Abstract
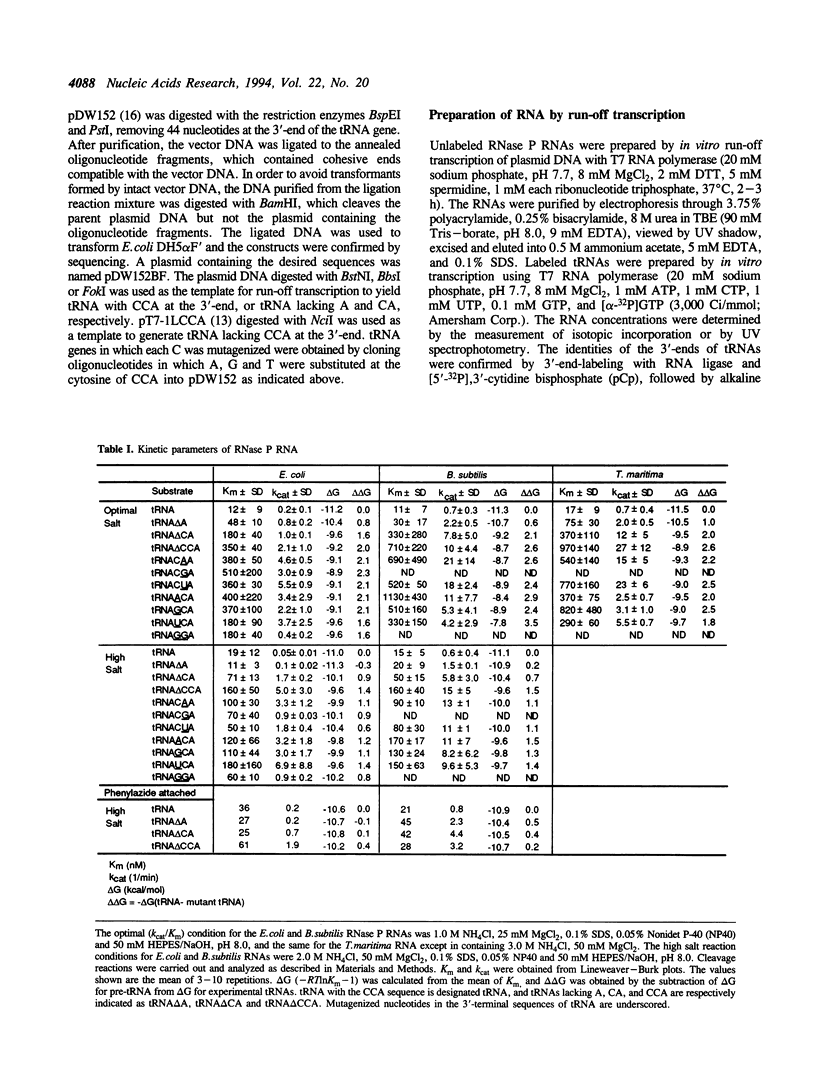
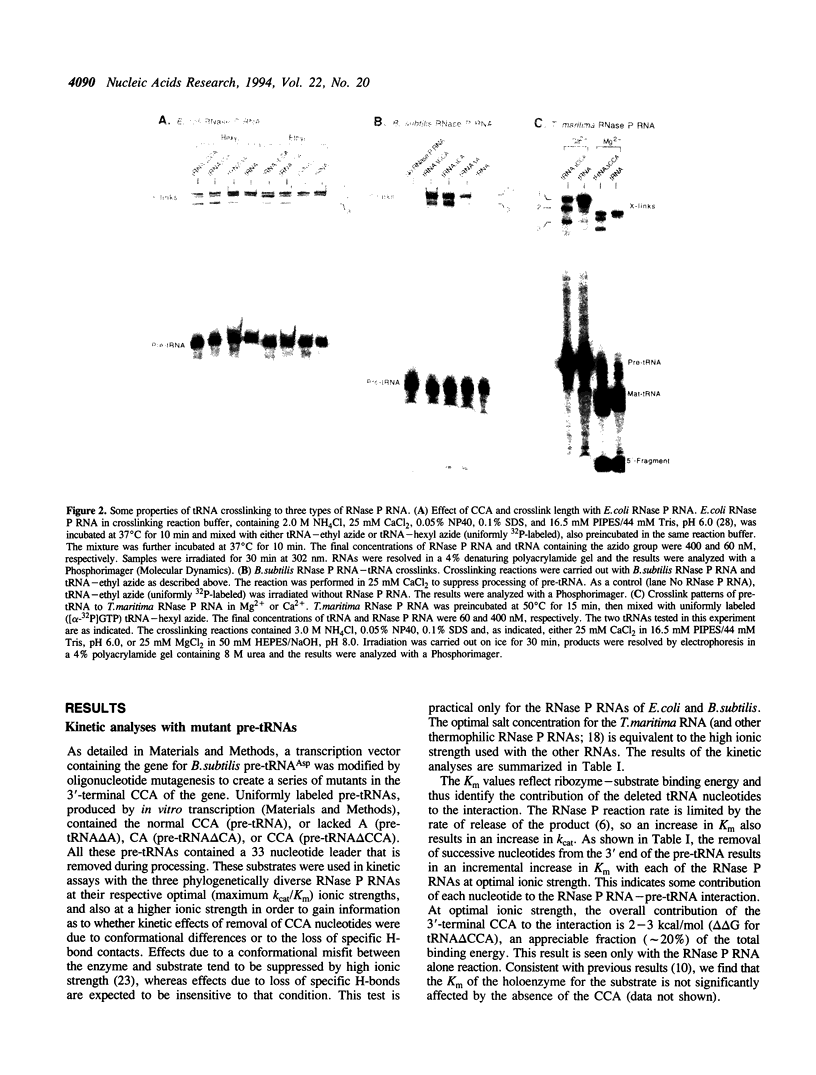
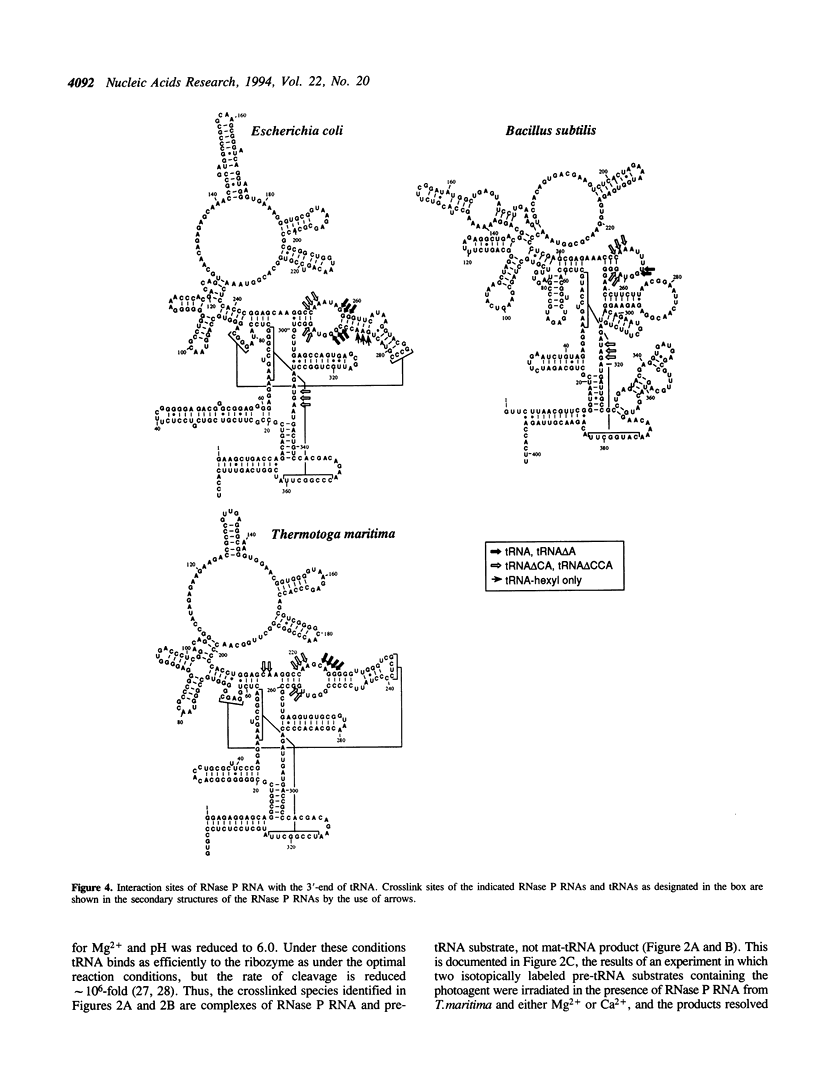
Ribonuclease P, which contains a catalytic RNA subunit, cleaves 5' precursor-specific sequences from pre-tRNAs. It was previously shown that the RNase P RNA optimally cleaves substrates which contain the mature, 3'-terminal CCA of tRNA. In order to determine the contributions of those individual 3'-terminal nucleotides to the interaction, pre-tRNAs that have CCA, only CC or C or are without CCA at the 3'-end were synthesized by run-off transcription, tested as substrates for cleavage by RNase P RNA and used in photoaffinity crosslinking experiments to examine contact sites in the ribozyme. In order to generalize the results, analyses were carried out using three different bacterial RNase P RNAs, from Escherichia coli, Bacillus subtilis and Thermotoga maritima. At optimal (Kcat/Km) ionic strength (1 M NH4+/25 mM Mg2+), Km increases incrementally 3- to 10-fold upon stepwise removal of each nucleotide from the 3'-end. At high ionic strength (2 M NH4+/50 mM Mg2+), which suppresses conformational effects, removal of the 3'-terminal A had little effect on Km, indicating that it is not a specific contact. Analysis of the deletion and substitution mutants indicated that the C residues act specially; their contribution to binding energy at high ionic strength (approximately 1 kcal/mol) is consistent with a non-Watson-Crick interaction, possibly irregular triple-strand formation with some component of the RNase P RNA. In agreement with previous studies, we find that the RNase P holoenzyme in vitro does not discriminate between tRNAs containing or lacking CCA. The structural elements of the three RNase P RNAs in proximity to the 3'-end of tRNA were examined by photoaffinity crosslinking. Photoagent-labeled tRNAs with 3'-terminal CCA, only CC or C, or lacking all these nucleotides were covalently conjugated to the three RNase P RNAs by irradiation and the sites of crosslinks were mapped by primer extension. The main crosslink sites are located in a highly conserved loop (probably an irregular helix) that is part of the core of the RNase P RNA secondary structure. The crosslinking results orient the CCA of tRNA with respect to that region of the RNase P RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Kirsebom L., Talbot S. Recent studies of ribonuclease P. FASEB J. 1993 Jan;7(1):7–14. doi: 10.1096/fasebj.7.1.7916700. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Haas E. S., Pace N. R. Characterization of ribonuclease P RNAs from thermophilic bacteria. Nucleic Acids Res. 1993 Feb 11;21(3):671–679. doi: 10.1093/nar/21.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin A. B., Pace N. R. Mapping the active site of ribonuclease P RNA using a substrate containing a photoaffinity agent. EMBO J. 1990 Dec;9(12):4111–4118. doi: 10.1002/j.1460-2075.1990.tb07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard U., Willis I., Söll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem. 1988 Feb 15;263(5):2447–2451. [PubMed] [Google Scholar]
- Carter B. J., Vold B. S., Hecht S. M. Control of the position of RNase P-mediated transfer RNA precursor processing. J Biol Chem. 1990 May 5;265(13):7100–7103. [PubMed] [Google Scholar]
- Easterbrook-Smith S. B., Wallace J. C., Keech D. B. Pyruvate carboxylase: affinity labelling of the magnesium adenosine triphosphate binding site. Eur J Biochem. 1976 Feb 2;62(1):125–130. doi: 10.1111/j.1432-1033.1976.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Gardiner K. J., Marsh T. L., Pace N. R. Ion dependence of the Bacillus subtilis RNase P reaction. J Biol Chem. 1985 May 10;260(9):5415–5419. [PubMed] [Google Scholar]
- Gardiner K., Pace N. R. RNase P of Bacillus subtilis has a RNA component. J Biol Chem. 1980 Aug 25;255(16):7507–7509. [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Structural requirements for processing of synthetic tRNAHis precursors by the catalytic RNA component of RNase P. J Biol Chem. 1988 Jan 15;263(2):652–657. [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., McClain W. H., Altman S. Cleavage of tRNA precursors by the RNA subunit of E. coli ribonuclease P (M1 RNA) is influenced by 3'-proximal CCA in the substrates. Cell. 1984 Aug;38(1):219–224. doi: 10.1016/0092-8674(84)90543-9. [DOI] [PubMed] [Google Scholar]
- Kahle D., Wehmeyer U., Krupp G. Substrate recognition by RNase P and by the catalytic M1 RNA: identification of possible contact points in pre-tRNAs. EMBO J. 1990 Jun;9(6):1929–1937. doi: 10.1002/j.1460-2075.1990.tb08320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Kirsebom L. A., Svärd S. G. Identification of a region within M1 RNA of Escherichia coli RNase P important for the location of the cleavage site on a wild-type tRNA precursor. J Mol Biol. 1993 Jun 5;231(3):594–604. doi: 10.1006/jmbi.1993.1312. [DOI] [PubMed] [Google Scholar]
- Knap A. K., Wesolowski D., Altman S. Protection from chemical modification of nucleotides in complexes of M1 RNA, the catalytic subunit of RNase P from E coli, and tRNA precursors. Biochimie. 1990 Nov;72(11):779–790. doi: 10.1016/0300-9084(90)90187-l. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Guerrier-Takada C., Altman S. Model substrates for an RNA enzyme. Science. 1987 Oct 23;238(4826):527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Smith D. Ribonuclease P: function and variation. J Biol Chem. 1990 Mar 5;265(7):3587–3590. [PubMed] [Google Scholar]
- Perreault J. P., Altman S. Important 2'-hydroxyl groups in model substrates for M1 RNA, the catalytic RNA subunit of RNase P from Escherichia coli. J Mol Biol. 1992 Jul 20;226(2):399–409. doi: 10.1016/0022-2836(92)90955-j. [DOI] [PubMed] [Google Scholar]
- Rayford R., Anthony D. D., Jr, O'Neill R. E., Jr, Merrick W. C. Reductive alkylation with oxidized nucleotides. Use in affinity labeling or affinity chromatography. J Biol Chem. 1985 Dec 15;260(29):15708–15713. [PubMed] [Google Scholar]
- Reich C., Olsen G. J., Pace B., Pace N. R. Role of the protein moiety of ribonuclease P, a ribonucleoprotein enzyme. Science. 1988 Jan 8;239(4836):178–181. doi: 10.1126/science.3122322. [DOI] [PubMed] [Google Scholar]
- Roberts R. W., Crothers D. M. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992 Nov 27;258(5087):1463–1466. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., McClain W. H. Three steps in conversion of large precursor RNA into serine and proline transfer RNAs. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1491–1495. doi: 10.1073/pnas.72.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Burgin A. B., Haas E. S., Pace N. R. Influence of metal ions on the ribonuclease P reaction. Distinguishing substrate binding from catalysis. J Biol Chem. 1992 Feb 5;267(4):2429–2436. [PubMed] [Google Scholar]
- Smith D., Pace N. R. Multiple magnesium ions in the ribonuclease P reaction mechanism. Biochemistry. 1993 May 25;32(20):5273–5281. doi: 10.1021/bi00071a001. [DOI] [PubMed] [Google Scholar]
- Stark B. C., Kole R., Bowman E. J., Altman S. Ribonuclease P: an enzyme with an essential RNA component. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3717–3721. doi: 10.1073/pnas.75.8.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt C. K., Carter B. J., Payne R. C., Hecht S. M. Metal ion and substrate structure dependence of the processing of tRNA precursors by RNase P and M1 RNA. J Biol Chem. 1990 Dec 25;265(36):22513–22519. [PubMed] [Google Scholar]
- Tallsjö A., Kirsebom L. A. Product release is a rate-limiting step during cleavage by the catalytic RNA subunit of Escherichia coli RNase P. Nucleic Acids Res. 1993 Jan 11;21(1):51–57. doi: 10.1093/nar/21.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]