Abstract
Cardiovascular disease (CVD) and autoimmune diseases (ADs) are the first and third highest causes of death in the USA, respectively. Men have an increased incidence of the majority of CVDs, including atherosclerosis, myocarditis, dilated cardiomyopathy and heart failure. By contrast, nearly 80% of all ADs occur in women. However, in one category of ADs, rheumatic diseases, CVD is the main cause of death. Factors that link rheumatic ADs to CVD are inflammation and the presence of autoantibodies. In this review we will examine recent findings regarding sex differences in the immunopathogenesis of CVD and ADs, explore possible reasons for the increased occurrence of CVD within rheumatic ADs and discuss whether autoantibodies, including rheumatoid factor, could be involved in disease pathogenesis.
Keywords: autoimmune disease, cardiovascular disease, pathogenesis, rheumatic disease, sex differences
Cardiovascular diseases (CVDs) and autoimmune diseases (ADs) are two of the highest causes of death in the USA [1,2]. Men have an increased incidence and severity of most CVDs, including atherosclerosis, myocardial infarction (MI), myocarditis, dilated cardiomyopathy (DCM) and heart failure, with the exception of hypertension, which is higher in women [1,3,4]. By contrast, nearly 80% of all ADs occur in women [2,5]. Although CVD occurs more frequently in women with rheumatic ADs such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis, myositis, Sjögren’s syndrome (SS) and antiphospholipid syndrome (APS) as compared with women without disease [6–8], several recent studies have shown that men with SLE are more likely to develop atherosclerosis [9–11]. Additionally, men with SLE develop more severe disease (increased lupus nephritis) and have a worse outcome (increased renal failure, MI and death) than women with SLE [12,13]. In RA, overall mortality due to CVD appears to increase in both sexes, yet male sex predicts cardiovascular events and death [11,14–16]. There is increasing evidence that autoimmunity plays an important role in the pathogenesis of inflammatory heart diseases such as atherosclerosis, myocarditis and DCM [6,17]. Both cell and antibody (Ab)-mediated pathology contribute to disease progression in ADs and CVDs [18]. In this review we will examine recent findings regarding sex differences in the immunopathogenesis of CVD and AD, and explore possible reasons for the increased occurrence of CVD within rheumatic ADs, including the pathogenic role for autoantibodies (autoAbs).
CVD is more prevalent in men
Sex hormones play a major role in the pathogenesis of CVD, as reflected by a higher incidence and severity of CVD in men than women across all age groups [1,19]. Estrogens are protective for heart physiology and prevent or delay CVD [19–21]. Evidence that the incidence of CVD increases in women as estrogen declines with age and menopause, further supports a protective role for estrogens in the heart. Sex differences in the incidence of CVD are also influenced by gender differences in cardiovascular risk factors and presentation of disease [22,23]. Although CVD occurs more frequently in men, it is also the leading cause of death in women, just as it is for men. For this reason it is critical that researchers and clinicians gain a better understanding of the factors that contribute to CVD in both men and women [24].
Sex differences in normal cardiovascular physiology
Sex differences exist in normal heart physiology and function. For example, men have larger hearts (left ventricular mass), cardiac contractility is greater in healthy women than age-matched men, myocardial mass is better preserved in women as they age, cardiac apoptosis is threefold higher in men compared with women as they age, women have smaller coronary vessels than men and pre-menopausal women have lower blood pressure but a faster resting heart rate than men [25,26]. Many other sex-specific differences exist in the hearts of apparently normal men and women according to microarray and proteomic analyses [27]. For example, in the aging female heart, hypertrophy, apoptosis and fibrosis are less pronounced than in the aging male heart [20]. These underlying sex differences in cardiac function directly influence the immune response to infection and injury in the heart.
Sex differences in the immune response
The immune system under normal and pathological conditions is extensively regulated by sex hormones [28]. Sex steroid hormone receptors such as estrogen receptor (ER)-α, ER-β rogen receptor and aromatase, the enzyme that converts androgens to estrogens, are expressed in vascular endothelial cells, vascular smooth muscle cells, cardiac fibroblasts and cardiomyocytes in humans and rodents [29]. Steroid receptors regulate gene expression by classic genomic actions as well as membrane-associated receptor signaling. Surface expression of sex steroid receptors in particular is likely to be important in influencing the immune response.
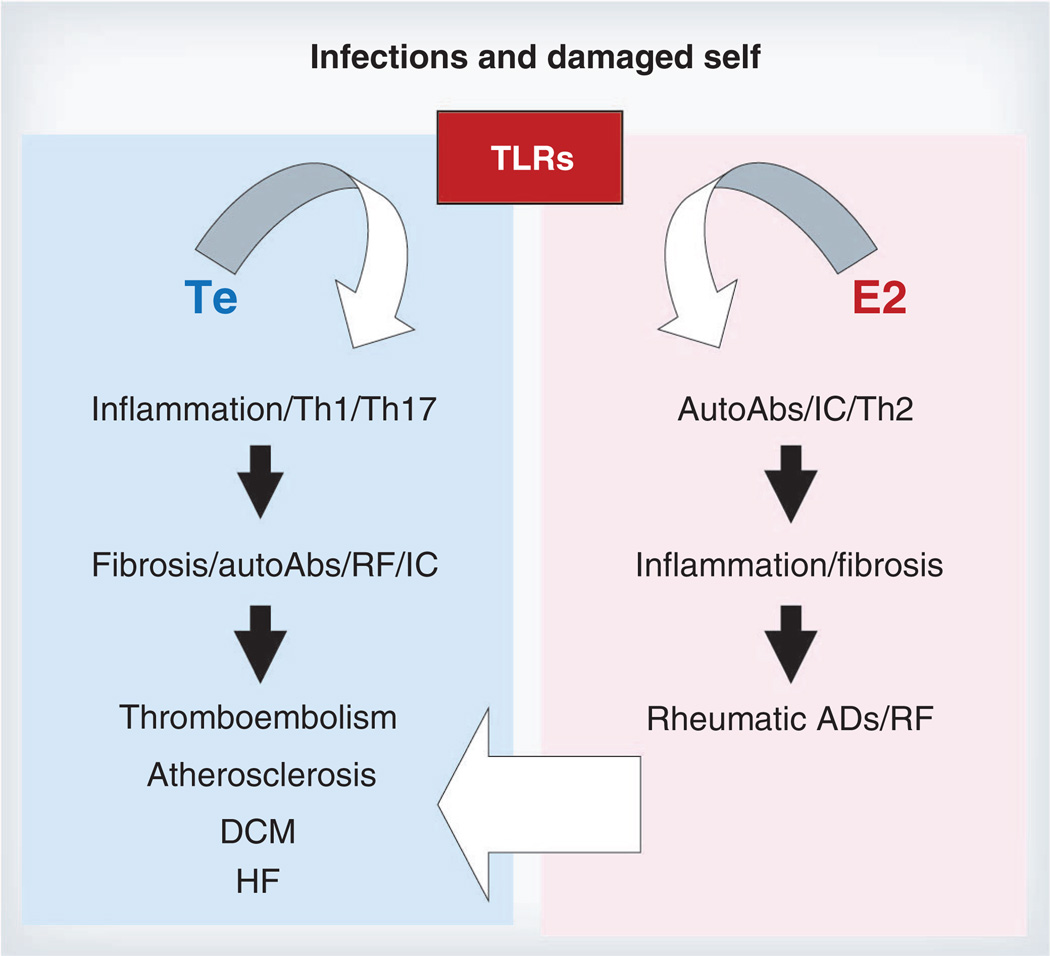
Estrogen is known to stimulate the immune system while androgens are recognized to be immunosuppressive [28,30]. However, descriptions of the role of androgens and estrogens on the immune response often do not take into account differences in the effect of sex hormones on the innate and adaptive arms of the immune response. This distinction between innate and adaptive immunity is critical in providing a framework for understanding how CVDs can be increased in men while ADs are increased in women [18]. Other important considerations include dose, timing and target organ effects of sex hormones [31]. Estrogen is well known to activate B cells, resulting in increased Ab and autoAb responses to infection, vaccines and autoantigens [5,28,31,32]. By contrast, androgens decrease B-cell maturation, reduce B-cell synthesis of Ab, and suppress autoAb production in humans with SLE [28]. Estrogen has been shown both in culture and in animal models to induce differentiation of CD11b+ dendritic cells (DCs) [33] and to increase Th1 and/ or Th17 responses, increasing inflammation via transcriptional activation of NF-κB [31,34,35]. Estrogens are also able to increase the regulatory arm of the adaptive immune response by enhancing tolerogenic DCs, IL-4-driven Th2 responses, TGF-β, PD-1 receptor expression on immune cells, Treg populations, and alternatively activated macrophages [5,31,36–40]. The capacity of estrogen to decrease cell-mediated immune responses by increasing regulatory mechanisms is probably due to its ability to inhibit components of the innate immune response including Toll-like receptors (TLRs) [18,28,37,41]. Far less research has been conducted on the role of androgens on immunity, but generally androgens have been found to increase Th1 responses [18,28,42]. Collectively, these data support the idea that the ability of estrogen to increase adaptive immune responses (e.g., autoAbs, autoreactive B and T cells) could increase ADs in women, while the ability of androgen to increase innate immune responses (e.g., TLRs, mast cells, macrophages) could increase inflammatory heart disease (Figure 1) [5,38,41,43–45]. Additionally, women may be particularly sensitive to lower circulating vitamin D levels, which may counteract some of the protective effects of estrogen and therefore increase AD in women [39].
Figure 1. Proposed role of sex hormones in regulating immune function during autoimmune and cardiovascular disease.
Toll-like receptors (TLRs) are activated by infections and damaged self. In men, Te promotes a proinflammatory Th1 and/or Th17-type immune response that leads to cardiac remodeling and fibrosis. In men, AutoAbs such as RF and ICs contribute to atherosclerosis and thrombosis and promote progression to DCM and HF following myocarditis. By contrast, E2 promotes a regulatory Th2 response in women that leads to increased autoAbs following infection and/or tissue damage. IC deposition in females promotes inflammation and fibrosis that leads to rheumatic AD. Additionally, a Th2-skewed response in females may allow infections to persist, resulting in increased RF, ICs and inflammation that predisposes women to premature cardiovascular disease.
AutoAb: Autoantibody; AD: Autoimmune disease; DCM: Dilated cardiomyopathy; E2: Estrogen; HF: Heart failure; IC: Immune complex; RF: Rheumatoid factor; Te: Testosterone; TLR: Toll-like receptor.
Sex differences in autoimmune heart disease
Autoimmune disease occurs when cell and Ab-mediated immune responses develop against self antigens, resulting in systemic or organ-specific damage [6,18]. For some time it has been appreciated that both genetic background and the environment contribute to the development of ADs [2]. A recent understanding of the interaction of genes with the environment has revealed the importance of epigenetic mechanisms and the influence of the X and Y chromosomes on disease pathogenesis (reviewed in [46–52]). Research in this area is progressing rapidly and will provide critical insight into the role of sex in determining the development of AD and CVD. The following sections focus on the influence of sex hormones on the immunopathology of disease.
Atherosclerosis
Approximately half of all deaths worldwide can be attributed to CVD and atherosclerosis, or coronary artery disease (CAD) in particular [1]. The pathology of atherosclerosis is characterized by atheromas or fibro-fatty plaques that protrude into and obstruct vessels and compromise blood flow to the heart (and other organs), resulting in ischemic heart disease [53,54]. MI (heart attack), cerebral infarction (stroke), aortic aneurysms and peripheral vascular disease are the major consequences of atherosclerosis. MI alone accounts for approximately 20–25% of all deaths in the USA [55]. The key processes in atherosclerosis are inflammation, lipid accumulation, intimal thickening and fibrosis [53]. Macrophages are the main inflammatory cell type in atherosclerotic plaques, with a 4:1 to 10:1 ratio of macrophages to T cells in human atherosclerotic lesions [53,54]. When macrophages engulf lipoprotein particles they become foam cells. The atherosclerotic plaque evolves to include a core of apoptotic and necrotic cells, cell debris and cholesterol crystals surrounded by inflammatory cells that include T cells and mast cells in addition to foam cells, vascular smooth muscle cells and a fibrous cap composed mostly of collagen [53]. Plaque growth can narrow the arterial lumen, but thrombosis at the site of plaque rupture is the proximal cause of MI. Deposition of autoAbs and/or immune complexes (ICs) at the plaque site may precipitate thromboembolism [6,56].
The presence of autoAbs and autoreactive T cells against oxidized low-density lipoprotein (oxLDL)/ low-density lipoprotein (LDL), Hsp60/65, β2-glycoprotein-I and/ or collagen suggest that atherosclerosis may be considered an AD [6,57,58]. T-cell clones reactive to oxLDL have been isolated from human plaques and LDL autoAbs are abundant in atherosclerosis patients [53,56,59]. In one study, autoAbs against oxLDL discriminated between atherosclerotic and control patients better than other lipoproteins such as total cholesterol or LDL levels [59]. A correlation exists between oxLDL–autoAb titer and the extent of atherosclerosis, cardiovascular complications and the risk of restenosis following angioplasty [6]. Elevated oxLDL/β2-glycoprotein-I autoAb complexes are associated with more severe CAD and predict a 3.5-fold increased risk for adverse outcomes, including arterial thrombosis [60]. Further evidence that atherosclerosis could be an AD comes from animal studies where atherosclerosis is accelerated after immunization with the autoantigens Hsp65 or β2-glycoprotein- I, or following transfer of β2-glycoprotein-I-autoreactive T cells [6]. Additionally, regulatory B and T cells, as well as intravenous administration of total IgG (IVIg), have been found to protect against atherosclerosis in mice, similar to their role in inhibiting ‘conventional’ ADs [6,61,62]. These findings indicate that autoAbs and autoreactive T cells participate in the pathogenesis of disease.
The innate immune response plays a major role in the initiation and development of atherosclerosis [45]. Macrophages and the endothelium of normal and atherosclerotic arteries express a broad range of TLRs including TLR1, 2, 3, 4, 5, 7 and 9. Signaling via the major TLR signaling adaptor protein, MyD88, is critically important for atherosclerosis development in mouse models of disease [63]. For example, oxLDL binds TLR2 and TLR4 resulting in activation of the inflammasome and IL-1β and IL-18 production, which generates a Th1 response (IL-18 is a potent inducer of IFN-γ) [53,64–67]. Thus, autoAb-ICs such as oxLDL/β2-glycoprotein-I activate macrophages to form foam cells, stimulate an innate proinflammatory response, and promote thromboembolism.
The prevalence and severity of atherosclerosis among individuals and groups is related to a number of major risk factors including age, sex, tobacco use and genetics. Men are at a far greater risk for developing atherosclerosis than women [1,19,68,69]. Although the increased incidence of CVD in men has been partially attributed to gender differences in smoking habits, recent studies on the effect of sex hormones on CVD indicate that testosterone may directly increase inflammation and cholesterol levels in men [19].
Human monocytes and macrophages express ER-α and ER-β, but in mice ER-α appears to only be expressed in macrophages [70]. Females are reported to have higher levels of ERs in their arteries than males, which decrease with age/menopause [19]. Similarly, androgen receptors are expressed at higher levels on/in macrophages in men than in women [71], and are present in mouse macrophages [70]. The androgen receptor was found to increase lipid loading in human macrophages [71], while estrogen prevented cholesterol accumulation in macrophages by reducing expression of the LDL scavenger receptor, CD36 [72,73]. Estrogen via ER-β signaling has been shown to regulate artery tone and blood pressure, while ER-α has been shown to mediate protection against vascular injury and atherosclerosis [19,74]. Additionally, estrogen has been found to decrease lipopolysaccharide-/TLR4-induced IL-1β, IL-6 and TNF-α production and NF-κB activation from splenic murine macrophages [75,76]. Studies examining whether sex differences exist in autoAbs during atherosclerosis are lacking. One study found that there were no sex differences in oxLDL levels in the sera of men and women that did not have CVD [77].
Males develop atherosclerotic plaques earlier and more extensively than females, suggesting that testosterone increases atherosclerosis in males [19,21]. This was confirmed in a study where treatment of atherosclerosis-prone male mice with testosterone increased atherosclerosis, while suppression of testosterone decreased atherosclerosis in male but not female mice [78]. However, men with CAD have lower circulating levels of testosterone than normal controls, suggesting that higher testosterone levels may be cardioprotective [79]. There could be several explanations for these findings. Higher concentrations of testosterone may be converted to cardioprotective estrogens by aromatase, circulating testosterone levels may not reflect cardiac level or function, or low testosterone may increase other cardiovascular risk factors. In support of this idea, low testosterone levels in men have been found to predict insulin resistance and the development of Type 2 diabetes and to be associated with high blood pressure [19], all of which are risk factors for CVD. However, studies to date suggest that estrogen protects against atherosclerosis, while androgens increase disease.
Myocarditis & dilated cardiomyopathy
Myocarditis, or inflammation of the myocardium, leads to a significant minority of DCM cases in the US [1,80–82]. DCM is the most common form of cardiomyopathy requiring a heart transplant [3,80]. Between 4 and 20% of sudden cardiovascular deaths among young adults, the military and athletes are due to myocarditis [82]. However, the true incidence and prevalence of myocarditis are unknown due to the lack of widely available, safe and accurate noninvasive diagnostic tests [83]. Viral infection is the most commonly identified cause of myocarditis in developed countries (Box 1), and other rare etiologies include bacteria, protozoa, toxins, drug reactions and sarcoidosis [82,84]. Most cases of suspected myocarditis are not linked to a specific cause [85]. Coxsackievirus B3 (CVB3) infection, a common cause of myocarditis, has recently been implicated in up to 40% of patients who died suddenly of MI [86], suggesting a possible etiologic link between myocarditis and MI. Infections are believed to induce or trigger autoimmunity [87–89]. Similar to atherosclerosis, myocarditis and DCM occur more frequently in men than women [3,4]. A recent study of myocarditis/acute DCM patients found that myocardial recovery and transplant-free survival were significantly worse in men – driven by a marked difference in survival [4].
Box 1. Infectious causes of myocarditis.
Viral
Bacterial
Chlamydia
Cholera
Mycoplasma
Neisseria
Salmonella
Staphylococcus
Streptococcus
Tetanus
TB
Spirochetes
Leptospirosis
Lyme disease
Relapsing fever
Syphilis
Protozoa
Chagas disease
Leishmaniasis
Malaria
†Frequent cause of myocarditis.
Adapted from [84].
Myocarditis by definition is inflammation of the myocardium. However, in most clinical cases and animal models, myocardial inflammation also involves the pericardium. Similar to findings in clinical biopsies of myocarditis patients [3], the primary infiltrate found in mouse models of myocarditis consists of macrophages and neutrophils with lower levels of T cells, B cells, mast cells and DCs [41,90,91]. Interestingly, the cellular composition during acute myocarditis is similar to the infiltrate of atherosclerotic plaques. NK cells, CD8+ T cells and γδT cells, needed for antiviral defense, are also important in the early cellular response in viral animal models of myocarditis [92–94]. Regulatory mechanisms such as Tim-3+ CD4+ T cells, alternatively activated macrophages and Tregs are elevated at the later stage of acute myocarditis, resulting in dispersion of acute inflammation [41,94–96]. Several weeks later, in susceptible strains of mice, a low-level inflammation re-emerges that is associated with myocyte necrosis, fibrosis and DCM [88]. Acute myocarditis is characterized by a predominantly Th1 and/or Th17 response [97–100]. However, only mice that respond to infection or self antigen with a Th2 response, such as BALB/c and A/J strains, develop the chronic stage of myocarditis associated with fibrosis and DCM [18,88,101].
The innate immune response to infection is critical to the development of CVDs, including myocarditis, DCM, heart failure and atherosclerosis [102]. Activation of innate sensors such as TLRs and the inflammasome result in distinct cytokine profiles that drive the adaptive immune response. TNF-α, IL-1β and IL-18 have all been shown to play an important role in myocarditis by inducing myocyte hypertrophy, contractile dysfunction, myocyte apoptosis, and contributing to extracellular matrix (ECM) remodeling – a critical step in the progression from myocarditis to DCM [103–106]. Out of all the TLRs that have been described so far in humans and mice, TLR4 is unique in its ability to work with the inflammasome to produce bioactive IL-1β and IL-18 in the heart [104,107]. TLR2 and TLR4 signaling increase TNF levels and TLR2 can act with TLR4 to increase IL-1β levels [107]. TLR2 and TLR4, in particular, are able to induce an immune response to damaged self proteins including cardiac myosin [102,108]. Tlr4 mRNA expression has been found to be higher in patients with myocarditis than controls and to correlate with viral RNA levels in the heart [109]. Myocarditis patients with active viral replication had higher levels of TLR4 that was associated with lower systolic function. We found that TLR4-deficient mice develop reduced inflammation and lower IL-1β and IL-18 levels in the heart during acute CVB3 myocarditis [104]. IL-1β is known to induce cardiac remodeling that leads to fibrosis, DCM and heart failure following acute myocarditis [103,106,110,111]. The importance of TLR4 signaling in a strictly autoimmune model of myocarditis was demonstrated by Nishikubo et al. where TLR4 signaling was found to be necessary to mount a Th1-type immune response [112]. We have shown that TLR4 is upregulated in macrophages and mast cells during the innate immune response to CVB3 as early as 12 h after infection and during acute CVB3 myocarditis [41]. A number of TLRs important in the response to viral infections (e.g., TLR3, TLR7, TLR9) and their downstream adaptors, MyD88 and TRIF, have been shown to be important in protecting against myocarditis in animal models of viral myocarditis [101,102,113–116]. The data so far suggests that viral-specific TLRs, such as TLR3 and TLR9, protect against myocarditis while TLR2 and TLR4, which are activated by infection and damaged self, increase disease.
Huber and colleagues were the first to describe that male mice have increased CVB3-induced myocarditis compared with females [117]. The amplified inflammatory response in males is not due to increased viral replication, which is similar between the sexes [41,118]. Innate immune responses to CVB3 are critical to sex differences in acute CVB3 myocarditis. In a mouse model of CVB3 myocarditis using purified CVB3, males had a higher cardiac infiltration of γδT cells, macrophages and T cells than female mice and an increased Th1 response [119]. By contrast, female mice had a higher protective Th2 response and more Tregs [119–121]. Similarly, in a mouse model where CVB3 and heart proteins were injected, males experienced increased cardiac TLR4+ CD11b+ inflammation including macrophages, neutrophils, mast cells and DCs and a predominant Th1 response, while females had increased B cells, inhibitory Tim-3+ CD4+ T cells, Tregs and a Th2 response [41,95]. We demonstrated that the predominant Th1 response in male mice was due to TLR4-derived IL-18, originally named IFN-γ-inducing factor, rather than to a classical IL-12/ STAT4-induced Th1 response [122]. Gonadectomy reduces CD11b+ inflammation, and specifically macrophages, in the heart of male mice during acute myocarditis and causes a reversal of the Th1 response in males to a Th2 response with increased Tim-3+ T cells and Treg [43]. To our surprise, TLR4 was only expressed on alternatively activated macrophages, cells that require IL-4 or a Th2 response [43,96]. These findings suggest that IL-1β produced by TLR4+ alternatively activated macrophages in males in susceptible Th2-responding BALB/c and A/J mouse strains is critical for the induction of fibrosis that leads to DCM.
TLR4 expression is significantly elevated on macrophages and mast cells in the spleen and peritoneum of male BALB/c mice compared with females as early as 12 h after infection with CVB3 and remains elevated in the heart of males with myocarditis [41]. We recently showed that, 12 h after CVB3 infection, male BALB/c mice upregulate genes in the spleen that are associated with the development of CVD and heart failure [44], indicating the importance of the innate inflammatory response in the progression of disease. This idea is supported by a clinical study that found that inflammation was the best predictor of progression from myocarditis to DCM in patients [123]. Additionally, in patients with acute myocarditis, men have been found to have more myocardial fibrosis than women [124]. In mice, testosterone administration was able to increase myocardial inflammation and fibrosis in males but not females in a MI model [125].
Although the role of inflammation in the pathogenesis of myocarditis and DCM is clear, the role for autoAbs in the pathogenesis of disease is less clear. AutoAbs are produced in response to cardiac injury and/or molecular mimicry to pathogens and thus serve as markers of injury [126,127]. Additionally, autoAbs may stimulate the immune response via ICs or directly alter cardiac or immune function by stimulating receptors including the β-adrenergic receptor or the M2 muscarinic receptor [128–130]. Circulating antimyosin autoAbs are found in patients with myocarditis, DCM, Chagas disease, Kawasaki disease, rheumatic fever and following MI [129]. In myocarditis/DCM patients, antimyosin Abs were found to bind to the S2 region of cardiac myosin and to activate β-adrenergic receptors, which control heart rate and contractility [131,132]. Overstimulation of this receptor can lead to cardiomyocyte death, ECM remodeling and heart failure in animal models [133]. These data suggest that autoAbs contribute to disease progression; however, whether sex differences exist in autoAbs or antimyosin isotypes is yet to be investigated. The majority of research on sex differences in CVD has centered on the role of sex hormones in heart failure.
Heart failure
Heart failure is the end consequence of a number of inflammatory cardiovascular conditions including atherosclerosis, myocarditis and chronic DCM. Most cases of heart failure are caused by reduced myocardial contractile function (systolic dysfunction), which occurs during ischemic injury, pressure or volume overload and during DCM. However, heart failure can also occur because of an inability to relax or fill the ventricle during diastole, in part due to myocardial fibrosis in the setting of preserved systolic function. Patients with chronic heart failure who have elevated levels of inflammatory mediators have a worse prognosis [134]. Antiviral treatments such as IFN-β may reduce myocarditis and heart failure in animal models and possibly patients, implying that viral infections are an important cause of myocarditis cases that lead to DCM and heart failure [123,135]. Male sex is an important risk factor for developing inflammatory DCM and heart failure, just as it is a risk factor for atherosclerosis and myocarditis.
Men with heart failure have a worse prognosis than women and a higher mortality [20]. The incidence of heart failure increases dramatically in women postmenopause, with the strongest predictor of mortality in women being age, whereas in men it is New York Heart Association (NYHA) classification [20]. Men and women with heart failure present with different clinical manifestations. In the Euro Heart Survey, men were found to develop systolic heart failure whereas more often women had preserved ejection fraction and diastolic heart failure [136]. Hypertension, obesity and diabetes are more likely to be risk factors in women, while CAD and DCM are risk factors in men [20,68]. In animal models of heart failure and in end-stage failing human hearts, gene-expression profiles reveal an increase in ECM remodeling genes in males compared with females [124,125,137], similar to myocarditis and DCM.
Thus, a common theme in all autoimmune heart diseases is the importance of innate activation of the immune response via TLRs (e.g., TLR2 and TLR4), the induction of proinflammatory and profibrotic cytokines (e.g., Th1 and IL-1β) and remodeling that leads to fibrosis, dilation and heart failure. Testosterone has been associated with, or shown to increase, all of these factors in clinical studies and/or animal models of disease.
Why are ADs more prevalent in women?
ADs affect approximately 8% of the population, and of those individuals approximately 78% are women [2,138]. There are a number of hypotheses to explain this gender difference, including the effect of sex hormones, microchimerism, genes on X or Y chromosomes, X chromosome inactivation and differing responses to environmental factors (reviewed in [5,18,46–48,139– 141]). Estrogen may directly increase ADs in women by elevating autoAbs and amplifying autoreactive T- and B-cell responses [18]. One unanswered question is why CVD is increased in rheumatic diseases including SLE, RA, systemic sclerosis and SS. These are more prevalent in women; SLE has an incidence of 9:1, SS 9:1, systemic sclerosis 4:1, RA 2–3:1, and myositis 2:1 in women versus men [6,7,9,14,18,139,142,143]. A better understanding of how sex hormones influence disease pathogenesis may provide clues to help answer these questions.
Systemic lupus erythematosus
SLE is a common multisystem autoimmune disease of unknown etiology that often presents as a photosensitive rash, polyarthritis, and/or renal disease. SLE occurs more frequently in women than men, at a ratio of 9:1, with an onset of disease at 20–30 years of age [12,18,144–146]. Infections such as Epstein–Barr virus, parvovirus B19 (PVB19) and CMV have been associated with the development of SLE [146,147]. SLE is a disease of multiple immune system abnormalities (Box 2). Factors involved in disease pathogenesis include defects in antigen presentation (e.g., HLA DR/DQ), poor clearance of ICs and/or apoptotic material by macrophages because of complement defects (e.g., CD11b, C1q, CRP, MBL, FcRs), transcriptional regulation of interferons (e.g., TLR7, TLR8, TLR9, IRF3, IRF5), elevated proinflammatory cytokines such as TNF, IL-6, and IFN-α/β, dysregulated B cells (e.g., BLyS), and elevated autoAbs (e.g., antinuclear Abs) [145–147].
Box 2. Risk factors common to atherosclerosis, systemic lupus erythematosus and rheumatoid arthritis.
Family history
Autoantibodies
Inflammation, e.g., CRP, complement activation
Infections, e.g., CMV
Toxins, e.g., tobacco smoke
Oxidative stress, e.g., oxLDL
Innate TLR activation e.g., IFN-α/β
Proinflammatory/profibrotic cytokines, e.g., TNF-α, IL-1β, IL-6 and osteopontin
Hyperlipidemia, e.g., LDL
Lp(a)
Hypertension
Stress
CRP: C-reactive protein; LDL: Low-density lipoprotein; Lp(a): Lipoprotein(a); oxLDL: Oxidized low-density lipoprotein; TLR: Toll-like receptor.
Data taken from [11].
Some studies suggest that women with SLE have abnormally high levels of active estrogen metabolites that could increase disease [146,148]. Recently, Wang et al. showed that polymorphisms in ER genes were associated with SLE, providing a mechanism for how estrogen metabolites could increase the risk for SLE in women [149]. Estrogen has been found to increase autoAbs and IC deposition in animal models of lupus [144,150]. Many lines of evidence also link elevated type I interferons IFN-α and IFN-β with worse SLE pathogenesis and women with SLE have higher levels of IFN-α than men [145,151]. TLR7, TLR8 and TLR9 are important in protection from viral infections, but are also activated by nucleic acid (i.e., nuclear antigens) and ICs, critical components of the elevated Ab response to damaged self found in SLE patients [152]. Interestingly, the genes for TLR7 and TLR8 are located on the X chromosome in humans and mice, which could contribute to elevated interferons in women with SLE [145]. Additionally, estrogen has been shown to activate DCs, which in the context of activation of TLRs by infections, may release interferons and activate macrophages [151–153]. Osteopontin is a cytokine involved in immune cell signaling and ECM remodeling that has been found to be overexpressed in biopsies from SLE patients [145] and is also elevated in heart diseases including CAD, MI, hypertrophy and myocarditis [154]. Interestingly, analysis by sex revealed that the relationship between osteopontin and SLE occurs only in men and not women with disease [155]. A summary of some of the known sex differences in SLE are listed in Box 3.
Box 3. Sex differences in systemic lupus erythematosus.
The prevalence of SLE is higher in women under 40 and the female to male ratio is 9:1.
SLE is often more severe in men than women.
Cardiovascular diseases such as atherosclerosis are more common in men than women with SLE.
Women with SLE are more likely to have malar rash, photosensitivity, oral ulcers, alopecia, Raynaud’s syndrome or arthralgia.
Men with SLE are more likely to have cardiovascular disease, peripheral vascular disease, myocardial infarction, thrombosis, neuropsychiatric symptoms or renal damage.
Men with SLE have poorer survival rates than women.
SLE: Systemic lupus erythematosus.
The major CVD found in SLE patients is atherosclerosis/vascular thrombosis. The risk of developing atherosclerosis/CAD in SLE patients is four- to eight-fold higher than in controls, and in young women the risk of MI is increased 50-fold [156]. However, in a recent study of 14,829 SLE patients, male SLE patients (10% of patients) were more likely to have cardiovascular and renal comorbidities compared with females [157]. Although SLE patients who develop atherosclerosis are more likely to be male and have classical CAD risk factors such as hypertension and obesity [9,158], recent studies suggest that increased CVD risk in SLE patients cannot be fully explained by CVD risk factors alone and that SLE disease-specific factors also play a role [159,160]. Rarely, patients with SLE develop pericarditis, myocarditis, endocarditis or valvular disease (Box 4) [156,161]. The major cause of death in SLE patients is premature atherosclerosis [156,162]. Very rarely, myocarditis associated with fibrosis in SLE patients may progress to DCM and heart failure. One post-mortem study of SLE patients revealed a high prevalence of pericarditis of 63% [161]. Vascular occlusion in SLE may be due to vasculitis (i.e., IC deposition), vasculopathy, atherosclerosis and/or thrombosis due to APS (Box 4). APS is associated with the presence of antiphospholipid autoAbs that induce vascular thrombosis and pregnancy loss. Although APS is most frequently associated with SLE, it can occur as an independent clinical entity [142]. Lupus anticoagulant is the antiphospholipid Ab that confers the greatest risk of thrombosis. In a prospective study, the presence of lupus anticoagulant was associated with thrombotic events (i.e., MI), but was not associated with subclinical atherosclerosis (i.e., carotid plaques) [163]. IC deposition may drive a local inflammatory response involving the vascular tissue, pericardium and/or myocardium, resulting in thrombosis [156,161]. oxLDL autoAbs may also be present in APS patients and antiphospholipid autoAbs have been found to accelerate the influx of oxLDL into macrophages further promoting atherosclerosis. Thus, the ability of autoAb/ICs in SLE to increase inflammation and trigger thromboembolic events may increase CVDs in women (Figure 1).
Box 4. Cardiac involvement in rheumatic autoimmune diseases.
Systemic lupus erythematosus
Atherosclerosis
Pericarditis
Myocarditis
Valve disease
Vascular thrombosis
Hypertension
Rheumatoid arthritis
Atherosclerosis
Pericarditis
Myocarditis
Valve disease
Vascular thrombosis
Scleroderma
Atherosclerosis
Vasculitis
Myocarditis
Myositis
Atherosclerosis
Pericarditis
Myocarditis
Valve disease
Congestive heart failure
Hypertension
Antiphospholipid syndrome
Thrombosis
Valve disease
Mural thrombi
Myositis: also called dermatomyositis; Scleroderma: also called systemic sclerosis.
Data taken from [199].
Rheumatoid arthritis
RA is a common inflammatory form of arthritis affecting approximately 1–2% of the population. The incidence of RA is two- to three-fold higher in females than males [18]. Anticyclic citrullinated protein Abs are an early biomarker of RA. Abs directed against peptides and proteins containing citrulline, a modified form of the amino acid arginine, are highly specific for RA [164]. Citrullination is a normal physiological process that occurs within cells undergoing cell death and citrullinated proteins are only released for recognition by the immune response if poor clearance of apoptotic or necrotic cells occurs [164]. RA patients also have elevated levels of oxLDL autoAbs in their sera [165]. Inflammation in the synovium during RA includes CD4+ T cells, macrophages, and mast cells with increased levels of TNF, IL-1β and IL-6 [166], a composition similar to that observed in atherosclerosis and myocarditis patients. Elevated levels of proinflammatory and profibrotic cytokines in RA patients contribute to increased inflammation and fibrosis. Unregulated autoAb production from B cells leads to IC deposition, which along with proinflammatory cytokines, recruits more inflammation and promotes ECM remodeling of the joint.
RA is more prevalent in women before age 50, but disease severity is greater in women after 50 years of age [31]. AutoAbs are known to increase with age, and so may contribute to increased IC deposition and damage in older women. The increased autoAb and IC response in women compared with men suggests a role for estrogen in the pathology of disease. However, many investigators have reported that estrogen protects against arthritis in animal models of RA [18,167]. Based on results found in animal models, recent clinical studies examined the effect of ER-α or ER-β agonists on RA. In two separate studies ER agonists were found to be ineffective at reducing the inflammatory response during RA [168,169]. What could account for the discrepancy between animal models and clinical RA? One possibility is that the acute inflammation induced by adjuvants in animal models may be effectively inhibited by estrogen, particularly if estrogen is administered during initiation of the innate immune response [18]. Evidence in support of a role for estrogen in promoting disease comes from a study that found that free estrogen levels in the synovial fluid of men with RA were increased twofold compared with control men, but were similar to estrogen levels in women with RA [170]. In addition, incidence rates of RA in men increase with age as androgen levels decrease and Th2 responses and autoAbs increase [171]. Overall, these findings suggest that estrogen increases RA-mediated pathology.
Numerous studies have shown that RA patients exhibit many of the traditional CVD risk factors, such as hypertension and hyperlipidemia, although several studies found no relationship [11,172]. For this reason it is not surprising that RA is associated with an increased cardiovascular morbidity and mortality compared with the normal population, which accounts for 30–50% of RA deaths [14,172]. There is a two- to three-fold increased risk for MI, congestive heart failure, sudden death and stroke in RA patients [166]. Several studies found that overall mortality and death due to CVD and ischemic heart disease in RA patients were increased in both sexes, but that male sex predicts cardiovascular events and death [14,15]. Although several studies have reported CVD is increased in both sexes with RA, few studies examined sex differences; a meta-analysis of RA patients found that only three out of 24 studies examined sex differences [15]. RA is likely to increase the risk of developing CVD by elevating vascular and myocardial inflammation and dyslipidemia. Similar to SLE, RA patients develop atherosclerosis and vascular thrombosis, and more rarely, pericarditis, myocarditis or endocarditis/valvular disease (Box 4). Evidence of the important role for elevated proinflammatory cytokines in disease pathogenesis comes from studies finding that treatment of RA patients with anti-TNF drugs reduces the chance of developing heart failure by 50% [134,173]. The inflammatory infiltrate in the joint of RA patients closely resembles the infiltrate in the atherosclerotic plaque and myocardium in inflammatory autoimmune diseases such as atherosclerosis and myocarditis. Importantly, the same pathogenic mechanisms that increase inflammation in the joint are likely to increase CVD in RA patients.
Sjögren’s syndrome
SS is characterized by chronic inflammation of the exocrine salivary and lacrimal glands, resulting in dry mouth and eyes [174]. SS can be considered a heterogeneous AD possessing both organ-specific and systemic features [175]. SS can occur as a primary condition or in association with other ADs such as RA and SLE. AutoAbs in SS include antinuclear Abs (ANA), particularly against ribonuclear proteins (e.g., anti-Ro/SSA), and rheumatoid factor (RF). The double name Ro/SSA derives from the description of these autoAbs by two different research groups – one to define a SLE patient (‘Ro’) and the other in association with SS (SS-A) [176]. Abs against Ro/SSA antigens have been detected in patients with SS, SLE, RA and fetal–maternal autoimmune syndromes, but their precise role in the pathogenesis of disease is unclear [176]. In humans and mouse models of the disease, the salivary gland infiltrate is composed of T cells, B cells, macrophages and DCs [174,177]. Similar to SLE, SS has a female predominance of 9:1. In the mouse model, females were found to have greater salivary gland inflammation and a more predominant Th2 and Th17 response and more B cells [174]. However, there are no studies that have specifically examined the role of sex hormones in disease pathogenesis. The true incidence of CVD among SS patients is not known but one study found a higher prevalence of subclinical atherosclerosis in patients compared with controls [178]. In this study, the presence of anti-Ro/SSA autoAbs, but not alterations in lipid profiles, was associated with subclinical atherosclerosis, suggesting a role for Abs in disease progression [142,178]. These data suggest that a similar increased CVD risk may exist for SS patients as occurs for other rheumatic ADs.
Systemic sclerosis
Systemic sclerosis (also called scleroderma) is considered the prototypic multisystem fibrotic disease that commonly affects the skin, lungs and kidneys [18,142]. Pathological characteristics of the human disease and animal models include overexpression of the profibrotic cytokine TGF-β and elevated numbers of mast cells, eosinophils and basophils – all of which are associated with Th2 responses. The role for autoAbs in the pathogenesis of disease is unknown, but approximately 95% of scleroderma patients are positive for ANA [179]. Although a relatively uncommon disease, it has the highest case-specific mortality of any of the autoimmune rheumatic diseases because of vascular and fibrotic complications. Patients with systemic sclerosis have a fourfold increase in mortality compared with the general population, with approximately a third of deaths due to CVDs, including atherosclerosis [142,180]. Patients with systemic sclerosis may rarely develop vasculitis or myocarditis (Box 4). A recent study found that systemic sclerosis was an independent risk factor for coronary calcification, in addition to the conventional risk factors for atherosclerosis [181]. Systemic sclerosis patients also have higher levels of lipoprotein (Lp[a]) and oxLDL autoAbs [142]. Although female predominance in systemic sclerosis patients ranges from 4:1 to 14:1, male gender is considered a factor determining poor prognosis [182]. Men with the disease were found to have more inflammatory myopathy, greater pulmonary artery pressure as assessed by echocardiography, and fewer anticentromere autoAbs than women [182]. Although more research is needed, the presence of mast cells, basophils, eosinophils and autoAbs strongly suggests a Th2-skewed immune response.
A role for RF?
The rheumatic diseases SLE, RA, myositis, SS, and systemic sclerosis are a group of inflammatory autoimmune disorders where autoAb and IC deposition induce tissue damage, inflammation and fibrosis [183]. Historically, RF was considered a primary pathogenic autoAb needed for the formation of ICs in RA [184]. RFs are autoAbs against the Fc region of Abs (e.g., IgM, IgG, IgA). As part of the normal immune response to infection, RF promotes complement fixation, clearance of ICs by macrophages, enhances antigen presentation and amplifies the avidity of IgG [185–187]. But RF can also participate in AD pathology by its ability to form ICs. RF occurs in 70–90% of RA, 60–80% of SS, 30% of SLE, 20% of myositis and 20% of systemic sclerosis patients [186,187]. When RF is present in RA patients, its presence predicts a more aggressive, destructive disease course [184]. RF levels also correlate with the severity of salivary gland damage in SS [186]. These data suggest that RF may contribute to the pathogenesis of rheumatic ADs, although the precise mechanisms involved remain unclear.
Many infections are known to transiently induce RF (Box 5) [186,187]. Interestingly, many of these infections are also associated with inflammatory heart diseases such as myocarditis (Box 1 & 5). The presence of RF may be useful and beneficial early in the course of a bacterial or viral infection [186–188]. RF levels are higher in secondary (e.g., 23%) compared with primary infections (e.g., 8%), and even higher in the sera of latently infected individuals (e.g., 37%) suggesting that individuals with higher RF may not be clearing infections [186]. Increased Th2 responses in females may inhibit clearance of bacterial or viral infections. Numerous infections, particularly viral and bacterial infections, have been associated with rheumatic ADs (Box 6) [189]. There is a considerable overlap in the infections that induce RF (Box 5), rheumatic ADs (Box 6) and myocarditis (Box 1). It would be interesting to determine whether RF could be a biomarker for infections that induce or promote autoimmune and CVDs.
Box 5. Examples of infections known to induce rheumatoid factor.
Bacterial
Viral
Parasitic
Trypanosoma cruzi†
Toxoplasmosis
Onchocerciasis
Malaria
†Infections that induce myocarditis in humans and/or animal models.
Adapted from [186].
Box 6. Infections associated with rheumatic autoimmune diseases.
Systemic lupus erythematosus
Epstein–Barr virus
Parvovirus B19
CMV
Salmonella
HIV
Escherichia coli
Rheumatoid arthritis
Epstein–Barr virus
Parvovirus B19
CMV
Human T-lymphotrophic virus
Mycobacteria
E. coli
Scleroderma
Epstein–Barr virus
Parvovirus B19
CMV
Human T-lymphotrophic virus
HIV
Heliobacter pylori
Myositis
Coxsackievirus
Parvovirus B19
Toxoplasma
Human T-lymphotrophic virus
HIV
Sjögren’s syndrome
Epstein–Barr virus
Coxsackievirus
CMV
Human T-lymphotrophic virus
HIV
Hepatitis C virus
Myositis: also called dermatomyositis; Scleroderma: also called systemic sclerosis.
Data taken from [200].
Considering the importance of infections and innate TLR activation in the pathogenesis of cardiovascular and rheumatic ADs [45,53,102,190], it is perhaps not surprising that several studies have found that RF is associated with increased all-cause mortality and CVD mortality in RA patients (although a stronger relationship may exist for CVD mortality and anticyclic citrullinated protein autoAbs) [11,191,192]. Goodson et al. found that after adjusting for age and sex, CVD mortality association in newly diagnosed patients with inflammatory polyarthritis was strongest in the subgroup of patients who were RF-positive at baseline [193]. In recent-onset inflammatory polyarthritis patients, male sex and RF positivity were predictors of early and later mortality [194]. It has been known for some time that ICs not only increase autoimmune heart disease but also serve as an independent risk factor for MI [195]. Importantly, a recent study found that elevated RF increased the risk for ischemic heart disease in men from the general population, but not women [196]. More men than women were positive for RF (23.9% men vs 11% women, mutually adjusted odds ratio: 2.9 [95% CI: 1.6–5.3] vs 1.3 [95% CI: 0.6–2.8]; p < 0.001, respectively) [196]. RF autoAb titers have also been found to be higher in men versus women with RA and SS even though these diseases are more prevalent in women [186–198]. The relatively higher levels of RF in rheumatic ADs such as RA and SS, where RF occurs in 80% of patients, may predispose women to develop CVD and/or indicate that drivers of disease, such as infection, are elevated. More studies are needed to determine whether sex hormones directly influence RF levels and whether RF is involved in the pathogenesis of autoimmune and/or CVDs.
Expert commentary
This review has focused on the role of sex hormones in regulating immune function during AD and CVD. TLRs are activated by infections and damaged self. In men, testosterone promotes a proinflammatory Th1/Th17 response that leads to cardiac remodeling and fibrosis (Figure 1). In men, autoAbs including RF and ICs contribute to atherosclerosis and thrombosis and promote the progression to DCM and heart failure following myocarditis. By contrast, estrogen promotes a regulatory Th2 response in women that leads to increased autoAbs following infection and/or tissue damage (Figure 1). IC deposition in females promotes inflammation and fibrosis that leads to AD. Additionally, Th2-skewed responses in females may allow infections to persist resulting in increased RF, ICs and inflammation that predisposes women to premature atherosclerosis.
Although the question of why CVDs occur more often in men is somewhat easier to address, the answer to why ADs are increased in women remains controversial. Even though estrogen has a well-established ability to activate B cells and increase Ab/autoAb levels that require a Th2-type immune response, few studies have examined the role of sex hormones on autoAb levels or IC-mediated pathology during autoimmune or CVDs. However, the critical role that IC deposition plays in driving inflammation and fibrosis suggests that estrogen may promote ADs in women. Additionally, a Th2-skewed immune response in females may allow infections to persist, resulting in increased production of RF in women which, together with high levels of other autoAbs, could increase thromboembolism and CVD in women. A better understanding of the influence of sex hormones on autoAbs and ICs should provide important information on why CVD is increased in patients with rheumatic ADs. Future studies need to address the pathogenesis of sex-specific responses of the immune system to CVD and AD.
Five-year view
A greater understanding of the importance of sex differences in the pathogenesis of CVD and rheumatic ADs is beginning to be realized. In the next 5 years, investigators who previously analyzed their data by controlling for sex will now also study sex differences. Findings from the study of sex differences will provide critical information concerning the pathogenesis of rheumatic disease, AD and CVD. More research is required to establish whether RF is increased in men with CVD and whether sex hormones such as testosterone regulate its levels. New techniques to study gene–environment interactions will rapidly increase our understanding of the role of infections and sex hormones in the pathogenesis of inflammatory heart disease. Understanding epigenetic mechanisms and the influence of X and Y chromosomes on disease pathogenesis will complement the study of the effect of sex hormones on the immune response. Breakthroughs in these areas will provide a better understanding of CVD risk factors between sexes and how to prevent and treat CVD and AD.
Key issues.
Cardiovascular diseases are more prevalent in men, and autoimmune diseases more prevalent in women.
Autoantibodies are important in the pathogenesis of cardiovascular and autoimmune diseases but information on sex differences in autoantibodies is lacking.
Estrogen activates B cells and increases autoantibodies, Tregs and Th2 responses that protect against heart disease but may lead to increased immune complex deposition during autoimmune disease.
Testosterone elevates components of the innate immune response following infection or injury that increase cardiac inflammation and fibrosis, leading to atherosclerosis, dilated cardiomyopathy and heart failure.
Cardiovascular disease is the leading cause of death in rheumatic autoimmune diseases.
Infections known to induce rheumatoid factor are implicated in the pathogenesis of rheumatic autoimmune diseases and inflammatory heart disease.
A small number of studies suggest that rheumatoid factor is elevated in men in the general population and in men with rheumatic or cardiovascular diseases.
Acknowledgments
D Fairweather and MJ Coronado were supported by funding from the NIH (R01 HL087033 to D Fairweather).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;104:276–308. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson DL, Gange SJ, Rose NR, Graham NMH. Epidemiology and estimated population burden of selected autoimmune disease in the United States. Clin. Immunol. Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3. Cooper LT., Jr Myocarditis. N. Engl. J.Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028.. •• Comprehensive review of myocarditis and dilated cardiomyopathy in human patients and animal models.
- 4. McNamara DM, Starling RC, Cooper LT, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011;58:1112–1118. doi: 10.1016/j.jacc.2011.05.033.. •• The first study of myocarditis/early dilated cardiomyopathy patients to show that men with myocarditis have worse recovery and transplant-free survival than women.
- 5.Rubtsov A, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimm. Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussinovitch U, Shoenfeld Y. Autoimmunity and heart diseases: pathogenesis and diagnostic criteria. Arch. Immunol. Ther. Exp. 2009;57:95–104. doi: 10.1007/s00005-009-0013-1. [DOI] [PubMed] [Google Scholar]
- 7.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune disease. Nat. Clin. Pract. Rheum. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 8.Lago F, Gomez R, Conde J, Scotece M, Gomez-Reino JJ, Gualillo O. Cardiometabolic co-morbidities and rheumatic diseases: focus on the role of fat mass and adipokines. Arthritis Care Res. (Hoboken) 2011;63:1083–1090. doi: 10.1002/acr.20488. [DOI] [PubMed] [Google Scholar]
- 9.Haque S, Gordon C, Isenberg D, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. J. Rheumatol. 2010;37:322–329. doi: 10.3899/jrheum.090306. [DOI] [PubMed] [Google Scholar]
- 10.Urowitz MB, Gladman D, Ibanez D, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res. 2010;62:881–887. doi: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symmons DPM, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 12.Lu L-J, Wallace DJ, Ishimori ML, Scofield RH, Weisman MH. Male systemic lupus erythematosus: a review of sex disparities in this disease. Lupus. 2010;19:119–129. doi: 10.1177/0961203309350755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agmon-Levin N, Mosca M, Petri M, Shoenfeld Y. Systemic lupus erythematosus one disease or many? Autoimmun. Rev. 2011 doi: 10.1016/j.autrev.2011.10.020. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 14.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in northern Sweden. J. Rheumatol. 1997;24:445–451. [PubMed] [Google Scholar]
- 15.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthitis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 16.Innala L, Moller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res. Ther. 2011;13:R131. doi: 10.1186/ar3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallwellis-Opara A, Dorner A, Poller WC, et al. Autoimmunological features in inflammatory cardiomyopathy. Clin. Res. Cardiol. 2007;96:469–480. doi: 10.1007/s00392-007-0524-x. [DOI] [PubMed] [Google Scholar]
- 18.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathologic perspective. Am. J.Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale C, Mendelsohn ME, Rosano GMC. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 20. Regitz-Zagrosek V, Oertelt-Prignione S, Seeland U, Hetzer R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ. J. 2010;74:1265–1273. doi: 10.1253/circj.cj-10-0196.. •• An excellent review that highlights clinical data on sex differences in heart disease.
- 21.Kaushik M, Sontineni SP, Hunter C. Cardiovascular disease and androgens: a review. Int. J.Cardiol. 2010;142:8–14. doi: 10.1016/j.ijcard.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Lee LV, Foody JM. Women and heart disease. Cardiol. Clin. 2011;29:35–45. doi: 10.1016/j.ccl.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Tan YY, Gast GM, van der Schouw YT. Gender differences in risk factors for coronary heart disease. Maturitas. 2010;65:149–160. doi: 10.1016/j.maturitas.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Blauwet LA. Sex and race/ethnicity reporting in clinical trials: a necessity, not an option. J. Women’s Health. 2011;20:313–314. doi: 10.1089/jwh.2011.2744. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 26.Legato MJ, Leghe JK. Gender and the heart: sex-specific differences in the normal myocardial anatomy and physiology. In: Legato MJ, editor. Principles of Gender-Specific Medicine (2nd Edition) MA, USA: Elsevier; 2010. pp. 151–161. [Google Scholar]
- 27.Isensee J, Ruiz, Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend. Med. 2007;4 Suppl. B:S75–S95. doi: 10.1016/s1550-8579(07)80049-0. [DOI] [PubMed] [Google Scholar]
- 28.Lahita RG. Sex hormones and immune function. In: Legato MJ, editor. Principles of Gender-Specific Medicine (2nd Edition) MA, USA: Elsevier; 2010. pp. 615–626. [Google Scholar]
- 29.Regitz-Zagrosek V, Becher E, Mahmoodzadeh S, Schubert C. Sex steroid hormones. In: Bader M, editor. Cardiovascular Hormone Systems: From Molecular Mechanisms to Novel Therapeutics. Weinheim, Germany: Wiley-Blackwell; 2008. pp. 39–64. [Google Scholar]
- 30.Cutolo M. Hormones and cytokines: gender-specific effects. In: Legato MJ, editor. Principles of Gender-Specific Medicine (2nd Edition) MA, USA: Elsevier; 2010. pp. 592–596. [Google Scholar]
- 31. Straub RH. The complex role of estrogens in inflammation. Endocrine Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001.. •• Comprehensive review of the literature on the role of estrogen in immune function.
- 32.Cook IF. Sexual dimorphism of humoral immunity with vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 33.Carreras E, Turner S, Frank MB, et al. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115:238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan D, Dai R, Karpuzoglu E, Ahmed SA. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur. J.Immunol. 2010;40:2549–2556. doi: 10.1002/eji.201040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir. Res. 2010;30:166. doi: 10.1186/1465-9921-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia X, Zhang S, Yu Y, et al. Effects of estrogen replacement therapy on estrogen receptor expression and immunoregulatory cytokine secretion in surgically induced menopausal women. J. Reprod. Immunol. 2009;81:89–96. doi: 10.1016/j.jri.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce Th2 and tolergenic responses. Nat. Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 38.Dinesh RK, Hahn BH, Singh RP. PD-1, gender and autoimmunity. Autoimm. Rev. 2010;9:583–587. doi: 10.1016/j.autrev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stuve O. The increasing incidence and prevalence of female multiple sclerosis – a critical analysis of potential environmental factors. Autoimm. Rev. 2011;10:495–502. doi: 10.1016/j.autrev.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Papenfuss TL, Powell ND, McClain MA, et al. Estriol generates tolergenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 2011;186:3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frisancho-Kiss S, Davis SE, Nyland JF, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 42.Giron-Gonzalez JA, Moral FJ, Elvira J, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared to women. Eur. J. Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 43.Frisancho-Kiss S, Coronado MJ, Frisancho JA, et al. Gonadectomy of male BALB/c mice increases Tim-3+ alternatively activated M2 macrophages, Tim-3+ T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav. Immun. 2009;23:649–657. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onyimba JA, Coronado MJ, Garton AE, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol. Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 2009;50:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teuscher C, Noubade R, Spach K, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc. Natl. Acad. Sci. USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozcelik T. X chromosome inactivation and female predisposition to autoimmunity. Clinic Rev. Allerg. Immunol. 2008;34:348–351. doi: 10.1007/s12016-007-8051-0. [DOI] [PubMed] [Google Scholar]
- 48.Selmi C. The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract. Res. Clin. Rheum. 2008;22:913–922. doi: 10.1016/j.berh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemos B, Branco AT, Hartl DL. Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc. Natl. Acad. Sci. USA. 2010;107:15826–15831. doi: 10.1073/pnas.1010383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renaudineau Y, Youinou P. Epigenetics and autoimmunity, with special emphasis on methylation. Keio. J. Med. 2011;60:10–16. doi: 10.2302/kjm.60.10. [DOI] [PubMed] [Google Scholar]
- 53. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001.. •• Excellent recent review on the role of inflammation in the pathogenesis of atherosclerosis with an emphasis on the importance of innate immunity.
- 54.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 55.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 56.Laczik R, Szodoray P, Veres K, et al. Assessment of IgG antibodies to oxidized LDL in patients with acute coronary syndrome. Lupus. 2011;20:730–735. doi: 10.1177/0961203311398884. [DOI] [PubMed] [Google Scholar]
- 57.Shi G-P. Immunomodulation of vascular diseases: atherosclerosis and autoimmunity. Eur. J. Vasc. Endovasc. Surg. 2010;39:485–494. doi: 10.1016/j.ejvs.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dart ML, Jankowska-Gan E, Huang G, et al. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circ. Res. 2010;107:1106–1116. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergmark C, Wu R, de Faire U, Lefvert AK, Swedenborg J. Patients with early-onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 1995;15:441–445. doi: 10.1161/01.atv.15.4.441. [DOI] [PubMed] [Google Scholar]
- 60.Greco TP, Conti-Kelly AM, Anthony JR, et al. Oxidized-LDL/beta(2)-glycoprotein I complexes are associated with disease severity and increased risk for adverse outcomes in patients with acute coronary syndromes. Am. J. Clin. Pathol. 2010;133:737–743. doi: 10.1309/AJCP88WVRDRDFBAS. [DOI] [PubMed] [Google Scholar]
- 61.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 62.Van Leeuwen M, Damoiseaux J, Duijvestijn A, Cohen JW, Cohen Tervaert JW. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimm. Rev. 2009;9:53–57. doi: 10.1016/j.autrev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 64.Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 65.Elhage R, Jawien J, Rudling M, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 66.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakao S, Tanabe N, Tatsumi K. The estrogen paradox in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L435–L438. doi: 10.1152/ajplung.00057.2010. [DOI] [PubMed] [Google Scholar]
- 69.Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat. Rev. Drug Discov. 2006;5:425–438. doi: 10.1038/nrd2032. [DOI] [PubMed] [Google Scholar]
- 70.Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin. Sci. 2010;119:493–513. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- 71.McCrohon JA, Death AK, Nakhla S, et al. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation. 2000;101:224–226. doi: 10.1161/01.cir.101.3.224. [DOI] [PubMed] [Google Scholar]
- 72.Wilson ME, Sengoku T, Allred KF. Estrogen prevents cholesteryl ester accumulation in macrophages induced by the HIV protease inhibitor ritonavir. J. Cell Biochem. 2008;103:1598–1606. doi: 10.1002/jcb.21546. [DOI] [PubMed] [Google Scholar]
- 73.Badeau RM, Metso J, Wahala K, Tikkanen MJ, Jauhiainen M. Human macrophage cholesterol efflux potential is enhanced by HDL-associated 17beta-estradiol fatty acyl esters. J. Steroid Biochem. Mol. Biol. 2009;116:44–49. doi: 10.1016/j.jsbmb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J. Clin. Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Change MD. Estradiol down-regulates LPS-induced cytokine production and NFκB activation in murine macrophages. Am. J. Reprod. Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 76.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ. Res. 2001;89:823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 77.Miller MA, Strazzullo P, Karanam S, Cappuccio FP. Ethnic variation in levels of circulation IgG autoantibodies to oxidized low-density lipoprotein. Atherosclerosis. 2009;203:126–136. doi: 10.1016/j.atherosclerosis.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 78.Von Dehn G, von Dehn O, Volker W, et al. Atherosclerosis in apolipoprotein E-deficient mice is decreased by the suppression of endogenous sex hormones. Horm. Metab. Res. 2001;33:110–114. doi: 10.1055/s-2001-12405. [DOI] [PubMed] [Google Scholar]
- 79.English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur. Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 80.Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: an overview. Am. Fam. Physician. 2009;79:778–784. [PMC free article] [PubMed] [Google Scholar]
- 81.Grzybowski J, Bilinska ZT, Ruzyllo W, et al. Determinants of prognosis of nonischemic dilated cardiomopathy. J. Card. Fail. 1996;2:77–85. doi: 10.1016/s1071-9164(96)80026-1. [DOI] [PubMed] [Google Scholar]
- 82.Gupta S, Markham DW, Drazner MH, Mammen PPA. Fulminant myocarditis. Nat. Clin. Practice. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 83.Schultheiss HP, Kuhl U, Cooper LT. The management of myocarditis. Eur. Heart J. 2011;32:2616–2625. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 84.Blauwet LA, Cooper LT. Myocarditis. Prog. Cardiovasc. Dis. 2010;52:274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schultheiss HP, Kuhl U. Why is diagnosis of infectious myocarditis such a challenge? Expert Rev. Anti Infect. Ther. 2011;9(12):1093–1095. doi: 10.1586/eri.11.135. [DOI] [PubMed] [Google Scholar]
- 86.Andreoletti L, Venteo L, Douche-Aourik F, et al. Active coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J. Am. Coll. Cardiol. 2007;50:2207–2214. doi: 10.1016/j.jacc.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 87.Huber SA. Autoimmunity in coxsackievirus B3 induced myocarditis. Autoimmunity. 2006;39:55–61. doi: 10.1080/08916930500484906. [DOI] [PubMed] [Google Scholar]
- 88.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–122. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellis NM, Kurahara DK, Vohra H, et al. Priming the immune system for heart disease: a perspective on group A streptococci. J. Infect. Dis. 2010;202:1059–1067. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 90.Huber SA, Job LP. Cellular immune mechanisms in coxsackievirus group B, type 3 induced myocarditis in BALB/c mice. Adv. Exp. Med. Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- 91.Cihakova D, Barin JG, Afanasyeva M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am. J. Pathol. 2008;172:1195–1208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J. Autoimm. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 93.Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. J. Virol. 2002;76:10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huber SA. T lymphocytes kill T regulatory cells through CD1d. Immunology. 2010;131:202–209. doi: 10.1111/j.1365-2567.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frisancho-Kiss S, Nyland JF, Davis SE, et al. Cutting Edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 96.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J. Autoimm. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber SA. Cd1d expression on hemopoietic cells promotes CD4+ Th1 response in coxsackievirus B3 induced myocarditis. Virology. 2006;352:226–236. doi: 10.1016/j.virol.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 98.Noutsias M, Rohde M, Goldner K, et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur. J. Heart Fail. 2011;13:611–618. doi: 10.1093/eurjhf/hfr014. [DOI] [PubMed] [Google Scholar]
- 99.Yuan J, Cao AL, Yu M, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J. Clin. Immunol. 2010;30:226–234. doi: 10.1007/s10875-009-9355-z. [DOI] [PubMed] [Google Scholar]
- 100.Baldeviano GC, Barin JG, Talor MV, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 101.Abston ED, Coronado MJ, Bucek A, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 vs. TRIF in progression to chronic disease. Clin. Dev. Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ. Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936.. •• Important review detailing studies on the role of innate activation and Toll-like receptors, in particular, on cardiovascular disease including atherosclerosis, myocardial infarction, myocarditis and heart failure.
- 103.Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, Rose NR. LPS promotes CB3-induced myocarditis in resistant B10.A mice. Cell. Immunol. 1991;136:219–233. doi: 10.1016/0008-8749(91)90396-s. [DOI] [PubMed] [Google Scholar]
- 104.Fairweather D, Yusung S, Frisancho S, et al. IL-12Rβ1 and TLR4 increase IL-1β and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 105.Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit. Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 106.Blyszczuk P, Kania G, Dieterle T, et al. Myeloid differentiation factor88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ. Res. 2009;105:912–920. doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- 107.Vallejo JG. Role of Toll-like receptors in cardiovascular diseases. Clin. Sci. 2011;121:1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 108. Zhang P, Cox CJ, Alvarez KM, Cunningham MW. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J. Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861.. •• The first report to show that Toll-like receptors in humans and rodents are activated by cardiac myosin. These findings highlight the importance of the innate immune response to damaged-self in the pathogenesis of autoimmune heart disease.
- 109.Satoh M, Nakamura M, Akatsu T, et al. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin. Sci. (London) 2003;104:577–584. doi: 10.1042/CS20020263. [DOI] [PubMed] [Google Scholar]
- 110.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am. J. Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fairweather D, Frisancho-Kiss S, Njoku DB, et al. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis and heart failure by increasing macrophages, IL-1β and immune complex deposition in the heart. J. Immunol. 2006;176:3516–3524. doi: 10.4049/jimmunol.176.6.3516. [DOI] [PubMed] [Google Scholar]
- 112.Nishikubo K, Imanaka-Yoshida K, Tamaki S, et al. Th1-type immune responses by Toll-like receptor 4 signaling are required for the development of myocarditis in mice with BCG-induced myocarditis. J. Autoimm. 2007;29:146–153. doi: 10.1016/j.jaut.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 113.Gorbea C, Makar KA, Pauschinger M, et al. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J. Biol. Chem. 2010;285:23208–23223. doi: 10.1074/jbc.M109.047464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riad A, Westermann D, Escher F, et al. Myeloid differentiation factor-88 contributues to TLR9-mediated modulation of acute coxsackievirus B3-induced myocarditis in vivo. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H2024–H2031. doi: 10.1152/ajpheart.01188.2009. [DOI] [PubMed] [Google Scholar]
- 115.Pagni PP, Truab S, Demaria O, Chasson L, Alexopoulou L. Contribution of TLR7 and TLR9 signaling to the susceptibility of MyD88-deficient mice to myocarditis. Autoimmunity. 2010;43:275–287. doi: 10.3109/08916930903509056. [DOI] [PubMed] [Google Scholar]
- 116.Riad A, Westermann D, Zietsch C, et al. TRIF is a critical survival factor in viral cardiomyopathy. J. Immunol. 2011;186:2561–2570. doi: 10.4049/jimmunol.1002029. [DOI] [PubMed] [Google Scholar]
- 117. Huber SA, Job LP, Woodruff JF. Sex-related differences in the pattern of coxsackievirus B3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect. Immun. 1981;32:68–73. doi: 10.1128/iai.32.1.68-73.1981.. •• Huber and colleagues were the first to show that myocarditis is more severe in male compared to female mice with viral myocarditis.
- 118.Robinson DP, Huber SA, Moussawi M, et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med. Microbiol. Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 120.Huber SA. Coxsackievirus B3-induced mycoarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378:292–298. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li K, Xu W, Guo Q, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 2009;105:353–364. doi: 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- 122.Frisancho-Kiss S, Nyland JF, Davis SE, et al. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 123. Kindermann I, Kindermann N, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:e493. doi: 10.1161/CIRCULATIONAHA.108.769489.. •• A long-term study of myocarditis patients showing that inflammation is the best predictor of who will progress to dilated cardiomyopathy. These findings confirmed studies in animal models of myocarditis that indicated that inflammation during acute myocarditis was responsible for the remodeling that leads to later dilation.
- 124.Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart. 2009;95:1925–1930. doi: 10.1136/hrt.2008.164061. [DOI] [PubMed] [Google Scholar]
- 125.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2043–H2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 126.Wolfgram LJ, Beisel KW, Rose NR. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J. Exp. Med. 1985;161:1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fairweather D, Abston ED, Coronado MJ. Biomarkers of heart failure in myocarditis and dilated cardiomyopathy. In: Cihakova D, editor. Myocarditis. Rijeka, Croatia: InTech Open Access Publisher; 2011. pp. 323–348. [Google Scholar]
- 128.Nussinovitch U, Shoenfeld Y. The clinical significance of anti-beta-1 adrenergic receptor autoantibodies in cardiac disease. Clin. Rev. Allergy Immunol. 2010 doi: 10.1007/s12016-010-8228-9. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 129.Nussinovitch U, Shoenfeld Y. The clinical and diagnostic significance of anti-myosin autoantibodies in cardiac disease. Clin. Rev. Allergy Immunol. 2011 doi: 10.1007/s12016-010-8229-8. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 130.Nussinovitch U, Shoenfeld Y. The diagnostic and clinical significance of anti-muscarinic receptor autoantibodies. Clin. Rev. Allergy Immunol. 2011 doi: 10.1007/s12016-010-8235-x. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 131.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoreceptors in human idiopathic dilated cardiomyopathy. Circ. Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 132.Mascaro-Blanco A, Alvarez K, Yu X, et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008;41:442–453. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jahns R, Boivin V, Hein L, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robinson T, Smith A, Channer KS. Reversible heart failure: the role of inflammatory activation. Postgrad. Med. J. 2011;87:110–115. doi: 10.1136/pgmj.2010.100123. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y-X, da Cunha V, Vincelette J, et al. Antiviral and myocyte protective effects of murine interferon-β and -α2 in coxsackievirus B3-induced myocarditis and epicarditis in BALB/c mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 136.Regitz-Zagrosek V, Seeland U. Sex and gender differences in myocardial hypertrophy and heart failure. Wien Med. Wochenschr. 2011;161:109–116. doi: 10.1007/s10354-011-0892-8. [DOI] [PubMed] [Google Scholar]
- 137.Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail. Rev. 2007;12:293–300. doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- 138.The Autoimmune Diseases Coordinating Committee. DC, USA: National Institutes of Health; 2005. Progress in Autoimmune Disease Research, Report to Congress. [Google Scholar]
- 139. Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimm. Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001.. •• Excellent review on sex differences in autoimmune disease.
- 140.McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- 141.Brooks WH, Le Dantec C, Pers JO, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J. Autoimmun. 2010;34:J207–J219. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 142.Toms TE, Panoulas VF, Kitas GD. Dyslipidaemia in rheumatological autoimmune diseases. Open Cardiov. Med. J. 2011;5:64–75. doi: 10.2174/1874192401105010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kung M, Stork S, Angermann CE. Cardiovascular comorbidity in rheumatic disease. Does sex play a role? Herz. 2005;30:512–521. doi: 10.1007/s00059-005-2717-2. [DOI] [PubMed] [Google Scholar]
- 144.Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412–415. doi: 10.1177/0961203308090026. [DOI] [PubMed] [Google Scholar]
- 145.Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clinic. Rev. Allerg. Immunol. 2011;40:42–49. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Petri M. Systemic lupus erythematosus: 2006 update. J. Clin. Rheumatol. 2006;12:37–40. doi: 10.1097/01.rhu.0000200420.67262.04. [DOI] [PubMed] [Google Scholar]
- 147.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimm. Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Abdou NI, Rider V. Gender differences in autoimmune diseases: immune mechanisms and clinical applications. In: Legato MJ, editor. Principles of Gender-Specific Medicine (2nd Edition) MA, USA: Elsevier; 2010. pp. 858–591. [Google Scholar]
- 149.Wang J, Nuite M, McAlindon TE. Association of estrogen and aromatase gene polymorphisms with systemic lupus erythematosus. Lupus. 2010;19:734–740. doi: 10.1177/0961203309359517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu L-J, Wallace DJ, Ishimori ML, Scofield RH, Weisman MH. Male systemic lupus erythematosus: a review of sex disparities in this disease. Lupus. 2010;19:119–129. doi: 10.1177/0961203309350755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum. 2008;58:2113–2119. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr. Opin. Rheumatol. 2009;21:471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 153.Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J. Immunol. 2008;180:1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- 154.Szalay G, Sauter M, Haberland M, et al. Osteopontin: a fibrosis-related marker molecule in cardiac remodeling of enterovirus myocarditis in the susceptible host. Circ. Res. 2009;104:851–859. doi: 10.1161/CIRCRESAHA.109.193805. [DOI] [PubMed] [Google Scholar]
- 155.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10:487–494. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus. 2005;14:683–686. doi: 10.1191/0961203305lu2200oa. [DOI] [PubMed] [Google Scholar]
- 157.Crosslin KL, Wiginton KL. Sex differences in disease severity among patients with systemic lupus erythematosus. Gend. Med. 2011;8:365–371. doi: 10.1016/j.genm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 158.Kiani AN, Magder L, Petri M. Coronary calcium in systemic lupus erythamatosus is associated with traditional cardiovascular risk factors, but not with disease activity. J. Rheumatol. 2008;35:1300–1306. [PubMed] [Google Scholar]
- 159.Karp I, Abrahamowicz M, Fortin PR, et al. Recent corticosteroid use and recent disease activity: independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum. 2008;59:169–175. doi: 10.1002/art.23352. [DOI] [PubMed] [Google Scholar]
- 160.Petri MA, Kiani AN, Christopher-Stine L, Magder LS. Lupus atherosclerosis prevention study (LAPS) Ann. Rheum. Dis. 2011;70(5):760–765. doi: 10.1136/ard.2010.136762. [DOI] [PubMed] [Google Scholar]
- 161.Ashrafi R, Garg P, McKay E, Gosney J, Chuah S, Davis G. Aggressive cardiac involvement in systemic lupus erythematosus: a case report and a comprehensive literature review. Cardiol. Res. Pract. 2011;2011:578390. doi: 10.4061/2011/578390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ippolito A, Petri M. An update on mortality in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2008;26(5 Suppl. 51):S72–S79. [PubMed] [Google Scholar]
- 163.Petri M. The lupus anticoagulant is a risk factor for myocardial infarction (but not atherosclerosis): Hopkins Lupus Cohort. Thromb. Res. 2004;114:593–595. doi: 10.1016/j.thromres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 164.Van Venrooij WJ, van Beers JJBC, Pruijn GJM. Anti-CCP antibodies: the past, the present and the future. Nat. Rev. Rheumatol. 2011;7:391–398. doi: 10.1038/nrrheum.2011.76. [DOI] [PubMed] [Google Scholar]
- 165.Steiner G, Urowitz M. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin. Arthritis Rheum. 2009;38:372–381. doi: 10.1016/j.semarthrit.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 166.Bartoloni E, Alunno A, Luccioli F, et al. Atherosclerotic vascular damage and rheumatoid arthritis: a complex but intriguing link. Expert Rev. Cardiovasc. Ther. 2010;8:1309–1316. doi: 10.1586/erc.10.123. [DOI] [PubMed] [Google Scholar]
- 167.Nielsen RH, Christiansen C, Stolina M, Karsdal MA. Oestrogen exhibits type II collagen protective effects and attenuates collagen-induced arthritis in rats. Clin. Exp. Immunol. 2008;152:21–27. doi: 10.1111/j.1365-2249.2008.03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Vollenhoven RF, Houbiers JG, Buttgereit F, et al. The selective estrogen receptor alpha agonist Org 37663 induces estrogenic effects but lacks antirheumatic activity: a Phase IIa trial investigating efficacy and safety of Org 37663 in postmenopausal female rheumatoid arthritis patients receiving stable background methotrexate or sulfasalazine. Arthitis Rheum. 2010;62:351–358. doi: 10.1002/art.27196. [DOI] [PubMed] [Google Scholar]
- 169.Cutolo M. Selective estrogen receptor agonism lacks clinical benefit in rheumatoid arthritis: comment on the article by van Vollenhoven et al. Arthitis Rheum. 2010;62:3832–3833. doi: 10.1002/art.27722. [DOI] [PubMed] [Google Scholar]
- 170.Castagnetta LA, Carruba G, Franata OM, et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J. Rheumatol. 2003;30:2597–2605. [PubMed] [Google Scholar]
- 171.Olsen NJ, Kovacs WJ. Hormones, pregnancy and rheumatoid arthritis. J. Gend. Specif. Med. 2002;5:28–37. [PubMed] [Google Scholar]
- 172.Kramer HR, Giles JT. Cardiovascular disease risk in rheumatoid arthritis: progress, debate, and opportunity. Arthritis Care Res. 2011;63:484–499. doi: 10.1002/acr.20386. [DOI] [PubMed] [Google Scholar]
- 173.Dixon WG, Watson KD, Lunt M, et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthitis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cihakova D, Talor MV, Barin JG, et al. Sex differences in a murine model of Sjorgren syndrome. Ann. N.Y. Acad. Sci. 2009;1173:378–383. doi: 10.1111/j.1749-6632.2009.04760.x. [DOI] [PubMed] [Google Scholar]
- 175.Baldini C, Tlarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjögren’s syndrome: a critical review. J. Autoimm. 2011 doi: 10.1016/j.jaut.2011.12.006. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 176.Defendenti C, Atzeni F, Spina MF, et al. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimm. Rev. 2011;10:150–154. doi: 10.1016/j.autrev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 177.Manoussakis MN, Boiu S, Korkolopoulou P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 178.Vaudo G, Bocci EB, Schoenfeld Y, et al. Precocious intima-media thickening in patients with primary Sjögren’s syndrome. Arthritis Rheum. 2005;52:3890–3897. doi: 10.1002/art.21475. [DOI] [PubMed] [Google Scholar]
- 179.Shanmugam VK, Swistowski DR, Saddic N, Wang H, Stten VD. Comparison of indirect immunofluorescence and multiplex antinuclear antibody screening in systemic sclerosis. Clin. Rheumatol. 2011;30:1363–1368. doi: 10.1007/s10067-011-1766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systemic review and meta-analysis. Arthritis Rheum. 2011;63:2078–2090. doi: 10.1002/art.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mok MY, Lau CS, Chiu SS, et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. 2011;63:1387–1395. doi: 10.1002/art.30283. [DOI] [PubMed] [Google Scholar]
- 182.Nguyen C, Berezne A, Baubet T, et al. Association of gender with clinical expression, quality of life, disability, and depression and anxiety in patients with systemic sclerosis. PLoS ONE. 2011;6:e17551. doi: 10.1371/journal.pone.0017551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dube SR, Fairweather D, Pearson W, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom. Med. J. Biobehav. Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 185.Carson DA, Chen PP, Fox RI, et al. Rheumatoid factor and immune networks. Ann. Rev. Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- 186. Newkirk MM. Rheumatoid factors: host resistance or autoimmunity? Clin. Immunol. 2002;104:1–13. doi: 10.1006/clim.2002.5210.. •• Comprehensive review that lists infections that are known to induce rheumatoid factor.
- 187.Dorner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr. Opin. Rheumatol. 2004;16:246–253. doi: 10.1097/00002281-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 188.Christensen P, Schroder AK. Possible role of microbial IgG Fc-binding proteins in rheumatic arthritis. Agents Actions. 1990;29:88–94. doi: 10.1007/BF01964728. [DOI] [PubMed] [Google Scholar]
- 189.Shoenfeld Y, Rose NR, editors. Infection and Autoimmunity. Amsterdam, The Netherlands: Elsevier; 2004. [Google Scholar]
- 190.Santegoets KCM, van Bon L, van den Berg WB, Wenink MH, Radstake TRDJ. Toll-like receptors in rheumatic diseases: are we paying a high price for our defense against bugs? FEBS Letters. 2011;585:3660–3666. doi: 10.1016/j.febslet.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 191.Tomasson G, Aspelund T, Jonsson T, Valdimarsson H, Felson DT, Gudnason V. Effect of rheumatoid factor on mortality and coronary heart disease. Ann. Rheum. Dis. 2010;69:1649–1654. doi: 10.1136/ard.2009.110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 193.Goodson NJ, Symmons DP, Scott DG, Bunn D, Lunt M, Silman AJ. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 194.Naz SM, Farraqher TM, Bunn DK, Symmons DP, Bruce IN. The influence of age at symptom onset and length of followup on mortality in patients with recent-onset inflammatory polyarthritis. Arthritis Rheum. 2008;58:985–989. doi: 10.1002/art.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–2581. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 196. Edwards CJ, Syddall H, Goswami R, et al. The autoantibody rheumatoid factor may be an independent risk factor for ischemic heart disease in men. Heart. 2007;93:1263–1267. doi: 10.1136/hrt.2006.097816.. •• Demonstrated that rheumatoid factor levels are elevated in men compared to women in the general population and that rheumatoid factor predicts the development of ischemic heart disease in men but not women.
- 197. Wolfe F, Cathey MA, Roberts FK. The latex test revisited: rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum. 1991;34:951–960. doi: 10.1002/art.1780340804.. •• Comprehensive review that describes the functions of rheumatoid factor and provides graphs of the percentage of patients positive for rheumatoid factor in a number of rheumatic diseases.
- 198.Anaya J-M, Liu GT, D’Souza E, Ogawa N, Luan X, Talal N. Primary Sjögren’s syndrome in men. Ann. Rheum. Dis. 1995;54:748–751. doi: 10.1136/ard.54.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doria A, Pauletto P, editors. The Heart in Systemic Autoimmune Diseases. Amsterdam, The Netherlands: Elsevier; 2004. [Google Scholar]
- 200.Shoenfeld Y, Rose NR, editors. Infection and Autoimmunity. Amsterdam, The Netherlands: Elsevier; 2004. [Google Scholar]