Abstract
We report that neuronal overexpression of the endogenous inhibitor of calpains, calpastatin (CAST), in a mouse model of human Alzheimer’s disease (AD) β-amyloidosis, the APP23 mouse, reduces β-amyloid pathology and Aβ levels when comparing aged, double transgenic (tg) APP23/CAST with APP23 mice. Concurrent with Aβ plaque deposition, aged APP23/CAST mice show a decrease in the steady-state brain levels of the amyloid precursor protein (APP) and APP C-terminal fragments when compared to APP23 mice. This CAST-dependent decrease in APP metabolite levels was not observed in single tg CAST mice expressing endogenous APP or in younger, Aβ plaque predepositing APP23/CAST mice. We also determined that the CAST-mediated inhibition of calpain activity in the brain is greater in the CAST mice with β-amyloid pathology than in non-APP tg mice, as demonstrated by a decrease in calpain-mediated cytoskeleton protein cleavage. Moreover, aged APP23/CAST mice have reduced ERK1/2 activity and tau phosphorylation when compared to APP23 mice. In summary, in vivo calpain inhibition mediated by CAST transgene expression reduces Aβ pathology in APP23 mice, with our findings further suggesting that APP metabolism is modified by CAST overexpression as the mice develop β-amyloid pathology. Our results indicate that the calpain system in neurons is more responsive to CAST inhibition under conditions of β-amyloid pathology, suggesting that in the disease state neurons may be more sensitive to the therapeutic use of calpain inhibitors.
Keywords: calpain, calpastatin, APP, Aβ, Alzheimer’s disease
Introduction
Disruption of multiple proteolytic systems contributes to Alzheimer’s disease (AD) pathobiology, including alterations in the enzymes responsible for β-amyloid (Aβ) generation and clearance and dysfunction of the lysosomal system (Mathews, et al., 2002a, Nixon, et al., 2000). Calpains have also shown evidence of hyperactivity in human AD tissue (Grynspan, et al., 1997, Liu, et al., 2005, Nixon, 2003, Saito, et al., 1993). The calpain family is a group of Ca2+-activated, cytosolic, neutral pH, cysteine proteases (Huang and Wang, 2001) which modulate, probably indirectly, the localization and proteolytic processing of the amyloid precursor protein (APP) in cultured cells (Mathews, et al., 2002b). The most abundantly expressed calpains are m-calpain and μ-calpain, which are distinguished by their different affinities for Ca2+, each of which forms a functional heterodimer with the shared regulatory calpain small subunit 1 (Capn4) (Croall and DeMartino, 1991, Sorimachi, et al., 1997). In addition to their Ca2+-dependence, calpains are regulated by cytosol-to-membrane translocation and an endogenous inhibitor, calpastatin (CAST). The CAST protein consists of four calpain-inhibitory domains that are subjected to calpain cleavage and terminal inactivation by caspase (Croall and DeMartino, 1991, Goll, et al., 2003, Maki, et al., 1991, Sorimachi, et al., 1997, Wang, et al., 1998).
Activation of calpains in AD is evidenced by increased levels of activated μ-calpain (Saito, et al., 1993) and m-calpain (Grynspan, et al., 1997) in neurons. The role of the calpain system in normal brain function and in pathological conditions has also been examined in various mouse models with modified calpain and CAST expression. While genetic deletion of either the m-calpain large subunit or the single calpain small subunit is lethal (Arthur, et al., 2000, Takano, et al., 2005), deletion of the μ-calpain large subunit does not result in an apparent gross phenotype (Azam, et al., 2001, Grammer, et al., 2005). Mice lacking CAST, while showing decreased locomotor activity and a decreased acoustic startle response, have no change in hippocampal-dependent memory function (Nakajima, et al., 2008). CAST overexpressing mice similarly do not have gross memory deficits (Higuchi, et al., 2005, Takano, et al., 2005), arguing that CAST deletion or overexpression produces, in general, mild effects in the normal mouse brain (Rao, et al., 2008).
The role of the calpain system in AD pathobiology has been recently explored in β-amyloid depositing mouse models. In Tg2576 (Vaisid, et al., 2007) and in APP/PS1 mice (Liang, et al., 2010), calpain appears to be activated and CAST diminished, consistent with reports in human AD tissue (Grynspan, et al., 1997, Liu, et al., 2005, Nixon, 2003, Saito, et al., 1993). In a mouse overexpressing wild-type APP that does not develop β-amyloid pathology, calpains also appear to be activated in neurons (Kuwako, et al., 2002). In APP/PS1 mice, chronic calpain inhibition has been shown to reduce amyloid plaque burden and improve memory and synaptic transmission (Liang, et al., 2010, Trinchese, et al., 2008). This is in contrast to previous results from multiple laboratories showing that the acute pharmacological inhibition of calpains in cell culture systems dramatically increases Aβ42 generation (Klafki, et al., 1996, Mathews, et al., 2002b, Yamazaki, et al., 1997, Zhang, et al., 1999), which is thought to be a more pathological Aβ species. Here, we examined brain APP metabolite levels in mice overexpressing CAST in neurons compared to wild-type mice, as well as CAST overexpressing mice crossed to the β-amyloid depositing APP23 line. While CAST-overexpression-mediated changes were not seen in otherwise wild-type mice, the development of β-amyloid pathology in the APP23 mice corresponded to both an increased sensitivity of the calpain system to CAST inhibition and a reduction in APP metabolite levels.
Materials and methods
Transgenic mice
All experiments involving mice received prior approval from the Nathan Kline Institute Animal Care and use committee. Neuron-specific overexpression of human CAST is driven by the Thy-1.2 promoter as previously described (Rao, et al., 2008). APP23 mice, which overexpress Swedish-mutant human APP (K670N, M671L) under the Thy-1.2 promoter, develop β-amyloid plaque pathology after the first year of life (Sturchler-Pierrat, et al., 1997). The depositing APP23/CAST and APP23 mice used in this study were female 13-month old mice. All mice were maintained on a C57Bl6 background.
Brain Processing, Western blotting and Aβ ELISA measurements
Ten-percent (weight-to-volume) homogenates were prepared from a hemibrain lacking the olfactory bulb and cerebellum, and used for biochemical analyses (Schmidt, et al., 2005a). An aliquot of the homogenates was extracted in diethylamine (DEA) prior to sandwich ELISA to detect soluble murine Aβ in single tg CAST and wild-type mice as well as soluble human Aβ in the predepositing APP23/CAST and APP23 mice (Schmidt, et al., 2005b). DEA-extracts of all genotypes were also used for sAPP isolation prior to Western blot analyses as previously described (Morales-Corraliza, et al., 2009). In aged APP23/CAST and APP23 mice, an aliquot of the homogenate was extracted in formic acid for β-amyloid-associated Aβ quantitation (Schmidt, et al., 2005a, Schmidt, et al., 2005b). For Western blotting, proteins were sized by SDS-PAGE, transferred to polyvinylidene difluoride membrane (Mathews, et al., 2002b), and incubated with antibodies as previously described (Morales-Corraliza, et al., 2009). For unbiased analysis of Aβ species, Aβ in brain homogenates was resolved by urea/SDS-PAGE as previously described (Klafki, et al., 1996) prior to Western blotting analysis.
Antibodies
Antibody C1/6.1 recognizes the carboxyl-terminal cytoplasmic domain of APP (Mathews, et al., 2002b), and m3.2 recognizes residues 10–15 of murine Aβ, also detecting murine APP, sAPPα and Aβ (Morales-Corraliza, et al., 2009). 22C11 was purchased from Millipore (Temecula, CA) and detects APP N-terminal in both human and mouse epitopes. Monoclonal antibody 6E10 (Covance, Princeton, NJ), which recognizes residues 1–16 of human Aβ, was used to detect human sAPPα and Aβ. The antibody 4G8 (Covance, Princeton, NJ) recognizes residues 17–24 of Aβ and was used to detect Aβ and APP. Mouse monoclonal antibody 6A1 detects sAPPβ containing the Swedish mutation but not the wild-type sequence (Morales-Corraliza, et al., 2009). Neprilysin was detected with the monoclonal antibody 56C6 (CD10) (Novacastra, Newcastle, UK), and IDE with the rabbit polyclonal antibody IDE1 ((Qiu, et al., 1998); a gift of Dr. Dennis Selkoe). Cytoskeletal proteins and kinases were detected with antibodies against MAP1 (Clone HM-1; Chemicon, Temecula, CA), MAP2 (antibody 18-1; (Rao, et al., 2008)) and αII-spectrin (MAB1622; Chemicon, Temecula, CA), CDK5 (C-8:sc-173; Santa Cruz Biotechnology, Santa Cruz, CA), GSK3β (27C10; Cell Signaling Technology, Danver, MA), and ERK1/2 and Phospho-ERK1/2 (9102 and 9101; Cell Signaling Technology, Danver, MA). Phosphorylated tau was detected using PHF1 (Chemicon International, Temecula, CA; phospho-epitope at Ser396/404) and CP13 (a gift of Dr. Dennis Selkoe; phospho-epitope at Ser202), while T57120 (BD Biosciences, San Jose, CA), MN37 (a gift of Dr. Dennis Selkoe) and tau1 (Chemicon International, Temecula, CA) were used to detect total tau independent of its phosphorylation state. Phosphorylated α-synuclein was detected using a phospho-Ser129-specific α-synuclein antibody (Abcam, Cambridge, MA). Syn-1 antibody (BD Biosciences, San Jose, CA) was used for phospho-independent α-synuclein recognition. Activated calpain II immunolabeling was done using the rabbit polyclonal antibody C24 (Rao, et al., 2008).
Thioflavin S Staining
β-amyloid plaque burden was visualized by florescence microscopy of 35-μm thick vibratome sections prepared from formalin-fixed hemibrains previously stained with Thioflavin S as described in (Mi, et al., 2007). Thioflavin labeling was quantitated by the program AxioVision 4.6 (n=5 in APP23/CAST and n=5 in APP23 mice; 5 sections per mouse).
Immunocytochemistry
Vibratome sections were blocked with 10% horse serum (Invitrogen, Carlsbad, CA) at room temperature and incubated with the primary antibody C24 (Rao, et al., 2008) overnight at 4° C. Sections were immunostained using a biotinylated goat anti-rabbit secondary antibody and Vectastain ABC kit (both products, Vector Laboratories, Burlingame, CA).
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry
Brain homogenates were immunoprecipitated with a combination of 6E10 and 4G8 antibodies (Covance, Princeton, NJ) using Dynabead M-280 sheep anti-mouse IgG (Invitrogen, Carlsbad, CA) (Tomidokoro, et al., 2010). Proteins were eluted with C4 ZipTip (Millipore, Billerica, MA) in 90% acetonitrile and 0.1% trifluoracetic acid (TFA), mixed 50:50 (v:v) with 10 mg/ml α-Cyano-4-hydroxycinnamic acid in 0.1% TFA and 50% acetonitrile, and air dried. Samples were analyzed using a Bruker Autoflex MALDI-TOF mass spectrometer (Bruker Daltonics, Billerica MA) in positive ion linear mode using standard operating conditions at the NYU Protein Mass Spectrometry Core for Neuroscience.
Statistical Analysis
Western blots were quantitated using ImageJ (http://rsb.info.nih.gov). ELISA measurements were analyzed using a non-parametric Mann-Whitney u test. All data were plotted on GraphPad Prism v.5 (GraphPad Software, San Diego, CA, USA) for statistical analysis. Throughout, results are expressed as the mean ± SEM.
Results
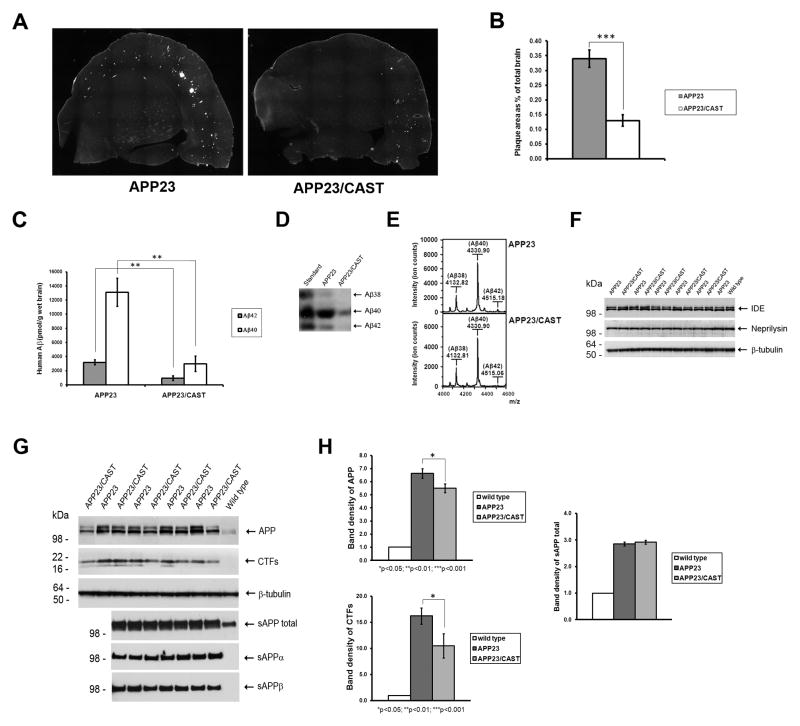
To explore the relationship between neuronal calpain function and AD pathology, we crossed human CAST overexpressing tg mice (showing ~7-times the levels of the endogenous CAST expression in the brain (Rao, et al., 2008)) with an amyloid precursor protein (APP) overexpressing tg line that develops β-amyloid plaques (APP23 mice; (Sturchler-Pierrat, et al., 1997)). Brain plaque pathology in littermate 13-month-old female APP23/CAST and APP23 double tg was visualized by Thioflavin S staining, with the area occupied by plaque in the double tg mice decreased by ~60% (plaque area as % of total brain: 0.13%±0.02 in APP23/CAST mice versus 0.34%±0.03 in APP23 mice; p<0.001) (Figure 1A–B). ELISA measurements of formic acid-extractable Aβ in the APP23/CAST double tg versus the APP23 single tg mice showed a decrease of 70%±11 for deposited human Aβ42 (p<0.01) and a similar decrease for human Aβ40 levels (77%±15; (p<0.01)) (Figure 1C). Similar decrease in co-deposited murine Aβ40 and Aβ42 was also observed by sandwich ELISA (decrease of 61%±22 in Aβ42 (p<0.05) and 63%±22 in Aβ40 (p<0.05); Supplemental Figure 1A). Thus, the Aβ burden is reduced in aged APP23 mice overexpressing CAST, with the ratio of Aβ42 to Aβ40 remaining similar (APP23/CAST: 4.7±0.3 and APP23: 4.1±0.5; p>0.05).
Figure 1. CAST overexpression decreases APP metabolite levels in depositing APP23/CAST mouse brain. (A–B).
Brain sections of depositing 13-month-old APP23/CAST and APP23 mice were used to visualize and quantify β-amyloid plaque area by Thioflavin S staining. (C) Formic acid-extractable human Aβ40 and Aβ42 levels were measured by sandwich ELISA. (D) Analysis of Aβ38, Aβ40 and Aβ42 using Western blots as previously described by Klafki et al. (Klafki, et al., 1996) based upon migration of Aβ standards. (E) MALDI-TOF mass spectrometry of brain homogenates immunoprecipitated simultaneously with 6E10 and 4G8 antibodies. (F) Neprilysin and IDE levels are shown by Western blot analysis of brain homogenates. (G) By Western blot, total proteins probed for APP and CTFs; soluble brain extracts lacking membrane-associated proteins probed for sAPP total, sAPPα and sAPPβ, as indicated (Morales-Corraliza, et al., 2009). (H) Graphic representation of the analysis of the band density of the blots of APP, CTFs and sAPP total. *p<0.05; ** p<0.01; ***p<0.001.
Since inhibiting calpain activity has been shown to change the ratio of Aβ40 and Aβ42 generated by APP overexpressing cells in culture (Klafki, et al., 1996, Mathews, et al., 2002b, Yamazaki, et al., 1997, Zhang, et al., 1999), we examined Aβ in these mice using urea SDS-PAGE in order to detect all Aβ species (Figure 1D). In agreement with our β-amyloid ELISA findings (Figure 1C), we observed a decrease in Aβ band densities in APP23/CAST versus APP23 mice, with the relative ratios of the three predominant Aβ species (Aβ38, Aβ40 and Aβ42) not showing changes. Additionally, we analyzed Aβ peptides by MALDI-TOF mass spectrometry. While this technique does not allow for the direct quantification of different Aβ species (Tomidokoro, et al., 2010), the ratio between the peak intensity of the Aβ species reflects the relative abundance of the peptides. No differences in the relative abundance of Aβ species were seen when comparing APP23/CAST with APP23 mice (Figure 1E), consistent with the ELISA measurements (Figure 1C) and the urea SDS-PAGE analysis (Figure 1D).
Given this decrease in β-amyloid in the APP23/CAST mice, we next determined the levels of two Aβ degrading enzymes within the brain: neprilysin and insulin degrading enzyme (IDE). No changes in protein levels in these proteases were observed when comparing APP23/CAST with APP23 mice (Figure 1F). APP levels in the brain of 13-month-old Aβ-plaque depositing APP23/CAST mice, however, showed a decrease of 25%±7 compared to APP23 mice ((p<0.05); Figure 1G–H). Consistent with this reduction in APP, APP C-terminal fragments (CTF) levels were also decreased in APP23/CAST mice by 36%±12 ((p<0.05); Figure 1G–H). We determined that the highly stable secreted APP (sAPP) metabolites (Morales-Corraliza, et al., 2009) showed no changes between APP23/CAST and littermate APP23 mice, either when probing with an antibody that detect both sAPPα and sAPPβ (sAPP total) or antibodies that only detect sAPPα or sAPPβ (Figure 1G). Relative levels of APP, CTFs and sAPP are shown graphically in the Figure 1H.
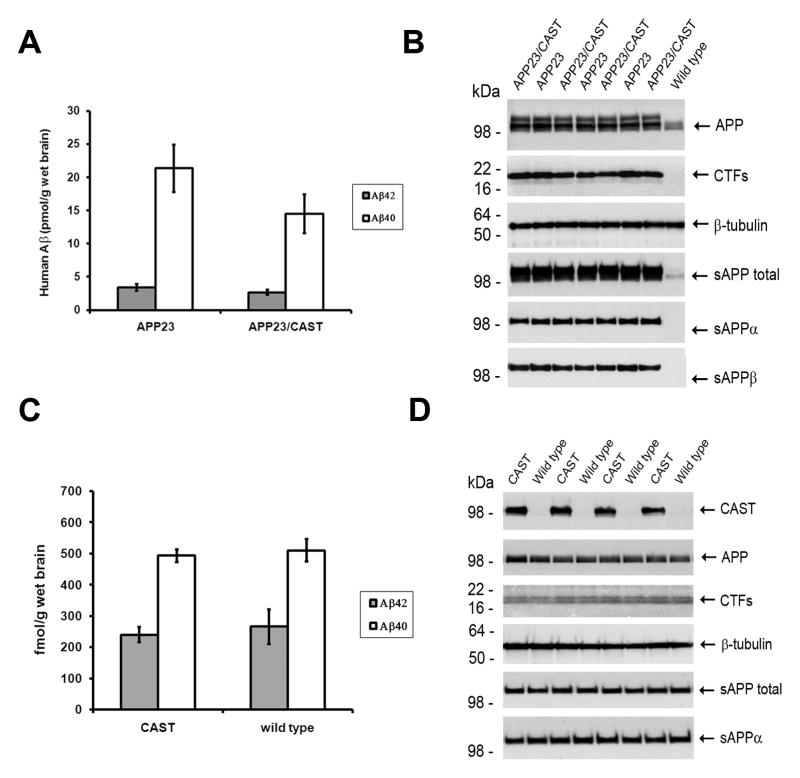
We extended our study on CAST overexpression in vivo by examining APP metabolite levels in CAST mice without Aβ-plaque deposition by examining younger (4-month old), predepositing APP23/CAST mice compared to APP23 mice and by comparing CAST single tg to wild-type mice. Predepositing APP23/CAST mice did not show a significant change in human Aβ brain levels compared to littermate APP23 mice (Figure 2A). At this age, no changes in the levels of APP, CTFs, sAPP total, sAPPα and sAPPβ were seen (Figure 2B), which is in contrast to the reduction in APP and CTF levels in the 13-month-old mice (Figure 1G–H). This suggests that calpastatin expression mediated changes in neuronal APP processing are not prominent until the mice are older and beginning to show brain β-amyloid pathology. To further examine this result, we analyzed at various ages APP metabolite levels in CAST mice expressing only the endogenous murine APP and therefore without Aβ accumulation or plaque pathology. At multiple ages from 4 to 24 months in single CAST tg mice (shown in Figure 2C–D are findings from 18–24 month old mice; other ages are shown in Supplement Figure 1B–D), no changes were seen in APP metabolite levels – including APP, CTFs, sAPP total, sAPPα and Aβ – when compared to littermate non-tg mice (Figure 1I–H).
Figure 2. CAST overexpression does not modify APP metabolism in predepositing 4-month-old APP23 and wild-type mouse brain. (A).
Soluble human Aβ40 and Aβ42 levels and (B) additional APP metabolites levels, including APP, CTFs, sAPP total, sAPPα and sAPPβ in predepositing 4-month-old APP23/CAST and APP23 mouse brain. (C) Soluble endogenous Aβ40 and Aβ42 levels and (D) additional APP metabolite levels in 18-month-old CAST and wild-type mice.
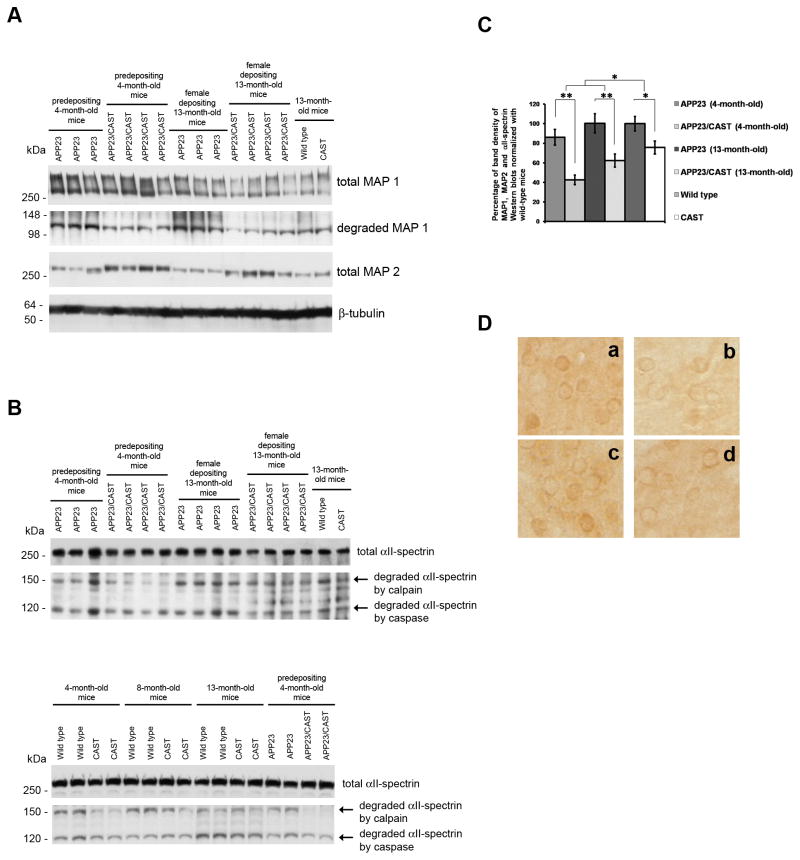
By Western blot analysis, we also characterized in APP23/CAST and APP23 mice, calpain-mediated cleavage of cytoskeleton proteins, including MAP1 and MAP2, and αII-spectrin (Fifre, et al., 2006, Pike, et al., 2001, Warren, et al., 2007). In Figure 3A, Western blot analysis of the total protein and calpain-specific breakdown products of MAP1 (Fifre, et al., 2006) showed lesser amounts of the breakdown products in APP23/CAST mice of either age when compared to APP23 littermates. Total MAP2 protein levels remaining in APP23/CAST were found to be greater than the levels seen in APP23 littermates, and these changes in MAP2 levels were not as great in single tg CAST compared to non-tg mice. Figure 3B shows that while αII-spectrin cleaved by caspase showed no significant changes between the two groups, calpain-mediated αII-spectrin breakdown product (Pike, et al., 2001, Warren, et al., 2007) levels were reduced in the APP23/CAST mice compared to single APP23 tg mice. Figure 3C shows a graphic representation of the analysis of the band density of total proteins and breakdown products of MAP2, MAP1 and αII-spectrin blots. Significant decreases in calpain activity were found in APP23/CAST and CAST mice compared to APP23 and non-tg mice, respectively. We also found greater CAST-mediated calpain inhibition when comparing APP23/CAST to APP23 mice than when CAST single tg were compared to non-tg mice (an apparent decrease in calpain activity of 41%±10 in APP23/CAST versus APP23 mice and 24%±7 in CAST versus non-tg mice; p<0.05 between the groups). This is consistent with calpain inhibition itself being greater in β-amyloid depositing mice expressing CAST (APP23/CAST versus APP23 mice) compared to CAST single tg versus non-tg mice. In Figure 3D, the immunolabeling pattern seen with an antibody that detects activated calpain II (C24; (Rao, et al., 2008)) is in agreement with our findings above on calpain activity indicated by cytoskeletal protein Western blotting (Figure 3A–C).
Figure 3. Assessment of calpain activity in APP23/CAST and APP23 mouse brain.
Characterization of calpain activity in APP23/CAST mice by Western blot analysis of calpain-mediated clearance/cleavage of cytoskeleton proteins (Fifre, et al., 2006, Pike, et al., 2001, Rao, et al., 2008, Warren, et al., 2007) (A) Blots show total protein remaining for MAP1 and MAP2 and the major breakdown products of MAP1. (B) Total protein remaining and the two major breakdown products of αII-spectrin, including a 120 kDa band corresponding to αII-spectrin cleaved by caspase and 150kDa band corresponding to αII-spectrin cleaved by calpain. (C) Graphic representation of the analysis of the band density of total proteins and breakdown products of MAP1, MAP2 and αII-spectrin in A and B. The percentage of band density of CAST single tg and non-tg was calculated based on these three blots as well as additional blots. (D) Immunolabeling with activated calpain II-specific antibody (antibody C24) of serial brain coronal sections of APP23 (a), APP23/CAST (b), wild-type (c) and CAST mice (d). *p<0.05; ** p<0.01; ***p<0.001.
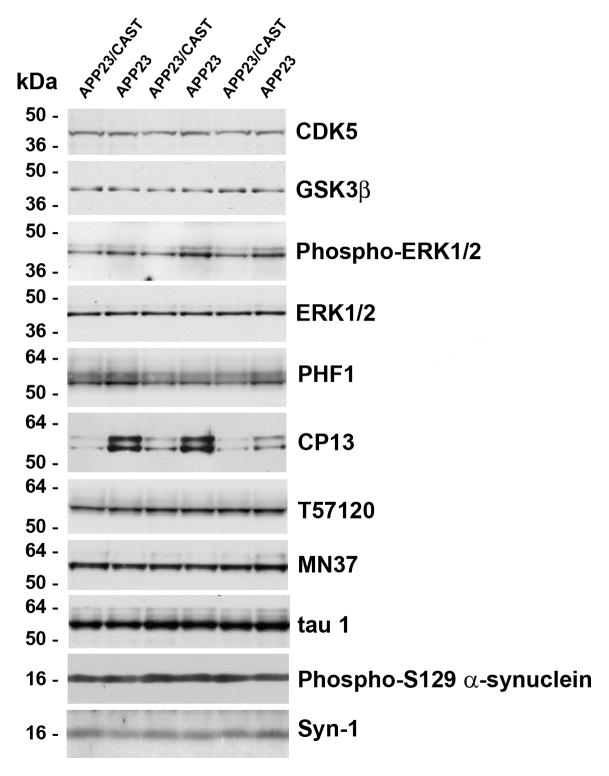
The phosphorylation of tau can be driven by Aβ accumulation in the brain (Gotz, et al., 2001, Lewis, et al., 2001), potentially by kinases whose activity can be modulated by calpains (Amadoro, et al., 2006, Hye, et al., 2005, Lee, et al., 2000, Veeranna, et al., 2004). Given the potential for aberrant activity of these kinases in AD, we determined the levels and activity/phosphorylation state of key tau kinases as well as tau using phospho-dependent and phospho-independent antibodies (Figure 4). While the level of the catalytic domain of CDK5 and level of GSK3β did not change, a decrease in phosphorylated ERK1/2 levels (active form compared to non-phosphorylated ERK1/2) was seen in APP23/CAST compared to APP23 mice (Figure 4). Additionally, the binding of two antibodies – PHF1 and CP13 – that recognize tau phospho-epitopes of ERK1/2 was found to be significantly reduced in APP23/CAST animals, consistent with a decrease in phosphorylated ERK1/2 levels. The phospho-independent tau antibodies T57120, MN37 and tau1 showed no differences in total tau levels between APP23/CAST and APP23 mice. This is in contrast to the calpain-mediated turnover of other cytoskeletal proteins as shown in Figure 3A–D, but consistent with the lack of tau-fragments seen in other mouse models of human β-amyloidosis (Liang, et al., 2010, Roberson, et al., 2007) and in agreement with the eventual accumulation of tau seen as paired-helical filament in human AD. To expand the potential disease relevance of these studies, we additionally examined the levels of phosphorylated α-synuclein (phospho-Ser129 α-synuclein) and total α-synuclein. No changes in α-synuclein signal were detected when comparing APP23/CAST with APP23 mice, consistent with the idea that the calpain system is particularly responsive to Aβ/β-amyloid accumulation, and that this appears to have downstream effects on tau phosphorylation.
Figure 4. CAST overexpression inhibits ERK1/2 dependent tau phosphorylation in APP23/CAST mouse brain.
Brain proteins levels of tau kinases and tau in 13-month-old depositing APP23/CAST compared to APP23 mice were determined by Western blotting. Levels of the catalytic domain of CDK5 (panel as indicated throughout) and GSK3β and levels of phospho-ERK1/2 (phospho-dependent antibody) compared to total ERK1/2 (probed with a phospho-independent antibody; see Methods). Levels of tau using two phosphorylation-dependent tau antibodies which recognize tau phospho-epitopes for ERK1/2: PHF1 (phospho-epitope at Ser396/404) and CP13 (phospho-epitope at Ser202) and three phospho-independent tau antibodies T57120, MN37 and tau1. Levels of α-synuclein using a phosphorylation-dependent α-synuclein antibody (which recognizes a phospho-epitope at Ser129) and a phospho-independent α-synuclein antibody (Syn-1).
Discussion
The pathological role of calpain hyperactivation following the unregulated increase in intracellular Ca2+ concentration that accompanies excitotoxicity has long been appreciated (Bartus, et al., 1995, Wang, 2000). This activation of calpains results in the cleavage of a number of neuronal substrates that can negatively affect neuronal structure, function, and survival (Siman and Noszek, 1988). However, the involvement of calpains in mediating neuronal vulnerability in chronic, long-term disease is less well understood. Multiple studies suggest that in post-mortem human AD brain the neuronal calpain system is upregulated (Grynspan, et al., 1997, Liu, et al., 2005, Nixon, 2003, Saito, et al., 1993), albeit these findings do not necessarily argue for a direct role for calpain activation in the disease. There are numerous studies, however, indicating that calpain activation may lead to pathological changes in tau phosphorylation both in vitro (Amadoro, et al., 2006, Chung, 2009, Lee, et al., 2000) and in vivo (Chung, 2009, Veeranna, et al., 2004), as well as alterations in APP metabolism in vitro (Klafki, et al., 1996, Mathews, et al., 2002b, Yamazaki, et al., 1997, Zhang, et al., 1999). The growing evidence from transgenic mouse models, including our study, that calpain activation can result from β-amyloid accumulation and/or altered Aβ levels argues that the calpain system responds to AD-related pathological changes in the brain (Liang, et al., 2010, Vaisid, et al., 2007), thus positioning calpains as having a role in both driving and responding to the disease.
We initiated this study because of the evidence from multiple groups, including ours (Klafki, et al., 1996, Mathews, et al., 2002b, Yamazaki, et al., 1997, Zhang, et al., 1999), that the acute pharmacological inhibition of calpains in cell culture systems dramatically increases Aβ42 generation, suggesting that inhibition of calpains in the brain might lead to greater β-amyloid pathology due to increased Aβ42 levels. In vivo, however, we now report the opposite, with CAST-transgene-overexpression-mediated calpain inhibition leading to a reduction in β-amyloid pathology while having no effect on the ratio of the various Aβ peptides detected in the brain. Indeed, we choose to use a model that develops β-amyloid pathology without artificial Aβ42-drive, such as the mutant PS1 expression in the APP/PS1Δ9 × CAST tg crosses described by Liang et al. (Liang, et al., 2010), in order to be able to detect a CAST-overexpression effect on Aβ C-terminal cleavage. In addition to the evidence we present that there are no changes in the C-terminal cleavage-site of the Aβ derived from the human Swedish APP, we did not detect changes in the murine Aβ40:Aβ42 ratio from the endogenous, wild-type APP in either single CAST tg mice or in the APP23/CAST and APP23 mice (see Figure 1 and 2 and Supplemental Figure 1).
We found that in Aβ depositing APP23/CAST mice, CAST overexpression leads to a decrease in multiple APP metabolite levels, including APP, CTFs, and Aβ. This reduction in brain APP metabolite levels with CAST overexpression did not occur either in wild-type mice throughout their life-span or in predepositing APP23 mice. Indeed, our findings suggest that only with β-amyloid pathology in the aged APP23 mice, CAST overexpression produces changes in APP metabolism. Additionally, changes in the activity of the calpain system by CAST observed in vivo in this study are consistent with those seen in human AD tissue (Grynspan, et al., 1997, Liu, et al., 2005, Nixon, 2003, Saito, et al., 1993) and in mouse models (Liang, et al., 2010, Vaisid, et al., 2007) subsequent to the formation of β-amyloid plaques, where calpains appear to be activated and CAST levels decreased. The influence of APP metabolism on calpain activity was also observed in a previous report in mice overexpressing wild-type APP that showed that, although these mice do not develop β-amyloid pathology, calpains are activated in neurons (Kuwako, et al., 2002). In this study, APP-induced calpain activation, which was sensitive to calpain inhibitors in vivo, was not seen in mice expressing an APP mutant that is not processed to produce Aβ. This provides further evidence that calpain activation in these models is dependent upon altered brain Aβ levels. By examining the levels of calpain substrates and cleavage-products, our findings show that the inhibition of calpain activity by CAST is greater in the aged APP23 mice with β-amyloid pathology (and apparent in the predepositing APP23 mice), when compared to single tg CAST mice, which is consistent with the idea that the calpain system is more responsive to CAST overexpression when perturbed by excess brain Aβ. That CAST overexpression-mediated modulation of calpain occurs under conditions of Aβ induced neuronal stress is also in agreement with the findings of Rao et al. (Rao, et al., 2008) showing that CAST overexpression inhibits pathologically elevated calpain activity in vivo following excitotoxicity, but less under baseline conditions. Our study supports the idea that, in vivo, there is cross-talk between developing Aβ pathology and the calpain/CAST system, which may show elevated specific activity to the disease that may render the calpain system more responsive to inhibition.
Our findings argue that restoring calpain homeostasis has multiple beneficial effects, including reducing β-amyloid accumulation and tau phosphorylation. While neither our study nor prior studies (Chung, 2009, Liang, et al., 2010) differentiate between reductions in tau phosphorylation resulting from less Aβ accumulation or directly from modulation of calpain activity, either mechanism is potentially beneficial in the disease. Pathologically important disruption of calpain activity, including dysregulation of tissue-specific calpain family members, can occur in a number of aging-related diseases, including type 2 diabetes, cataracts, muscular dystrophy, Parkinson’s disease, rheumatoid arthritis, ischemia, stroke and brain trauma, various platelet syndromes, hypertension, liver dysfunction and some types of cancer (Carragher, 2006, Zatz and Starling, 2005). When associated with a specific calpain family member, disease development and progression appears to be directly linked to altered calpain expression and/or activity. Currently, the evidence from mouse models would suggest that changes in calpain activity in AD correlate with developing Aβ pathology. Such vulnerability of the calpain system in AD would appear to offer an opportunity for therapeutic modulation, with the CAST overexpression systems suggesting that inhibition and/or restoration of more normal calpain activity has benefits both by reducing β-amyloid accumulation and potentially by reducing tau phosphorylation.
Supplementary Material
(A) Formic-acid extracted murine Aβ40 and Aβ42 levels were measured by sandwich ELISA in the APP23/CAST and APP23 mice also characterized in Figure 1. (B) Soluble endogenous Aβ40 and Aβ42 levels (C–D) APP metabolite levels (APP, CTFs, sAPP total and sAPPα) in 4 and 8 months old CAST and wild-type mice.
Acknowledgments
We would like to thank Mr. Steven Blais of the NYU Protein Mass Spectrometry Core for Neuroscience for MALDI-TOF analysis, Drs. Haung Yu and Karen Duff for the kind gift of the synuclein antibodies and Dr. Panaiyur Mohan for advice on the assessment of calpain activity in vivo. This work was supported by the Alzheimer’s Association (IIRG-07-60047 to P.M.M), the NINDS (NS045205 to P.M.M) and NIA (AG017617 to P.M.M, R.A.N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103(8):2892–7. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Molecular and cellular biology. 2000;20(12):4474–81. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse μ-calpain gene reveals an essential role in platelet function. Molecular and cellular biology. 2001;21(6):2213–20. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Elliott PJ, Hayward NJ, Dean RL, Harbeson S, Straub JA, Li Z, Powers JC. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995;17(4):249–58. doi: 10.1080/01616412.1995.11740322. [DOI] [PubMed] [Google Scholar]
- Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm Des. 2006;12(5):615–38. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB reports. 2009;42(8):467–74. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiological reviews. 1991;71(3):813–47. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T. Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis during soluble amyloid β-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3. J Biol Chem. 2006;281(1):229–40. doi: 10.1074/jbc.M507378200. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological reviews. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ42 fibrils. Science. 2001;293(5534):1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Grammer M, Kuchay S, Chishti A, Baudry M. Lack of phenotype for LTP and fear conditioning learning in calpain 1 knock-out mice. Neurobiol Learn Mem. 2005;84(3):222–7. doi: 10.1016/j.nlm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Grynspan F, Griffin WR, Cataldo A, Katayama S, Nixon RA. Active site-directed antibodies identify calpain II as an early- appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain research. 1997;763(2):145–58. doi: 10.1016/s0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tomioka M, Takano J, Shirotani K, Iwata N, Masumoto H, Maki M, Itohara S, Saido TC. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J Biol Chem. 2005;280(15):15229–37. doi: 10.1074/jbc.M500939200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7(8):355–62. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- Hye A, Kerr F, Archer N, Foy C, Poppe M, Brown R, Hamilton G, Powell J, Anderton B, Lovestone S. Glycogen synthase kinase-3 is increased in white cells early in Alzheimer’s disease. Neurosci Lett. 2005;373(1):1–4. doi: 10.1016/j.neulet.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Klafki H, Abramowski D, Swoboda R, Paganetti PA, Staufenbiel M. The carboxyl termini of β-amyloid peptides 1–40 and 1–42 are generated by distinct γ-secretase activities. J Biol Chem. 1996;271(45):28655–9. doi: 10.1074/jbc.271.45.28655. [DOI] [PubMed] [Google Scholar]
- Kuwako K, Nishimura I, Uetsuki T, Saido TC, Yoshikawa K. Activation of calpain in cultured neurons overexpressing Alzheimer amyloid precursor protein. Brain Res Mol Brain Res. 2002;107(2):166–75. doi: 10.1016/s0169-328x(02)00489-8. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405(6784):360–4. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Liang B, Duan BY, Zhou XP, Gong JX, Luo ZG. Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2010;285(36):27737–44. doi: 10.1074/jbc.M110.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280(45):37755–62. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- Maki M, Ma H, Takano E, Adachi Y, Lee WJ, Hatanaka M, Murachi T. Calpastatins: biochemical and molecular biological studies. Biomed Biochim Acta. 1991;50(4–6):509–16. [PubMed] [Google Scholar]
- Mathews PM, Guerra CB, Jiang Y, Grbovic OM, Kao BH, Schmidt SD, Dinakar R, Mercken M, Hille-Rehfeld A, Rohrer J, Mehta P, Cataldo AM, Nixon RA. Alzheimer’s disease-related overexpression of the cation-dependent mannose 6-phosphate receptor increases Aβ secretion: role for altered lysosomal hydrolase distribution in β-amyloidogenesis. J Biol Chem. 2002a;277(7):5299–307. doi: 10.1074/jbc.M108161200. [DOI] [PubMed] [Google Scholar]
- Mathews PM, Jiang Y, Schmidt SD, Grbovic OM, Mercken M, Nixon RA. Calpain activity regulates the cell surface distribution of amyloid precursor protein: inhibition of calpains enhances endosomal generation of β-cleaved C-terminal APP fragments. J Biol Chem. 2002b;277(39):36415–24. doi: 10.1074/jbc.M205208200. [DOI] [PubMed] [Google Scholar]
- Mi W, Pawlik M, Sastre M, Jung SS, Radvinsky DS, Klein AM, Sommer J, Schmidt SD, Nixon RA, Mathews PM, Levy E. Cystatin C inhibits β-amyloid deposition in Alzheimer’s disease mouse models. Nat Genet. 2007;39(12):1440–2. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- Morales-Corraliza J, Mazzella MJ, Berger JD, Diaz NS, Choi JH, Levy E, Matsuoka Y, Planel E, Mathews PM. In vivo turnover of tau and APP Metabolites in the brains of wild-type and Tg2576 mice: greater stability of sAPP in the β-amyloid depositing mice. PLoS One. 2009;4(9):e7134. doi: 10.1371/journal.pone.0007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Takao K, Huang SM, Takano J, Iwata N, Miyakawa T, Saido TC. Comprehensive behavioral phenotyping of calpastatin-knockout mice. Mol Brain. 2008;1(1):7. doi: 10.1186/1756-6606-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2(4):407–18. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochem Res. 2000;25(9–10):1161–72. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid αII-spectrin and calpain-cleaved αII-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. Journal of neurochemistry. 2001;78(6):1297–306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of β-amyloid-protein by degradation. J Biol Chem. 1998;273(49):32730–8. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Rao MV, Mohan PS, Peterhoff CM, Yang DS, Schmidt SD, Stavrides PH, Campbell J, Chen Y, Jiang Y, Paskevich PA, Cataldo AM, Haroutunian V, Nixon RA. Marked calpastatin (CAST) depletion in Alzheimer’s disease accelerates cytoskeleton disruption and neurodegeneration: neuroprotection by CAST overexpression. J Neurosci. 2008;28(47):12241–54. doi: 10.1523/JNEUROSCI.4119-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates β-amyloid-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90(7):2628–32. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Jiang Y, Nixon RA, Mathews PM. Tissue processing prior to protein analysis and β-amyloid quantitation. Methods Mol Biol. 2005a;299:267–78. doi: 10.1385/1-59259-874-9:267. [DOI] [PubMed] [Google Scholar]
- Schmidt SD, Nixon RA, Mathews PM. ELISA method for measurement of β-amyloid levels. Methods Mol Biol. 2005b;299:279–97. doi: 10.1385/1-59259-874-9:279. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1(4):279–87. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. The Biochemical journal. 1997;328(Pt 3):721–32. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94(24):13287–92. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tomioka M, Tsubuki S, Higuchi M, Iwata N, Itohara S, Maki M, Saido TC. Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. J Biol Chem. 2005;280(16):16175–84. doi: 10.1074/jbc.M414552200. [DOI] [PubMed] [Google Scholar]
- Tomidokoro Y, Rostagno A, Neubert TA, Lu Y, Rebeck GW, Frangione B, Greenberg SM, Ghiso J. Iowa variant of familial Alzheimer’s disease: accumulation of posttranslationally modified AβD23N in parenchymal and cerebrovascular amyloid deposits. The American journal of pathology. 2010;176(4):1841–54. doi: 10.2353/ajpath.2010.090636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. The Journal of clinical investigation. 2008;118(8):2796–807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisid T, Kosower NS, Katzav A, Chapman J, Barnoy S. Calpastatin levels affect calpain activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer’s disease. Neurochemistry international. 2007;51(6–7):391–7. doi: 10.1016/j.neuint.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Veeranna, Kaji T, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A, Rudnicki A, Amin N, Li BS, Pant HC, Hungund BL, Arancio O, Nixon RA. Calpain mediates calcium-induced activation of the ERK1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer’s disease. The American journal of pathology. 2004;165(3):795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23(1):20–6. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Archives of biochemistry and biophysics. 1998;356(2):187–96. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- Warren MW, Zheng W, Kobeissy FH, Cheng Liu M, Hayes RL, Gold MS, Larner SF, Wang KK. Calpain- and caspase-mediated αII-spectrin and tau proteolysis in rat cerebrocortical neuronal cultures after ecstasy or methamphetamine exposure. Int J Neuropsychopharmacol. 2007;10(4):479–89. doi: 10.1017/S1461145706007061. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Haass C, Saido TC, Omura S, Ihara Y. Specific increase in β-amyloid-protein 42 secretion ratio by calpain inhibition. Biochemistry. 1997;36(27):8377–83. doi: 10.1021/bi970209y. [DOI] [PubMed] [Google Scholar]
- Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005;352(23):2413–23. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- Zhang L, Song L, Parker EM. Calpain inhibitor I increases β-amyloid peptide production by inhibiting the degradation of the substrate of γ-secretase. Evidence that substrate availability limits β-amyloid peptide production. J Biol Chem. 1999;274(13):8966–72. doi: 10.1074/jbc.274.13.8966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Formic-acid extracted murine Aβ40 and Aβ42 levels were measured by sandwich ELISA in the APP23/CAST and APP23 mice also characterized in Figure 1. (B) Soluble endogenous Aβ40 and Aβ42 levels (C–D) APP metabolite levels (APP, CTFs, sAPP total and sAPPα) in 4 and 8 months old CAST and wild-type mice.