Abstract
Background
Cardiorenal syndrome (CRS) type 1 is characterized by a rapid worsening of cardiac function leading to acute kidney injury (AKI). An immune-mediated damage and alteration of immune response have been postulated as potential mechanisms involved in CRS type 1. In this pilot study, we examined the possible role of the immune-mediated mechanisms in the pathogenesis of this syndrome. The main objective was to analyze in vitro that plasma of CRS type 1 patients was able to trigger a response in monocytes resulting in apoptosis. The secondary aim was to evaluate TNF-α and IL-6 plasma levels of CRS type 1 patients.
Methods
Fifteen patients with acute heart failure (AHF) and CRS type 1 were enrolled and 20 healthy volunteers without AHF or AKI were recruited as control group. Plasma from these two groups was incubated with monocytes and, subsequently, cell apoptosis was evaluated. In addition, the activity of caspase-8 was assessed after 24 h incubation. Quantitative determination of TNF-α and IL-6 levels was performed.
Results
Plasma-induced apoptosis was significantly higher in CRS type 1 patients compared with healthy controls at 72 h (78 vs. 11%) and 96 h (81 vs. 11%). At 24 h, the activity of caspase-8 was significantly higher in monocytes incubated with plasma from the CRS type 1 group. TNF-α (2.39 vs. 28.49 pg/ml) and IL-6 (4.8 vs. 16.5 pg/ml) levels were significantly elevated in the CRS type 1 group (p < 0.01).
Conclusions
In conclusion, there is a defective regulation of monocyte apoptosis in CRS type 1 patients, and inflammatory pathways may have a central role in the pathogenesis of CRS type 1 and may be fundamental in damage to distant organs.
Key Words: Cardiorenal syndrome, Acute heart failure, Apoptosis, Acute kidney injury
Introduction
Heart performance and kidney function are closely interconnected and a synergistic relationship exists between these organs. Dysfunction of one organ often leads to a deterioration of function of the other one [1]. This clinical entity has been defined as cardiorenal syndrome (CRS). Recently, a new definition of CRS has been accepted and it includes a classification of the syndrome into 5 separate subtypes [1].
CRS type 1 or acute CRS is characterized by a rapid worsening of cardiac function leading to acute kidney injury (AKI). In the United States, more than 1 million patients present to hospitals with acute decompensated heart failure (ADHF) every year [2]. Approximately one-third of the ADHF patients develop AKI as defined by an increase in serum creatinine of ≥0.3 mg/dl [3]. In patients with cardiogenic shock, the incidence of AKI can exceed 70% [4]. Furthermore, patients who develop AKI after an acute cardiac event have a significantly high mortality risk [5]. Baseline chronic kidney disease (CKD), diabetes, prior heart failure, and initial presentation with hypertension are established risk predictors for CRS type 1 [3].
The pathophysiology of CRS type 1 is complex and poorly understood as it involves several factors which are interrelated [6]. Possible mechanisms of this spiraling dysfunction include an altered balance between nitric oxide and reactive oxygen species, systemic inflammation and apoptosis, activation of both the sympathetic nervous system and the renin-angiotensin-aldosterone system, and the paracrine and systemic actions of various substances such as endothelin, prostaglandins, vasopressin and natriuretic peptides [7, 8].
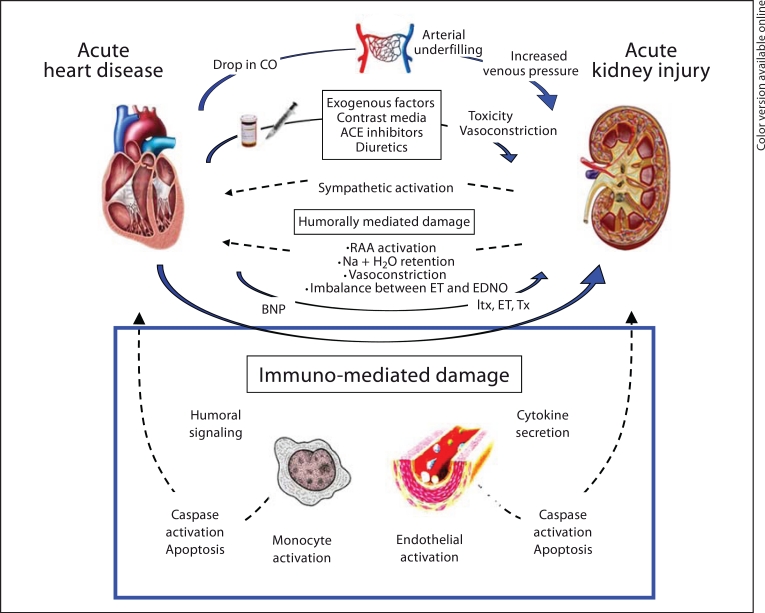
In addition, immune-mediated damage has been postulated as a potential mechanism involved in the pathogenesis of CRS [1], but it has not been demonstrated (fig. 1). Alterations in the immune response might include cytokine release and changes in immune cell functions including apoptosis [9]. Apoptosis is a normal physiological process of cell death, by which unwanted cells are eliminated from living organism during embryonic and adult development [10], and it plays a very important role especially in the immune system [11]. It is associated with the regulation of cellular homeostasis in organs and the elimination of damaged cells or of cells with deleterious activity from the host.
Fig. 1.
Diagram illustrating and summarizing the pathophysiological mechanisms hypothesized for CRS type 1.
There are multiple inducers and inhibitors of apoptosis which interact with specific receptors and transduce the signal by second messengers to program cell death. The apoptotic process can be initiated by 2 different mechanisms: the intrinsic and the extrinsic pathway. The intrinsic apoptotic pathway is triggered by toxic alterations inside the cell which modify the mitochondrial transmembrane potential and cause the release of cytochrome c into the cytoplasm [12]. The extrinsic pathway occurs if a member of the tumor necrosis factor superfamily death ligands, such as TNF-α or Fas-L, binds receptors to its cell surface, activating caspase-8 and consequently the caspase cascade [13].
Apoptosis is characterized by a variety of cellular changes including loss of membrane phospholipid asymmetry, chromatin condensation, mitochondrial swelling and DNA cleavage. The final result is a form of cell death that avoids the normal inflammatory response associated with necrosis. However, an alteration in the regulation of cell death by apoptosis may negatively affect the mechanism of host defense; in fact, this mechanism requires a fine balance between recruitment and death of immunocompetent cells, involving lymphocytes and monocytes [11]. Apoptosis is clearly necessary to maintain the health of the organism; dysregulation of cell death by excessive or defective apoptosis has been implicated in a variety of disease states.
In particular, a loss of immune cells by apoptosis is associated with physiologic changes that occur in several diseases. There are several links of evidence suggesting that apoptosis may play a role in the pathophysiology of immune dysfunction in uremia. In fact, a high degree of peripheral blood mononuclear cell (PBMC) apoptosis was observed in uremic patients and this is related to the severity of uremia [14]. Two different groups showed that accelerated PBMC apoptosis and high levels of proinflammatory cytokines are associated with sustained cell activation and chronic inflammation [15, 16]. The impaired cellular host defense is associated with an elevated degree of monocyte apoptosis in end-stage renal disease patients on long-term hemodialysis, CAPD, as well as those in predialytic uremia [17]. We have previously shown that uremic plasma could increase in vitro apoptosis rates in U937, a human monocytic cell line [18, 19]. Recent studies have investigated the immune-modulation in the failing human heart and have shown activation of inflammatory cytokines in the myocardium and peripheral monocytes leading to monocyte phenotype transition, myocyte apoptosis, and activation of matrix metalloproteinase [20, 21]. In addition, heart failure can also be considered an inflammatory state that may contribute to gradual toxic injury to renal cells, first sublethal but later lethal (apoptosis), culminating in permanent chronic kidney damage and functional loss [22, 23].
Moreover, experimental studies indicated that proinflammatory cytokines (TNF-α and IL-6) were associated with some molecular, clinical and physiology aspects of heart failure [24]; in addition, cytokines were released by leukocytes and renal tubular cells in the injured kidney, were important components of both the initiation and extension of inflammation and contributed to the pathogenesis, clinical manifestation and complications of AKI [25]. In this study, we conducted a pilot study to examine the possible role of the immune-mediated mechanisms in the pathogenesis of CRS type 1. The main objective was to analyze in vitro that plasma from CRS type 1 patients was able to trigger a response in human monocyte cells, resulting in apoptosis. The secondary aim of the study was evaluated the presence of cytokines in plasma of CRS type 1 patients to better understand the mechanisms of this syndrome.
Subjects and Methods
Subjects
We enrolled 15 patients (mean age 72.7 ± 16.6 years; 11 males, 4 females) who were admitted for acute heart failure (AHF), referred for nephrology consultation and diagnosed with CRS type 1 according to the definition (table 1). All patients were known to have CKD prior to the current admission; 4 of them were in CKD stage V, but not yet on renal replacement therapy (RRT). CRS type 1 was defined according to the current classification system [1]. Patients who had AHF of any cause and subsequently developed AKI were included. Patients who had AKI prior to the episode of AHF, or had any other potential causes of AKI, were excluded from the study. Causes of admission for AHF included non-ST segment elevation myocardial infarction (n = 2), excessive salt and fluid intake (n = 2), and hypertensive crisis (n = 1). The other 10 patients did not have a recognizable cause of AHF.
Table 1.
Baseline characteristics of CRS type 1 patients and clinical parameters
| Patient | Sex | Age years | CKD stage | Cause of CKD | Creatininea mg/dl | Ureaa mg/dl | AKI stagea | RRT | Prior myocardial infarction or revascularization | Prior history of cardiac disease |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 83 | V | uncertain | 4.9 | 150 | 1 | yes | yes | ICM |
| 2 | M | 80 | III | DM | 1.7 | 208 | 1 | no | no | AF |
| 3 | M | 71 | V | DM | 5.4 | 293 | 3 | yes | no | no |
| 4 | M | 28 | V | HTN | 7.3 | 270 | 3 | no | no | no |
| 5 | M | 81 | II | DM | 9.5 | 204 | 3 | yes | no | no |
| 6 | M | 71 | III | DM | 2.5 | 112 | 1 | yes | no | ICM |
| 7 | M | 85 | IV | uncertain | 7.8 | 195 | 3 | yes | no | no |
| 8 | F | 60 | IV | HTN | 4.2 | 254 | 3 | no | no | AF |
| 9 | M | 85 | V | uncertain | 5.9 | 120 | 3 | yes | yes | ICM |
| 10 | M | 48 | IV | DM | 4.8 | 211 | 3 | yes | no | AF |
| 11 | F | 84 | III | DM | 2.1 | 244 | 1 | no | no | no |
| 12 | M | 67 | IV | DM | 4.1 | 318 | 3 | yes | yes | ICM |
| 13 | F | 82 | IV | uncertain | 7.6 | 107 | 3 | yes | yes | ICM, HTN HD |
| 14 | F | 80 | IV | HTN | 2.0 | 95 | 1 | no | no | DCM |
| 15 | M | 88 | III | DM | 2.4 | 233 | 1 | no | yes | ICM |
a At enrollment. AF = Acute failure; DCM = dilated cardiomyopathy; DM = diabetes mellitus; HTN = hypertension; HTN HD = hypertensive heart disease; ICM = ischemic cardiomyopathy.
AKI was defined by Acute Kidney Injury Network (AKIN) criteria [26]. The cause of AKI was presumed to be related to the AHF after exclusion of other plausible causes based on a review of the clinical course. None of the patients had exposure to contrast media in the 72 h preceding AKI. The median baseline creatinine was 2.3 mg/dl [interquartile range (IQR) 1.6–4.0] with a median estimated glomerular filtration rate (eGFR) of 23.4 ml/min/1.73 m2 (IQR 14.9–38.2). The eGFR was calculated with the 4-variable standardized MDRD formula [27]. Median serum creatinine on admission was 6.5 mg/dl (IQR 3.0–7.3) and 9 patients required RRT. Based on the AKIN classification, 9 patients had AKI stage 3 although not all of them underwent RRT. The other 6 patients were classified as AKI stage 1. None of the patients had hypotension that required inotropic support prior to the diagnosis of AKI.
Twenty healthy volunteers (mean age 52.0 ± 7.7 years; 15 males, 5 females) without AHF or AKI were recruited as control group for this study.
Sample Collection
All patients were informed about the experimental protocol and the objectives of the study before providing informed consent and blood sample. Whole blood samples were collected from the recruited patients on admission into the nephrology ward. The blood sample was collected in a heparinized tube and subsequently centrifuged. Plasma was immediately separated from the blood cells and stored at −80°C until use. Control samples from healthy volunteers were processed in the same manners.
U937 Cell Culture
The human monocytic cell line U937 is a monocytic precursor cell line derived from a histiocytic lymphoma [28]. The U937 cells were grown in complete liquid phase medium (RPMI 1640; International PBI) supplemented with 10% heat-inactivated (30 min at 56°C) fetal calf serum, 2 mML-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma Chemical Co.). The U937 cells were maintained in a controlled atmosphere (5% CO2) incubator at 37°C and passaged every second or third day. Cell viability was 99%, as assessed by trypan blue exclusion.
Induction of Apoptosis
The U937 cells were plated at 1 × 105 cells per well in 96-well plates, and incubated with 50% RPMI 1640 medium (with 2 mML-glutamine, 100 IU/ml penicillin and 100 mg/ml streptomycin) and 50% heparinized plasma from CRS type 1 patients and healthy controls in standard condition (at 37°C in 5% CO2 for up to 24, 72, or 96 h). Before use in experiments, the U937 cells were washed twice in Dulbecco's PBS (without calcium and magnesium), pH 7.4.
Evaluation of Apoptosis
Analysis of DNA Morphological Changes
Apoptosis is characterized by DNA fragmentation, showinga ladder-like pattern, and nuclear fragmentation into several smallerfragments ranging in number from 2 to more than 20 per cell. Apoptotic cells showed cellular contraction, nuclear condensation and pyknosis. At the end of incubation, the cells were stained with 10 μM/ml Hoechst 33342 (Sigma ChemicalCo.) for 15 min at 37°C according to published procedures.
The quantitative analysis of apoptosis was performed by scoring the number of cells displaying the typical nuclear morphology, as measured by fluorescence microscopy [29, 30]. The level of apoptosis was evaluated after an incubation of 72 and 96 h and was expressed as percentage of the total cell population, counting at least 300 cells in 6 randomly selected fields at 100× magnification [31]. Each experiment was performed in triplicate.
Determination of Caspase-8 Activity
Caspase-8 is an intracellular cysteine protease which plays a central role in the initiation of apoptotic cascades. Caspase-8 activity was measured by the TruPoint Caspase-8 kit (PerkinElmer Life Sciences) with a fluorometric assay. The U937 cells incubated with plasma for 24 h were processed according to the manufacturer's instruction and finally caspase-8 activity was measured in cell lysates at 615 nm in the VICTOR3 Multilabel Plate Reader (PerkinElmer Life Sciences). The data were expressed as signal to background (S/B) ratio. The activity of caspase-8, assayed in triplicate wells, was determined as a fold median increase comparing the signal from cells treated with plasma from CRS type 1 patients to the signal from cells treated with plasma from the control group.
Cytokine Enzyme-Linked Immunosorbent Assay
The quantitative determination of TNF-α and IL-6 productions in the plasma of CRS type 1 patients and controls was performed by the Human Instant enzyme-linked immunosorbent assay (ELISA) kit (eBioscience). Measuring the concentration of cytokines was performed according to the manufacturer's protocol and instructions. Optical density was read using a VICTOR3 Multilabel Plate Reader (PerkinElmer Life Sciences) at 450 nm. The amount of TNF-α (in picograms per milliliter) and IL-6 (in picograms per milliliter) was calculated from the standard curve according to the manufacturer's protocol. All tests were performed in triplicate.
Statistical Analysis
The results were expressed as median and IQR. The Mann-Whitney U test was used for comparison between two groups. Pearson's rank order correlation coefficient (r) was used to test the correlation between variables. A p value <0.05 was considered significant. Statistical analysis was performed using the SPSS (version 15; SPSS Inc., Chicago, Ill., USA).
Results
Effect of Plasma on U937 Apoptosis
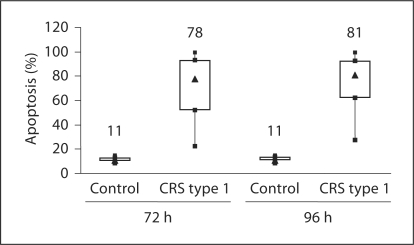
The U937 monocytes incubated with plasma from CRS type 1 patients showed significantly higher apoptosis rates (p < 0.001) compared with those incubated with plasma from controls. The level of apoptosis detected after 72 h incubation was 78% (IQR 52–93) in patients, while it was 11% (IQR 10–12.5) in the control group (fig. 2; table 2). After an incubation of 96 h, it was 81% (IQR 62–92) in patients versus 11% (IQR 10.5–13) in controls (fig. 2; table 2). There was no significant difference in the level of apoptosis comparing patients in AKI stage 1 to AKI stage 3.
Fig. 2.
Evaluation of percentage of apoptosis in U937 cells after incubation with plasma from CRS type 1 patients and healthy volunteers for 72 and 96 h.
Table 2.
Evaluation of apoptosis and caspase-8 activity in U937 cells after incubation with plasma from CRS type 1 patients and healthy volunteers
| CRS type 1 patients | Healthy controls | p value | |
|---|---|---|---|
| (n = 15) | (n = 20) | ||
| Plasma-induced apoptosis after 72 h, % | 78 (52.0–93.0) | 11 (10.0–12.5) | <0.001 |
| Plasma-induced apoptosis after 96 h, % | 81 (62.0–92.0) | 11 (10.5–13.0) | <0.001 |
| Caspase-8, S/B ratio | 0.91 (0.75–1.02) | 0.44 (0.41–0.52) | 0.01 |
Results (median with IQR) are given as percentage of apoptotic cells/field for apoptosis at 72 and 96 h or S/B ratio for caspase-8 activity at 24 h.
Our data showed a statistically significant association between baseline CKD stage V and apoptosis. At 72 h, plasma from patients in CKD stage V had a median apoptosis of 96% (IQR 83–100) as compared to 66% (IQR 46–85) in patients in CKD stages II–IV (p = 0.02). Similarly at 96 h, plasma from patients in CKD stage V had a median apoptosis of 96% (IQR 84–100) as compared to 73% (IQR 48–91) in patients in other CKD stages (p = 0.04).
Measurement of Caspase-8 Activity
The activity of caspase-8 was measured in U937 cells incubated for 24 h with heparinized plasma. In concordance with the apoptosis rate, U937 cells incubated with the plasma from CRS type 1 patients demonstrated a significantly higher caspase-8 activity compared to those of controls [0.91 (IQR 0.75–1.02) vs. 0.44 (IQR 0.41–0.52); p = 0.01] (table 2).
Cytokine Values in Plasma Samples
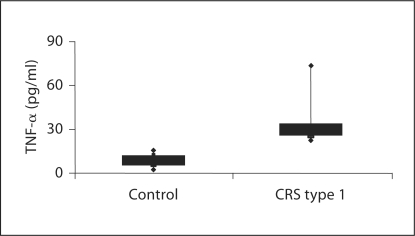
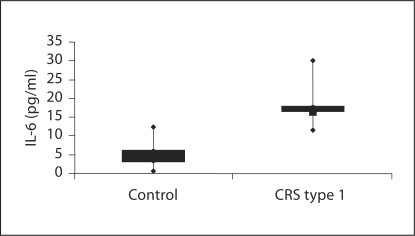
To examine potential mediators involved in the immune-mediated damage in CRS type 1 pathogenesis, TNF-α and IL-6 levels were measured by ELISA in the plasma of CRS type 1 patients and in controls. When compared with healthy control subjects, TNF-α levels were significantly elevated in the CRS type 1 group [28.49 pg/ml (IQR 25.05–33.43) vs. 2.39 pg/ml (IQR 1.47–4.10); p < 0.01] (fig. 3). Specifically, the median value of TNF-α was more than 10 times higher in CRS type 1 patients with respect to the control subjects. Furthermore, in CRS type 1 patients the proinflammatory cytokine IL-6 was significantly higher compared with that of the control group [4.8 pg/ml (IQR 2.97–6.154) vs. 16.5 pg/ml (IQR 16.22–17.89); p < 0.01 for both] (fig. 4). Specifically, the median value of IL-6 was more than 3 times increased in CRS type 1 patients with respect to the control subjects.
Fig. 3.
Evaluation of TNF-α in plasma from CRS type 1 patients and healthy volunteers.
Fig. 4.
Evaluation of IL-6 in plasma from CRS type 1 patients and healthy volunteers.
Discussion
It is postulated that CRS is due to a cellular and molecular crosstalk between heart and kidneys. Key physical, chemical and biological processes may be involved, including activation of the immune response, inflammation, release of cytokines and activation of apoptotic pathways (fig. 1). Furthermore, the role of innate immunity in acute tissue injury is well established, with engagement of complement, cytokines, and neutrophils [32].
In this pilot study, we examined the possible role of immune-mediated mechanisms involved in the pathogenesis of CRS type 1. Furthermore, we studied the in vitro response of monocytes, incubated with plasma from CRS type 1 patients, resulting in apoptosis, and we analyzed the plasma cytokine levels in these patients. Apoptosis occurs in both human and animal kidney during AKI and experimental evidence supports a pathogenic role for apoptosis in this process [33]. For example, cellular apoptosis has been demonstrated to occur alongside necrosis in experimental models of ischemia-reperfusion, toxin exposure, inflammation and septic AKI [33].
Numerous studies have reported that plasma levels of inflammatory markers and cytokines are increased in AKI and in heart failure. However, the multiple factors involved in the development of AKI during heart failure describe a pathogenesis of AKI accounting for multiple pathways. Recently, Goh et al. [34] proposed humoral signaling and the inflammatory pathways as new interesting mechanisms to explain distant organ damage and, in particular, kidney injury in heart failure. A marked pro-apoptotic activity was observed in the monocytes incubated with plasma from CRS type 1 patients. We found a significantly higher level of apoptosis in in vitro monocytes treated with CRS type 1 plasma compared to control group plasma. This finding suggests that AHF may partially affect the kidneys, the other organ involved in CRS, through a humoral signaling and an immunological way.
After 24 h, we studied caspase-8 activity and CRS type 1 induction of apoptosis to better understand the mechanism leading to apoptosis in the U937 cell line. Caspases play a central role in the initiation of the apoptotic cascade. When cells receive apoptosis-triggering signals through the activation of the death receptor, caspase-8 activation occurs in the cytoplasm. In fact, this protein is an upstream caspase and initiates a cascade of caspase activation, thereby leading to programmed cell death. We observed a 2-fold increase in caspase-8 in cells incubated with CRS type 1 plasma, which was associated with a higher extent of apoptotic death. This preliminary result suggests that the observed apoptotic death is more likely triggered by death receptor signaling.
It is likely that in U937 cells the observed apoptosis may be due to the presence of pro-apoptotic factors in the plasma from patients with CRS type 1. For this reason, we investigated cytokine levels in the plasma of these patients. We chose IL-6, which is a multifunctional cytokine that regulates immune responses and acute phase reactions, and TNF-α, which is a polypeptide cytokine produced by monocytes and macrophages and is a multipotent modulator of immune response. In fact, we observed a 3-fold higher level of IL-6 in plasma from CRS type 1 patients compared with that from controls and a 10-fold increase of TNF-α concentration in plasma from CRS type 1 patients. This finding suggests that CRS type 1 might have an inflammatory pattern due to various mediators that induce a pathological apoptosis in monocytes.
However, the observed increase in the levels of these inflammatory cytokines should not necessarily be interpreted as a central role for IL-6 and TNF-α in the pathogenesis of CRS type 1. It is possible that these elevated levels are dependent on a generalized inflammatory state. In addition, the single blood sampling for cytokine dosage could be influenced by the circadian rhythm they have and could be a limitation of our data. Therefore, other experiments are necessary to better understand the inflammatory aspects of CRS type 1 and the humoral signaling. Otherwise, the effect of the humoral signal for a distant organ could be proved an important contribution of the inflammatory pathway to the development of CRS type 1, and we can speculate that cytokines (TNF-α and interleukins) or other mediators, such as endothelin-1 and nitric oxide, play a role in the mechanism of CRS type 1. Other factors, such as the uremic milieu in AKI, may also be a trigger for apoptosis. In this pilot study, the severity of AKI did not appear to correlate with the apoptotic activity; however, the modest sample size precludes definitive conclusions.
We observed that plasma from CRS patients with advanced CKD at baseline triggered a higher apoptosis rate. This may be due to a greater accumulation of toxic factors during the acute event, as compared to patients who had a better baseline renal function. Abnormal apoptosis rates in chronic uremic patients have been previously reported by our group [18, 19]. Interestingly, the plasma of CRS type 1 subjects demonstrated a stronger apoptogenic effect compared with that reported in CKD patients from our prior studies (a more than 3-fold increase). This suggests that the markedly higher apoptotic level, compared with healthy controls, may be accounted for by different factors than baseline renal impairment and CKD. Indeed, in our CKD patients admitted with AHF, we postulate that an immune-mediated mechanism may play a role in the pathogenesis of CRS type 1, and the loss of the normal balance of the immune system may induce the deleterious effect of AHF on kidney function. Various factors, including inflammation and oxidative stress may also be implicated in an altered immune regulation and induce monocyte apoptosis, thus exacerbating tissue and organ damage. We might hypothesize that in a patient with AHF, oxidative stress, cytokine release and eventual derangement on the immune system may also directly lead to lethal renal cellular injury resulting in apoptosis, as well as myocardial contractile dysfunction and an increase in cardiomyocyte apoptosis.
This study explores the premise of an immune-mediated process in the pathophysiology of CRS type 1. Nevertheless, we acknowledge the limitations of the small sample size in this pilot study, which would preclude meaningful multivariate analysis. Our preliminary results can be considered hypothesis generating, and stimulate further exploration of novel pathophysiological mechanisms in CRS type 1. To better assess whether monocyte apoptosis is secondary to renal injury rather than the cause, we will require another control population, such as patients with AHF without AKI. We observed significant differences in apoptosis between CRS type 1 patients and healthy controls. Although these findings are provocative, the design of the study does not allow us to make conclusions about causality. Indeed, these changes in apoptotic rate could be secondary to renal injury rather than the cause. Such aspects could be clarified in future studies also evaluating AHF patients without CRS type 1. Nevertheless, investigations elucidating the paracrine signaling pathways and the molecular mechanism of apoptosis in CRS type 1 (death genes, signals and receptors) and determining the involved factors will better define the actual pathophysiological process.
In conclusion, we observed that plasma-induced apoptosis and caspase-8 activity were significantly higher in CRS type 1 patients; in addition, the plasma levels of IL-6 and TNF-α in CRS type 1 patients were significantly increased. These findings suggest that there is a defective regulation of monocyte apoptosis in these patients, and that an immune-mediated mechanism may play a role in the pathophysiology of this syndrome. Furthermore, these preliminary results suggest that inflammatory pathways may have a central role in the pathogenesis of CRS type 1 and may be fundamental in damage to distant organs.
This study adds to the current body of knowledge on CRS type 1 and provides hypotheses deserving further exploration in future studies.
Disclosure Statement
None of the authors has reported any conflicts of interest.
References
- 1.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA. Cardiorenal syndromes: pathophysiology to prevention. Int J Nephrol. 2011;2011:762590. doi: 10.4061/2011/762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17:2886–2891. doi: 10.1681/ASN.2006010063. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 6.Liang KV, Williams AW, Greene EL, Redfield MM. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med. 2008;36(1 suppl):S75–S88. doi: 10.1097/01.CCM.0000296270.41256.5C. [DOI] [PubMed] [Google Scholar]
- 7.Ronco C, House AA, Haapio M. Cardiorenal and renocardiac syndromes: the need for a comprehensive classification and consensus. Nat Clin Pract Nephrol. 2008;4:310–311. doi: 10.1038/ncpneph0803. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW, Nohria A, Mielniczuk L. Torrent or torment from the tubules? Challenge of the cardiorenal connections. J Am Coll Cardiol. 2005;45:2004–2007. doi: 10.1016/j.jacc.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Bongartz LG, Cramer MJ, Braam B. The cardiorenal connection. Hypertension. 2004;43:e14. doi: 10.1161/01.HYP.0000118521.06245.b8. [DOI] [PubMed] [Google Scholar]
- 10.Bright J, Khar A. Apoptosis: programmed cell death in health and disease. Biosci Rep. 1994;14:67–81. doi: 10.1007/BF01210302. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CR, Jarvis WD. Caspase-9 regulation: an update. Apoptosis. 2004;9:423–427. doi: 10.1023/B:APPT.0000031457.90890.13. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Malo A, Carracedo J, Ramirez R, Rodriguez-Benot A, Soriano S, Rodriguez M, Aljama P. Effect of uremia and dialysis modality on mononuclear cell apoptosis. J Am Soc Nephrol. 2000;11:936–942. doi: 10.1681/ASN.V115936. [DOI] [PubMed] [Google Scholar]
- 15.Schindler R, Boenisch O, Fischer C, Frei U. Effect of the hemodialysis membrane on the inflammatory reaction in vivo. Clin Nephrol. 2000;53:452–459. [PubMed] [Google Scholar]
- 16.Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 17.Heidenreich S, Schmidt M, Bachmann J, Harrach B. Apoptosis of monocytes cultured from long-term hemodialysis patients. Kidney Int. 1996;49:792–799. doi: 10.1038/ki.1996.110. [DOI] [PubMed] [Google Scholar]
- 18.D'Intini V, Bordoni V, Bolgan I, Bonello M, Brendolan A, Crepaldi C, Gastaldon F, Levin NW, Bellomo R, Ronco C. Monocyte apoptosis in uremia is normalized with continuous blood purification modalities. Blood Purif. 2004;22:9–12. doi: 10.1159/000074918. [DOI] [PubMed] [Google Scholar]
- 19.D'Intini V, Bordoni V, Fortunato A, Galloni E, Carta M, Galli F, Bolgan I, Inguaggiato P, Poulin S, Bonello M, Tetta C, Levin N, Ronco C. Longitudinal study of apoptosis in chronic uremic patients. Semin Dial. 2003;16:467–473. doi: 10.1046/j.1525-139x.2003.16101.x. [DOI] [PubMed] [Google Scholar]
- 20.Satoh M, Minami Y, Takahashi Y, Nakamura M. Immune modulation: role of the inflammatory cytokine cascade in the failing human heart. Curr Heart Fail Rep. 2008;5:69–74. doi: 10.1007/s11897-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 21.Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. doi: 10.1007/BF02938356. [DOI] [PubMed] [Google Scholar]
- 22.Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med. 2008;34:957–962. doi: 10.1007/s00134-008-1017-8. [DOI] [PubMed] [Google Scholar]
- 23.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 24.Baumgarten G, Knuefermann P, Mann DL. Cytokines as emerging targets in the treatment of heart failure. Trends Cardiovasc Med. 2000;10:216–223. doi: 10.1016/s1050-1738(00)00063-3. [DOI] [PubMed] [Google Scholar]
- 25.Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI) Clin Nephrol. 2011;76:165–173. doi: 10.5414/cn106921. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 29.de Cal M, Cruz DN, Corradi V, Nalesso F, Polanco N, Lentini P, Brendolan A, Tetta C, Ronco C. HLA-DR expression and apoptosis: a cross-sectional controlled study in hemodialysis and peritoneal dialysis patients. Blood Purif. 2008;26:249–254. doi: 10.1159/000122110. [DOI] [PubMed] [Google Scholar]
- 30.Bordoni V, Piroddi M, Galli F, de Cal M, Bonello M, Dimitri P, Salvatori G, Ranishta R, Levin N, Tetta C, Ronco C. Oxidant and carbonyl stress-related apoptosis in end-stage kidney disease: impact of membrane flux. Blood Purif. 2006;24:149–156. doi: 10.1159/000089452. [DOI] [PubMed] [Google Scholar]
- 31.Dini L, Coppola S, Ruzittu MT, Ghibelli L. Multiple pathways for apoptotic nuclear fragmentation. Exp Cell Res. 1996;223:340–347. doi: 10.1006/excr.1996.0089. [DOI] [PubMed] [Google Scholar]
- 32.Rabb H. Immune modulation of acute kidney injury. J Am Soc Nephrol. 2006;17:604–606. doi: 10.1681/ASN.2006010060. [DOI] [PubMed] [Google Scholar]
- 33.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh CY, Vizzi G, De Cal M, Ronco C. Cardiorenal syndrome: a complex series of combined heart/kidney disorders. Contrib Nephrol. 2011;174:33–45. doi: 10.1159/000329233. [DOI] [PubMed] [Google Scholar]