Abstract
Background
Best strategies to screen postmenopausal women for osteoporosis are not clear.
Objective
To identify the effectiveness and cost-effectiveness of various screening strategies.
Design
Individual-level state-transition cost-effectiveness model.
Data Sources
Published literature.
Target Population
U.S. women age 55 years and older.
Time Horizon
Lifetime.
Perspective
Payer.
Interventions
Multiple osteoporosis screening strategies composed of alternative tests, initiation ages, treatment thresholds, and rescreening intervals. Evaluated tests included central dual-energy x-ray absorptiometry (DXA); calcaneal quantitative ultrasonography (QUS), and the Simple Calculated Osteoporosis Risk Estimation (SCORE) tool. Oral bisphosphonate treatment was assumed.
Outcome Measures
Incremental cost-effectiveness ratios (2010 U.S. dollars per quality-adjusted life-year [QALY] gained).
Results of Base-Case Analysis
At all evaluated ages, screening was superior to not screening. In general, quality-adjusted life-days gained with screening tended to increase with age. At all initiation ages, the best strategy with an incremental cost-effectiveness ratio (ICER) of less than $50 000 per QALY was DXA screening with a T-score threshold of −2.5 or less for treatment and with follow-up screening every 5 years (that is, DXA −2.5 with rescreening every 5 years). Across screening initiation ages, the best strategy with an incremental cost-effectiveness ratio of less than $50 000 per QALY was initiation of screening at age 55 years by using DXA −2.5, with rescreening every 5 years. The best strategy with an ICER of less than $100 000 per QALY was initiation of screening at age 55 years by using DXA with a T-score threshold of −2.0 or less for treatment and then rescreening every 10 years. No other strategy that involved treatment of women with osteopenia (low bone mass) was cost-effective under the assumption of a willingness-to-pay of $100 000/QALY. Many other strategies, including strategies with SCORE or QUS prescreening, were also cost-effective, and in general the differences in effectiveness and costs between evaluated strategies was small.
Results of Sensitivity Analysis
Probabilistic sensitivity analysis did not reveal a consistently superior strategy.
Limitations
Data were primarily from white women. Screening initiation ages younger than 55 years were not examined. It was assumed that each woman identified for treatment was offered oral bisphosphonate therapy, with a base-case adherence rate of 50% and a 5-year on/off treatment pattern. Only osteoporotic fractures of the hip, vertebrae, and wrist were modeled.
Conclusions
Many strategies for postmenopausal osteoporosis screening are effective and cost-effective, including strategies involving screening initiation at age 55. No one single strategy is clearly best.
Primary Funding Source
The National Center for Research Resources.
In the United States, osteoporosis affects approximately 10 million people, 80% of whom are women (1, 2). Studies indicate that half of all postmenopausal women will have an osteoporotic fracture in their lifetime (2); potential consequences include short- and long-term morbidity, including nursing home placement. Osteoporosis-related costs approached $17 billion in 2005 (3) and may double or triple by 2040 (4). Although medical therapy can reduce the risk for fracture and is cost-effective (5–7), osteoporosis is often undiagnosed and untreated. Therefore, the US Preventive Services Task Force (USPSTF) recommends that women age 65 years and older be routinely screened (8).
Available screening methods include dual-energy x-ray absorptiometry of the femoral neck and lumbar spine (central DXA), calcaneal quantitative ultrasonography (QUS), and clinical risk assessment instruments for low bone mineral density (BMD) or fracture (2). DXA is considered the gold standard for diagnosing osteoporosis; trials of medical therapies to prevent fractures in persons without a history of fracture have used DXA to select participants for inclusion, and it is widely used in the United States. However, DXA testing typically requires travel to a referral center and is relatively expensive, with a median cost of $98 in 2010 (9). In contrast, QUS and risk assessment instruments are portable and less expensive; for example, QUS costs approximately $12, and the primary cost associated with risk assessment instruments is the brief time it takes to administer them.
Screening tests can be used to predict future fracture risk, and several have been shown to be cost-effective (10, 11). But there is lack of consensus regarding the best test or sequence of tests to use, the optimal age at which to initiate screening, the threshold for selection of individuals for treatment, and the interval for repeat screening. The most comprehensive clinical trial of osteoporosis screening in the United States, the Osteoporosis Population-Based Risk Assessment trial (12)—a randomized trial that evaluated 3 screening tests—had a short follow-up period (2 years), did not evaluate different ages to start or repeat screening, and did not report costs or cost-effectiveness. Similarly, models reported in the literature have not compared multiple tests, ages to initiate screening, treatment thresholds, or intervals for follow-up (10,11).
Our study modeled the results of multiple screening strategies for postmenopausal women at risk for osteoporotic fracture—directly comparing strategies involving various combinations of screening tests, screening initiation ages, treatment thresholds, and rescreening intervals—to identify the effectiveness and cost-effectiveness of different strategies.
Methods
We constructed an individual-level state-transition model of osteoporosis screening and treatment for community-dwelling US postmenopausal women. The model estimates quality-adjusted life-years (QALYs), costs in 2010 US dollars, and incremental cost-effectiveness ratios (ICERs) for the screening strategies described in Table 1; it uses a lifetime time horizon and payer perspective (includes direct societal costs). ICERs represent cost per QALY gained for a particular strategy compared to another strategy. The model allows direct comparison of multiple screening tests and sequences of tests, screening initiation ages, treatment thresholds, and repeat screening intervals. We followed recommendations of the Panel on Cost-Effectiveness in Health and Medicine (13) and used TreeAge Pro Suite 2009 (TreeAge Software, Williamstown, Massachusetts). We describe our methods briefly here and provide details in the Appendix and Appendix Tables, available at www.annals.org.
Table 1.
Descriptions of Screening Strategies Evaluated in the Cost-Effectiveness Models*
| Screening Strategy |
Description |
|---|---|
| No screening | No screening for prevention; treatment only if an osteoporotic fracture occurs |
| DXA −2.5 | DXA of the femoral neck and lumbar spine, with treatment if the T-score is −2.5 or less at either site |
| DXA −2.0 | DXA of the femoral neck and lumbar spine, with treatment if the T-score is −2.0 or less |
| DXA −1.5 | DXA of the femoral neck and lumbar spine, with treatment if the T-score is −1.5 or less |
| QUS −1.0 | Calcaneal QUS prescreening, with subsequent DXA screening if the QUS T-score is −1.0 or less and with treatment if the DXA T-score is −2.5 or less |
| QUS −0.5 | Calcaneal QUS prescreening, with DXA screening if the QUS T-score is −0.5 or less and with treatment if the DXA T-score is −2.5 or less |
| SCORE −2.5 | SCORE tool prescreening, with DXA screening if the SCORE result is 7 or greater and with treatment if the DXA T-score is −2.5 or less |
| SCORE NOF | SCORE prescreening, with DXA screening if the SCORE result is 7 or greater and with treatment if the DXA T-score is −2.0 or less; or DXA T-score −1.5 or less with an additional osteoporosis risk factor; or age ≥ 80 |
DXA = dual-energy x-ray absorptiometry; NOF = National Osteoporosis Foundation; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Each strategy was evaluated for initiation in postmenopausal women with no known history of osteoporotic fracture at 6 different ages (55, 60, 65, 70, 75, and 80 years of age) and with 3 different repeat screening intervals (1-time screening, rescreening every 5 years, or rescreening every 10 years).
Model Development
General Structure
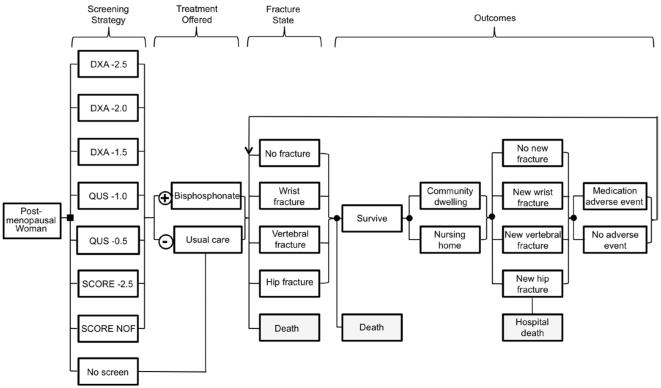
The Figure depicts a simplified representation of the model, in which cohorts of postmenopausal women are screened with DXA; prescreened with QUS before DXA; prescreened with the Simple Calculated Osteoporosis Risk Estimation (SCORE) tool before DXA; or are not screened. Each woman identified for treatment with a particular strategy is offered oral bisphosphonate therapy, and each who is not identified for treatment receives usual care only (calcium and vitamin D as used in the Fracture Intervention Trial study sample) (14). During each time period in the model, the woman may sustain a wrist, vertebral, or hip fracture; may survive or die with or without a fracture; may remain in the community or move to a nursing home; and may develop a medication-related adverse event. Hip fractures increase the risks for nursing home placement and short-term death. Fractures, nursing home placement, and medication-related adverse events incur direct costs and “disutility” (a decrease in QALYs). Table 2 summarizes model variable assumptions.
Figure.
Model schematic. This is a simplified and partial representation of the full model. The model evaluates 7 screening strategies, each of which was evaluated as a 1-time strategy, as a strategy repeated every 5 years, and as a strategy repeated every 10 years; additionally, no screening was also considered, resulting in a total of 22 screening options at each screening initiation age; these are described in more detail in Table 1. The screening result is either positive, in which case the individual is offered treatment with a bisphosphonate, or negative, in which case usual care of calcium and vitamin D is offered. We assume that at the time of initial screening individuals are in the “no fracture” state, with no known history of osteoporotic fracture. Whether on or off treatment, patients may experience a series of events over time, including a new osteoporotic fracture, the results of which may be death, transfer to a long-term care facility, or recovery. Patients may also experience medication adverse events. Individuals move through the outcomes and fracture states portions of the model on a 3-month cycle.
Table 2.
Key Model Parameter Assumptions
| Parameter | Base-Case Value |
Probabilistic Sensitivity Analysis Values (Range)* |
Data Source (Reference) |
|---|---|---|---|
| Screening test performance characteristics | |||
| QUS −0.5 prescreening sensitivity | 0.88 | 0.80–0.93 | Nayak et al (15) |
| QUS −0.5 prescreening specificity | 0.39 | 0.23–0.59 | Nayak et al (15) |
| QUS −1.0 prescreening sensitivity | 0.79 | 0.69–0.86 | Nayak et al (15) |
| QUS −1.0 prescreening specificity | 0.58 | 0.44–0.70 | Nayak et al (15) |
| SCORE −2.5 prescreening sensitivity | 0.937 | 0.883–0.991 | Brenneman et al (16) |
| SCORE −2.5 prescreening specificity | 0.238 | 0.096–0.38 | Brenneman et al (16) |
| SCORE NOF prescreening sensitivity† | 0.898 | 0.799–0.996 | Brenneman et al (16) |
| SCORE NOF prescreening specificity† | 0.348 | 0.237–0.458 | Brenneman et al (16) |
| Costs, 2010 $US ‡ | |||
| Alendronate, annual cost | 108 | 50–200§ | Red Book 2010 (37) (base-case value) |
| Nongeneric oral bisphosphonate, annual cost | 1500 | 750–2200§ | Red Book 2010 (37) (base-case value) |
| Hip fracture, direct medical costs | 22 528 | 11 264–33 792∥ | Gabriel et al (38) (base-case value) |
| Clinical vertebral fracture, direct medical costs | 9214 | 4607–13 821∥ | Gabriel et al (38) (base-case value) |
| Wrist fracture, direct medical costs | 5003 | 2502–7505∥ | Gabriel et al (38) (base-case value) |
| Nursing home care, annual cost | 74 846 | 60 000–90 000§ | GE Financial Nursing Home Cost of Care Survey (39) (base-case value) |
| Central DXA (CPT code 77080) | 97.71 | 60–120§ | Centers for Medicare & Medicaid Services (9) (base-case value) |
| Calcaneal QUS (CPT code 76977) | 11.80 | 5–18§ | Centers for Medicare & Medicaid Services (9) (base-case value) |
| SCORE risk assessment tool | 5 | 1–10§ | Assumed |
| Physician visit (CPT code 99213) | 66.74 | N/A | Centers for Medicare & Medicaid Services (9) (base-case value) |
| Omeprazole OTC, annual cost | 240 | N/A | Walmart Pharmacy Medication Finder (40) |
| Upper endoscopy (CPT code 43235) | 286.87 | N/A | Centers for Medicare & Medicaid Services (9) |
| Health state utility values | |||
| No fracture, age 55 y | 0.837 | 0.753–0.921¶ | Hanmer et al (41) (base-case value) |
| No fracture, age 65 y | 0.811 | 0.730–0.892¶ | Hanmer et al (41) (base-case value) |
| No fracture, age 75 y | 0.771 | 0.694–0.848¶ | Hanmer et al (41) (base-case value) |
| No fracture, age 85 y | 0.724 | 0.652–0.796¶ | Hanmer et al (41) (base-case value) |
| Hip fracture, first year/subsequent years (multiplier) |
0.797/0.9 | 0.717–0.877/0.81–0.99¶ | Brazier et al (46) (first-year multiplier); Brazier et al (42) (subsequent-years multiplier) |
| Vertebral fracture, first year/subsequent years (multiplier) |
0.82/0.931 | 0.740–0.902/0.840–1.0¶ | Kanis et al (47) (base-case values) |
| Wrist fracture, first year/subsequent years (multiplier) |
0.981/1.0 | 0.95–1.0/1.0§ | Dolan et al (44) (first-year multiplier); Brazier et al (42) (subsequent-years multiplier) |
| Nursing home residence (multiplier) | 0.4 | 0.2–0.6§ | Brazier et al (42) (base-case value) |
| Esophagitis (multiplier)** | 0.98 | NA | Fryback et al (45) (base-case value)†† |
| Esophageal ulcer (multiplier)** | 0.91 | NA | Fryback et al (45) (base-case value)†† |
| Relative risk for fracture during alendronate treatment | |||
| Hip fracture (history of prior vertebral fracture) | 0.49 | 0.34–0.64†† | Black et al (30) |
| Hip fracture (femoral neck T-score −2.5 or less) | 0.44 | 0.31–0.57†† | Cummings et al (14) |
| Hip fracture (lumbar spine T-score −2.5 or less) | 0.46 | 0.32–0.60†† | Karpf et al (27) |
| Vertebral fracture (history of prior vertebral fracture) |
0.53 | 0.37–0.69†† | Black et al (30) |
| Vertebral fracture (femoral neck T-score −2.5 or less) |
0.50 | 0.35–0.65†† | Cummings et al (14) |
| Vertebral fracture (lumbar spine T-score −2.5 or less) |
0.52 | 0.36–0.68†† | Liberman et al (28) |
| Vertebral fracture (femoral neck or lumbar spine T-score −2.0 or less and greater than −2.5) |
0.54 | 0.38–0.70†† | Cummings et al (14) |
| Vertebral fracture (femoral neck or lumbar spine T-score −1.5 or less and greater than −2.0) |
0.82 | 0.57–1.07†† | Cummings et al (14) |
| Wrist fracture (history of prior vertebral fracture) | 0.52 | 0.36–0.68†† | Black et al (30) |
| Wrist fracture (femoral neck T-score −2.5 or less) | 0.88 | 0.62–1.14†† | Cummings et al (14) |
| Wrist fracture (lumbar spine T-score −2.5 or less) | 0.39 | 0.27–0.51†† | Karpf et al (27) |
| Nonvertebral fracture (femoral neck or lumbar spine T-score greater than −2.5) |
1.0 | NA | Cummings et al (14) |
| Alendronate treatment | |||
| Adherence with treatment, % | 50 | 30–70§ | Solomon et al (19) (base-case value) |
| Length of treatment, y | 5 | NA | Black et al (20); Schwartz et al (21) |
| Admission to nursing home after hip fracture | |||
| Rate of admission | 0.60 | 0.42–0.78†† | Braithwaite et al (36) (base-case value) |
| Annual discount rate | |||
| Costs | 0.03 | NA | Weinstein et al (13) |
| Quality-adjusted life-years | 0.03 | NA | Weinstein et al (13) |
CPT = Current Procedural Terminology; DXA = dual-energy x-ray absorptiometry; NA = not applicable; OTC = over the counter; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation; NOF = National Osteoporosis Foundation.
Triangular probability distributions.
The sensitivity and specificity values assumed for this SCORE criterion are values reported for individuals with T-scores of −2.5 or less and T-scores greater than −2.5 meeting the treatment criteria combined; separate values were not presented in Brenneman et al. for this criterion for individuals with T-scores of −2.5 or less versus greater than −2.5. The use of these estimates may be a source of bias in the evaluation of the performance of this SCORE criterion, by underestimating the sensitivity and overestimating the specificity of SCORE for individuals with T-scores of −2.5 or less; and conversely overestimating sensitivity and underestimating specificity of SCORE for individuals with T-scores greater than −2.5. We do not know what the overall direction of this bias may be; but we believe that its magnitude would be small.
Costs not presented in 2010 $US inflated to 2010 $US using the Consumer Price Index for Medical Care.
Sensitivity analysis values assumed.
Sensitivity analysis values 50% lower and 50% higher than base-case value.
Sensitivity analysis values 10% lower and 10% higher than base-case value.
Surrogate utility value used.
Sensitivity analysis values 30% lower and 30% higher than base-case value.
Age at Initiation of Screening
We modeled initiation of screening at ages 55, 60, 65, 70, 75, and 80 years.
Screening Test Results
We used published data on the performance characteristics of QUS and the SCORE tool as prescreening tests before DXA as they apply to screening primarily in US postmenopausal women (15, 16). For each simulated individual, initial DXA T-score values were sampled from data from the Third National Health and Nutrition Examination Survey at the femoral neck and reference data from a DXA manufacturer (Hologic, Inc., Bedford, Massachusetts) at the lumbar spine (17).
Treatment
We assumed that all women with positive screening results or those who sustained an osteoporotic fracture of the hip, vertebrae (clinically identified), or wrist were initially offered generic alendronate, the least expensive and most cost-effective treatment for osteoporosis, at a dose of 70 mg once weekly (5, 18). In base-case analysis, we assumed that treatment adherence was 50% (19). Of the 50% of individuals initially adherent to alendronate treatment, we assumed that half were switched to another more expensive oral bisphosphonate within the first month of therapy and remained adherent. For individuals adherent to bisphosphonate treatment, we assumed treatment for 5-years periods (20, 21), followed by a drug holiday for 5 years given current concerns about long-term bisphosphonate use (22–24), and that this pattern of 5 years of treatment on/off continued for the remainder of an individual’s lifetime in the absence of a medication adverse event.
Fracture Rates
For women not receiving alendronate, we based fracture rates on data from the Study of Osteoporotic Fractures (25). To predict future fracture probabilities dependent on the women’s age, femoral neck or lumbar spine BMD, and presence or absence of a history of fracture, we used logistic regression equations developed from Study of Osteoporotic Fractures data (Black DM. Personal communication. December 2005). We assumed that 100% of hip and wrist fractures and 35% of vertebral fractures were clinically apparent (26). For women receiving oral bisphosphonate treatment with no history of osteoporotic fracture, we based relative risk for fracture on data from the Fracture Intervention Trial, the Alendronate Phase III Osteoporosis Treatment Study Group, and a meta-analysis (14, 27–29). For individuals who sustained a fracture, we based future fracture relative risk on data from the Fracture Intervention Trial for women with previous fractures (30). We assumed that after bisphosphonate cessation there was a linear decline in fracture risk reduction benefit over 5 years to return to rates in the absence of therapy (31).
Mortality Rates
We used rates from US national vital statistics tables for age-stratified mortality in women (32). We assumed increased mortality after hip fracture (33) and used age-specific data for mortality after hip fractures during hospitalization and the first year after fracture (34).
Nursing Home Characteristics
We used published data to determine nursing home admission rates, length of stay, and mortality rates for postmenopausal women and patients with hip fracture (34, 35); 60% of the latter patients were assumed to be admitted to a nursing home after hospitalization (36). Rates of discharge from nursing homes back to the community were obtained from actuarial data (35, 36).
Costs
We included direct costs of screening tests, oral bisphosphonate treatment, physician visits, fracture-related treatment, nursing home stay, and adverse events. For base-case analysis, we assumed an annual generic alendronate cost of $108 and an annual cost for a more expensive nongeneric oral bisphosphonate of $1500 (37). We obtained costs for fracture-related treatment and other medical services from Medicare diagnosis-related group and Current Procedural Terminology reimbursement rates or published studies on osteoporosis-related costs (9, 38). Costs for fracture-related treatment were obtained from a study that estimated incremental direct medical costs in the year after osteoporotic fracture (38). We based nursing home costs on a national nursing home insurance survey (39). We obtained costs for over-the-counter omeprazole from a low-cost pharmacy (40).
Utilities
For baseline health state utility values, we used data from a nationally representative noninstitutionalized sample of elderly women (41). We modeled disutility associated with fractures, nursing home residence, and medication adverse events using data from several sources (42–47).
Discounting
Future costs and benefits were discounted at 3% annually (13).
Adverse Events
Esophagitis and esophageal ulceration rates were obtained from alendronate clinical trials (30).
Analyses
We performed base-case and probabilistic sensitivity analyses separately for all screening initiation ages. Probabilistic sensitivity analyses involved the characterization of uncertain key model inputs as probability distributions and were performed to evaluate the effects of joint input parameter uncertainty, in addition to individual patient variability, on the model results. For each age at screening initiation, we identified strategies with the highest probabilities of producing the most QALYs at 2 willingness-to-pay thresholds commonly cited in the literature: $50 000/QALY and $100 000/QALY. We then developed a separate model in which we included the top 3 strategies for these willingness-to-pay categories as well as all of the nondominated strategies from base-case analysis at each age. For this “best strategies” model that directly compared different screening initiation ages, we used the same 55-year-old cohort to examine screening started at age 55 years versus older ages. We performed base-case and probabilistic sensitivity analyses, as well as additional sensitivity analyses for the “best strategies” model, in which we evaluated different assumptions for critical model parameters, including fracture risk (50% higher and 50% lower); medication adherence (high adherence rate of 70%); costs (high costs [upper limit of sensitivity analysis range] for all screening tests, bisphosphonates, fractures, and nursing home care); and bisphosphonate adverse events (100 times rates reported in clinical trials).
Model Validation
To validate our model, we compared the model’s predictions about life expectancy and fractures with actual outcomes reported in US data sources.
Role of the Funding Source
This study was supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and the National Institute of Diabetes and Digestive and Kidney Diseases. None of the funding organizations or sponsors had any role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; the preparation, review, or approval of the manuscript; or the decision to publish the manuscript.
Results
Model Validation
Our model predicted that the mean life expectancy for 65-year-old women without screening would be 19.4 years. This is close to the figure of 19.8 years reported for women of this age in 2006 (48). Our model predicted that 49% of 65-year-old women without screening would incur 1 or more fractures over their lifetime. Our predicted percentage for vertebral fracture (27%) was close to that reported in a previous study of US postmenopausal women (28%) (49). The percentage of 65-year-old women in our model who experienced a hip fracture before age 90 was 17%, similar to the percentage reported in a study of Medicare beneficiaries who would have a fracture by age 90 (16%) (50). Our lifetime estimates for hip fracture and wrist fracture (23% and 17%, respectively) were higher than estimates reported in the study of Medicare beneficiaries who would have a fracture by age 90 (16% and 9%, respectively), based on 1986–1990 data (50). However, the differences in percentages may have occurred because women’s life expectancies have increased by 1.3 years since 1988 and 29% of the women in our model lived to be at least 90 years old.
Analysis of Strategies by Age
Base-Case Analysis
At all screening initiation ages, all screening strategies were more effective (that is, resulted in more QALYs) than no screening; in addition, no screening was more expensive than multiple screening strategies at screening initiation ages of 65 years and older, and thus screening “dominated” no screening at ages 65 and older.
The cost-effectiveness of screening strategies varied widely (Table 3), but at all initiation ages the best strategy with an ICER of less than $50 000 per QALY was DXA screening with a T-score threshold of −2.5 or less for treatment and with follow-up screening every 5 years (that is, DXA −2.5 with rescreening every 5 years). From ages 55 through 75, the incremental cost per QALY gained with this strategy decreased as the screening initiation age increased. Assuming a willingness-to-pay of $100 000 per QALY, DXA −2.5 with rescreening every 5 years remained the best strategy at ages 60 and older; at age 55, DXA −2.0 with rescreening every 10 years was the best strategy with an ICER of less than $100 000/QALY. Several strategies involving QUS −1.0 prescreening or SCORE −2.5 prescreening were more cost-effective than screening initiation with DXA at ages 55 through 65, with ICERs of less than $20 000 per QALY.
Table 3.
Base-Case Analysis Results at Each Screening Initiation Age*
| Incremental Cost-Effectiveness Ratio of the Strategy (Average Quality-Adjusted Life-Days Added), by Screening Initiation Age† |
||||||
|---|---|---|---|---|---|---|
| Screening Strategy | 55 y | 60 y | 65 y | 70 y | 75 y | 80 y |
| No screening | Baseline‡ | Baseline‡ | − | − | − | − |
| QUS −1.0 one time | − (5.3) | 2300 (7.7) | Baseline‡ (10.9) | − (14.8) | − (17.5) | − (17.8) |
| QUS −1.0 with rescreening every 10 y |
5980 (11.8) | 4130 (13.4) | 3010 (15.6) | − (18.2) | − (19.7) | − (18.8) |
| QUS −1.0 with rescreening every 5 y | 13 410 (13.7) | 17 780 (15.9) | 3320 (17.6) | − (20.7) | − (21.9) | − (20.8) |
| QUS −0.5 one time | − (6.0) | − (8.5) | − (12.0) | − (16.2) | − (19.5) | − (19.7) |
| QUS −0.5 with rescreening every 10 y |
− (12.7) | − (14.3) | − (16.6) | − (19.5) | − (21.7) | − (20.7) |
| QUS −0.5 with rescreening every 5 y | − (14.2) | − (16.4) | − (18.3) | − (21.6) | − (23.4) | − (22.3) |
| SCORE −2.5 one time | 3560 (6.4) | − (9.0) | − (12.7) | − (17.1) | − (20.8) | − (21.1) |
| SCORE −2.5 with rescreening every 10 y |
8020 (13.1) | 14 090 (14.8) | − (17.3) | 4530 (20.4) | − (22.8) | − (21.9) |
| SCORE −2.5 with rescreening every 5 y |
19 550 (14.5) | − (16.7) | − (18.7) | − (22.1) | − (24.2) | − (23.2) |
| SCORE NOF one time | − (13.7) | − (16.2) | − (18.5) | − (21.4) | − (23.7) | 234 900 (24.9) |
| SCORE NOF with rescreening every 10 y |
− (17.3) | − (18.8) | − (20.4) | − (22.6) | − (24.5) | NA§ |
| SCORE NOF with rescreening every 5 y |
− (18.0) | − (19.6) | − (21.0) | − (23.4) | − (25.3) | NA§ |
| DXA −2.5 one time | − (6.9) | − (9.6) | − (14.6) | Baseline‡ (18.3) | Baseline‡ (22.1) | Baseline‡ (22.4) |
| DXA −2.5 with rescreening every 10 y |
− (13.7) | − (15.4) | − (18.0) | 4600 (21.3) | 3010 (23.9) | 1640 (23.1) |
| DXA −2.5 with rescreening every 5 years |
30 360 (14.8) | 28 230 (17.1) | 23 090 (19.1) | 16 670 (22.8) | 9720 (25.0) | 19 550 (24.0) |
| DXA −2.0 one time | − (11.6) | − (14.8) | − (18.3) | − (22.3) | − (25.2) | − (24.2) |
| DXA −2.0 with rescreening every 10 y |
96 020 (16.7) | 110 950 (18.8) | 110 210 (20.7) | − (23.5) | 152 950 (25.9) | − (24.3) |
| DXA −2.0 with rescreening every 5 y | − (17.1) | − (18.9) | − (20.9) | 202 290 (23.8) | − (26.0) | 222 700 (24.5) |
| DXA −1.5 one time | − (15.7) | − (18.3) | − (20.8) | − (23.8) | − (26.2) | − (24.6) |
| DXA −1.5 with rescreening every 10 y |
194 610 (18.3) | − (19.8) | − (21.5) | − (24.2) | − (26.4) | − (24.7) |
| DXA −1.5 with rescreening every 5 y | 301 640 (18.5) | 246 170 (20.1) | 285 840 (21.7) | 307 300 (24.3) | 200 460 (26.6) | − (24.7) |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; NOF = National Osteoporosis Foundation; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Strategies are not ranked in order of increasing costs or efficacy in all columns. Instead, the same order is used across ages to facilitate comparisons. Dashes represent dominated strategies. Dominated strategies were found to be less efficacious and more expensive than another strategy (strict dominance) or to have an incremental cost-effectiveness ratio that is greater than that of the next, more effective and more expensive alternative (extended dominance).
Ratios represent cost per quality-adjusted life-year gained for a particular strategy compared to the next less costly nondominated strategy, and are expressed in terms of 2010 $US per quality-adjusted life-year, rounded off to the nearest 10. Values in parentheses are presented in quality-adjusted life-days (discounted), not total life-days or quality-adjusted life-years; the numbers shown represent the average quality-adjusted life-days added with the screening method shown compared to no screening.
Least expensive strategy.
We assumed that all individuals age 80 years and older would test positive on SCORE and be offered treatment in accordance with the SCORE NOF criteria; therefore, SCORE NOF was evaluated as a 1-time screening test in this age group.
At all initiation ages of 75 years or younger, the most effective strategy was DXA screening with a T-score threshold of −1.5 or less for treatment and with follow-up screening every 5 years (that is, DXA −1.5 with rescreening every 5 years). However, this strategy was expensive, with ICERs in the $200 000 to $300 000/QALY range. At a screening initiation age of 80 years, the most effective strategy was the SCORE National Osteoporosis Foundation (NOF) strategy, with which we assume that all individuals of this age are offered treatment; however, this strategy was also expensive, with an ICER of $234 900/QALY.
The average difference between the most effective strategy and no screening at each screening initiation age ranged from 18.5 to 26.6 quality-adjusted life-days. In general, quality-adjusted life-days gained with screening tended to increase with age.
All screening strategies that involved treatment of individuals prescreened with QUS using a T-score threshold of −0.5 and most strategies involving SCORE prescreening with the NOF criteria for treatment (except at age 80 years) were less effective and more expensive than other evaluated options.
Probabilistic Sensitivity Analysis
The strategies most frequently ranked among the top 3 in willingness-to-pay categories of $50 000/QALY and $100 000/QALY at various screening initiation ages in probabilistic sensitivity analysis included the use of DXA −2.5 with rescreening every 5 years or 10 years; QUS −1.0 prescreening with rescreening every 5 years; DXA −2.0 with rescreening every 5 years or 10 years; and SCORE −2.5 prescreening with rescreening every 5 years. These were among the strategies included in the best-strategies model to identify the optimal age of screening initiation. No individual strategy was ranked first in greater than 22% of the analyses at any screening initiation age at either willingness-to-pay threshold.
Comparison of Best Strategies Across Screening Ages
Base-Case Analysis
The best strategy with an ICER of less than $50 000 per QALY was initiating DXA −2.5 screening at age 55 and rescreening every 5 years (Table 4). Assuming a willingness-to-pay of $100 000 per QALY, initiating DXA −2.0 screening at age 55 with rescreening every 10 years was the best strategy. The most effective strategy across initiation ages was initiating DXA −1.5 screening at age 55 and rescreening every 5 years. However, this strategy was very expensive, with an ICER of $696 710/QALY. Several strategies involving SCORE −2.5 prescreening or QUS −1.0 prescreening were more cost-effective than strategies involving screening initiation with DXA, with ICERs of less than $30 000 per QALY. All of the most effective strategies involved screening initiation at age 55 years (Table 4).
Table 4.
Base-Case Analysis Results for Best Strategies Model Across Screening Initiation Ages*
| Screening Strategy | Lifetime Cost, 2010 $US† |
Bisphosphonate Therapy (%)‡ |
Hip Fracture (%)§ |
Quality-Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|---|---|
| SCORE −2.5 initiated at age 80 y with rescreening every 5 y | 57 360 | 27.7 | 21.5 | 14.0477 | NA |
| SCORE −2.5 initiated at age 70 y with rescreening every 10 y | 57 400 | 28.4 | 20.8 | 14.0593 | 3200 |
| QUS −1.0 initiated at age 60 y with rescreening every 10 y | 57 420 | 27.9 | 20.5 | 14.0655 | 3650 |
| SCORE −2.5 initiated at age 60 y with rescreening every 10 y | 57 450 | 28.5 | 20.3 | 14.0689 | 8490 |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 57 580 | 29.1 | 20.0 | 14.0749 | 21 850 |
| SCORE −2.5 initiated at age 55 y with rescreening every 5 y | 57 650 | 29.5 | 19.9 | 14.0773 | 26 750 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 57 680 | 29.6 | 19.8 | 14.0780 | 45 450 |
| DXA−2.0 initiated at age 55 y with rescreening every 10 y | 58 180 | 31.9 | 19.5 | 14.0833 | 94 210 |
| DXA −2.0 initiated at age 55 y with rescreening every 5 y | 58 380 | 32.8 | 19.4 | 14.0842 | 217 980 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 58 970 | 34.8 | 19.3 | 14.0863 | 278 530 |
| DXA −1.5 initiated at age 55 y with rescreening every 5 y | 59 170 | 35.5 | 19.3 | 14.0866 | 696 710 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy. For this “best strategies” analysis, we used the same 55-year-old cohort to examine screening started at age 55 years versus older ages.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Percentage of individuals ever taking bisphosphonate treatment during lifetime.
Percentage of individuals sustaining a hip fracture during lifetime.
Sensitivity Analyses
The results of sensitivity analyses are shown in the Appendix Tables. In general, varying several critical parameter assumptions resulted in the same strategies being among the most effective, but with a change in their ICERs. In the individual sensitivity analyses in which costs were high, adverse event rates were high, and fracture risk was low, none of the strategies involving treatment of individuals with DXA T-scores greater than −2.5 (osteopenia or low bone mass) were cost-effective, assuming a willingness-to-pay of $100 000/QALY. In the high-fracture-risk sensitivity analysis, strategies involving treatment of osteopenia had lower ICERs than in the base-case analysis; strategies of DXA −2.0 screening initiation at age 55 and rescreening every 10 years as well as DXA −2.0 screening initiation at age 55 and rescreening every 5 years had ICERs less than $100 000/QALY. Results for the high-medication-adherence sensitivity analysis were similar to those of the base-case analysis.
Probabilistic sensitivity analysis did not reveal a dominant strategy at willingness-to-pay thresholds of $50 000 or $100 000/QALY; no individual strategy was ranked first in greater than 11% of the analyses at either willingness-to-pay threshold (Appendix Table 1), indicating that many of the strategies are close in costs and effectiveness.
Discussion
Our results demonstrated that all evaluated osteoporosis screening strategies, including the use of central DXA, or the combination of QUS or SCORE prescreening and DXA screening, are effective for postmenopausal women, and most are well within typical thresholds used for establishing a strategy as cost-effective. In addition, no screening is more expensive and less effective than multiple screening strategies at screening initiation ages of 65 years and older; thus, starting screening at age 65 and older “dominated” no screening. In a direct comparison of screening initiation ages from 55 through 80, the best strategy with an ICER of less than $50 000 per QALY was initiating screening at age 55 with DXA −2.5 and rescreening every 5 years. Several strategies involving SCORE −2.5 prescreening or QUS −1.0 prescreening were more cost-effective than strategies involving screening initiation with DXA, with ICERs of less than $30 000 per QALY. We found that screening continues to be effective and cost-effective at older ages, such as 80 years, and that in general, quality-adjusted life-days gained with screening tend to increase with increasing age. In addition, strategies involving screening with DXA, rather than QUS or SCORE prescreening, were most effective, although the differences between strategies were on the order of quality-adjusted life-days.
Current USPSTF guidelines recommend routine screening for women 65 years of age or older, as well as routine screening for women age 60 years or older who have additional osteoporosis risk factors (8). Current NOF guidelines recommend screening for all women age 65 years and older and for younger postmenopausal women with risk factors for osteoporosis (51). Our results suggest that it would be more effective and of good value to routinely screen all postmenopausal women with central DXA starting at age 55, and offer treatment if a DXA T-score is −2.5 or less. For women who test negative with these criteria, repeating screening every 5 years is better than repeating it every 10 years or one-time screening. If the willingness-to-pay is $100 000/QALY, then screening initiation at age 55 and treatment of individuals with DXA T-scores of −2.0 or less could be considered, with a rescreening interval of 10 years. If the willingness-to-pay is less than $30 000 per QALY, various strategies involving SCORE −2.5 prescreening or QUS −1.0 prescreening before DXA could be considered.
In general, our results do not indicate a clear superiority of a repeat screening interval of 5 years versus every 10 years. It is likely that the best repeat screening interval may vary according to previous DXA T-scores. Sensitivity analyses of individual important model parameters indicated that the strategies involving DXA screening remained most effective when changing these parameter values; however, the incremental cost-effectiveness of these strategies varied with different assumptions. In general, the cost-effectiveness of initiating screening at age 55 with DXA, treating if the T-score is −2.5 or less, and rescreening every 5 years for women who test negative was robust to these sensitivity analyses, giving us confidence in the effectiveness and value of this strategy.
Probabilistic sensitivity analysis revealed that no single strategy emerged as clearly best at willingness-to-pay thresholds of $50 000/QALY or $100 000/QALY, when considering joint input parameter uncertainty of multiple parameters. This finding, as well as our finding of an average difference in effectiveness between various screening strategies on the order of several quality-adjusted life-days, indicates that the differences between strategies are likely to be small; for example, the difference in average quality-adjusted life expectancy among the nondominated strategies in our best strategies analysis ranged from several hours to 14 quality-adjusted life days, and the difference in average lifetime costs ranged from $20 to $1810. However, further value of information research should be done to identify the key variables affecting the relative cost-effectiveness of strategies and to obtain greater certainty in the values of these variables. Given the relatively small difference between various osteoporosis screening strategies and that no one strategy is clearly superior when considering parameter uncertainty, patient preferences should play an important role in the choice of screening strategy. For women with limited access to DXA or those who prefer not to travel for DXA screening if possible, our findings show that use of the SCORE tool or QUS for prescreening are reasonable alternatives. Because other studies have shown the Osteoporosis Self-Assessment Tool and Osteoporosis Risk Assessment Instrument to perform similarly to the SCORE tool, we expect that these too may be acceptable alternatives (52–54).
Our finding that osteoporosis screening may be an even better value in older postmenopausal women than younger postmenopausal women is consistent with a previously conducted limited cost-effectiveness analysis of bone densitometry screening (10). Positive screening results tended to increase with age because of age-associated decline in bone mineral density and increased fracture risk.
Previous research indicates that more postmenopausal women have DXA T-scores in the osteopenic or low bone mass range (−1.0 to −2.5) than in the osteoporotic range (−2.5 or less) and that about half of fractures occur in those with T-scores −2.5 or greater (51). However, although we found that initiation of screening at age 55 with treatment of individuals with DXA T-scores of −2.0 or less and repeat screening in 10 years may be cost-effective, assuming a willingness-to-pay of $100 000/QALY, this finding was not robust to sensitivity analysis of individual important model parameters. Thus, like others (55, 56), we did not find compelling evidence that treating women with osteopenia and no history of osteoporotic fracture is cost-effective. This may not be the case if other osteoporosis risk factors, such as use of glucocorticoids or diseases known to cause bone loss, were considered.
Investigators have suggested using future absolute fracture risk over a particular time period to select individuals for treatment, for example with FRAX (Fracture Risk Assessment Tool) (57). However, a major limitation of applying a future fracture risk paradigm to identify individuals for treatment is that there is currently no direct prospective evidence of treatment efficacy based on individuals with increased fracture risk who are not known to have low BMD. Additionally, some evidence suggests that women selected for osteoporosis therapy on the basis of fracture risk rather than low DXA BMD may not benefit similarly (58). Thus, we chose a conservative approach of not assuming treatment efficacy for individuals identified as having increased fracture risk but who did not have low BMD; we did not evaluate FRAX rather than speculating about treatment efficacy. Once treatment efficacy data become available for individuals identified for treatment with FRAX and other fracture risk prediction tools, we plan to compare them to the other options evaluated in our model.
Our model has several limitations. First, it includes data for hip, vertebral, and wrist fractures only because these are the most common osteoporotic fractures, and good data concerning the efficacy of treatment on risk for these fractures are available from clinical trials. If treatment is effective in preventing other fractures, the screening strategies evaluated would be more cost-effective. Second, we did not evaluate screening initiation ages younger than 55; it is possible that osteoporosis screening may be effective and cost-effective at even younger ages than 55. Third, our evaluation of SCORE and QUS prescreening tools only considered 2 and 1 subsequent DXA T-score thresholds for treatment, respectively; thus, we did not evaluate all possible combinations of these prescreening tools with DXA. In addition, we did not evaluate different screening intervals as a function of the initial DXA T-score result; the best rescreening interval may differ depending on the previous DXA T-scores. Furthermore, the studies from which we obtained data on test performance characteristics of SCORE and QUS used a regional sample of women from the greater Seattle area (16) and several different populations for whom data were combined with a meta-analysis (15), respectively; we make the assumption that the performance characteristics of SCORE and QUS in US postmenopausal women would be the same as that found in these study populations. The test performance characteristics assumed for SCORE prescreening with the NOF criteria for treatment may have biased our results for this strategy. Moreover, the Study of Osteoporotic Fractures has associated healthy volunteer bias, which may have biased our estimates of fracture rates for postmenopausal US women downward. Additionally, our analyses did not incorporate additional medical care costs from living longer because of osteoporosis screening, or indirect costs for women in the younger age groups in our analyses. However, we expect the costs of added life days would be small because age of death was very similar in the screening and no screening model arms. Finally, most model inputs were based on data from white women and may be less applicable to other women.
Our study also had notable strengths. We simultaneously compared a variety of screening tests, initiation ages, repeat screening intervals, and treatment thresholds. A clinical trial comparing this range of options would be expensive and impractical because it would require extensive numbers of participants and lifetime follow-up. Furthermore, the model created for this study will allow future investigations of new screening tools/strategies, other osteoporosis medications, different repeat screening intervals, or the impact of changes in key parameter values over time. In conclusion, multiple osteoporosis screening strategies are effective and cost-effective for postmenopausal women, including strategies involving screening initiation at age 55. In general, the differences in average effectiveness and costs between evaluated strategies are small. Expansion of osteoporosis screening could improve health outcomes at a reasonable cost.
Acknowledgments
The authors thank Hau Liu, MD, MPH, MBA, and Kaleb Michaud, PhD, for assistance with development of the cost-effectiveness model and Dennis Black, PhD, for providing logistic regression equations to predict women’s future fracture probabilities developed from Study of Osteoporotic Fractures data.
Grant Support: By grant KL2 RR024154 from the National Center for Research Resources (a component of the National Institutes of Health) and National Institutes of Health Roadmap for Medical Research (Dr. Nayak), grant 1R01AR060809-01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr. Nayak), and by grant K24 DK062895 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. Greenspan).
Appendix: Model Structure, Development, and Analysis
Model Structure
We constructed an individual-level state-transition model of osteoporosis screening and treatment for postmenopausal women. The Figure depicts a simplified representation of the model. The screening strategies evaluated in the model include no screening (treatment only if osteoporotic fracture occurs); central DXA; QUS; and the SCORE tool. Table 1 describes the strategies, and Table 2 summarizes input parameter assumptions.
For each simulated woman, we assigned initial DXA T-score values at the femoral neck and lumbar spine. The various screening test results and the natural history of BMD T-scores and future fracture risk follow from these initial scores. After screening, each woman with a positive result is offered oral bisphosphonate treatment, and each with a negative result receives usual care only (calcium and vitamin D). The model assumes that each screened woman has no known history of an osteoporotic hip, vertebral, or wrist fracture; thus, each woman initially starts the model in a “no prior fracture” state.
During each cycle in the model, each individual woman may survive or die. If she survives, she may remain in the community or go to a nursing home, and she may avoid a fracture or may sustain a hip, vertebral, or wrist fracture. If she sustains a hip fracture, she may or may not die during hospitalization. If she survives a hip fracture, she may be discharged to a nursing home. Additionally, during each cycle, she may or may not experience an adverse event from bisphosphonate therapy. Occurrences of events, such as medication treatment, fractures, nursing home placement, and adverse events, are recorded with tracker variables.
After completing a cycle in the model, the woman starts the next cycle. If she did not sustain an osteoporotic fracture in the earlier cycle, she starts this subsequent cycle in the “no prior fracture” state. But if she sustained a fracture, she starts this cycle in the fracture state of the most severe previous fracture incurred (hip > vertebral > wrist fracture).
Each cycle length is 3 months, and individuals continue cycling through the model until death occurs.
Model Development
Screening Strategies
We evaluated the use of DXA screening strategies with T-score thresholds of −2.5 or less, −2.0 or less, and −1.5 or less to offer treatment. These are thresholds for which treatment has been recommended by various organizations (24, 51, 59, 60).
We evaluated the use of calcaneal QUS as a prescreening test prior to DXA. We evaluated QUS T-score thresholds of −1.0 or less and −0.5 or less to select individuals for DXA testing, with treatment offered if subsequent DXA T-score was −2.5 or less. These are thresholds for which reasonable tradeoff exists between sensitivity and specificity estimates for prescreening (15).
We chose to evaluate the SCORE tool as representative of a simple validated risk assessment tool used to identify individuals who may have low BMD values for subsequent DXA testing. Several studies have demonstrated that various simple risk assessment tools for prescreening US postmenopausal women have similar performance characteristics (52–53). We chose to include SCORE in our model rather than another similar risk assessment tool because good-quality data were available for the performance of the SCORE tool in a regional sample of US postmenopausal women without a previous osteoporosis diagnosis (16). Risk factors assessed with the SCORE tool include age, weight, race, the presence or absence of rheumatoid arthritis, a history of minimal trauma fracture after age 45 years, and a history of estrogen therapy; a different number of points are assigned to various components, as shown in Appendix Table 1 (53). We evaluated use of SCORE with a threshold of 7 or more points to identify individuals for DXA testing and evaluated 2 different subsequent thresholds for treatment recommendation: 1) DXA T-score −2.5 or less and 2) DXA T-score −2.0 or less or DXA T-score −1.5 or less with an additional osteoporosis risk factor or age 80 years or older, in accordance with National Osteoporosis Foundation intervention criteria reported by Brenneman and colleagues (16).
We used estimates of QUS and SCORE diagnostic test performance obtained from the largest studies we could find on the performance of these tests as prescreening tools before DXA done in the population of interest for our model, and that defined osteoporosis or osteopenia using DXA findings at the femoral neck or lumbar spine rather than just the femoral neck (15, 16). We used the SCORE and DXA thresholds presented in these studies; which included only 2 DXA T-score thresholds for treatment used with the SCORE tool (−2.5 or less and −2.0 or less); and one with QUS (−2.5 or less). Thus, not all possible combinations of DXA with QUS or SCORE prescreening were evaluated with our model.
Model Parameter Estimates Selection
Comprehensive PubMed literature searches were conducted for all data estimates, using text words for each parameter of interest, to identify the highest-quality data and largest studies done in postmenopausal US women for each estimate in our model. We preferentially used data from large clinical trials or meta-analyses when possible. We also checked multiple references for each parameter to evaluate for consistency in estimates. Where estimates were not available specifically for US postmenopausal women, we considered estimates from other similar populations. One of our authors who is an osteoporosis expert was consulted on the choice of parameter values if there was any discrepancy or questions about the literature. Although we tried to find the most recent high-quality data for each estimate, for some of the parameter estimates we found a lack of relevant recent studies.
Assignment of Values on Screening Tests
For each simulated individual, we assigned initial DXA T-score values at the femoral neck and lumbar spine by sampling from normal distributions by age based on 2 sources. The data that we used for the femoral neck values came from the Third National Health and Nutrition Examination Survey and pertained to non-Hispanic white women in the United States (17). The data that we used for the lumbar spine values came from a DXA manufacturer (Hologic, Inc., Bedford, Massachusetts) and pertained to white women. On the basis of published data, we incorporated correlations between femoral neck and lumbar spine values (r = 0.603) and modeled the average annual change in each woman’s T-scores at the femoral neck and lumbar spine (17, 61). We assumed constant, linear decrement in T-scores over time.
Because QUS and SCORE were evaluated as prescreening tools followed by DXA screening, we assigned results on the basis of the sensitivity and specificity estimates for calcaneal QUS prescreening reported in a recent meta-analysis (15), and the sensitivity and specificity estimates for SCORE prescreening obtained from a US population of postmenopausal women (16). To do this, we randomly sampled from uniform distributions with cutoffs based on these sensitivity and specificity values for SCORE and QUS for each individual, with knowledge of their sampled DXA T-scores, to determine whether these prescreening tests would have identified individuals as requiring subsequent DXA testing or not.
Treatment
We assumed that all individuals, whether receiving bisphosphonate treatment or not, were taking calcium and vitamin D but that the calcium and vitamin D did not provide additional protection against fracture. We assumed that everyone who had a positive screening test result or sustained an osteoporotic fracture was offered alendronate 70mg weekly therapy; we assumed that 50% of individuals initially offered alendronate subsequently switched to another non-generic oral bisphosphonate within the first month of treatment. In base-case analysis, we assumed that adherence with bisphosphonate treatment was 50% (19, 62). We assumed that the 50% of individuals who were initially adherent continued to receive treatment for the duration of recommended therapy in the absence of side effects requiring discontinuation, and that individuals who were initially nonadherent were nonadherent for the entire period of recommended therapy. Individuals who were initially nonadherent were not rescreened and continued to be nonadherent in the future unless they sustained an osteoporotic fracture; in that case we assumed that they then had a 50% chance of being newly adherent. We assumed that nonadherent individuals did not incur the costs or fracture reduction benefits of alendronate therapy. We assumed that individuals receiving bisphosphonates were treated for 5-year intervals (20, 21) and then took a drug holiday for 5 years (22–24), and that they continued 5 year on/off treatment for the remainder of their lives unless they experienced a side effect. We assumed that individuals did not experience a decline in their T-scores at the femoral neck or lumbar spine during the years on treatment. We assumed that during the 5-year drug holiday the T-scores declined at the same rate as the untreated population.
Fracture Rates
For women not receiving bisphosphonate therapy, we based fracture rates on data from the Study of Osteoporotic Fractures (25). To predict future fracture probabilities dependent on the women’s age, femoral neck or lumbar spine BMD, and presence or absence of prior fracture, we used logistic regression equations developed from Study of Osteoporotic Fractures data (Black DM. Personal communication. December 2005). These logistic regression fracture risk equations for hip, vertebral, and wrist fractures included 2 separate equations for each of these types of fractures with predictors of 1) age, femoral neck T-score, and prior vertebral fracture and 2) age, lumbar spine T-score, and prior vertebral fracture, respectively. In women without a history of fracture, we used fracture risk estimates based on the lower of the T-scores from the femoral neck or lumbar spine.
For women receiving bisphosphonate therapy, we determined the relative risk for fracture on the basis of data from the Fracture Intervention Trial, the Alendronate Phase III Osteoporosis Treatment Study Group, and a meta-analysis (14, 27–29); fracture risk varied depending on fracture history and BMD T-scores. We assumed that after bisphosphonate cessation, the risk for fracture returned to rates in the absence of therapy over 5 years, with a linear decline of medication benefit (31).
Costs
We included costs of screening tests, bisphosphonate therapy, osteoporotic fractures (hip, vertebral, and wrist), nursing home stay, physician visits, and adverse events in our analyses. All costs were presented in 2010 US dollars, and only direct costs were included. If costs were not presented in 2010 US dollars, we inflated them to 2010 costs based on the US Consumer Price Index for Medical Care (63). Costs for fracture-related treatment were obtained from a study in which incremental direct medical costs in the year after osteoporotic fracture were estimated; costs for all health services and procedures provided were included with several exceptions, specifically outpatient prescription drugs, durable medical equipment, transportation, and nursing home care (38). We assumed that vertebral fractures that were not clinically apparent did not generate any costs. We assumed that individuals who were diagnosed with osteoporosis incurred the cost of an additional physician visit per year. We discounted future costs by 3% annually (13).
Utilities
For baseline health state utility values, we used data from a nationally representative noninstitutionalized civilian sample of elderly women in the United States, with utility scores derived by using the EuroQol-5D instrument with US scoring (41). To model disutility associated with fractures, nursing home residence, and medication adverse events, we used data from studies that applied time trade-off or standard gamble methods when these data were available (42–44). Otherwise, we used values determined from expert opinion or surrogate values for similar health states (42, 45). We assumed that if a woman had previously had both a hip fracture and a vertebral fracture, she would incur utility decrements associated with both (64), with the utilities multiplied. If she sustained a fracture at the same site on multiple occasions, she would only suffer the utility decrement associated with the most recent fracture. If she fractured both her hip and wrist or if she fractured both her wrist and vertebrae, she would suffer the utility decrement associated with the more severe of the fractures. We assumed that both clinical and morphometric vertebral fractures were associated with utility decrements. We modeled long-term disutility associated with hip and vertebral fractures that persisted for the remainder of an individual’s life, but no long-term disutility associated with wrist fractures (42, 47). We discounted future utility values by 3% annually (13).
Adverse Events
We assumed that women who experienced esophageal ulceration discontinued alendronate and had an upper endoscopy done (Medicare CPT code 43235; cost, $286.87) (9). We also assumed that they incurred the costs of having 2 additional outpatient physician visits ($133.48) and the use of proton-pump inhibitor therapy for 1 year ($240) (9, 40). Thus, the total cost associated with the adverse event of esophageal ulceration was $660.35. We assumed that women who experienced esophagitis continued treatment but incurred the costs of having an additional physician visit ($66.74) and the use of proton-pump inhibitor therapy for 1 year ($240) (9, 40); thus, the total cost for the side effect of esophagitis was $306.74.
Analyses
Table 2 shows the fixed parameter values used for base-case analyses and the range of values used for probabilistic sensitivity analyses. Probabilistic sensitivity analyses involved the characterization of uncertain key model inputs as triangular probability distributions and were performed to evaluate the effects of joint input parameter uncertainty, in addition to individual patient variability, on the model results. We modeled the initiation of screening at ages 55, 60, 65, 70, 75, and 80 years. For base-case analyses, we ran the model with 1 million trials, simulating the screening of 1 million individuals. For the probabilistic sensitivity analyses at each of the 6 screening initiation ages and the model comparing best strategies across screening initiation ages, we conducted 500 simulations and 2000 trials per simulation.
Appendix Table 2 shows model validation results for women initiating screening at age 65. Appendix Table 3 shows results from the base-case analysis of the best strategies model for all included strategies. Appendix Table 4 shows sensitivity analysis results from the best strategies model, assuming 50% higher hip, vertebral, and wrist fracture rates than the base-case model. Appendix Table 5 shows sensitivity analysis results from the best strategies model, assuming 50% lower hip, vertebral, and wrist fracture rates than the base-case model. Appendix Table 6 shows sensitivity analysis results from the best strategies model, assuming 70% bisphosphonate medication compliance. Appendix Table 7 shows sensitivity analysis results from the best strategies model, assuming high screening test; bisphosphonate; fracture-related; and nursing home costs (high values of the range for these costs shown in Table 2). Appendix Table 8 shows sensitivity analysis results from the best strategies model, assuming 100 times baseline adverse drug event rates. Appendix Table 9 shows probabilistic sensitivity analysis results for the best strategies model.
Appendix Table 1.
Simple Calculated Osteoporosis Risk Estimation Tool Variables and Scoring
| Variable | Points Scored |
|---|---|
| Race (not black) | 5 |
| Age | 3 times first digit of age in years |
| Rheumatoid arthritis | 4 |
| History of low-trauma fracture after age 45 y (hip, wrist, or rib) |
4 per low-trauma fracture (maximum 12) |
| Weight | −1 × weight in pounds/10 |
| Estrogen therapy | 1 point if never used |
Appendix Table 2.
Model Validation for Women Initiating Screening at Age 65
| Parameter | Model Estimate | Literature Estimate | Source (Reference) |
|---|---|---|---|
| Life expectancy, y | 19.4 | 19.8 | Heron et al (48) |
| Hip fractures by age 90 y, % | 17 (23 lifetime) | 16 | Barrett et al (50) |
| Vertebral fractures, % | 27 (lifetime) | 28 (by age 94 y) | Cummings et al (49) |
| Wrist fractures, % | 17 (lifetime) | 9 (by age 90 y) | Barrett et al (50) |
Appendix Table 3.
Base-Case Analysis Results for All Strategies Included in Best Strategies Model*
| Screening Strategy | Lifetime Costs, $† |
Bisphosphonate Therapy During Lifetime, %‡ |
Hip Fracture During Lifetime, %§ |
Quality- Adjusted Life- Years |
Incremental Cost- Effectiveness Ratio†∥ |
|---|---|---|---|---|---|
| 80 SCORE prescreen (DXA −2.5) repeat 5 | 57 360 | 27.7 | 21.5 | 14.0477 | NA |
| 65 QUS prescreen threshold −1 (DXA −2.5) one time | 57 390 | 24.0 | 21.7 | 14.0539 | – |
| 75 SCORE prescreen (DXA −2.5) repeat 5 | 57 400 | 28.8 | 21.0 | 14.0551 | – |
| 70 SCORE prescreen (DXA −2.5) repeat 10 | 57 400 | 28.4 | 20.8 | 14.0593 | 3200 |
| No screening | 57 410 | 22.4 | 22.9 | 14.0373 | – |
| 70 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 57 410 | 28.9 | 20.7 | 14.0597 | – |
| 60 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 57 420 | 27.9 | 20.5 | 14.0655 | 3650 |
| 70 SCORE prescreen (DXA −2.5) repeat 5 | 57 420 | 29.3 | 20.6 | 14.0614 | – |
| 80 SCORE prescreen (NOF) one time | 57 420 | 28.7 | 21.4 | 14.0487 | – |
| 65 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 57 430 | 27.9 | 20.7 | 14.0620 | – |
| 60 SCORE prescreen (DXA −2.5) repeat 10 | 57 450 | 28.5 | 20.3 | 14.0689 | 8490 |
| 55 SCORE prescreen (DXA −2.5) One Time | 57 450 | 22.7 | 22.0 | 14.0552 | – |
| 65 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 57 460 | 29.1 | 20.4 | 14.0659 | – |
| 70 DXA −2.5 one time | 57 460 | 25.6 | 21.3 | 14.0563 | – |
| 80 DXA −2.5 repeat 5 | 57 470 | 27.9 | 21.4 | 14.0481 | – |
| 80 DXA −2.5 repeat 10 | 57 470 | 27.4 | 21.5 | 14.0478 | – |
| 80 DXA −2.5 one time | 57 470 | 26.6 | 21.6 | 14.0474 | – |
| 70 DXA −2.5 repeat 10 | 57 480 | 28.6 | 20.7 | 14.0602 | – |
| 65 QUS prescreen threshold −0.5 (DXA −2.5) repeat 5 | 57 480 | 29.4 | 20.3 | 14.0671 | – |
| 75 DXA −2.5 one time | 57 480 | 26.5 | 21.3 | 14.0533 | – |
| 75 DXA −2.5 repeat 10 | 57 490 | 28.2 | 21.0 | 14.0545 | – |
| 60 DXA −2.5 repeat 10 | 57 490 | 28.7 | 20.3 | 14.0701 | – |
| 70 DXA −2.5 repeat 5 | 57 500 | 29.4 | 20.5 | 14.0618 | – |
| 75 DXA −2.5 repeat 5 | 57 500 | 28.9 | 20.9 | 14.0556 | – |
| 60 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 57 500 | 29.1 | 20.2 | 14.0706 | – |
| 55 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 57 500 | 27.8 | 20.4 | 14.0695 | – |
| 65 DXA −2.5 repeat 10 | 57 500 | 28.7 | 20.4 | 14.0661 | – |
| 65 DXA −2.5 repeat 5 | 57 550 | 29.6 | 20.2 | 14.0687 | – |
| 55 SCORE prescreen (DXA −2.5) repeat 10 | 57 560 | 28.4 | 20.2 | 14.0729 | – |
| 60 DXA −2.5 repeat 5 | 57 570 | 29.6 | 20.0 | 14.0735 | – |
| 55 DXA −2.5 repeat 10 | 57 580 | 28.6 | 20.1 | 14.0741 | – |
| 55 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 57 580 | 29.1 | 20.0 | 14.0749 | 21 850 |
| 55 SCORE prescreen (DXA −2.5) repeat 5 | 57 650 | 29.5 | 19.9 | 14.0773 | 26 750 |
| 80 DXA −2.0 repeat 5 | 57 660 | 29.6 | 21.3 | 14.0489 | – |
| 55 DXA −2.5 repeat 5 | 57 680 | 29.6 | 19.8 | 14.0780 | 45 450 |
| 75 DXA −2.0 repeat 10 | 57 720 | 30.7 | 20.8 | 14.0568 | – |
| 70 DXA −2.0 repeat 10 | 57 790 | 31.6 | 20.4 | 14.0638 | – |
| 70 DXA −2.0 repeat 5 | 57 850 | 32.3 | 20.4 | 14.0639 | – |
| 65 DXA −2.0 repeat 10 | 57 930 | 31.9 | 20.0 | 14.0721 | – |
| 60 DXA −2.0 repeat 10 | 57 990 | 32.0 | 19.7 | 14.0774 | – |
| 65 DXA −2.0 repeat 5 | 58 020 | 32.7 | 20.0 | 14.0723 | – |
| 75 DXA −1.5 repeat 5 | 58 020 | 32.9 | 20.8 | 14.0575 | – |
| 60 DXA −2.0 repeat 5 | 58 150 | 32.9 | 19.7 | 14.0781 | – |
| 55 DXA −2.0 repeat 10 | 58 180 | 31.9 | 19.5 | 14.0833 | 94 210 |
| 70 DXA −1.5 repeat 5 | 58 190 | 34.3 | 20.3 | 14.0648 | – |
| 55 DXA −2.0 repeat 5 | 58 380 | 32.8 | 19.4 | 14.0842 | 217 980 |
| 65 DXA −1.5 repeat 5 | 58 490 | 35.1 | 19.9 | 14.0736 | – |
| 60 DXA −1.5 repeat 5 | 58 770 | 35.5 | 19.6 | 14.0796 | – |
| 55 DXA −1.5 repeat 10 | 58 970 | 34.8 | 19.3 | 14.0863 | 278 530 |
| 55 DXA −1.5 repeat 5 | 59 170 | 35.5 | 19.3 | 14.0866 | 696 710 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; NOF = National Osteoporosis Foundation; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are ranked in order of increasing costs. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Percentage of individuals ever taking bisphosphonate treatment during lifetime.
Percentage of individuals sustaining a hip fracture during lifetime.
Dashes represent dominated strategies. Dominated strategies are strategies that were found to be less efficacious and more expensive than another strategy (strict dominance) or to have an incremental cost-effectiveness ratio that is greater than that of the next, more effective, and more expensive alternative (extended dominance).
Appendix Table 4.
Sensitivity Analysis Results for Best Strategies Model; 50% Higher Fracture Rates*
| Screening Strategy | Lifetime Cost, $† |
Quality- Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|
| SCORE −2.5 initiated at age 60 y with rescreening every 10 y | 61 320 | 13.9307 | NA |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 61 370 | 13.9404 | 5770 |
| SCORE −2.5 initiated at age 55 y with rescreening every 5 y | 61 420 | 13.9436 | 12 880 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 61 430 | 13.9445 | 17 560 |
| DXA −2.0 initiated at age 55 y with rescreening every 10 y | 61 810 | 13.9534 | 42 070 |
| DXA −2.0 initiated at age 55 y with rescreening every 5 y | 62 000 | 13.9554 | 99 760 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 62 500 | 13.9597 | 113 330 |
| DXA −1.5 initiated at age 55 y with rescreening every 5 y | 62 680 | 13.9611 | 134 030 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Appendix Table 5.
Sensitivity Analysis Results for Best Strategies Model; 50% Lower Fracture Rates*
| Screening Strategy | Lifetime Cost, $† |
Quality- Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|
| No screening | 52 540 | 14.1859 | NA |
| SCORE −2.5 initiated at age 80 y with rescreening every 5 y | 52 710 | 14.1935 | 21 260 |
| SCORE −2.5 initiated at age 70 y with rescreening every 10 y | 52 930 | 14.2008 | 31 170 |
| QUS −1.0 initiated at age 60 y with rescreening every 10 y | 53 090 | 14.2042 | 46 380 |
| SCORE −2.5 initiated at age 60 y with rescreening every 10 y | 53 190 | 14.2061 | 48 990 |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 53 400 | 14.2088 | 79 810 |
| SCORE −2.5 initiated at age 55 y with rescreening every 5 y | 53 510 | 14.2097 | 127 650 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 53 550 | 14.2099 | 151 080 |
| DXA −2.0 initiated at age 55 y with rescreening every 10 y | 54 140 | 14.2121 | 269 880 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 55 050 | 14.2132 | 831 330 |
| DXA −1.5 initiated at age 55 y with rescreening every 5 y | 55 280 | 14.2133 | 2 849 790 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Appendix Table 6.
Sensitivity Analysis Results for Best Strategies Model; 70% Bisphosphonate Adherence*
| Screening Strategy | Lifetime Cost, $† |
Quality- Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|
| SCORE −2.5 initiated at age 80 y with rescreening every 5 y | 57 280 | 14.0624 | NA |
| SCORE −2.5 initiated at age 70 y with rescreening every 10 y | 57 300 | 14.0776 | 1220 |
| SCORE −2.5 initiated at age 60 y with rescreening every 10 y | 57 390 | 14.0902 | 6920 |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 57 560 | 14.0989 | 20 040 |
| SCORE −2.5 initiated at age 55 with rescreening every 5 y | 57 640 | 14.1021 | 23 530 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 57 660 | 14.1028 | 28 470 |
| DXA −2.0 initiated at age 55 y with rescreening every 10 y | 58 290 | 14.1096 | 93 940 |
| DXA −2.0 initiated at age 55 y with rescreening every 5 y | 58 550 | 14.1107 | 229 760 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 59 330 | 14.1125 | 415 750 |
| DXA −1.5 initiated at age 55 y with rescreening every 5 y | 59 590 | 14.1130 | 535 040 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Appendix Table 7.
Sensitivity Analysis Results for Best Strategies Model; High Costs*
| Screening Strategy | Lifetime Cost $† |
Quality- Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|
| SCORE −2.5 initiated at age 80 y with rescreening every 5 y | 70 500 | 14.0478 | NA |
| SCORE −2.5 initiated at age 70 y with rescreening every 10 y | 70 560 | 14.0594 | 5460 |
| QUS −1.0 initiated at age 60 y with rescreening every 10 y | 70 610 | 14.0655 | 7440 |
| SCORE −2.5 initiated at age 60 y with rescreening every 10 y | 70 650 | 14.0689 | 13 020 |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 70 830 | 14.0750 | 30 290 |
| SCORE −2.5 initiated at age 55 y with rescreening every 5 y | 70 920 | 14.0774 | 37 110 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 70 960 | 14.0780 | 53 260 |
| DXA −2.0 initiated at age 55 y with rescreening every 10 y | 71 660 | 14.0833 | 131 410 |
| DXA −2.0 initiated at age 55 y with rescreening every 5 y | 71 930 | 14.0843 | 298 520 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 72 760 | 14.0864 | 393 380 |
| DXA −1.5 initiated at age 55 y with rescreening every 5 y | 73 050 | 14.0867 | 911 200 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Appendix Table 8.
Sensitivity Analysis Results for Best Strategies Model; 100 Times Adverse Drug Event Rates*
| Screening Strategy | Lifetime Cost $† |
Quality- Adjusted Life-Years Accrued |
Incremental Cost- Effectiveness Ratio† |
|---|---|---|---|
| SCORE −2.5 initiated at age 80 y with rescreening every 5 y | 57 360 | 14.0424 | NA |
| SCORE −2.5 initiated at age 75 y with rescreening every 5 y | 57 390 | 14.0451 | 6 130 |
| QUS −1.0 initiated at age 70 y with rescreening every 5 y | 57 440 | 14.0476 | 19 170 |
| SCORE −2.5 initiated at age 70 with rescreening every 5 y | 57 470 | 14.0488 | 22 570 |
| QUS −1.0 initiated at age 65 y with rescreening every 5 y | 57 530 | 14.0510 | 26 940 |
| QUS −0.5 initiated at age 65 y with rescreening every 5 y | 57 550 | 14.0517 | 28 830 |
| QUS −1.0 initiated at age 60 y with rescreening every 5 y | 57 590 | 14.0529 | 37 540 |
| QUS −1.0 initiated at age 55 y with rescreening every 5 y | 57 710 | 14.0554 | 45 860 |
| DXA −2.5 initiated at age 55 y with rescreening every 5 y | 57 820 | 14.0570 | 69 540 |
| DXA −2.0 initiated at age 55 y with rescreening every 10 y | 58 210 | 14.0588 | 225 590 |
| DXA −1.5 initiated at age 55 y with rescreening every 10 y | 58 820 | 14.0588 | 7 327 770 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Strategies are described in Table 1. Costs are expressed in 2010 $US, and incremental cost-effectiveness ratios represent cost per quality-adjusted life-year gained for each strategy compared to the next less costly nondominated strategy.
Costs and incremental cost-effectiveness ratios are rounded off to the nearest 10.
Appendix Table 9.
Probabilistic Sensitivity Analysis Results for Best Strategies Model
| Screening Strategy | Probability Most Cost-Effective | |
|---|---|---|
| Willingness to Pay $50 000/QALY |
Willingness to Pay $100 000/QALY |
|
| No screening | 0.002 | 0.000 |
| 65 DXA −2.5 repeat 5 | 0.016 | 0.014 |
| 65 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 0.012 | 0.002 |
| 80 SCORE prescreen (DXA −2.5) repeat 5 | 0.002 | 0.002 |
| 60 DXA −2.5 repeat 5 | 0.070 | 0.040 |
| 70 DXA −2.5 repeat 5 | 0.008 | 0.002 |
| 75 DXA −2.5 repeat 5 | 0.000 | 0.000 |
| 80 DXA −2.5 repeat 5 | 0.002 | 0.000 |
| 55 SCORE prescreen (DXA −2.5) repeat 5 | 0.068 | 0.058 |
| 55 DXA −2.5 repeat 5 | 0.072 | 0.062 |
| 55 DXA −2.5 repeat 10 | 0.062 | 0.036 |
| 60 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 0.030 | 0.028 |
| 60 SCORE prescreen (DXA −2.5) repeat 10 | 0.044 | 0.016 |
| 75 DXA −2.5 repeat 10 | 0.006 | 0.002 |
| 75 SCORE prescreen (DXA −2.5) repeat 5 | 0.018 | 0.002 |
| 70 DXA −2.5 repeat 10 | 0.004 | 0.000 |
| 55 SCORE prescreen (DXA −2.5) repeat 10 | 0.046 | 0.026 |
| 55 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 0.030 | 0.024 |
| 55 DXA −1.5 repeat 10 | 0.022 | 0.068 |
| 55 DXA −2.0 repeat 10 | 0.082 | 0.108 |
| 55 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 0.060 | 0.052 |
| 55 DXA −2.0 repeat 5 | 0.044 | 0.104 |
| 55 DXA −1.5 repeat 5 | 0.012 | 0.080 |
| 60 DXA −1.5 repeat 5 | 0.012 | 0.024 |
| 60 DXA −2.0 repeat 10 | 0.042 | 0.080 |
| 60 DXA −2.0 repeat 5 | 0.018 | 0.038 |
| 60 DXA −2.5 repeat 10 | 0.046 | 0.014 |
| 55 SCORE prescreen (DXA −2.5) one time | 0.010 | 0.010 |
| 60 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 0.020 | 0.008 |
| 65 DXA −2.0 repeat 5 | 0.010 | 0.020 |
| 65 DXA −1.5 repeat 5 | 0.000 | 0.010 |
| 65 QUS prescreen threshold −0.5 (DXA −2.5) repeat 5 | 0.026 | 0.012 |
| 65 QUS prescreen threshold −1 (DXA −2.5) repeat 10 | 0.020 | 0.010 |
| 65 DXA −2.0 repeat 10 | 0.004 | 0.018 |
| 65 DXA −2.5 repeat 10 | 0.020 | 0.008 |
| 70 DXA −2.0 repeat 5 | 0.002 | 0.000 |
| 70 DXA −1.5 repeat 5 | 0.000 | 0.000 |
| 65 QUS prescreen threshold −1 (DXA −2.5) one time | 0.004 | 0.002 |
| 70 DXA −2.5 one time | 0.002 | 0.000 |
| 70 SCORE prescreen (DXA −2.5) repeat 10 | 0.008 | 0.000 |
| 70 QUS prescreen threshold −1 (DXA −2.5) repeat 5 | 0.010 | 0.004 |
| 70 DXA −2.0 repeat 10 | 0.008 | 0.004 |
| 70 SCORE prescreen (DXA −2.5) repeat 5 | 0.018 | 0.006 |
| 75 DXA −1.5 repeat 5 | 0.000 | 0.000 |
| 80 SCORE prescreen (NOF) one time | 0.004 | 0.002 |
| 75 DXA −2.5 one time | 0.002 | 0.000 |
| 75 DXA −2.0 repeat 10 | 0.000 | 0.004 |
| 80 DXA −2.0 repeat 5 | 0.000 | 0.000 |
| 80 DXA −2.5 repeat 10 | 0.002 | 0.000 |
| 80 DXA −2.5 one time | 0.000 | 0.000 |
DXA = dual-energy x-ray absorptiometry; NA = not applicable; NOF = National Osteoporosis Foundation; QALY = quality-adjusted life-year; QUS = quantitative ultrasonography; SCORE = Simple Calculated Osteoporosis Risk Estimation.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health.
Potential Conflicts of Interest: Consultancies: S.L. Greenspan (Merck & Co. Inc.); Grants received: S.L. Greenspan (Merck & Co. Inc.).
References
- 1.National Osteoporosis Foundation . About Osteoporosis: Fast Facts. National Osteoporosis Foundation; Washington, DC: 2005. [Google Scholar]
- 2.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–41. doi: 10.7326/0003-4819-137-6-200209170-00015. [PMID: 12230356] [DOI] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [PMID: 17144789] [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services . Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. [Google Scholar]
- 5.Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med. 2006;166:1209–17. doi: 10.1001/archinte.166.11.1209. [PMID: 16772249] [DOI] [PubMed] [Google Scholar]
- 6.Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14:605–15. [PMID: 18778176] [PubMed] [Google Scholar]
- 7.Schousboe JT. Cost-effectiveness modeling research of pharmacologic therapy to prevent osteoporosis-related fractures. Curr Rheumatol Rep. 2007;9:50–6. doi: 10.1007/s11926-007-0022-1. [PMID: 17437668] [DOI] [PubMed] [Google Scholar]
- 8.U.S. Preventive Services Task Force Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526–8. doi: 10.7326/0003-4819-137-6-200209170-00014. [PMID: 12230355] [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services [on 2 March 2011];National Physician Fee Schedule. Accessed at http://www.cms.hhs.gov/PFSlookup/
- 10.Schousboe JT, Ensrud KE, Nyman JA, Melton LJ, 3rd, Kane RL. Universal bone densitometry screening combined with alendronate therapy for those diagnosed with osteoporosis is highly cost-effective for elderly women. J Am Geriatr Soc. 2005;53:1697–704. doi: 10.1111/j.1532-5415.2005.53504.x. [PMID: 16181168] [DOI] [PubMed] [Google Scholar]
- 11.Kraemer DF, Nelson HD, Bauer DC, Helfand M. Economic comparison of diagnostic approaches for evaluating osteoporosis in older women. Osteoporos Int. 2006;17:68–76. doi: 10.1007/s00198-005-1922-4. [PMID: 15889313] [DOI] [PubMed] [Google Scholar]
- 12.Lacroix AZ, Buist DS, Brenneman SK, Abbott TA., 3rd Evaluation of three population-based strategies for fracture prevention: results of the osteoporosis population-based risk assessment (OPRA) trial. Med Care. 2005;43:293–302. doi: 10.1097/00005650-200503000-00012. [PMID: 15725986] [DOI] [PubMed] [Google Scholar]
- 13.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PMID: 8849754] [PubMed] [Google Scholar]
- 14.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [PMID: 9875874] [DOI] [PubMed] [Google Scholar]
- 15.Nayak S, Olkin I, Liu H, Grabe M, Gould MK, Allen IE, et al. Meta-analysis: accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144:832–41. doi: 10.7326/0003-4819-144-11-200606060-00009. [PMID: 16754925] [DOI] [PubMed] [Google Scholar]
- 16.Brenneman SK, Lacroix AZ, Buist DS, Chen YT, Abbott TA., 3rd Evaluation of decision rules to identify postmenopausal women for intervention related to osteoporosis. Dis Manag. 2003;6:159–68. doi: 10.1089/109350703322425509. [PMID: 14570384] [DOI] [PubMed] [Google Scholar]
- 17.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–89. doi: 10.1007/s001980050093. [PMID: 9850356] [DOI] [PubMed] [Google Scholar]
- 18.Mobley LR, Hoerger TJ, Wittenborn JS, Galuska DA, Rao JK. Cost-effectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate. Med Decis Making. 2006;26:194–206. doi: 10.1177/0272989X06286478. [PMID: 16525173] [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–9. doi: 10.1001/archinte.165.20.2414. [PMID: 16287772] [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [PMID: 17190893] [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, et al. FLEX Research Group Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010;25:976–82. doi: 10.1002/jbmr.11. [PMID: 20200926] [DOI] [PubMed] [Google Scholar]
- 22.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–65. doi: 10.1210/jc.2009-1947. [PMID: 20173017] [DOI] [PubMed] [Google Scholar]
- 23.Fraser LA, Vogt KN, Adachi JD, Thabane L. Fracture risk associated with continuation versus discontinuation of bisphosphonates after 5 years of therapy in patients with primary osteoporosis: a systematic review and meta-analysis. Ther Clin Risk Manag. 2011;7:157–66. doi: 10.2147/TCRM.S19385. [PMID: 21691586] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis: executive summary of recommendations. Endocr Pract. 2010;16:1016–9. doi: 10.4158/ep.16.6.1016. [PMID: 21216723] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [PMID: 7862179] [DOI] [PubMed] [Google Scholar]
- 26.Melton LJ, 3rd, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–9. doi: 10.1007/BF01623271. [PMID: 8481586] [DOI] [PubMed] [Google Scholar]
- 27.Karpf DB, Shapiro DR, Seeman E, Ensrud KE, Johnston CC, Jr, Adami S, et al. Prevention of nonvertebral fractures by alendronate. A meta-analysis. Alendronate Osteoporosis Treatment Study Groups. JAMA. 1997;277:1159–64. [PMID: 9087473] [PubMed] [Google Scholar]
- 28.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [PMID: 7477143] [DOI] [PubMed] [Google Scholar]
- 29.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture Intervention Trial. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jcem.85.11.6953. [PMID: 11095442] [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [PMID: 8950879] [DOI] [PubMed] [Google Scholar]
- 31.Tosteson AN, Jönsson B, Grima DT, O’Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12:849–57. doi: 10.1007/s001980170036. [PMID: 11716188] [DOI] [PubMed] [Google Scholar]
- 32.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PMID: 18274319] [PubMed] [Google Scholar]
- 33.Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15:38–42. doi: 10.1007/s00198-003-1490-4. [PMID: 14593451] [DOI] [PubMed] [Google Scholar]
- 34.Office of Technology Assessment . Hip fracture outcomes in people age 50 and over - background paper. US Congress, Office of Technology Assessment; Washington, DC: Jul, 1994. OTA-BP-H-120. [Google Scholar]
- 35.Corliss GR, Lucas R, Newton M, Tillman K. Long-term care experience committee intercompany study 1984-2001. Society of Actuaries; Nov, 2004. pp. 1–70. 4th report. [Google Scholar]
- 36.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–70. doi: 10.1046/j.1532-5415.2003.51110.x. [PMID: 12588580] [DOI] [PubMed] [Google Scholar]
- 37.Red Book. Thomson Reuters; Montvale, NJ: 2010. [Google Scholar]
- 38.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–30. doi: 10.1007/s001980200033. [PMID: 12035765] [DOI] [PubMed] [Google Scholar]
- 39.GE Financial Nursing Home Cost of Care Survey. GE Financial Assurance Holdings Inc.; Richmond, VA: 2003. [Google Scholar]
- 40. [on 16 December 2010];Walmart Pharmacy Medication Finder. Accessed at http://medicationfinder.walmart.com/mapd/MedicationFinder.jsp.
- 41.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [PMID: 16855127] [DOI] [PubMed] [Google Scholar]
- 42.Brazier JE, Green C, Kanis JA, Committee Of Scientific Advisors International Osteoporosis Foundation A systematic review of health state utility values for osteoporosis-related conditions. Osteoporos Int. 2002;13:768–76. doi: 10.1007/s001980200107. [PMID: 12378365] [DOI] [PubMed] [Google Scholar]
- 43.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15:1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [PMID: 10893688] [DOI] [PubMed] [Google Scholar]
- 44.Dolan P, Torgerson D, Kakarlapudi TK. Health-related quality of life of Colles’ fracture patients. Osteoporos Int. 1999;9:196–9. doi: 10.1007/s001980050136. [PMID: 10450406] [DOI] [PubMed] [Google Scholar]
- 45.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [PMID: 8483408] [DOI] [PubMed] [Google Scholar]
- 46.Brazier JE, Kohler B, Walters S. A prospective study of the health related quality of life impact of hip fracture. ScHARR, University of Sheffield; Sheffield: 2000. [Google Scholar]
- 47.Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15:20–6. doi: 10.1007/s00198-003-1463-7. [PMID: 14593450] [DOI] [PubMed] [Google Scholar]
- 48.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PMID: 19788058] [PubMed] [Google Scholar]
- 49.Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445–8. [PMID: 2818106] [PubMed] [Google Scholar]
- 50.Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52:243–9. doi: 10.1016/s0895-4356(98)00167-x. [PMID: 10210242] [DOI] [PubMed] [Google Scholar]
- 51.National Osteoporosis Foundation . Clinician’s Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC: 2008. [Google Scholar]
- 52.Geusens P, Hochberg MC, van der Voort DJ, Pols H, van der Klift M, Siris E, et al. Performance of risk indices for identifying low bone density in postmenopausal women. Mayo Clin Proc. 2002;77:629–37. doi: 10.4065/77.7.629. [PMID: 12108600] [DOI] [PubMed] [Google Scholar]
- 53.Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP, Canadian Multicentre Osteoporosis Study Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA. 2001;286:57–63. doi: 10.1001/jama.286.1.57. [PMID: 11434827] [DOI] [PubMed] [Google Scholar]
- 54.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. Osteoporotic Fractures Research Group BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–54. doi: 10.1359/jbmr.2003.18.11.1947. [PMID: 14606506] [DOI] [PubMed] [Google Scholar]
- 55.Schousboe JT, Nyman JA, Kane RL, Ensrud KE. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med. 2005;142:734–41. doi: 10.7326/0003-4819-142-9-200505030-00008. [PMID: 15867405] [DOI] [PubMed] [Google Scholar]
- 56.Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Introduction. Osteoporos Int. 1998;8(Suppl 4):S7–80. [PMID: 10197173] [PubMed] [Google Scholar]
- 57.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–9. doi: 10.1007/s00198-004-1780-5. [PMID: 15616758] [DOI] [PubMed] [Google Scholar]
- 58.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Hip Intervention Program Study Group Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [PMID: 11172164] [DOI] [PubMed] [Google Scholar]
- 59.North American Menopause Society Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause. 2006;13:340–67. doi: 10.1097/01.gme.0000222475.93345.b3. [PMID: 16735931] [DOI] [PubMed] [Google Scholar]
- 60.American College of Obstetricians and Gynecologists. Women’s Health Care Physicians ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 50, January 2003. Obstet Gynecol. 2004;103:203–16. [PMID: 14704265] [PubMed] [Google Scholar]
- 61.Lu Y, Genant HK, Shepherd J, Zhao S, Mathur A, Fuerst TP, et al. Classification of osteoporosis based on bone mineral densities. J Bone Miner Res. 2001;16:901–10. doi: 10.1359/jbmr.2001.16.5.901. [PMID: 11341335] [DOI] [PubMed] [Google Scholar]
- 62.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–61. doi: 10.4065/80.7.856. [PMID: 16007889] [DOI] [PubMed] [Google Scholar]
- 63.U.S. Consumer Price Index for Medical Care for All Urban Consumers. U.S. Department of Labor Bureau of Labor Statistics; [Google Scholar]
- 64.Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ., 3rd Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12:1042–9. doi: 10.1007/s001980170015. [PMID: 11846331] [DOI] [PubMed] [Google Scholar]