Abstract
Cytomegalovirus (CMV) infection enhances expression of several cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), granulocyte macrophage colony-stimulating factor (GM-CSF), and IL-8, to the benefit of virus replication and dissemination. However, the stimulus for certain cytokine production remains unclear. CMV encodes a series of proteins that alter and/or mimic functions of leukocyte migration, activation, and cytokine responses. Our study revealed that human CMV (HCMV)-encoded UL128 protein, which contains signal peptides and has similar amino acid sequences to the CC chemokine, recruits monocytes as human β chemokine (microphage inflammatory protein 1α). Using RNA interference technology, we constructed an HCMV (UL128+/UL128−)-infected tissue cell (MRC-5) and peripheral blood mononuclear cell (PBMC) co-culture system. We measured 6 cytokine levels (IL-2, IL-4, IL-6, IL-10, TNF-α, and interferon-γ [IFN-γ]) in the supernatant, and found significantly elevated IL-6 and elevated TNF-α levels in the HCMV UL128+-infected group. Conversely, we observed decreased levels in the UL128-knockout supernatant. PBMCs presented with UL128 (50 ng/mL) demonstrated better cell viability than the UL128-absent group. Finally, the MAPK/ERK pathway was found to be involved in UL128 induction of cell proliferation. Selective induction of cytokine expression indicates that HCMV-encoded UL128 is a potent inducer of several inflammatory mediators.
Introduction
Human cytomegalovirus (HCMV) is a widespread herpesvirus that causes many infection-related birth defects, and may trigger severe disease in immunocompromised individuals (1,2). Recent work has revealed the potential role of HCMV in the development of various inflammatory diseases, such as vascular diseases, autoimmune diseases, hepatitis, interstitial pneumonia, gastrointestinal disease, and atherosclerosis (3,4). During the early stage of virus infection, host inflammatory cells such as leukocytes and monocytes may migrate to and infiltrate organ tissues (5). The interplay between local tissue cells and inflammatory cells may be the initial trigger of HCMV-induced pathology.
After replicating in host cells and establishing lifelong infection, HCMV has evolved multiple strategies to interact with the host immune system (6). HCMV immune modulatory gene products are potential modifiers of the host immune system (7,8). Many of these genes are duplicated by the host during the long process of co-evolution between virus and host. These gene products include G protein-coupled receptors, MHC-I molecules, and chemokines, which provide a rich microenvironment that leads to increased virus replication and dissemination (9).
Chemokines are a group of small molecules with chemotactic properties that recruit inflammatory cells to sites of inflammation, and thereby play a vital role in the host immune system (6). Chemokine domains are defined by the presence of four cysteines in highly-conserved positions. The four kinds of chemokines (C, CC, CXC, and CX3C) are classified based on the arrangement of 1 or 2 N-terminal cysteine residues (10). HCMV encodes a UL128 protein containing signal peptides that has similar amino acid sequences to the CC chemokine (Table 1). UL128 has been shown to be involved in HCMV entry into epithelial and endothelial cells by binding with membrane protein gH/gL (11). The complex of UL128-131A has been recognized as a determinant of infection in monocytes (4,12). The precise function of UL128 on host immune cells remains unknown. Recent evidence shows that HCMV may upregulate cellular gene products associated with monocyte extravasation and inflammation, such as adhesion molecules, inflammatory cytokines, chemokines, and chemokine receptors in a PI(3)K-dependent manner (13,14). Our objective was to determine if HCMV-encoded UL128 interferes with the initiating steps in the host immune response, or if it modulates the function of host inflammatory cells. First we constructed soluble recombinant UL128 protein to confirm that UL128 has the potential to recruit peripheral blood mononuclear cells (PBMCs) in vitro. Next we created an HCMV-infected tissue cell and PBMC co-culture system. We then compared cytokine levels in samples infected with HCMV (UL128+) and HCMV (UL128−). Additional impacts of UL128 on cell proliferation and the related transduction pathway were also assessed in these experiments.
Table 1.
Comparison of N-terminal Amino Acid Sequence Between Human β Chemokine MIP-1α and Different Species of CMV
 |
CCMV, chimpanzee cytomegalovirus; SCMV, simian cytomegalovirus. The four cysteine residues (red) characteristic of β-chemokine are in box.
Materials and Methods
Expression of recombinant UL128 protein
The UL128 gene sequence position 1243–2003 was selected from the GenBank (accession no. GU574790.1), and the gene product was cloned using the pIRES-AcGFP vector (Qiagen, Valencia, CA), with a 6-histag fused to its N-terminus. Recombinant UL128 protein was obtained through His·Bind purification columns (Novagen, Darmstadt, Germany). The specific UL128 polyclonal antibody was collected from immunized New Zealand rabbits.
Chemotaxis assay
Human recombinant chemokine macrophage inflammatory protein-1α (MIP-1α; R&D Systems, Minneapolis, MN) dissolved in RPMI 1640 was used as a positive control. Cell migration was evaluated by a 24-well Boyden chamber with 5-μm pore size polycarbonate filters. Each sample was assayed in triplicate, and the number of cells that migrated to the lower chamber was counted in five visual fields for each well. Data are shown as means for three replicates±standard deviation (SD).
Western blot analysis
Western blot analysis was carried out using 6× His Rabbit Polyclonal Antibody (cat. no. S2917; Epitomics, Burlingame, CA) to confirm the presence of UL128 protein. The purified recombinant UL128-histag from SDS PAGE was transferred to a PVDF membrane. The membrane was blocked in 5% defatted milk dissolved in PBST (PBS containing 0.5% Tween-20) with gentle shaking at room temperature for 2 h. The membrane was incubated in a 1:1000 dilution of rabbit anti-his polyclonal antibody in blocking buffer with gentle shaking at room temperature for 1 h, then at 4°C overnight. After washing with PBST three times for 10 min each, the membrane was incubated in a 1:2000 dilution of sheet anti-rabbit IgG horseradish peroxidase (HRP) conjugated as secondary antibody (cat. no. 3053-1; Epitomics) in blocking buffer, with gentle shaking at room temperature for 1 h.
Cell lines and virus infection
A human embryonic fibroblast cell line (MRC-5) was obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in an incubator with a humidified atmosphere containing 5% CO2 at 37°C and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 4.5 g/L glucose, 1% penicillin/streptomycin, and 10% fetal bovine serum. Culture media was changed every 24 h. Cells were passaged every 2 or 3 d when they grew a full monolayer. The HCMV strain AD169 was used throughout the entire experiment. Viral titers were determined by cytopathic effect on MRC-5 cells. TCID50 (the highest dilution of virus that caused the death of 50% of the cells) was used in this experiment.
UL128 gene knockout
RNA interference
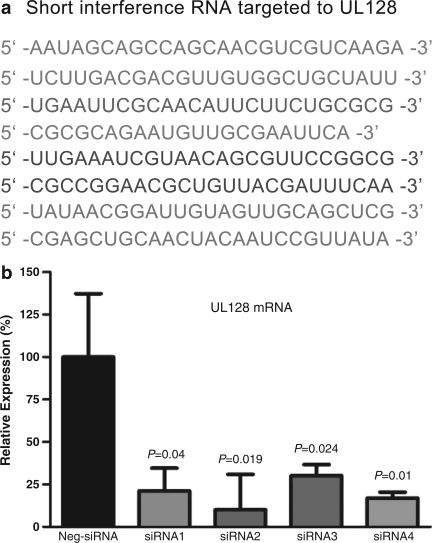
Four duplexes of UL128 small interference RNA (siRNA) were designed and synthesized by Invitrogen. Negative control siRNA (Stealth™; Invitrogen, Carlsbad, CA) was transfected into MRC-5 cells using Lipofectamin™ RNAiMAX (Invitrogen), according to the protocols provided by the manufacturer. A fluorescein-labeled, double-stranded RNA duplex with the same length, charge, and configuration as standard siRNA was used to assess the transfection efficiency. To verify the result of UL128 gene knockout, UL128 mRNA levels were determined using real-time polymerase chain reaction (PCR), and the most efficient short interference RNA duplex was selected for the follow-up experiments (Fig. 1). MRC-5 cells (1×106/well) were seeded in 24-well plates and starved for 24 h before siRNA transfection. RNA duplexes were transfected with 1 μL lipofectamine per well and incubated at 37°C for 6 h. Cell monolayers were washed once with DMEM, then replaced with fresh medium. The efficacy of transfection was assessed using fluorescence microscopy. Cells were inoculated with HCMV at TCID50 and the virus was allowed to absorb for 120 min at 37°C, and then viral inoculum medium was replaced by fresh maintenance media. To confirm whether the suppression of UL128 mRNA in MRC-5 cells indeed affected UL128 protein expression, membrane proteins were extracted 24 h post-infection from UL128-knockout cells and HCMV-normal infected cells.
FIG. 1.
Knockout of UL128 by short interference RNA (siRNA). A significant reduction of the UL128 transcript relative to a non-targeting control siRNA was detected in MRC-5 cells transfected with the above siRNA sequences shown in (a). (b) Fold differences and subsequent percent gene expression levels were calculated using the comparative CT (2−▵▵CT) method. Data were analyzed using one-way analysis of variance for comparison among groups, followed by pairwise comparisons using Tukey 95% confidence intervals.
Quantitative real-time PCR
MRC-5 with or without siRNA was infected with HCMV at the same time and total RNA was extracted using the RNeasy kit (Qiagen). Total RNA (2 mg) was reverse transcribed to cDNA using the Fermentas First Strand Synthesis kit (Invitrogen) with oligo-dT primer. In the real-time PCR step, reactions were performed in triplicate with 1 mg cDNA per reaction. Primers specific for UL128 were designed using Primerdesign software and synthesized by Shanghai Sangon Biological Engineering Technology & Services Corporation, Shanghai, P.R. China. UL128 mRNA copies were measured by the SYBR Premix Ex Taq™ kit (cat. no. DRR420; TaKaRa, Shiga, Japan) using the ABI 7500 real-time PCR system. The UL128 overlapping primers were as follows: forward primer 5′-GCTGAGATTCGCGGGATCGT-3′; reverse primer 5′-GTACTGCGCCTTGTCGTTCA-3′. GAPDH was used as an endogenous control: forward primer 5′-GAAGGTGAAGGGTCGGAGTC-3’; reverse primer 5′-GAAGATGGTGATGGGATTC-3′. The reactions were performed at 95°C for 5 min, followed by 35 cycles of PCR (94°C, 15 sec; 60°C, 30 sec). The relative changes in gene expression were calculated using the following formula: fold change in gene expression, 2–▵▵Ct=2–{▵Ct(siRNA)–▵Ct(control)}, where ▵Ct=Ct(UL128 or control) – Ct(GAPDH), and Ct represents threshold cycle number.
Flow cytometric (FCM) analysis
PBMCs (2×105 cells/well) co-cultured with MRC-5 cells were infected with HCMV (UL128+/UL128−). Supernatants harvested at different time points (18, 24, 48, 72, and 96 h) were centrifuged for 5 min at 1000 g before cytokine detection. The levels of the cytokines IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α were determined in 50 μL of washout medium, using the Cytometric Bead Array Human Th1/Th2 Cytokine Kit II, purchased from BD Pharmingen (San Jose, CA) (cat. no. 551809). Cytokine titers were expressed as pg/mL, as calculated by reference to standard curves constructed with known amounts of recombinant cytokines.
Cell proliferation and activation of signal transduction pathways
PBMCs (2×104/well) were seeded in 96-well plates. The vitality of PBMCs with or without UL128 was measured using MTT cell proliferation and the Cytotoxicity Detection Kit (cat. no. KGA311; KeyGEN, Shanghai, P.R. China). PBMCs (2×106/well) were stimulated with UL128 at 50 ng/mL for 30 min, 60 min, and 24 h at 37°C, and lysed in 2× RIPA buffer containing protease and phosphatase inhibitors. Then 20 μg of total protein was run on a 10% SDS PAGE and immunoblotted with antibodies against phosphorylated ERK, and ERK.
Statistical analyses
Results from real-time PCR and FCM were derived from at least three independent experiments. Statistical significance between siRNA and control groups was evaluated with one-way analysis of variance (ANOVA), followed by the Dunnett post-hoc test using Prism3 GraphPad software. Significance level was calculated using an assigned confidence interval of 95%, and p<0.05 was considered significant.
Results
Expression, purification, and identification of the recombinant UL128 protein
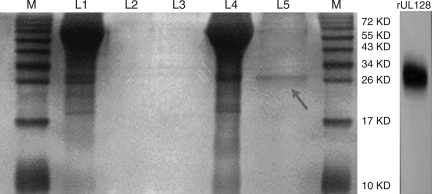
E. coli DH5α transformed with pIRES-AcGFP-UL128 was cultured in LB medium supplemented with 50 μg/mL kanamycin for growth at 37°C until the logarithmic phase (at an OD 600 of 0.8), and induced by isopropyl-b-D-thiogalactoside (IPTG) at a final concentration of 1.0 mM for 4 h. Purification of the His-tagged UL128 protein was performed with a Ni2+-NTA resin column. The solubility of the recombinant UL128 protein was performed by SDS-PAGE and silver stain analysis (Fig. 2). To confirm the presence of the UL128-His protein, Western blotting was performed using anti-His antibody.
FIG. 2.
The silver stain of isolated proteins. (M, marker; lane 1, the lysate of CHO transfected with empty vector pIRES-AcGFP; lane 2, elute from the lysate of CHO transfected with empty vector pIRES-AcGFP; lane 3, blank; lane 4, the lysate of CHO transfected with pIRES-AcGFP-UL128; lane 5, elute from the lysate of CHO transfected with pIRES-AcGFP-UL128).
Recombinant UL128 can recruit PBMCs in vitro
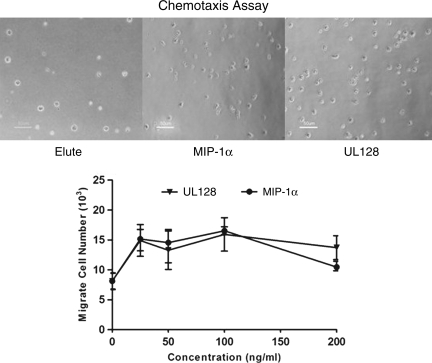
We tested the ability of UL128 to induce migration of human PBMCs using a Boyden chamber, and compared the results with human MIP-1α protein, which is a member of β-chemokine. We found that recombinant UL128 is able to recruit PBMCs equally compared to MIP-1α protein in vitro (Fig. 3).
FIG. 3.
Recombinant UL128 recruits peripheral blood mononuclear cells (PBMCs) in vitro. Migration assays were conducted by adding human PBMCs to the upper chamber of a 24-well plate with a pore size of 5 μm. The lower chamber was loaded with increasing concentrations of chemokine. The chemotactic index was calculated by the number of migrated cells divided by the random migration±standard deviation. Data were collected from three independent experiments (MIP-1α, macrophage inflammatory protein-1α).
UL128 treatment or HCMV infection of PBMCs increases cytokine release
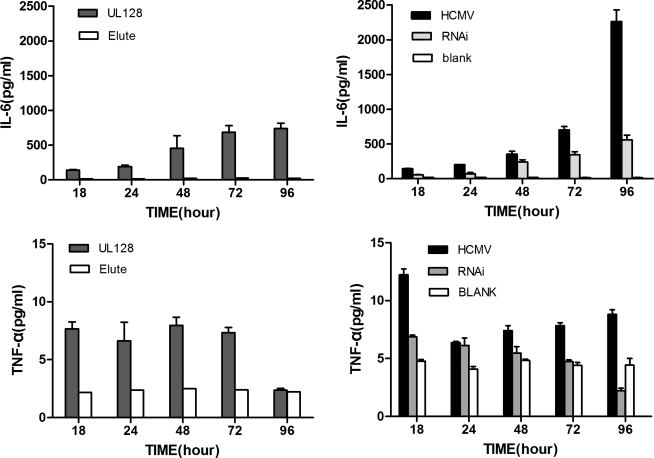
To assess cytokine production, supernatants from PBMC culture medium in the presence or absence of UL128 were collected at different time points (Table 2). IL-6 and TNF-α expression were increased in the UL128-treated group as well as the HCMV-infected group. Levels of TNF-α peaked at 18 h post-infection, and then gradually decreased afterwards, while levels of IL-6 continued to increase over time. Knockout of UL128 led to decreasing levels of IL-6 and TNF-α (Fig. 4). Expression of IL-2 was observed to be a slightly elevated in the HCMV/MRC-5/PBMC supernatant compared to the control group (MRC-5/PBMC, p=0.02), but no significant difference was observed between the UL128-treated group (UL128/PBMC) and the control supernatant. Levels of IL-4, IL-10, and IFN-γ were found to be in the normal range in all experiment groups.
Table 2.
Supernatant Levels of IL-6/TNF-α in the Different Experimental Groups
| |
IL-6 |
||||
|---|---|---|---|---|---|
| Group | 18 h | 24 h | 48 h | 72 h | 96 h |
| Negative control | 14.7±2.4 | 16.1±2.5 | 16.1±1.7 | 18.8±2.0 | 24.6±6.8 |
| HCMV (UL128+) | 130.9±14.8ns | 200.9±14.8** | 387.8±69.4*** | 721.0±68.1*** | 2075.7±168.2*** |
| HCMV (UL128−) | 57.9±11.2ns | 90.0±11.1ns | 224.93±23.0** | 369.2±26.7*** | 625.1±79.2*** |
| |
TNF-α |
||||
|---|---|---|---|---|---|
| Group | 18 h | 24 h | 48 h | 72 h | 96 h |
| Negative control | 4.0±1.4 | 3.7±1.0 | 3.9±1.2 | 4.0±1.4 | 3.9±1.5 |
| HCMV (UL128+) | 11.2±1.4*** | 7.9±2.2** | 8.6±2.3** | 4.0±2.3ns | 8.2±0.6** |
| HCMV (UL128−) | 6.1±1.0*** | 6.1±0.9ns | 5.7±0.6ns | 4.5±0.5ns | 2.8±0.7*** |
p<0.05; **p<0.01; ***p<0.001; ns, not significant.
Cytokine levels are shown as mean pg/mL±standard deviation; p values of negative controls versus HCMV (UL128+), and HCMV (UL128+) versus HCMV (UL128−) are shown.
HCMV, human cytomegalovirus; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
FIG. 4.
UL128 treatment or human cytomegalovirus (HCMV) infection of peripheral blood mononuclear cells (PBMCs) increases levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). PBMCs were pre-treated with 50 ng/mL UL128 or elute from CHO transfected with empty vector pIRES-AcGFP. HCMV infection group: MRC-5 cells were cultured in 24-well plates and infected with HCMV. PBMCs were then co-cultured with MRC-5 cells. RNAi group: 100 nM of UL128 siRNA duplexes were transfected into MRC-5 cells, and seeded with HCMV, and lastly co-cultured with PBMCs. Blank: MRC-5 cells and a PBMC co-culture system without HCMV infection. Supernatants from these groups were collected at 18, 24, 48, 72, and 96 h, and cytokine levels were determined by flow cytometry. Data are representative of at least 3 independent experiments.
UL128 promotes PBMC proliferation by activating the MAPK/ERK signaling pathway
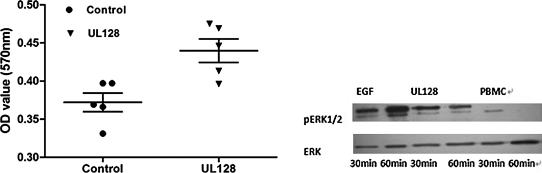
Both UL128-treated (50 ng/mL) and UL128-untreated PBMCs were cultured for 72 h. Cell proliferation measurements were then performed by MTT assay. Equivalent endothelial growth factor (EGF) served as a positive control. Significant differences were found between the UL128/EGF-treated group and the negative control group (p<0.001). The MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway) pathway plays an important role in the transmission of cell signals to the cell nucleus that influence the expression of genes regulating cell growth, proliferation, and apoptosis (15). We examined the effects of UL128 on MAPK activity by measuring phosphorylation of extracellular signal-regulated kinase (pERK1/2). Western blot analysis showed that treatment with UL128 led to an increase in ERK1/2 phosphorylation of PBMCs (Fig. 5).
FIG. 5.
UL128 promotes PBMC proliferation through the MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase signaling) pathway. Peripheral blood mononuclear cells (PBMCs) cultured in the presence or absence of UL128 (50 ng/mL) for 72 h were analyzed by a MTT Cell Proliferation Assay Kit. A significant difference was found between the UL128-treated group and the negative control group (p<0.001). All the data shown represent the mean±standard deviation. Western blot analysis was done to detect ERK and pERK1/2 activation using antibody that specifically recognizes ERK and phosphorylated forms of ERK1/2. Lysates made from PBMCs (2×106cells/mL in the presence of 1% fetal bovine serum) were incubated for 30 and 60 min with UL128 (50 ng/mL). Endothelial growth factor (EGF) treatment of PBMCs and isolated PBMCs cultured for 30 or 60 min served as positive and negative controls, respectively (OD, optical density).
Discussion
There are two general strategies that CMV employs to modulate the host immune response. First, CMV alters cell function by infecting or modulating the cells with which they interact. Second, CMV promotes the secretion of cytokines and chemokines that act upon these cells. Chronic infection caused by this virus may induce a microenvironment called “smoldering inflammation,” which may correlate with the development of many inflammatory disorders (16). CMV infection increases the expression of cytokines such as TNF-α, IL-6, and IL-8 (17,18). Observations of clinical samples showed that the increased serum levels of TNF-α seen in patients with sepsis and atopic dermatitis were related to activation of latent HCMV (19,20). Expression of IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) are involved in HCMV-mediated angiogenesis, and may contribute to the development of HCMV-associated vascular diseases (21). These studies indicate that inflammation may play a central role in the pathogenesis of HCMV, and reactivation of HCMV is apparently dependent on immune activation. The precise stimuli for these cytokine elevations remain unclear.
Several viral genes have been found to be involved in host immune modulation. The HCMV UL146 gene produces a viral chemokine (vCXCL-1), which was shown to bind exclusively to CXCR2, and can induce calcium flux and chemotaxis of neutrophils that may contribute to increased viral dissemination and virulence (22). A previous study showed that the HCMV UL128 gene was highly conserved in all clinical strains (23). The UL128 protein has a similar structure to the CC chemokine, and contains signal peptides that support its potential role in immune modulation.
Based on these previous studies, we analyzed the chemotaxis assay using recombinant UL128 protein, and found that UL128 was able to recruit PBMCs similarly to human MIP-1α protein in vitro. MIP-1α is one of the human CC chemokines that modulates host acute or chronic inflammation by recruiting proinflammatory cells to sites of infection. It is produced by several immune cells, such as lymphocytes, monocytes, macrophages, and neutrophils. It has been theorized that upregulation of monocyte migration and immune mediators by HCMV infection is required for dissemination of the virus, and therefore could promote chronic inflammatory diseases associated with HCMV infection (12). Previous studies have demonstrated the importance of leukocyte infiltration in the initiation of the inflammatory cascade, and the extent of the inflammatory response is an important determinant of the outcome of disease (24). To further explore the impact of UL128 on the host immune response, we compared supernatant cytokine levels of HCMV- and HCMV (UL128 knockout)-infected MRC-5 cells and PBMCs in a co-culture system. Of the 6 cytokines included in this analysis, significantly higher IL-6 and slightly higher TNF-α levels were found in the HCMV (UL128+)-infected group. These levels were decreased in the UL128-knockout supernatant. UL128-treated PBMCs had increased levels of IL-6, as well as enhanced expression of TNF-α. These results suggest that HCMV-encoded UL128 protein could selectively induce cytokine expression, and is involved in the host innate immune response. Upregulation of certain cytokines has been shown to be associated with increased infectivity of virus. A previous study showed that HCMV infection of PDCs could enable NK cells to induce CD69 expression, which resulted in enhanced production of inflammatory cytokines (TNF-α/IFN-α), but impaired NK cells' killing abilities, which facilitated reactivation and replication of HCMV (24). TNF-α has also been proven to induce HCMV IE promoter activity in myeloid cells (4,26). In our virus-infected tissue cell and PBMC co-culture system, both TNF-α and IL-6 expression were triggered by the HCMV-encoded protein UL128, and levels of these proinflammatory cytokines were reduced by knockout of UL128 gene expression.
Proinflammatory cytokines stimulate diverse receptors and can induce cellular proliferation by activation of certain signal transduction pathways. Since UL128 protein has the ability to promote expression of TNF-α and IL-6 in vitro, we examined the impact of UL128 protein on PBMC proliferation, and demonstrated that UL128 (50 ng/mL) presentation to PBMCs induced better cell viability than the UL128-absent group. The MAPK/ERK transduction pathway was found to be involved in this process. More recently, several transduction pathways were found to be closely connected with cytokine-modulated cellular proliferation. In prostate epithelial cells, translocation of NF-κB to the nucleus could be stimulated by the IL-6/ERK transduction pathway, and activation of NF-κB could regulate IL-6 expression (27,28). Another recent study claimed that HCMV-encoded US28 protein increases the secretion of IL-6 via the NF-κB pathway, and induces NIH 3T3 cells to adopt a proliferative phenotype by activating the IL-6-STAT3 axis (29). Based on our study, we deduce that HCMV-encoded UL128 protein may improve PBMC viability through upregulation of IL-6, and thus activates the IL-6/ERK pathway. Sustained activation of PBMCs may contribute to the excessive proinflammatory cytokine production, and may give rise to inflammatory disorders.
In summary, HCMV-encoded UL128 protein acts similarly to the human CC chemokine, both structurally and functionally. UL128 recruits PBMCs to the site of infection, and enhances expression of TNF-α and IL-6, leading to virus replication and dissemination. The MAPK/ERK transduction pathway was involved in UL128 induction of PBMC proliferation, which may facilitate virus latency and cell-cell transference. Selective induction of cytokine expression indicates that HCMV-encoded UL128 protein may act as a potent inducer of several inflammatory mediators, and provides evidence that a specific inflammatory macroenvironment is essential for viral replication and dissemination. Failure to control this process may lead to aggravated inflammation in affected tissues, and induce inflammation disease. As a result, further research should be done to explore the capacity of the inactivation of certain proinflammatory cytokines as a treatment of various inflammatory disorders associated with HCMV infection.
Acknowledgments
We gratefully acknowledge Dr. Jimmy Beck for generous instruction and critical reading of this manuscript. This work was supported by the National Natural Science Foundation of China (grant no. 81071337), and the Natural Science Foundation of Zhejiang Province (grant no. Z2110006).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Onorato IM. Morens DM. Martone WJ. Epidemiology of cytomegaloviral infections: recommendations for prevention and control. Rev Infect Dis. 1985;7:479–497. doi: 10.1093/clinids/7.4.479. [DOI] [PubMed] [Google Scholar]
- 2.Foler KB. Stagno S. Pass RF. Britt WJ. Boll TJ. Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 3.Scalzo AA. Corbett AJ. Rawlinson WD. Scott GM. Degli-Esposti MA. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol Cell Biol. 2007;85:46–54. doi: 10.1038/sj.icb.7100013. [DOI] [PubMed] [Google Scholar]
- 4.Sodergerg-Nauclea C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J. Quyyumi AA. Norman JJE. Csako G. Epstein SE. Cytomegalovirus in the pathogenesis of atherosclerosis: the role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol. 1999;34:1738–1743. doi: 10.1016/s0735-1097(99)00410-6. [DOI] [PubMed] [Google Scholar]
- 6.Crane MJ. Hokeness-Antonelli KL. Salazar-Mather TP. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands. J Immunol. 2009;183:2810–2817. doi: 10.4049/jimmunol.0900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelson S. Consequences of human cytomegalovirus mimicry. Human Immunol. 2004;65:465–475. doi: 10.1016/j.humimm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Loewendorf A. Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J Intern Med. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 10.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 11.Ryckman BJ. Chase MC. Johnson DC. HCMV gH/gL/UL128–131 interferes with virus entry into epithelial cells: Evidence for cell type-specific receptors. Proc Natl Acad Sci USA. 2008;105:14118–14123. doi: 10.1073/pnas.0804365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straschewski S. Patrone M. Walther P. Gallina A. Mertens T. Frascaroli G. Protein pUL128 of human cytomegalovirus is necessary for monocyte infection and blocking of migration. J Virol. 2011;85:5150–5158. doi: 10.1128/JVI.02100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MS. Bentz GL. Smith PM. Bivins ER. Yurochko AD. HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. J Leukoc Biol. 2004;76:65–76. doi: 10.1189/jlb.1203621. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RA. Wang X. Ma XL. Huong SM. Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: Inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshet Y. Seger R. The MAP Kinase Signaling Cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–39. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- 16.Juhn SK. Jung MK. Hoffman MD. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–138. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagneaux L. Delforge A. Snoeck R, et al. Imbalance in production of cytokines by bone marrow stromal cells following cytomegalovirus infection. J Infect Dis. 1996;174:913–919. doi: 10.1093/infdis/174.5.913. [DOI] [PubMed] [Google Scholar]
- 18.Murayama T. Mukaida N. Sadanari H. Yamaguchi N. Khalid SA. The immediate early gene 1 product of human cytomegalovirus is sufficient for up-regulation of interleukin-8 gene expression. Bio Chem Bioph Res Co. 2000;279:298–304. doi: 10.1006/bbrc.2000.3923. [DOI] [PubMed] [Google Scholar]
- 19.Docke WD. Kiessling C. Worm M, et al. Subclinical activation of latent cytomegalovirus (CMV) infection and anti-CMV immune response in patients with atopic dermatitis. Br J Dermatol. 2003;148:954–963. doi: 10.1046/j.1365-2133.2003.05263.x. [DOI] [PubMed] [Google Scholar]
- 20.Kutza AST. Muhl E. Hackstein H. Kirchner H. Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentini S. Luganini A. Dell'Oste V, et al. Human cytomegalovirus productively infects lymphatic endothelial cells and induces a secretome that promotes angiogenesis and lymphangiogenesis through interleukin-6 and granulocyte-macrophage colony-stimulating factor. J Gen Virol. 2011;92:650–660. doi: 10.1099/vir.0.025395-0. [DOI] [PubMed] [Google Scholar]
- 22.Lüttichau HR. The cytomegalovirus UL146 gene product vCXCL1 targets both CXCR1 and CXCR2 as an agonist. J Biol Chem. 2010;285:9137–9146. doi: 10.1074/jbc.M109.002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhengrong S. Yaohua J. Qiang R. Gengfu X. Rong H. Ying Q. Yanping M. Genetic variability of human cytomegalovirus UL131A, UL130, UL128 genes in strains from congenitally infected infants. J Wuhan Univ. 2006;52:783–788. [Google Scholar]
- 24.Miller-Kittrell M. Jiqing S. Penfold M. Richmond A. Sparer TE. Functional characterization of chimpanzee cytomegalovirus chemokine, vCXCL-1CCMV. Virology. 2007;364:454–465. doi: 10.1016/j.virol.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cederarva M. Soderberg-Nauclera C. Odeberg J. HCMV infection of PDCs deviates the NK cell response into cytokine-producing cells unable to perform cytotoxicity. Immunobiology. 2009;214:331–341. doi: 10.1016/j.imbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ritter T. Brandt C. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokines. 2000;12:1163–1170. doi: 10.1006/cyto.2000.0689. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Berriguete G. Prieto A. Fraile B. Bouraoui Y. Relationship between IL-6/ERK and NF-κB: a study in normal and pathological human prostate gland. Eur Cytokine Netw. 2010;21:241–250. doi: 10.1684/ecn.2010.0211. [DOI] [PubMed] [Google Scholar]
- 28.Ruzek MC. Miller AH. Opal SM. Pearce BD. Biron CA. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraile-Ramos A. Lira SA. Söderberg-Nauclér C. Martine J, et al. HCMV-Encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;58:1–10. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]