Abstract
Introduction
Chronic kidney disease (CKD) is a widespread invalidating condition, leading to erythropoietin deficiency and decreased cardiovascular performance. Darbepoetin-α and epoetin-α are extensively used to correct renal anemia. The aim of this study was to evaluate cardiological outcomes in two groups of CKD patients treated with erythropoiesis-stimulating agents (ESA: 20 μg darbepoetin-α weekly vs. 2,000 IU epoetin-α thrice weekly) with an unconventional 1:300 conversion ratio.
Methods
The study was designed as a single center, retrospective, observational study. One hundred stage IV CKD patients were selected. Hemoglobin (Hb), hematocrit, C-reactive protein, pro-brain natriuretic peptide (BNP) and basal echocardiograms were monitored every 3 months.
Results
Darbepoetin-α was significantly more effective in increasing Hb levels after 3 (p < 0.0001), 6 (p < 0.0001), 9 (p < 0.01) and 12 months (p < 0.01) compared to epoetin-α. The optimal Hb target level (11 g/dl < Hb < 12 g/dl) was completely reached after 1 year of treatment with darbepoetin-α and in 70% of the patients treated with epoetin-α (p < 0.01). Cardiovascular performance (left ventricular end-diastolic volume, ejection fraction and pro-BNP) was significantly improved after darbepoetin-α treatment at the 6- and 12-month follow-ups compared to epoetin-α. Discussion: Despite the limitations of a retrospective observational study, these results encourage nephrologists to test the 1:300 darbepoetin/epoetin conversion ratio in ‘easy’ patients, and aggressive protocols for the treatment of anemia in CKD patients are avoided. Darbepoetin-α appeared effective in anemia correction, improving cardiovascular performance in a significantly higher proportion than epoetin. At low doses, on the other hand, it has to be borne in mind that a treatment regimen with only one submaximal administration per week may increase patient compliance and adherence to therapy, explaining in part the observed results.
Key Words: Chronic kidney disease, Darbepoetin-α, Epoetin-α, Hemodialysis
Introduction
Chronic kidney disease (CKD) is a condition affecting public health due to the potential to cause not only progressive loss of kidney function but also major cardiovascular complications, with high costs in terms of invalidity, working capacity and pharmacoeconomics [1, 2].
Continuous nephron loss interacts with the cardiovascular system in many different ways, and progressive erythropoietin (EPO) deficiency and consequent development of anemia is one of the most significant CKD manifestations possibly associated with a worse renal and overall prognosis [3, 4].
Left ventricular hypertrophy, a compensatory response to anemia and hypertension, is very frequently linked to renal disease, with an estimated prevalence of 39% in CKD stages III and IV, and nearly 74% at the start of dialysis [5, 6, 7]. In addition to being a compensatory system, left ventricular hypertrophy is a crucial step in CKD-related cardiovascular remodeling and uremic cardiomyopathy [5, 6, 7]. The nephrologist is then called to prevent it, mainly via correction of anemia and control of blood pressure treatment.
This paper aimed to answer the question of whether erythropoiesis-stimulating agent (ESA) therapy can modify cardiac parameters during a 1-year treatment period.
EPO is involved in cardiac remodeling not only through anemia correction, but also by many pleiotropic effects, typically at increased dosages. To minimize this second role, we enrolled CKD patients treated either with darbepoetin-α or epoetin-α at low, stable doses for 12 months. In a retrospective model, we evaluated the effect of this treatment on renal anemia and cardiovascular parameters.
Materials and Methods
The study was conducted as retrospective, single center study on clinically stable EPO-naïve adult patients in CKD stage IV according to the National Kidney Foundation guidelines routinely referred to Outpatient Ambulatory Units.
The hospital charts of all patients treated with ESA between January 2009 and April 2010 were retrieved and reviewed. For each patient, clinical history data were available for a 12-month period.
A total of 1,245 patients were evaluated. Inclusion criteria were:
(1) stable CKD stage IV (estimated glomerular filtration rate, GFR, <30 and >15 ml/min);
(2) anemia (hemoglobin, Hb, <11 g/dl) at the first visit;
(3) anemia (Hb <11 g/dl) and transferrin saturation >25% at the 2nd visit (oral iron supplementation was regularly prescribed at the first visit);
(4) weekly EPO dose >6,000 IU or 20 μg;
(5) stable EPO dose during the 12-month follow-up period.
A total of 122 charts were selected and reviewed; 22 charts were excluded because of inadvertent discontinuation of ESA treatment for at least 4 weeks (hospital admission in 9 cases and lack of treatment compliance in 13 cases).
As patients were participating concomitantly in another research project, blood sampling and transthoracic echocardiography were performed in every patient at 3-month intervals. Blood samples were routinely collected and stored at −80°C. N-terminal prohormone B-type natriuretic peptide (pro-BNP) levels were determined in every blood sample. All echocardiograms were performed at ultrasonography departments by an operator blinded to the study.
Epoetin-α (Eprex®, Janssen-Cilag) and darbepoetin-α (Aranesp®, Amgen) were provided in open label, commercial forms directly to the patient from routine administrative channels and self-administered through subcutaneous injection.
Statistical Analysis
All statistical analyses were conducted with SAS, release 8.1 (SAS Institute). The primary endpoint was the change in mean Hb during the 12-months retrospective observational window. Secondary endpoints included percentage of Hb responders (Hb ≥11 g/dl), the mean change in hematocrit (Hct), C-reactive protein (CRP), pro-BNT and cardiac indices: left ventricular end diastolic volume (LVEDV), right ventricular end diastolic volume (RVEDV), ejection fraction (EF) and arterial blood pressure.
Efficacy data were assessed using a modified intent-to-treat group consisting of all treated patients who had baseline Hb and at least one other measurement at 3, 6, 9 or 12 months. Descriptive statistics were calculated for all efficacy endpoints and values were expressed as means ± SD. Normal (Gaussian) distribution of all parameters was assessed using diagnostic plots and the Shapiro-Wilk test to define the appropriate statistical test to be applied to study endpoints. When evaluating parameters with a Gaussian distribution, comparisons were carried out using unpaired t test between groups and paired t test for intragroup comparisons. In groups that do not show a normal distribution, corrections in t statistics and degrees of freedom were made. For those parameters with non-Gaussian distribution, comparisons were performed by non-parametric methods using the Wilcoxon rank-sum test between the groups and the Wilcoxon signed-rank test within each group.
In order to assess homogeneity between the two treatments (darbepoetin/epoetin), cardiological parameters were investigated at baseline by means of the above-described tests. If a significant situation of non-homogeneity was discovered, the impact on efficacy was investigated and the appropriate action taken (e.g. application of a multivariate model). Spearman's correlation was performed to determine linear correlations between Hb change and the other study endpoint changes at the follow-ups. Pearson's χ2 test has been applied to assess the treatment effect on Hb response (≥11 g/dl). All p values reported are two sided and p < 0.05 was considered significant.
Results
Underlying pathologies leading to CKD, related concomitant treatments and demographic features observed at baseline were homogenously distributed among both groups (table 1).
Table 1.
Demographic and baseline characteristics at 3 months
| Darbepoetin-α | Epoetin-α | p value | |
|---|---|---|---|
| (50 patients) | (50 patients) | ||
| Patients, n | 1.000 | ||
| Male | 30 (60%) | 30 (60%) | |
| Female | 20 (40%) | 20 (40%) | |
| Age, years | 0.88 | ||
| Mean ± SD | 50.68 ± 4.51 | 50.82 ± 4.74 | |
| Range | 45–60 | 45–60 | |
| Patients with underlying morbidities, n | |||
| Chronic glomerulonephritis of unknown origin | 20 | 20 | |
| Nephroangiosclerosis in hypertensive stage II–IIIa | 16 | 14 | |
| Polycystic kidney disease | 14 | 16 | |
| Hypertension | 18 | 22 | |
| Patients receiving concomitant therapy, n | |||
| Calcium antagonists | 8 | 12 | |
| Calcium antagonists + doxazosin | 10 | 10 | |
| Hb, g/dl | 0.169 | ||
| Mean ± SD | 10.52 ± 0.22 | 10.46 ± 0.18 | |
| Range | 10.20–10.90 | 10.00–10.70 | |
| Hct, % | 0.061 | ||
| Mean ± SD | 30.58 ± 1.57 | 30.40 ± 1.15 | |
| Range | 27.00–32.00 | 29.00–32.00 | |
| Serum phosphorus, mg/dl | 0.339 | ||
| Mean ± SD | 4.77 ± 0.31 | 4.81 ± 0.32 | |
| Range | 4.00–5.30 | 4.00–5.50 | |
| CRP, mg/l | 0.309 | ||
| Mean ± SD | 8.12 ± 3.65 | 8.94 ± 3.70 | |
| Range | 2.00–16.00 | 2.00–19.00 | |
| Pro-BNP, pg/ml | 0.748 | ||
| Mean ± SD | 384.42 ± 36.11 | 383.50 ± 43.30 | |
| Range | 313.00–479.00 | 264.00–479.00 | |
| LVEDV, ml | 0.015 | ||
| Mean ± SD | 53.54 ± 2.57 | 54.16 ± 1.66 | |
| Range | 50.00–59.00 | 51.00–57.00 | |
| RVEDV, ml | 0.637 | ||
| Mean ± SD | 22.14 ± 1.14 | 22.02 ± 1.19 | |
| Range | 20.00–24.00 | 20.00–24.00 | |
| EF, % | 0.007 | ||
| Mean ± SD | 50.28 ± 3.07 | 49.28 ± 2.01 | |
| Range | 43.00–54.00 | 44.00–52.00 | |
| Mean arterial pressure, mm Hg | 0.476 | ||
| Mean ± SD | 124.12 ± 1.92 | 124.38 ± 1.64 | |
| Range | 120.00–129.00 | 121.00–128.00 |
a Definition according to the classification of the International Society of Hypertension.
The prescription ratio in darbepoetin-α- and epoetin-α-treated patients was 1:300 (mean 1:285, median 1:300) instead of the ‘classic’ 1:200 ratio reported in the European ESA data sheet [9].
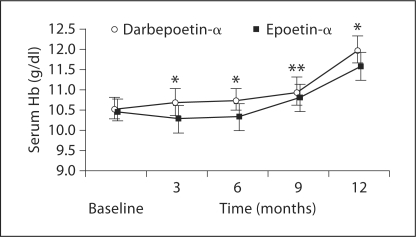
Darbepoetin-α-treated patients showed a significantly faster increase in Hb levels compared with the epoetin-α group (fig. 1). The stronger and faster response was also supported by the percentage of patients responding to treatment. At 3 months, 10 (20%) patients reached the optimal Hb target (>11 g/dl) in the darbepoetin-α group, but no patient in the epoetin-α group. At 6 months, 11 patients (22%) on darbepoetin-α and none on epoetin-α reached this target (table 2). At the following visits (9 and 12 months), the number of responders in the darbepoetin-α group steadily increased but not in the epoetin-α group.
Fig. 1.
Mean Hb concentration (±SD) in patients in CKD stage IV and secondary anemia due to EPO deficiency who were treated with 20 μg darbepoetin-α weekly or 2,000 IU epoetin-α thrice weekly during the follow-up (* p < 0.0001; ** p < 0.05).
Table 2.
Number of patients reaching the Hb target (Hb >11 g/dl) during treatment with darbepoetin-α (20 µg/week) or epoetin-α (2,000 IU thrice weekly)
| Darbepoetin-α | Epoetin-α | Total | p value | |
|---|---|---|---|---|
| Month 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Month 6 | 10 (20.0%) | 0 (0.0%) | 10 (10.0%) | 0.0005 |
| Month 9 | 17 (34.0%) | 11 (22.0%) | 28 (28.0%) | 0.2653 |
| Month 12 | 50 (100.0%) | 36 (72.0%) | 86 (86.0%) | <0.0001 |
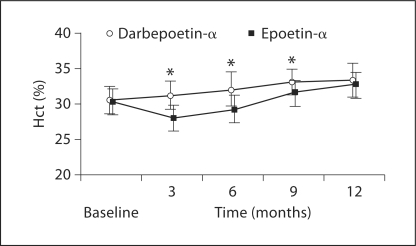
The mean Hct values observed at the different time points mirrored the mean Hb changes obtained with the two treatment strategies (fig. 2).
Fig. 2.
Mean Hct concentration (±SD) at quarterly visits in patients in CKD stage IV treated with darbepoetin-α 20 μg/weekly and epoetin-α 2,000 IU/thrice weekly (* p < 0.0001).
GFR showed a slight reduction between the first and the last control values, with a mean loss of 2.5 ml/min.
Regarding cardiac parameters, both treatments induced reductions in left heart volumes. Darbepoetin-α induced a stronger reduction in LVEDV (reductions: 4.15 and 7.57% after 6 and 12 months of darbepoetin-α therapy compared to 2.88 and 4.96% after 6 and 12 months of epoetin-α, respectively). Following 6 and 12 months of darbepoetin-α therapy, EF improved by 4.03 and 9.53% compared to 3.12 and 5.98% following epoetin-α treatment, respectively). Mean pro-BNP level was 236.68 ± 43.94 pg/ml (−37.76%) after 6 months and 170.84 ± 39.85 pg/ml (−54.91%) after 1 year of treatment with darbepoetin-α. Reduction in cardiac polypeptide was less evident during and after epoetin-α treatment: 270.44 ± 36.38 (−28.84%) and 195.44 ± 33.13 (−48.35%) at 6 and 12 months, respectively. No difference in right cardiac performance and arterial blood pressure was noted during and after 1 year of treatment (table 3).
Table 3.
Cardiovascular parameters in the study patients
| Treatments | Baseline | 6 months | 12 months | |
|---|---|---|---|---|
| LVEDV, ml | darbepoetin-α | 53.54 ± 2.57 | 51.30 ± 2.31) | 49.44 ± 1.54 |
| epoetin-α | 54.16 ± 1.66 | 52.60 ± 1.92) | 51.44 ± 1.43 | |
| p < 0.001 | p < 0.001 | |||
| RVEDV, ml | darbepoetin-α | 22.14 ± 1.14 | 22.20 ± 1.03 | 22.40 ± 1.12 |
| epoetin-α | 22.02 ± 1.19 | 22.02 ± 1.19 | 22.20 ± 1.21 | |
| NS | NS | |||
| EF, % | darbepoetin-α | 50.2 ± 83.07 | 52.26 ± 2.56 | 54.92 ± 1.31 |
| epoetin-α | 49.2 ± 82.01 | 50.80 ± 1.75 | 52.1 ± 81.48 | |
| p < 0.01 | p < 0.0001 | |||
| Pro-BNP, pg/ml | darbepoetin-α | 384.42 ± 36.11 | 236.6 ± 843.94 | 170.84 ± 39.85 |
| epoetin-α | 383.50 ± 43.30 | 270.44 ± 36.38 | 195.44 ± 33.13 | |
| p < 0.01 | p < 0.05 | |||
| Mean arterial pressure, mm Hg | darbepoetin-α | 124.12 ± 1.92 | 124.29 ± 1.45 | 124.34 ± 1.45 |
| epoetin-α | 124.3 ± 81.64 | 123.86 ± 1.54 | 124.96 ± 1.54 | |
| NS | NS | |||
Cardiovascular parameters (means ± SD) in patients treated with 20 μg darbepoetin-α weekly or 2,000 IU epoetin-α thrice weekly at baseline and 6 and 12 months.
The mean CRP blood level reductions after 6 and 12 months were 3.93 ± 0.43 (−39.18%, p < 0.0001) and 2.49 ± 0.69 mg/l (−63.50%, p < 0.0001) in the darbepoetin-α group, respectively. At the same time points, the mean CRP blood levels with epoetin-α were 4.17 ± 0.29 (−43.91%, p < 0.0001) and 2.81 ± 0.54 mg/l (−63.83%, p < 0.0001), respectively.
The safety profile of both human recombinant EPO-treated groups monitored during the observation period and reported in the hospital charts was consistent with that reported in other studies.
Discussion
In agreement with our current practice, the classic switch rule ‘1 μg darbepoetin = 200 IU epoetin’ was replaced by a more moderate ‘instinctive’ 1:300 ratio. In our experience, this ratio helps to avoid overestimation of the darbepoetin dose needed to treat anemia and to keep hemoglobin levels constant.
Bock et al. [9] reported similar experience with patients switched from epoetin-α to darbepoetin-α, for whom mean Hb level remained constant, even if the mean final conversion ratio of darbepoetin/epoetin was 1:336. Hirai et al. [10, 11] confirmed this observation in a similar analysis: in 104 end-stage renal disease patients on hemodialysis, the conversion ratio was 1:350.5. Nevertheless, in both papers Hb concentrations were determined during a shorter evaluation period (24 weeks) than in our study (1 year).
The clinical experience reviewed in this retrospective analysis confirms the efficacy of long-term darbepoetin-α at a lower dose, especially when particular attention is paid to iron repletion, and transferrin saturation is >25%.
Apart from the faster and significant increase in Hb and Hct levels, this report is one of the few published reports on changes in cardiovascular parameters (echocardiographic patterns) in patients with chronic renal failure treated with EPO (darbepoetin-α and epoetin-α).
Left heart function and parameters apparently improved after correction of anemia, being more pronounced following darbepoetin-α than epoetin-α. LVEDV significantly decreased after 9 and 12 months, and EF significantly increased at the same follow-ups, while mean pro-BNP levels significantly decreased. Both effects were stronger following darbepoetin-α treatment.
The reduction in pro-BNP observed in our patients probably reflects left ventricular remodeling during the 1-year time window. It is in fact well known that treatment with human recombinant EPO to correct anemia, even in patients having Hb levels lower than normal, beneficially affects cardiovascular performance and renal function in patients with hemodialysis-dependent end-stage renal disease [10, 11, 12] as well as in patients with chronic heart failure [13, 14, 15]. This finding is further supported by the reduction in GFR in light of an expected increase in BNP levels. In other words, despite the small reduction in GFR potentially leading to reduced BNP clearance, BNP decreased.
Conclusions
In conclusion, despite the retrospective design of this observational study (without randomization and crossover), these results may encourage nephrologists to test the 1:300 darbepoetin/epoetin conversion ratio in ‘easy’ patients and avoid aggressive protocols for the treatment of anemia in CKD patients. In fact, 20 μg darbepoetin-α per week appeared effective in anemia correction and seemed to improve cardiovascular performance in a significantly higher proportion than 2,000 IU epoetin thrice weekly in patients with optimal iron balance. On the other hand, it has to be borne in mind that a treatment regimen restricted to only one administration per week may increase patient compliance and adherence to therapy, explaining in part our results.
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 2.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 3.Nurko S. Anemia in chronic kidney disease: causes, diagnosis, treatment. Clev Clin J Med. 2006;73:289–297. doi: 10.3949/ccjm.73.3.289. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation: IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2008. [DOI] [PubMed]
- 5.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 6.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 8.Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, Egrie J. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 9.Bock HA, Hirt-Minkowski P, Brunisholz M, Keusch G, Rey S, von Albertini B, Swiss EFIXNES trial investigators Darbepoetin alpha in lower-than-equimolar doses maintains haemoglobin levels in stable haemodialysis patients converting from epoetin alpha/beta. Nephrol Dial Transplant. 2008;23:301–308. doi: 10.1093/ndt/gfm579. [DOI] [PubMed] [Google Scholar]
- 10.Hirai T, Nakashima A, Shiraki N, Takasugi N, Yorioka N. Dose conversion ratio one year after switching from epoetin alpha to darbepoetin alpha in Japanese hemodialysis patients. Int J Artif Organs. 2010;33:283–289. [PubMed] [Google Scholar]
- 11.Hirai T, Sugiya N, Nakashima A, Takasugi N, Yorioka N. Switching from epoetin alpha to darbepoetin alpha in Japanese hemodialysis patients: dose conversion ratio. Nephron Clin Pract. 2009;111:c81–c86. doi: 10.1159/000183843. [DOI] [PubMed] [Google Scholar]
- 12.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anaemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg DS, Wexler D, Sheps Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anaemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg DS, Wexler D, Blum M. The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18:141–146. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]