Abstract
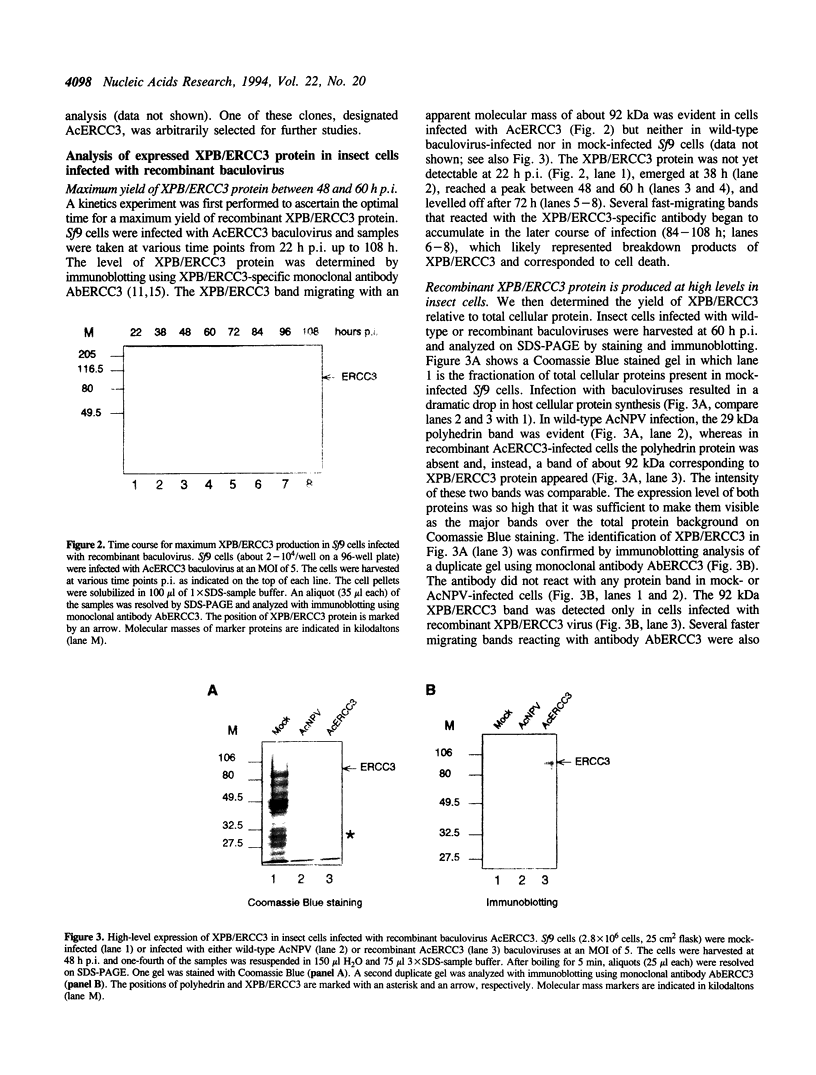
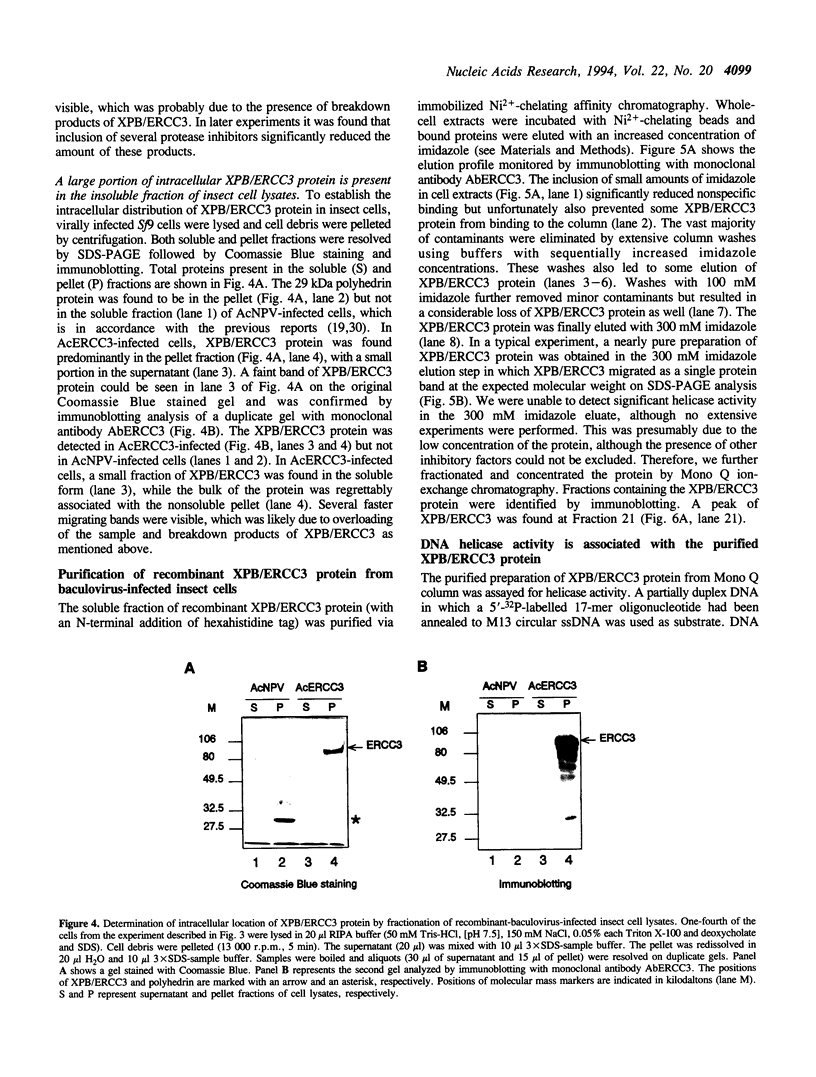
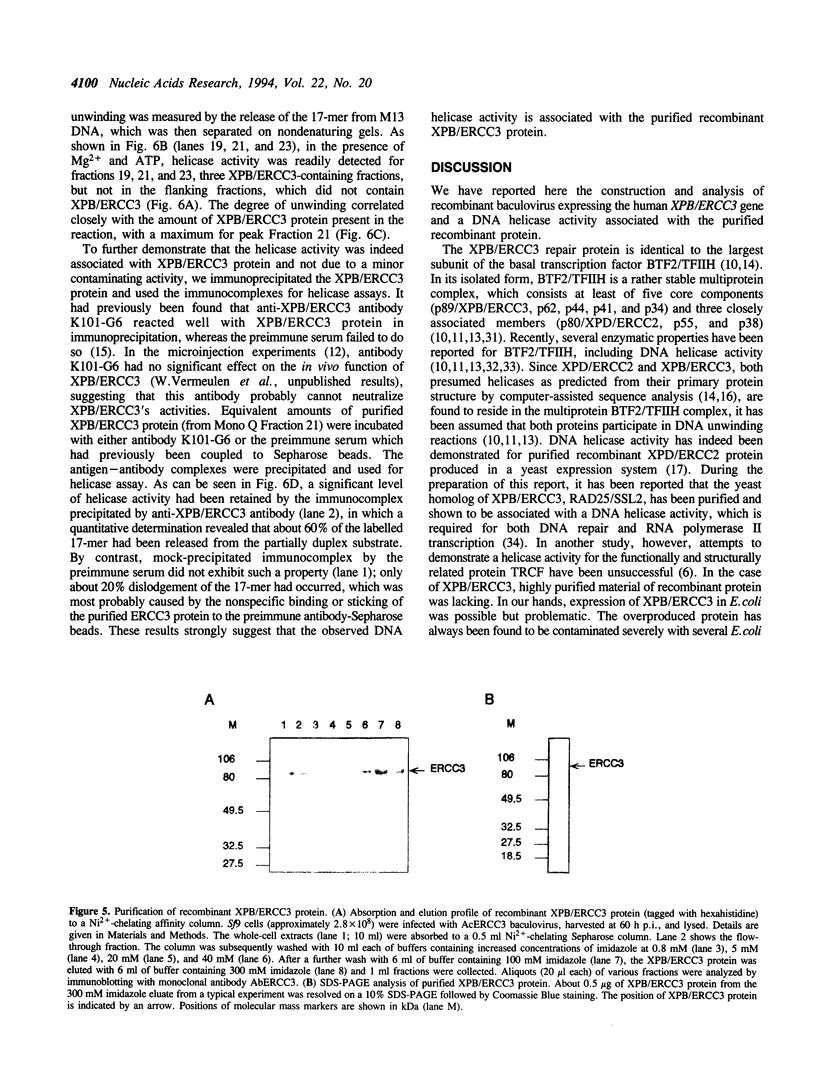
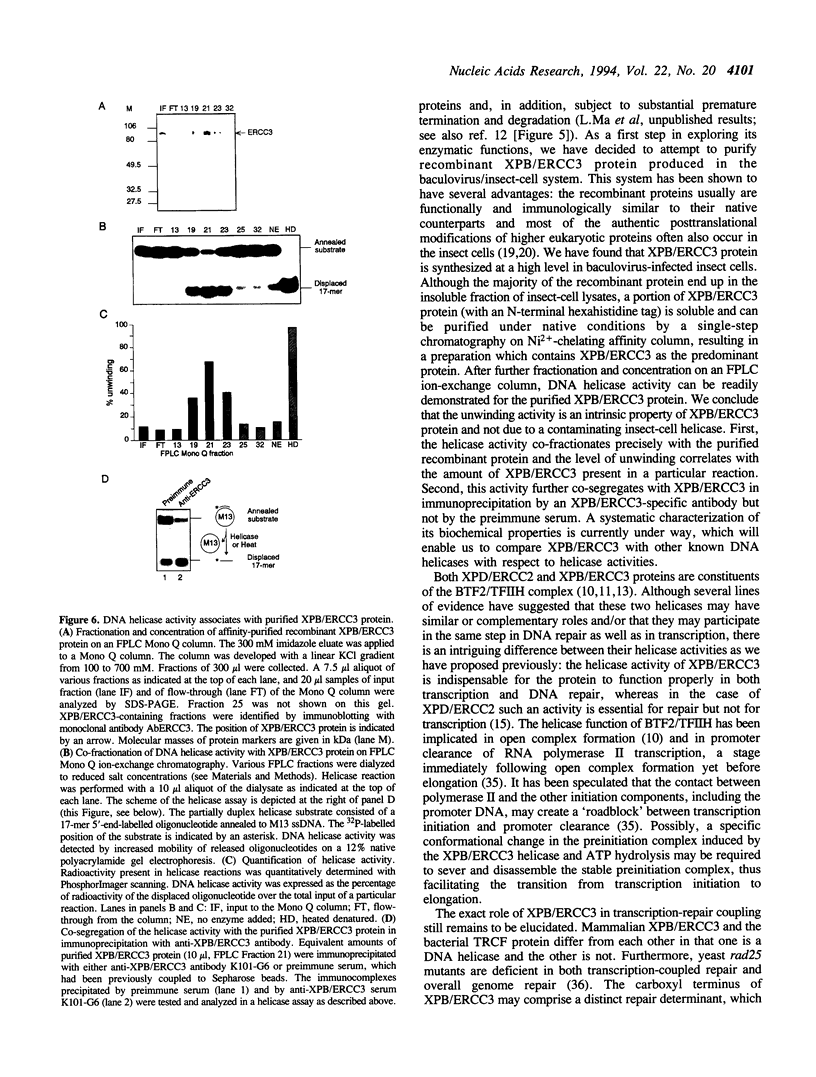
The XPB/ERCC3 gene corrects the nucleotide excision-repair defect in the human hereditary disease xeroderma pigmentosum group B and encodes the largest subunit of the basal transcription factor BTF2/TFIIH. The primary sequence of the XPB/ERCC3 protein features the hallmarks of seven helicase motifs found in many known and putative helicases or helicase-related proteins. Recently, the multiprotein BTF2/TFIIH complex has been found to be associated with DNA helicase activity. To explore the properties and functions of XPB/ERCC3, we have used the baculovirus/insect-cell expression system to produce recombinant protein. We report here the construction and analysis of recombinant baculovirus expressing XPB/ERCC3. The XPB/ERCC3 protein is synthesized at a relatively high level in baculovirus-infected insect cells. While the majority of XPB/ERCC3 end up in the insoluble fraction of insect cell lysates, a minor fraction of recombinant protein is present in soluble form which can be purified under native conditions. We have found that a DNA helicase activity is associated with the purified XPB/ERCC3 protein, suggesting that XPB/ERCC3 may function as a DNA helicase in local unwinding of DNA template both in the context of transcription and nucleotide excision repair.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Wood R. D. Repair of UV-damaged DNA by mammalian cells and Saccharomyces cerevisiae. Curr Opin Genet Dev. 1994 Apr;4(2):212–220. doi: 10.1016/s0959-437x(05)80047-4. [DOI] [PubMed] [Google Scholar]
- Biggerstaff M., Szymkowski D. E., Wood R. D. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 1993 Sep;12(9):3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma D., Hoeijmakers J. H. The molecular basis of nucleotide excision repair syndromes. Mutat Res. 1994 May 1;307(1):15–23. doi: 10.1016/0027-5107(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Reardon J. T., Ansari A., Huang J. C., Zawel L., Ahn K., Sancar A., Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Sancar A., Reinberg D. Where transcription meets repair. Cell. 1994 Apr 8;77(1):9–12. doi: 10.1016/0092-8674(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Tjian R. Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell. 1994 Apr 8;77(1):145–156. doi: 10.1016/0092-8674(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grossman L., Thiagalingam S. Nucleotide excision repair, a tracking mechanism in search of damage. J Biol Chem. 1993 Aug 15;268(23):16871–16874. [PubMed] [Google Scholar]
- Guzder S. N., Sung P., Bailly V., Prakash L., Prakash S. RAD25 is a DNA helicase required for DNA repair and RNA polymerase II transcription. Nature. 1994 Jun 16;369(6481):578–581. doi: 10.1038/369578a0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P., Mellon I. Stranded in an active gene. Curr Biol. 1993 Jan;3(1):67–69. doi: 10.1016/0960-9822(93)90156-i. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Humbert S., van Vuuren H., Lutz Y., Hoeijmakers J. H., Egly J. M., Moncollin V. p44 and p34 subunits of the BTF2/TFIIH transcription factor have homologies with SSL1, a yeast protein involved in DNA repair. EMBO J. 1994 May 15;13(10):2393–2398. doi: 10.1002/j.1460-2075.1994.tb06523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Weeda G., Jochemsen A. G., Bootsma D., Hoeijmakers J. H., van der Eb A. J. Molecular and functional analysis of the XPBC/ERCC-3 promoter: transcription activity is dependent on the integrity of an Sp1-binding site. Nucleic Acids Res. 1992 Jan 25;20(2):217–224. doi: 10.1093/nar/20.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Westbroek A., Jochemsen A. G., Weeda G., Bosch A., Bootsma D., Hoeijmakers J. H., van der Eb A. J. Mutational analysis of ERCC3, which is involved in DNA repair and transcription initiation: identification of domains essential for the DNA repair function. Mol Cell Biol. 1994 Jun;14(6):4126–4134. doi: 10.1128/mcb.14.6.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani C., Sugasawa K., Yanagisawa J., Sonoyama T., Ui M., Enomoto T., Takio K., Tanaka K., van der Spek P. J., Bootsma D. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23. EMBO J. 1994 Apr 15;13(8):1831–1843. doi: 10.1002/j.1460-2075.1994.tb06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Noteborn M. H., de Boer G. F., Kant A., Koch G., Bos J. L., Zantema A., van der Eb A. J. Expression of avian leukaemia virus env-gp85 in Spodoptera frugiperda cells by use of a baculovirus expression vector. J Gen Virol. 1990 Nov;71(Pt 11):2641–2648. doi: 10.1099/0022-1317-71-11-2641. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Roeder R. G. Regulation of TFIIH ATPase and kinase activities by TFIIE during active initiation complex formation. Nature. 1994 Mar 10;368(6467):160–163. doi: 10.1038/368160a0. [DOI] [PubMed] [Google Scholar]
- Peeper D. S., Parker L. L., Ewen M. E., Toebes M., Hall F. L., Xu M., Zantema A., van der Eb A. J., Piwnica-Worms H. A- and B-type cyclins differentially modulate substrate specificity of cyclin-cdk complexes. EMBO J. 1993 May;12(5):1947–1954. doi: 10.1002/j.1460-2075.1993.tb05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeper D. S., Zantema A., Dowdy S. F., van der Eb A. J. Expression, purification, and functional characterization of adenovirus 5 and 12 E1A proteins produced in insect cells. Virology. 1992 Oct;190(2):733–745. doi: 10.1016/0042-6822(92)90911-8. [DOI] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F. Polyhedrin structure. J Gen Virol. 1986 Aug;67(Pt 8):1499–1513. doi: 10.1099/0022-1317-67-8-1499. [DOI] [PubMed] [Google Scholar]
- Roy R., Schaeffer L., Humbert S., Vermeulen W., Weeda G., Egly J. M. The DNA-dependent ATPase activity associated with the class II basic transcription factor BTF2/TFIIH. J Biol Chem. 1994 Apr 1;269(13):9826–9832. [PubMed] [Google Scholar]
- Sancar A., Tang M. S. Nucleotide excision repair. Photochem Photobiol. 1993 May;57(5):905–921. doi: 10.1111/j.1751-1097.1993.tb09233.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Moncollin V., Roy R., Staub A., Mezzina M., Sarasin A., Weeda G., Hoeijmakers J. H., Egly J. M. The ERCC2/DNA repair protein is associated with the class II BTF2/TFIIH transcription factor. EMBO J. 1994 May 15;13(10):2388–2392. doi: 10.1002/j.1460-2075.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schmitt J., Hess H., Stunnenberg H. G. Affinity purification of histidine-tagged proteins. Mol Biol Rep. 1993 Oct;18(3):223–230. doi: 10.1007/BF01674434. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Sung P., Bailly V., Weber C., Thompson L. H., Prakash L., Prakash S. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993 Oct 28;365(6449):852–855. doi: 10.1038/365852a0. [DOI] [PubMed] [Google Scholar]
- Sung P., Higgins D., Prakash L., Prakash S. Mutation of lysine-48 to arginine in the yeast RAD3 protein abolishes its ATPase and DNA helicase activities but not the ability to bind ATP. EMBO J. 1988 Oct;7(10):3263–3269. doi: 10.1002/j.1460-2075.1988.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Watkins J. F., Prakash L., Prakash S. Negative superhelicity promotes ATP-dependent binding of yeast RAD3 protein to ultraviolet-damaged DNA. J Biol Chem. 1994 Mar 18;269(11):8303–8308. [PubMed] [Google Scholar]
- Sweder K. S., Hanawalt P. C. The COOH terminus of suppressor of stem loop (SSL2/RAD25) in yeast is essential for overall genomic excision repair and transcription-coupled repair. J Biol Chem. 1994 Jan 21;269(3):1852–1857. [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Zantema A., Schrier P. I., Davis-Olivier A., van Laar T., Vaessen R. T., van der EB A. J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985 Nov;5(11):3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren A. J., Appeldoorn E., Odijk H., Yasui A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 1993 Sep;12(9):3693–3701. doi: 10.1002/j.1460-2075.1993.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren A. J., Vermeulen W., Ma L., Weeda G., Appeldoorn E., Jaspers N. G., van der Eb A. J., Bootsma D., Hoeijmakers J. H., Humbert S. Correction of xeroderma pigmentosum repair defect by basal transcription factor BTF2 (TFIIH). EMBO J. 1994 Apr 1;13(7):1645–1653. doi: 10.1002/j.1460-2075.1994.tb06428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]