD
atopic dermatitis
- BBB
blood-brain barrier
- Bcl10-Malt1
B cell lymphoma 10-mucosal-associated lymphoid tissue 1
- BDNF
brain-derived neurotrophic factor
- CRH
corticotropin-releasing hormone
- CRHR
corticotropin-releasing hormone receptor
- Drp1
dynamin related protein 1
- EAE
experimental allergic encephalomyelitis
- FcεRI
high affinity surface receptors for IgE
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- hCBMCs
human umbilical cord-derived mast cells
- HPA
hypothalamic-pituitary-adrenal
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- LT
leukotriene
- MBP
myelin basic protein
- MCP-1
monocyte chemoattractant protein-1
- MS
multiple sclerosis
- MMP
matrix metalloproteinase
- NGF
nerve-growth factor
- NK
neurokinin
- NT
neurotensin
- PACAP
pituitary adenylate cyclase activating polypeptide
- PAF
platelet activating factors
- PAR
protease activated receptors
- PI3-K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome ten
- RANTES
regulated upon activation normal T cell expressed and secreted
- RBL
rat basophil leukemia
- SCF
stem cell factor
- SF-1α
stromal cell-derived factor-1 alpha
- SLPI
secretory leukocyte protease inhibitor
- SP
substance P
- TGFβ
transforming growth factor β
- TLR
toll-like receptors
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
- Ucn
urocortin
- UCP2
uncoupling protein 2
- VEGF
vascular endothelial growth factor
- VIP
vasoactive intestinal peptide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
TCT is the inventor of US patents 6,635,625; 6,641,806; 6,645,482; 6,689,748; 6,984,667 and EPO 1365777 covering the role of mast cells in inflammatory diseases, US 6,020,305 covering stress-induced skin diseases, as well as US patent application 11/214,831 and 12/861,152 covering the treatment of multiple sclerosis and brain inflammation.
References
- 1.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U.S.A. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitamura Y, Ito A. Mast cell-committed progenitors. Proc Natl Acad Sci U.S.A. 2005;102:11129–11130. doi: 10.1073/pnas.0505073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 5.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev.Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 8.Aloe L, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Research. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 9.Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807–1820. [PubMed] [Google Scholar]
- 10.Metz M, Botchkarev VA, Botchkareva NV, Welker P, Tobin DJ, Knop J, Maurer M, Paus R. Neurotrophin-3 regulates mast cell functions in neonatal mouse skin. Exp.Dermatol. 2004;13:273–281. doi: 10.1111/j.0906-6705.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 11.Lorentz A, Hoppe J, Worthmann H, Gebhardt T, Hesse U, Bienenstock J, Bischoff SC. Neurotrophin-3, but not nerve growth factor, promotes survival of human intestinal mast cells. Neurogastroenterol.Motil. 2007;19:301–308. doi: 10.1111/j.1365-2982.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 12.Tal M, Liberman R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neurosci Lett. 1997;221:129–132. doi: 10.1016/s0304-3940(96)13318-8. [DOI] [PubMed] [Google Scholar]
- 13.Conti P, Pang X, Boucher W, Letourneau R, Reale M, Barbacane RC, Thibault J, Theoharides TC. Impact of Rantes and MCP-1 chemokines on in vivo basophilic mast cell recruitment in rat skin injection model and their role in modifying the protein and mRNA levels for histidine decarboxylase. Blood. 1997;89:4120–4127. [PubMed] [Google Scholar]
- 14.Cima K, Vogelsinger H, Kahler CM. Sensory neuropeptides are potent chemoattractants for human basophils in vitro. Regul.Pept. 2010;160:42–48. doi: 10.1016/j.regpep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses:comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 16.Molderings GJ. Mast cell function in physiology and pathophysiology. BIOTREND Reviews. 5:2010. [Google Scholar]
- 17.Redegeld FA, Nijkamp FP. Immunoglobulin free light chains and mast cells: pivotal role in T-cell-mediated immune reactions? Trends Immunol. 2005;24:181–185. doi: 10.1016/s1471-4906(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 18.Redegeld FA, van der Heijden MW, Kool M, Heijdra BM, Garssen J, Kraneveld AD, Van Loveren H, Roholl P, Saito T, Verbeek JS, Claassens J, Koster AS, Nijkamp FP. Immunoglobulin-free light chains elicit immediate hypersensitivity-like responses. Nat Med. 2002;8:694–701. doi: 10.1038/nm722. [DOI] [PubMed] [Google Scholar]
- 19.Theoharides TC. Mast cell: a neuroimmunoendocrine master player. Int J Tissue React. 1996;18:1–21. [PubMed] [Google Scholar]
- 20.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Goetzl EJ, Cheng PPJ, Hassner A, Adelman DC, Frick OL, Speedharan SP. Neuropeptides, mast cells and allergy: novel mechanisms and therapeutic possibilities. Clin Exp Allergy. 1990;20:3–7. doi: 10.1111/j.1365-2222.1990.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 22.Foreman JC. Peptides and neurogenic inflammation. Brain Res Bull. 1987;43:386–398. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- 23.Janiszewski J, Bienenstock J, Blennerhassett MG. Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol. 1994;267:C138–C145. doi: 10.1152/ajpcell.1994.267.1.C138. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- 25.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 26.Carraway R, Cochrane DE, Lansman JB, Leeman SE, Paterson BM, Welch HJ. Neurotensin stimulates exocytotic histamine secretion from rat mast cells and elevates plasma histamine levels. J Physiol. 1982;323:403–414. doi: 10.1113/jphysiol.1982.sp014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bienenstock J, Tomioka M, Matsuda H, Stead RH, Quinonez G, Simon GT, Coughlin MD, Denburg JA. The role of mast cells in inflammatory processes: evidence for nerve mast cell interactions. Int Arch Allergy Appl Immunol. 1987;82:238–243. doi: 10.1159/000234197. [DOI] [PubMed] [Google Scholar]
- 28.De Simone R, Alleva E, Tirassa P, Aloe L. Nerve growth factor released into the bloodstream following intraspecific fighting induces mast cell degranulation in adult male mice. Brain Behav Immun. 1990;4:74–81. doi: 10.1016/0889-1591(90)90008-e. [DOI] [PubMed] [Google Scholar]
- 29.Seebeck J, Kruse ML, Schmidt-Choudhury A, Schmidt WE. Pituitary adenylate cyclase activating polypeptide induces degranulation of rat peritoneal mast cells via high-affinity PACAP receptor-independent activation of G proteins. Ann NY Acad Sci. 1998;865:141–146. doi: 10.1111/j.1749-6632.1998.tb11172.x. [DOI] [PubMed] [Google Scholar]
- 30.Odum L, Petersen LJ, Skov PS, Ebskov LB. Pituitary adenylate cyclase activating polypeptide (PACAP) is localized in human dermal neurons and causes histamine release from skin mast cells. Inflamm Res. 1998;47:488–492. doi: 10.1007/s000110050363. [DOI] [PubMed] [Google Scholar]
- 31.Goetzl EJ, Chernov T, Renold F, Payan DG. Neuropeptide regulation of the expression of immediate hypersensitivity. J Immunol. 1985;135:802s–805s. [PubMed] [Google Scholar]
- 32.Fewtrell CMS, Foreman JC, Jordan CC, Oehme P, Renner H, Stewart JM. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. Journal of Physiology. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toyoda M, Makino T, Kagoura M, Morohashi M. Immunolocalization of substance P in human skin mast cells. Arch Dermatol Res. 2000;292:418–421. doi: 10.1007/s004030000149. [DOI] [PubMed] [Google Scholar]
- 34.Xiang Z, Nilsson G. IgE receptor-mediated release of nerve growth factor by mast cells. Clin Exp Allergy. 2000;30:1379–1386. doi: 10.1046/j.1365-2222.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 35.Allakhverdi Z, Comeau MR, Jessup HK, yoon BP, Breuer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cell in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;19:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bashyam B. TSLP-tickled mast cells. J Exp Med. 2007;204:209. [Google Scholar]
- 37.Dvorak AM. New aspects of mast cell biology. Int Arch Allergy Immunol. 1997;114:1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 38.Serafin WE, Austen KF. Mediators of immediate hypersensitivity reactions. N Engl J Med. 1987;317:30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LB. Mediators of human mast cells and human mast cell subsets. Ann Allergy. 1987;58:226–235. [PubMed] [Google Scholar]
- 40.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 41.Holgate ST. The role of mast cells and basophils in inflammation. Clin Exp Allergy. 2000;30:28–32. doi: 10.1046/j.1365-2222.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 42.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 43.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 45.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 46.Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, DeSouza EB. Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Charmers DT, DeSouza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovenberg TW, Chalmers DT, Liu C, DeSouza EB. CRF2α and CRF2β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinol. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 50.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 51.Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 52.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF 2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karalis K, Louis JM, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 54.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 55.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Ito N, Sugawara K, Bodo E, Takigawa M, van, Beek N, Ito T, Paus R. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol. 2010;130:995–1004. doi: 10.1038/jid.2009.387. [DOI] [PubMed] [Google Scholar]
- 58.Bradding P, Okayama Y, Howarth PH, Church MK, Holgate ST. Heterogeneity of human mast cells based on cytokine content. J Immunol. 1995;155:297–307. [PubMed] [Google Scholar]
- 59.Levi-Schaffer F, Austen KF, Gravallese PM, Stevens RL. Co-culture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci USA. 1986;83:6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci USA. 1999;96:8080–8085. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bischoff SC, Sellge G, Manns MP, Lorentz A. Interleukin-4 induces a switch of human intestinal mast cells from proinflammatory cells to Th2-type cells. Int Arch.Allergy Immunol. 2001;124:151–154. doi: 10.1159/000053695. [DOI] [PubMed] [Google Scholar]
- 62.Ochi H, DeJesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci USA. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 64.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 65.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur.J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat.Rev.Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rottem M, Mekori YA. Mast cells and autoimmunity. Autoimmun Rev. 2005;4:21–27. doi: 10.1016/j.autrev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 68.O’Connor W, Jr., Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat.Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 69.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 70.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav M, Goetzl EJ. Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity. Ann.N.Y.Acad.Sci. 2008;1144:83–89. doi: 10.1196/annals.1418.020. [DOI] [PubMed] [Google Scholar]
- 72.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcγRI: additive effects of C3a. Clin Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Kraneveld AD, Kool M, van Houwelingen AH, Roholl P, Solomon A, Postma DS, Nijkamp FP, Redegeld FA. Elicitation of allergic asthma by immunoglobulin free light chains. Proc Natl Acad Sci U.S.A. 2005;102:1578–1583. doi: 10.1073/pnas.0406808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur.J Immunol. 2007;37:434–444. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 75.Bachelet I, Levi-Schaffer F. Mast cells as effector cells: a co-stimulating question. Trends Immunol. 2007;28:360–365. doi: 10.1016/j.it.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 77.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 78.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Theoharides TC, Kempuraj D, Iliopoulou BP. Mast cells, T cells, and inhibition by luteolin: implications for the pathogenesis and treatment of multiple sclerosis. Advances in Experimental Medicine and Biology. 2007;601:423–430. doi: 10.1007/978-0-387-72005-0_45. [DOI] [PubMed] [Google Scholar]
- 80.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolferg A, Theoharides TC. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T cell-induced mast cell activation: a role for microparticles released from activated T cells. J Immunol. 2010;185:4206–4212. doi: 10.4049/jimmunol.1000409. [DOI] [PubMed] [Google Scholar]
- 82.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp.Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, Chen SS. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC.Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novak N, Bieber T, Kraft S. Immunoglobulin E-bearing antigen-presenting cells in atopic dermatitis. Curr.Allergy Asthma Rep. 2004;4:263–269. doi: 10.1007/s11882-004-0069-2. [DOI] [PubMed] [Google Scholar]
- 85.Poncet P, Arock M, David B. MHC class II-dependent activation of CD4+ T cell hybridomas by human mast cells through superantigen presentation. J Leukoc.Biol. 1999;66:105–112. doi: 10.1002/jlb.66.1.105. [DOI] [PubMed] [Google Scholar]
- 86.Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 87.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat.Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 88.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat.Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Current Opinions in Immunology. 2010;22:73–77. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimoto T. Basophils as T(h)2-inducing antigen-presenting cells. Int Immunol. 2010;22:543–550. doi: 10.1093/intimm/dxq052. [DOI] [PubMed] [Google Scholar]
- 91.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat.Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat.Rev.Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frossi B, Gri G, Tripodo C, Pucillo C. Exploring a regulatory role for mast cells: ‘MCregs’? Trends Immunol. 2010;31:97–102. doi: 10.1016/j.it.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Theoharides TC. Mast cells and precursor protein molecules. Perspect Biol Med. 1981;24:499–502. doi: 10.1353/pbm.1981.0019. [DOI] [PubMed] [Google Scholar]
- 96.Cochrane DE, Carraway RE, Feldberg RS, Boucher W, Gelfand JM. Stimulated rat mast cells generate histamine-releasing peptide from albumin. Peptides. 1993;14:117–123. doi: 10.1016/0196-9781(93)90018-c. [DOI] [PubMed] [Google Scholar]
- 97.Carraway RE, Mitra SP, Ferris CF. Pepsin treatment of mammalian plasma generates immunoreactive and biologically active neurotensin-related peptides in micromolar concentrations. Endocrinology. 1986;119:1519–1526. doi: 10.1210/endo-119-4-1519. [DOI] [PubMed] [Google Scholar]
- 98.Cochrane DE, Carraway RE, Boucher W, Feldberg RS. Rapid degradation of neutotensin by stimulated rat mast cells. Peptides. 1991;12:1187–1194. doi: 10.1016/0196-9781(91)90193-s. [DOI] [PubMed] [Google Scholar]
- 99.Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, Wada E, Wada K, Zacharias S, Mohanasundaram UM, Faix JD, Abrink M, Pejler G, Pearl RG, Tsai M, Galli SJ. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat.Med. 2008;14:392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hultner L, Ehrenreich H. Mast cells and endothelin-1: a life-saving biological liaison? Trends Immunol. 2005;26:235–238. doi: 10.1016/j.it.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Damerau B, Lege L, Oldigs HD, Vogt W. Histamine release, formation of prostaglandin-like activity (SRS-C) and mast cell degranulation by the direct lytic factor (DLF) and phospholipase A of cobra venom. Naunyn Schmiedebergs Arch.Pharmacol. 1975;287:141–156. doi: 10.1007/BF00510446. [DOI] [PubMed] [Google Scholar]
- 102.Liu CS, Chen JM, Chang CH, Chen SW, Teng CM, Tsai IH. The amino acid sequence and properties of an edema-inducing Lys-49 phospholipase A2 homolog from the venom of Trimeresurus mucrosquamatus. Biochim Biophys Acta. 1991;1077:362–370. doi: 10.1016/0167-4838(91)90552-b. [DOI] [PubMed] [Google Scholar]
- 103.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 104.Woolley DE. The mast cell in inflammatory arthritis. N Engl J Med. 2003;348:1709–1711. doi: 10.1056/NEJMcibr023206. [DOI] [PubMed] [Google Scholar]
- 105.Harvima IT, Viinamäki H, Naukkarinen A, Paukkonen K, Neittaanmäki H, Horsmanheimo M. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychotherapy and Psychosomatics. 1993;60:168–176. doi: 10.1159/000288690. [DOI] [PubMed] [Google Scholar]
- 106.Özdamar SO, Seckin D, Kandemir B, Turanlt AY. Mast cells in psoriasis. Dermatology. 1996;192:190. doi: 10.1159/000246359. [DOI] [PubMed] [Google Scholar]
- 107.Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 108.Jayapal M, Tay HK, Reghunathan R, Zhi L, Chow KK, Rauff M, Melendez AJ. Genome-wide gene expression profiling of human mast cells stimulated by IgE or FcepsilonRI-aggregation reveals a complex network of genes involved in inflammatory responses. BMC.Genomics. 2006;7:210. doi: 10.1186/1471-2164-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carraway RE, Cochrane DE, Boucher W, Mitra SP. Structures of histamine-releasing peptides formed by the action of acid proteases on mammalian albumin(s) J Immunol. 1989;143:1680–1684. [PubMed] [Google Scholar]
- 110.Schmidlin F, Bunnett NW. Protease-activated receptors: how proteases signal to cells. Curr Opin Pharmacol. 2001;1:575–582. doi: 10.1016/s1471-4892(01)00099-6. [DOI] [PubMed] [Google Scholar]
- 111.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 112.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 113.Theoharides TC, Douglas WW. Secretion in mast cells induced by calcium entrapped within phospholipid vesicles. Science. 1978;201:1143–1145. doi: 10.1126/science.684435. [DOI] [PubMed] [Google Scholar]
- 114.Van Loveren H, Kops SK, Askenase PW. Different mechanisms of release of vasoactive amines by mast cells occur in T cell-dependent compared to IgE-dependent cutaneous hypersensitivity responses. Eur J Immunol. 1984;14:40–47. doi: 10.1002/eji.1830140108. [DOI] [PubMed] [Google Scholar]
- 115.Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphologic changes reflecting secretion in rat dura mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 116.Letourneau R, Pang X, Sant GR, Theoharides TC. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Br J Urol. 1996;77:41–54. doi: 10.1046/j.1464-410x.1996.08178.x. [DOI] [PubMed] [Google Scholar]
- 117.Dvorak AM, McLeod RS, Onderdonk A, Monahan-Earley RA, Cullen JB, Antonioli DA, Morgan E, Blair JE, Estrella P, Cisneros RL, Silen W, Cohen Z. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int Arch Allergy Immunol. 1992;99:74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- 118.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 119.Tamir H, Theoharides TC, Gershon MD, Askenase PW. Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H] serotonin. J Cell Biol. 1982;93:638–647. doi: 10.1083/jcb.93.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc.Natl.Acad Sci U.S.A. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 122.Benyon R, Robinson C, Church MK. Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. Br J Pharmacol. 1989;97:898–904. doi: 10.1111/j.1476-5381.1989.tb12030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levi-Schaffer F, Shalit M. Differential release of histamine and prostaglandin D2 in rat peritoneal mast cells activated with peptides. Int Arch Allergy Appl Immunol. 1989;90:352–357. doi: 10.1159/000235052. [DOI] [PubMed] [Google Scholar]
- 124.van Haaster CM, Engels W, Lemmens PJMR, Hornstra G, van der Vusse GJ, Heemskerk JWM. Differential release of histamine and prostaglandin D2 in rat peritoneal mast cells; roles of cytosolic calcium and protein tyrosine kinases. Biochim Biophys Acta. 1995;1265:79–88. doi: 10.1016/0167-4889(94)00210-6. [DOI] [PubMed] [Google Scholar]
- 125.Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 126.Marquardt DL, Alongi JL, Walker LL. The phosphatidylinositol 3-kinase inhibitor wortmannin blocks mast cell exocytosis but not IL-6 production. J Immunol. 1996;156:1942–1945. [PubMed] [Google Scholar]
- 127.Gagari E, Tsai M, Lantz CS, Fox LG, Galli SJ. Differential release of mast cell interleukin-6 via c-kit. Blood. 1997;89:2654–2663. [PubMed] [Google Scholar]
- 128.Cao J, Curtis CL, Theoharides TC. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol.Pharmacol. 2006;69:998–1006. doi: 10.1124/mol.105.019539. [DOI] [PubMed] [Google Scholar]
- 129.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 130.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukoc.Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 131.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br.J Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin TJ, Issekutz TB, Marshall JS. Human mast cells transmigrate through human umbilical vein endothelial monolayers and selectively produce IL-8 in response to stromal cell-derived factor-1 alpha. J Immunol. 2000;165:211–220. doi: 10.4049/jimmunol.165.1.211. [DOI] [PubMed] [Google Scholar]
- 133.Fischer M, Harvima IT, Carvalho RF, Moller C, Naukkarinen A, Enblad G, Nilsson G. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J.Clin.Invest. 2006;116:2748–2756. doi: 10.1172/JCI24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukocyte Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 135.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 136.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 137.Okayama Y. Mast cell-derived cytokine expression induced via Fc receptors and Toll-like receptors. Chem.Immunol Allergy. 2005;87:101–110. doi: 10.1159/000087574. [DOI] [PubMed] [Google Scholar]
- 138.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, Ikeda S, Okumura K, Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc.Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 139.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 140.Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 141.Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 142.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 145.Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 146.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 147.Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol.Chem. 2005;280:6610–6620. doi: 10.1074/jbc.M412126200. [DOI] [PubMed] [Google Scholar]
- 148.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lessmann E, Leitges M, Huber M. A redundant role for PKC-epsilon in mast cell signaling and effector function. Int Immunol. 2006;18:767–773. doi: 10.1093/intimm/dxl012. [DOI] [PubMed] [Google Scholar]
- 150.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 151.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 152.Stempelj M, Ferjan I. Signaling pathway in nerve growth factor induced histamine release from rat mast cells. Inflamm.Res. 2005;54:344–349. doi: 10.1007/s00011-005-1364-7. [DOI] [PubMed] [Google Scholar]
- 153.Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol. 2006;148:208–215. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Forssell J, Sideras P, Eriksson C, Malm-Erjefalt M, Rydell-Tormanen K, Ericsson PO, Erjefalt JS. Interleukin-2-inducible T cell kinase regulates mast cell degranulation and acute allergic responses. Am.J Respir.Cell Mol.Biol. 2005;32:511–520. doi: 10.1165/rcmb.2004-0348OC. [DOI] [PubMed] [Google Scholar]
- 155.Seow CJ, Chue SC, Wong WS. Piceatannol, a Syk-selective tyrosine kinase inhibitor, attenuated antigen challenge of guinea pig airways in vitro. Eur.J Pharmacol. 2002;443:189–196. doi: 10.1016/s0014-2999(02)01534-0. [DOI] [PubMed] [Google Scholar]
- 156.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int.Arch.Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 157.Oka T, Hori M, Tanaka A, Matsuda H, Karaki H, Ozaki H. IgE alone-induced actin assembly modifies calcium signaling and degranulation in RBL-2H3 mast cells. Am.J Physiol Cell Physiol. 2004;286:C256–C263. doi: 10.1152/ajpcell.00197.2003. [DOI] [PubMed] [Google Scholar]
- 158.Gonzalez-Espinosa C, Odom S, Olivera A, Hobson JP, Martinez ME, Oliveira-Dos-Santos A, Barra L, Spiegel S, Penninger JM, Rivera J. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J Exp.Med. 2003;197:1453–1465. doi: 10.1084/jem.20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp.Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells1. J Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 161.Rivera J. Adaptors discriminate mast-cell cytokine production from eicosanoid production and degranulation. Trends Immunol. 2006;27:251–253. doi: 10.1016/j.it.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 162.Klemm S, Gutermuth J, Hultner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. The Bcl10-Malt1 complex segregates Fc epsilon RI-mediated nuclear factor kappa B activation and cytokine production from mast cell degranulation. J Exp.Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–7995. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- 164.Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- 165.Karra L, Berent-Maoz B, Ben-Zimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Current Opinions in Immunology. 2009;21:708–714. doi: 10.1016/j.coi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 166.Andrasfalvy M, Peterfy H, Toth G, Matko J, Abramson J, Kerekes K, Vamosi G, Pecht I, Erdei A. The beta subunit of the type I Fcepsilon receptor is a target for peptides inhibiting IgE-mediated secretory response of mast cells. J Immunol. 2005;175:2801–2806. doi: 10.4049/jimmunol.175.5.2801. [DOI] [PubMed] [Google Scholar]
- 167.Theoharides TC, Patra P, Boucher W, Letourneau R, Kempuraj D, Chiang G, Jeudy S, Hesse L, Athanasiou A. Chondroitin sulfate inhibits connective tissue mast cells. Br J Pharmacol. 2000;131:1039–1049. doi: 10.1038/sj.bjp.0703672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Davis BJ, Flanagan BF, Gilfillan AM, Metcalfe DD, Coleman JW. Nitric oxide inhibits IgE-dependent cytokine production and Fos and Jun activation in mast cells. J Immunol. 2004;173:6914–6920. doi: 10.4049/jimmunol.173.11.6914. [DOI] [PubMed] [Google Scholar]
- 169.Conti P, Kempuraj D, Kandere K, Gioacchino MD, Barbacane RC, Castellani ML, Felaco M, Boucher W, Letourneau R, Theoharides TC. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 170.He SH, Xie H, Zhang XJ, Wang XJ. Inhibition of histamine release from human mast cells by natural chymase inhibitors. Acta Pharmacol.Sin. 2004;25:822–826. [PubMed] [Google Scholar]
- 171.Tagen M, Elorza A, Boucher W, Kepley CL, Shirihai O, Theoharides TC. Mitochondrial uncoupling protein 2 (UCP2) inhibits mast cell activation and reduces histamine content. J Immunol. 2009;183:6313–6319. doi: 10.4049/jimmunol.0803422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang B, Alysandratos KD, Angelidou A, Kempuraj D, Tagen M, Vasiadi M, Asadi S, Theoharides TC. TNF secretion from human mast cells is regulated by mitochondrial dynamics and mitochondrial uncoupling protein 2 (UCP2) J Immunol. 2010;184:11. [Google Scholar]
- 173.Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, Vasiadi M, Alexandra Katsarou-Katsari, Miao B, Weng Z, Leeman SE, Kalogeromitros D, Theoharides TC. Human mast cell degranulation and granule-stored TNF secretion require mitochondrial translocation to exocytosis sites-relevance to atopic dermatitis. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Middleton E, Jr., Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 175.Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo CL, Theoharides TC. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fiorani M, Guidarelli A, Blasa M, Azzolini C, Candiracci M, Piatti E, Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J Nutr.Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 177.Katsarou-Katsari A, Filippou A, Theoharides TC. Effect of stress and other psychological factors on the pathophysiology and treatment of dermatoses. Int J Immunopathol Pharmacol. 1999;12:7–11. [PubMed] [Google Scholar]
- 178.Church MK, Clough GF. Human skin mast cells:in vitro and in vivo studies. Ann Allergy Asthma Immunol. 1999;83:471–475. doi: 10.1016/S1081-1206(10)62853-0. [DOI] [PubMed] [Google Scholar]
- 179.Alysandratos KD, Angelidou A, Vasiadi M, Zhang B, Kalogeromitros D, Katsarou-Katsari A, Theoharides TC. Increased affected skin gene expression and serum levels of thymic stromal lymphopoietin in atopic dermatitis. Ann Allergy Asthma Immunol. 2010;105:403–404. doi: 10.1016/j.anai.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R., Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat.Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 181.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–e460. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 182.Kimata H. Enhancement of allergic skin wheal responses and in vitro allergen-specific IgE production by computer-induced stress in patients with atopic dermatitis. Brain Behav.Immun. 2003;17:134–138. doi: 10.1016/s0889-1591(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 183.Kimata H. Enhancement of allergic skin wheal responses in patients with atopic eczema/dermatitis syndrome by playing video games or by a frequently ringing mobile phone. Eur.J Clin.Invest. 2003;33:513–517. doi: 10.1046/j.1365-2362.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 184.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 185.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 186.Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Letters. 1995;374:113–116. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 187.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 188.Skofitsch G, Zamir N, Helke CJ, Savitt JM, Jacobowitz DM. Corticotropin-releasing factor-like immunoreactivity in sensory ganglia and capsaicin sensitive neurons of the rat central nervous system: colocalization with other neuropeptides. Peptides. 1985;6:307–318. doi: 10.1016/0196-9781(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 189.Merchenthaler I, Hynes MA, Vingh S, Schally AV, Petrusz P. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat spinal cord. Brain Res. 1983;275:373–377. doi: 10.1016/0006-8993(83)91001-6. [DOI] [PubMed] [Google Scholar]
- 190.Kubler A, Rothacher G, Knappertz VA, Kramer G, Nink M, Beyer J, Lehnert H. Intra and extracerebral blood flow changes and flushing after intravenous injection of human corticotropin-releasing hormone. Clin Invest. 1994;72:331336–336. doi: 10.1007/BF00252822. [DOI] [PubMed] [Google Scholar]
- 191.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its pro-inflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 192.Crompton R, Clifton VL, Bisits AT, Read MA, Smith R, Wright IM. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J Clin Endocrinol Metab. 2003;88:5427–5432. doi: 10.1210/jc.2003-030377. [DOI] [PubMed] [Google Scholar]
- 193.Clifton VL, Crompton R, Smith R, Wright IM. Microvascular effects of CRH in human skin vary in relation to gender. J Clin Endocrinol Metab. 2002;87:267–270. doi: 10.1210/jcem.87.1.8149. [DOI] [PubMed] [Google Scholar]
- 194.Papadopoulou N, Kalogeromitros D, Staurianeas NG, Tiblalexi D, Theoharides TC. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol. 2005;125:952–955. doi: 10.1111/j.0022-202X.2005.23913.x. [DOI] [PubMed] [Google Scholar]
- 195.Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–231. doi: 10.1159/000069516. [DOI] [PubMed] [Google Scholar]
- 196.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaneko K, Kawana S, Arai K, Shibasaki T. Corticotropin-releasing factor receptor type 1 is involved in the stress-induced exacerbation of chronic contact dermatitis in rats. Exp Dermatol. 2003;12:47–52. doi: 10.1034/j.1600-0625.2003.120106.x. [DOI] [PubMed] [Google Scholar]
- 198.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin and substance P: A link to neurogenic skin disorders. Brain Behav Immunity. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 199.Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, Theoharides TC. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of CRH receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 200.Katsarou-Katsari A, Filippou A, Theoharides TC. Stress and inflammatory dermatoses. Int J Immunopathol Pharmacol. 1999;12:7–11. [PubMed] [Google Scholar]
- 201.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol.Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 202.Harvima RJ, Viinamäki H, Harvima IT, Naukkarinen A, Savolainen L, Aalto M-L, Horsmanheimo M. Association of psychic stress with clinical severity and symptoms of psoriatic patients. Acta Derm.Venereol.(Stockh.) 1996;76:467–471. doi: 10.2340/0001555576467471. [DOI] [PubMed] [Google Scholar]
- 203.Tagen M, Stiles L, Kalogeromitros D, Gregoriou S, Kempuraj D, Makris M, Donelan J, Vasiadi M, Staurianeas NG, Theoharides TC. Skin corticotropin-releasing hormone receptor expression in psoriasis. J Invest Dermatol. 2007;127:1789–1791. doi: 10.1038/sj.jid.5700757. [DOI] [PubMed] [Google Scholar]
- 204.Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch.Dermatol Res. 2008;300:461–476. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- 205.Saraceno R, Kleyn CE, Terenghi G, Griffiths CE. The role of neuropeptides in psoriasis. Br.J Dermatol. 2006;155:876–882. doi: 10.1111/j.1365-2133.2006.07518.x. [DOI] [PubMed] [Google Scholar]
- 206.Remröd C, Lonne-Rahm S, Nordliond K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299:85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- 207.Naukkarinen A, Jarvikallio A, Lakkakorpi J, Harvima IT, Harvima RJ, Horsmanheimo M. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180:200–205. doi: 10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 208.Kawana S, Liang Z, Nagano M, Suzuki H. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J Dermatol Sci. 2006;42:47–54. doi: 10.1016/j.jdermsci.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 209.Kandere-Grzybowska K, Gheorghe D, Priller J, Esposito P, Huang M, Gerard N, Theoharides TC. Stress-induced dura vascular permeability does not develop in mast cell-deficient and neurokinin-1 receptor knockout mice. Brain Res. 2003;980:213–220. doi: 10.1016/s0006-8993(03)02975-5. [DOI] [PubMed] [Google Scholar]
- 210.Leeman SE, Ferguson SL. Substance P: an historical perspective. Neuropeptides. 2000;34:249–254. doi: 10.1054/npep.2000.0826. [DOI] [PubMed] [Google Scholar]
- 211.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J.Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 212.Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch.Dermatol.Res. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- 213.Al’Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ. Neuropeptides and general neuronal marker in psoriasis--an immunohistochemical study. Clin.Exp.Dermatol. 1995;20:384–389. doi: 10.1111/j.1365-2230.1995.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 214.Peters EM, Kuhlmei A, Tobin DJ, Muller-Rover S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 215.Paus R, Heinzelmann T, Robicsek S, Czarnetzki BM, Maurer M. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Arch.Dermatol.Res. 1995;287:500–502. doi: 10.1007/BF00373436. [DOI] [PubMed] [Google Scholar]
- 216.Liu JY, Hu JH, Zhu QG, Li FQ, Sun HJ. Substance P receptor expression in human skin keratinocytes and fibroblasts. Br J Dermatol. 2006;155:657–662. doi: 10.1111/j.1365-2133.2006.07408.x. [DOI] [PubMed] [Google Scholar]
- 217.Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, Sung KJ, Armstrong CA, Ansel JC. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R) Exp Dermatol. 2000;9:42–52. doi: 10.1034/j.1600-0625.2000.009001042.x. [DOI] [PubMed] [Google Scholar]
- 218.Sato S, Kume K, Ito C, Ishii S, Shimizu T. Accelerated proliferation of epidermal keratinocytes by the transgenic expression of the platelet-activating factor receptor. Arch Dermatol Res. 1999;291:614–621. doi: 10.1007/s004030050463. [DOI] [PubMed] [Google Scholar]
- 219.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, Saito H, Ra C, Okayama Y. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin.Immunol. 2010;125:1137–1145. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 220.Nestle FO, Kaplan DH, Barker J. Psoriasis. N.Engl.J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 221.Xu D, Jiang H, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie ANJ, Mclinnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proceedings of the National Academy of Sciences. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Castellani ML, Kempuraj DJ, Salini V, Vecchiet J, Tete S, Ciampoli C, Conti F, Cerulli G, Caraffa A, Antinolfi P, Theoharides TC, De, Amicis D, Perrella A, Cuccurullo C, Boscolo P, Shaik Y. The latest interleukin: IL-33 the novel IL-1-family member is a potent mast cell activator. J Biol Regul Homeost Agents. 2009;23:11–14. [PubMed] [Google Scholar]
- 223.Pushparaj PN, Tay HK, H’ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc.Natl.Acad.Sci.U.S.A. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 225.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 226.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGFsecretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dimitriadou V, Rouleau A, Trung Tuong MD, Newlands GJF, Miller HRP, Luffau G, Schwartz J-C, Garbarg M. Functional relationships between sensory nerve fibers and mast cells of dura mater in normal and inflammatory conditions. Neuroscience. 1997;77:829–839. doi: 10.1016/s0306-4522(96)00488-5. [DOI] [PubMed] [Google Scholar]
- 228.Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mast cell-neuron interactions in vitro and in vivo. Brain Res. 1999;849:1–15. doi: 10.1016/s0006-8993(99)01855-7. [DOI] [PubMed] [Google Scholar]
- 229.Dimitriadou V, Aubineau P, Taxi J, Seylaz J. Ultrastructural evidence for a functional unit between nerve fibers and type II cerebral mast cells in the cerebral vascular wall. Neuroscience. 1987;22:621–630. doi: 10.1016/0306-4522(87)90358-7. [DOI] [PubMed] [Google Scholar]
- 230.Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976;115:377–393. doi: 10.1016/0006-8993(76)90356-5. [DOI] [PubMed] [Google Scholar]
- 231.Ibrahim MZ. The mast cells of the mammalian central nervous system. Part I. Morphology, distribution and histochemistry. J Neurol Sci. 1974;21:431–478. doi: 10.1016/0022-510x(74)90044-6. [DOI] [PubMed] [Google Scholar]
- 232.Pollard H, Bischoff S, Llorens-Cortes C, Schwartz JC. Histidine decarboxylase and histamine in discrete nuclei of rat hypothalamus and the evidence for mast cells in the median eminence. Brain Res. 1976;118:509–513. doi: 10.1016/0006-8993(76)90322-x. [DOI] [PubMed] [Google Scholar]
- 233.Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J Neurochem. 1985;44:1943–1947. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- 234.Lambracht-Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Dev Brain Res. 1990;56:151–159. doi: 10.1016/0165-3806(90)90077-c. [DOI] [PubMed] [Google Scholar]
- 235.von During M, Bauersachs M, Bohmer B, Veh RW, Andres KH. Neuropeptide Y- and substance P-like immunoreactive nerve fibers in the rat dura mater encephali. Anat Embryol (Berl) 1990;182:363–373. doi: 10.1007/BF02433496. [DOI] [PubMed] [Google Scholar]
- 236.Esposito P, Chandler N, Kandere-Grzybowska K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 237.Esposito P, Gheorghe D, Kandere K, Pang X, Conally R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- 238.De Vreis HE, Kuiper J, de Boer AG, Van Berkel TJC, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–155. [PubMed] [Google Scholar]
- 239.Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DPE, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- 240.Moor ACE, de Vries HE, de Boer AG, Breimer DD. The blood-brain barrier and multiple sclerosis. Biochem Pharmacol. 1994;47:1717–1724. doi: 10.1016/0006-2952(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 241.Kwon EE, Prineas JW. Blood-brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. J Neuropathol Exp Neurol. 1994;53:625–636. doi: 10.1097/00005072-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 242.Syndulko K, Tourtellotte WW, Conrad AJ, Izuierdo G, Multiple Sclerosis Study Group. Alpha Interferon Study Group Trans-blood-brain-barrier albumin leakage and comparisons of intrathecal IgG synthesis calculations in multiple sclerosis patients. J Neuroimmunol. 1993;46:185–192. doi: 10.1016/0165-5728(93)90248-w. [DOI] [PubMed] [Google Scholar]
- 243.Mohr DC, Goodkin DE, Bacchetti P, Boudewyn AC, Huang L, Marrietta P, Cheuk W, Dee B. Psychological stress and the subsequent appearances of new brain MRI lesions in MS. Neurology. 2000;55:55–61. doi: 10.1212/wnl.55.1.55. [DOI] [PubMed] [Google Scholar]
- 244.Olsson Y. Mast cells in plaques of multiple sclerosis. Acta Neurol Scand. 1974;50:611–618. doi: 10.1111/j.1600-0404.1974.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 245.Krüger PG, Bo L, Myhr KM, Karlsen AE, Taule A, Nyland HI, Mork S. Mast cells and multiple sclerosis: a light and electron microscopic study of mast cells in multiple sclerosis emphasizing staining procedures. Acta Neurol Scand. 1990;81:31–36. doi: 10.1111/j.1600-0404.1990.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 246.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 247.Bomprezzi R, Ringner M, Kim S, Bittner ML, Khan J, Chen Y, Elkahloun A, Yu A, Bielekova B, Meltzer PS, Martin R, McFarland HF, Trent JM. Gene expression profile in multiple sclerosis patients and healthy controls: identifying pathways relevant to disease. Hum Mol Genet. 2003;12:2191–2199. doi: 10.1093/hmg/ddg221. [DOI] [PubMed] [Google Scholar]
- 248.Lu C, Diehl SA, Noubade R, Ledoux J, Nelson MT, Spach K, Zachary JF, Blankenhorn EP, Teuscher C. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:18967–18972. doi: 10.1073/pnas.1008816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC. Definitive characterization of rat hypothalamic mast cells. Neuroscience. 1996;73:889–902. doi: 10.1016/0306-4522(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 250.Shanas U, Bhasin R, Sutherland AK, Silverman A-J, Silver R. Brain mast cells lack the c-kit receptor: immunocytochemical evidence. J Neuroimmunol. 1998;90:207–211. doi: 10.1016/s0165-5728(98)00137-4. [DOI] [PubMed] [Google Scholar]
- 251.Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145:18–26. doi: 10.1016/j.jneuroim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 252.Silver R, Silverman A-J, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- 253.Wilhelm M, Silver R, Silverman AJ. Central nervous system neurons acquire mast cell products via transgranulation. Eur.J Neurosci. 2005;22:2238–2248. doi: 10.1111/j.1460-9568.2005.04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Evidence that brain mast cells can modulate neuroinflammatory responses by tumor necrosis factor-α production. Neuroreport. 1998;9:95–98. doi: 10.1097/00001756-199801050-00019. [DOI] [PubMed] [Google Scholar]
- 255.Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-α transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137–141. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 256.Klinkert WEF, Kojima K, Lesslauer W, Rinner W, Lassmann H, Wekerle H. TNF-α receptor fusion protein prevents experimental auto-immune encephalomyelitis and demyelination in Lewis rats: An overview. J Neuroimmunol. 1997;72:163–168. doi: 10.1016/s0165-5728(96)00183-x. [DOI] [PubMed] [Google Scholar]
- 257.Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- 258.Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 259.Malamud V, Vaaknin A, Abramsky O, Mor M, Burgess LE, Ben-Yehudah A, Lorberboum-Galski H. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-alpha, IL-6 and IL-1 beta: possible relevance to multiple sclerosis. J Neuoimmunol. 2003;138:115–122. doi: 10.1016/s0165-5728(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 260.Bunnett NW. Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin Thromb.Hemost. 2006;32(Suppl 1):39–48. doi: 10.1055/s-2006-939553. [DOI] [PubMed] [Google Scholar]
- 261.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 262.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 264.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cell rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 265.Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a crucial re-appraisal. Trends Immunol. 2004;25:132–137. doi: 10.1016/j.it.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 266.Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- 267.Pedotti R, De Voss JJ, Steinman L, Galli SJ. Involvement of both ‘allergic’ and ‘autoimmune’ mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 2003;24:479–484. doi: 10.1016/s1471-4906(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 268.Robbie-Ryan M, Brown M. The role of mast cells in allergy and autoimmunity. Curr Opin Immunol. 2002;14:728–733. doi: 10.1016/s0952-7915(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 269.Brown MA, Tanzola M, Robbie-Ryan M. Mechanisms underlying mast cell influence on EAE disease course. Mol Immunol. 2002;38:1373–1378. doi: 10.1016/s0161-5890(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 270.Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol. 2001;167:4008–4016. doi: 10.4049/jimmunol.167.7.4008. [DOI] [PubMed] [Google Scholar]
- 271.Theoharides TC, Dimitriadou V, Letourneau RJ, Rozniecki JJ, Vliagoftis H, Boucher WS. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain demyelination: changes resembling early stages of demyelination. Neuroscience. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-j. [DOI] [PubMed] [Google Scholar]
- 272.Griffin DE, Mendoza QP. Identification of the inflammatory cells present in the central nervous system of normal and mast cell-deficient mice during Sindbis virus encephalitis. Cell Immunol. 1986;97:454–459. doi: 10.1016/0008-8749(86)90414-4. [DOI] [PubMed] [Google Scholar]
- 273.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Theoharides TC, Weinkauf C, Conti P. Brain cytokines and neuropsychiatric disorders. Journal of Clinical Psychopharmacology. 2004;24:577–581. doi: 10.1097/01.jcp.0000148026.86483.4f. [DOI] [PubMed] [Google Scholar]
- 275.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 276.Laham RJ, Li J, Tofukuji M, Post M, Simons M, Sellke FW. Spatial heterogeneity in VEGF-induced vasodilation: VEGF dilates microvessels but not epicardial and systemic arteries and veins. Ann Vasc Surg. 2003;17:245–252. doi: 10.1007/s10016-001-0299-x. [DOI] [PubMed] [Google Scholar]
- 277.Theoharides TC, Konstantinidou A. Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci. 2007;12:1615–1628. doi: 10.2741/2174. [DOI] [PubMed] [Google Scholar]
- 278.Theoharides TC, Spanos CP, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos G. Stress-induced intracranial mast cell degranulation. A corticotropin releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- 279.Bugajski AJ, Chlap Z, Gadek-Michalska A, orycz J, Bugajski J. Degranulation and decrease in histamine levels of thalamic mast cells coincides with corticosterone secretion induced by compound 48/80. Inflamm Res. 1995;44(Supp.1):S50–S51. doi: 10.1007/BF01674391. [DOI] [PubMed] [Google Scholar]
- 280.Gadek-Michalska A, Chlap Z, Turon M, Bugajski J, Fogel WA. The intracerebroventicularly administered mast cells degranulator compound 48/80 increases the pituitary-adrenocortical activity in rats. Agents Actions. 1991;32:203–208. doi: 10.1007/BF01980874. [DOI] [PubMed] [Google Scholar]
- 281.Matsumoto I, Inoue Y, Shimada T, Aikawa T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J Exp Med. 2001;194:71–78. doi: 10.1084/jem.194.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kjaer A, Larsen PJ, Knigge U, Jorgensen H, Warberg J. Neuronal histamine and expression of corticotropin-releasing hormone, vasopressin and oxytocin in the hypothalamus: relative importance of H1 and H2 receptors. Eur J Endocrinol. 1998;139:238–243. doi: 10.1530/eje.0.1390238. [DOI] [PubMed] [Google Scholar]
- 283.Lytinas M, Kempuraj D, Kandere K, Huang M, Madhappan B, Christodoulou S, Athanassiou A, Theoharides TC. Human mast cells synthesize and secrete corticotropin-releasing hormone (CRH) which triggers them to secrete IL-6. Endocrinology. 2003 [Google Scholar]
- 284.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Goodin DS, Ebers GC, Johnson KP, Rodriguez M, Sibley WA, Wolinsky JS. The relationship of MS to physical trauma and psychological stress. Neurology. 1999;52:1737–1745. doi: 10.1212/wnl.52.9.1737. [DOI] [PubMed] [Google Scholar]
- 286.Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis:case control evidence of a relationship. J Chronic Dis. 1982;35:821–831. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 287.Ackerman KD, Stover A, Heyman R, Anderson BP, Houck PR, Frank E, Rabin BS, Baum A, Robert Ader New Investigator award Relationship of cardiovascular reactivity, stressful life events, and multiple sclerosis disease activity. Brain Behav Immun. 2003;17:141–151. doi: 10.1016/s0889-1591(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 288.Buljevac D, Hop WC, Reedeker W, Janssens AC, van der Meche FG, van Doorn PA, Hintzen RQ. Self reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ. 2003;327:646. doi: 10.1136/bmj.327.7416.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Li J, Johansen C, Bronnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark. Neurology. 2004;62:726–729. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- 290.Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328:731. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gold SM, Mohr DC, Huitinga I, Flachenecker P, Sternberg EM, Heesen C. The role of stress-response systems for the pathogenesis and progression of MS. Trends Immunol. 2005;26:644–652. doi: 10.1016/j.it.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 292.Heesen C, Schulz H, Schmidt M, Gold S, Tessmer W, Schulz KH. Endocrine and cytokine responses to acute psychological stress in multiple sclerosis. Brain Behav Immun. 2002;16:282–287. doi: 10.1006/brbi.2001.0628. [DOI] [PubMed] [Google Scholar]
- 293.Lalive PH, Burkhard PR, Chofflon M. TNF-alpha and psychologically stressful events in healthy subjects: potential relevance for multiple sclerosis relapse. Behav Neurosci. 2002;116:1093–1097. doi: 10.1037//0735-7044.116.6.1093. [DOI] [PubMed] [Google Scholar]
- 294.Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc Res. 2003;59:241–249. doi: 10.1016/s0008-6363(03)00340-7. [DOI] [PubMed] [Google Scholar]
- 295.Chandler N, Jacobson S, Connolly R, Esposito P, Theoharides TC. Acute stress shortens the time of onset of experimental allergic encephalomyelitis (EAE) in SJL/J mice. Brain Behav Immun. 2002;16:757–763. doi: 10.1016/s0889-1591(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 296.Teunis MA, Heijnen CJ, Sluyter F, Bakker JM, Van Dam AM, Hof M, Cools AR, Kavelaars A. Maternal deprivation of rat pups increases clinical symptoms of experimental autoimmune encephalomyelitis at adult age. J Neuroimmunol. 2002;133:30–38. doi: 10.1016/s0165-5728(02)00351-x. [DOI] [PubMed] [Google Scholar]
- 297.Benou C, Wang Y, Imitola J, VanVlerken L, Chandras C, Karalis KP, Khoury SJ. Corticotropin-releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2005;174:5407–5413. doi: 10.4049/jimmunol.174.9.5407. [DOI] [PubMed] [Google Scholar]
- 298.Jeyaraju DV, Cisbani G, Pellegrini L. Calcium regulation of mitochondria motility and morphology. Biochim Biophys Acta. 2009;1787:1363–1373. doi: 10.1016/j.bbabio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 299.Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJ. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- 300.Bugajski AJ, Chiap Z, Gadek-Michalska A, Bugajski J. Effect of isolation stress on brain mast cells and brain histamine levels in rats. Agents Actions. 1994;41:C75–C76. doi: 10.1007/BF02007774. [DOI] [PubMed] [Google Scholar]
- 301.Cirulli F, Pistillo L, De Acetis L, Alleva E, Aloe L. Mast cells increase in the central nervous system of adult male mice following chronic subordination stress. Soc for Neurosci. 1997;23:714. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- 302.Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR. Terfenadine-ketoconazole interaction: pharmacokinetic and electrocardiographic consequences. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- 303.Zappulla JP, Arock M, Mars LT, Liblau RS. Mast cells: new targets for multiple sclerosis therapy? J Neuroimmunol. 2002;131:5–20. doi: 10.1016/s0165-5728(02)00250-3. [DOI] [PubMed] [Google Scholar]
- 304.Hendriks JJ, de Vries HE, van der Pol SM, van den Berg TK, van Tol EA, Dijkstra CD. Flavonoids inhibit myelin phagocytosis by macrophages; a structure-activity relationship study. Biochem Pharmacol. 2003;65:877–885. doi: 10.1016/s0006-2952(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 305.Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S, Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 306.Hendriks JJ, Alblas J, van der Pol SM, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp.Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochemical Pharmacology. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 308.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation. 2010;7:3. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sternberg Z, Chadha K, Lieberman A, Drake A, Hojnacki D, Weinstock-Guttman B, Munschauer F. Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: additive effects of IFN-beta. J Neuroinflammation. 2009;6:28. doi: 10.1186/1742-2094-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Theoharides TC. Luteolin as a therapeutic option for multiple sclerosis. J Neuroinflammation. 2009;6:29. doi: 10.1186/1742-2094-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]