Abstract
Background
Recent evidence shows that despite high incidence of dementia in the very old, they exhibit significantly lower levels of AD neuropathology relative to younger persons with dementia. The levels and distributions of some synaptic proteins have been found to be associated with dementia severity, even in the oldest-old, but the molecular and functional nature of these deficits have not been studied in detail.
Objective
To assess the relationship of dementia with gene and protein expression of a panel of synaptic markers associated with different synaptic functions in young-, middle-, and oldest-old individuals.
Design
The protein and mRNA levels of seven synaptic markers (complexin-1, complexin-2, synaptophysin, synaptobrevin, syntaxin, SNAP-25, and septin-5) were compared in the brains of non-demented and demented individuals ranging from 70 to 103 years of age.
Participants
111 brains were selected to have either no significant neuropathology or only AD-associated pathology (neuritic plaques (NPs) and neurofibrillary tangles (NFTs)). The cohort was then stratified into tertiles as young-old (70-81 years-old), middle-old (82-88), and oldest-old (89-103).
Results
The brains of persons with dementia evidenced significantly lower levels of gene and protein expression of synaptic markers regardless of age. Importantly, dementia was associated with reductions in all measured synaptic markers irrespective of their role(s) in synaptic function.
Conclusions
Although other dementia-associated hallmarks of AD neuropathology (NPs and NFTs) become less prominent with increasing age, synaptic marker abnormalities in dementia remain constant with increasing age and may represent an independent substrate of dementia spanning all ages.
Keywords: synaptic proteins, gene expression, protein expression, Alzheimer’s disease, aging
INTRODUCTION
The oldest-old (OO, persons over the age of 85 years) are the fastest growing segment of our population. Currently numbering over 4 million, by 2050 about 19 million Americans will be 85 years of age or older(U.S.Census Bureau, 2008). About half of the OO will have dementia(Evans et al., 1989) with its accompanying disabilities. Most studies show a consistent increase in incidence of dementia with age, and extraordinarily high incident rates of over 18% for individuals above 90(Corrada et al., 2010). Nevertheless, the relationships of risk factors with Alzheimer’s disease (AD) typically found in younger elderly (such as diabetes(Schnaider et al., 2004), hypertension(Skoog et al., 1996), systemic chronic inflammation(Engelhart et al., 2004) are often weaker or even reversed eg.(Silverman et al., 2009; West et al., 2008) in the OO. Importantly, accumulating recent evidence from multiple studies and diverse cohorts has shown that the tight relationship between the extent of canonical lesions of AD, neuritic plaques (NP) and neurofibrillary tangles (NFT) and dementia severity or is measurably less pronounced, in the brains of very old people relative to the brains of younger elderly(Haroutunian et al., 2008; Head et al., 2007; Prohovnik et al., 2006; Savva et al., 2009). In addition, the transcriptome of dementia changes considerably with age(Katsel et al., 2009). These observations suggest that other, age-invariant neurobiological factors, must contribute to the neurobiology of dementia.
Effective neurotransmission requires an orchestrated series of interactions between various proteins that encapsulate neurotransmitters and exocytose them into the synaptic cleft. Since effective neurotransmission and neurotransmitter release must underlie normal cognitive function, impairments in the levels or functions of synaptic proteins are likely involved in the cognitive deficits of both young-old and oldest-old persons. Different proteins serve different functions within the synaptic boutons and include proteins associated with the vesicular membrane (e.g., synaptophysin, synaptotagmin); the presynaptic membranes, where they participate in the fusion of synaptic vesicles to the synaptic membrane promoting exocytosis (e.g., SNARE complex constituents SNAP-25, Syntaxin, Septin5, and Synaptobrevin); or reside in the presynaptic cytoplasm or other compartments, where they aid in fusion/secretory processes(Rizo and Rosenmund, 2008; Sudhof and Rothman, 2009) (e.g., NSF, Rab3a, Complexins). The levels and distributions of some synaptic proteins have been investigated in AD and they have been consistently shown to be reduced and associated with dementia (for review see(Honer, 2003)). However, the relationship of functionally different synaptic proteins with dementia and their involvement with dementia in young-old and oldest-old persons has not been studied extensively. Understanding the dementia-associated molecular and functional changes in synaptic proteins associated with different aspects of neurotransmitter release can provide insights into the mechanisms of dementia in young-old and oldest-old persons beyond that afforded by the study of individual markers.
We are aware of two studies that examined synaptic proteins in the context of extreme age and dementia(Head et al., 2007; Ubhi et al., 2010). Synaptophysin (but not postsynaptic density 95 nor growth-associated protein 43) protein levels were associated with cognitive function in the 90+ study(Head et al., 2007). Synaptophysin immunoreactivity was more strongly associated with dementia in the older age group (90+) of another study, both in AD and in Dementia with Lewy Bodies subjects(Ubhi et al., 2010). In the present study, we undertook both a protein and an mRNA expression analysis of a broad panel of seven synaptic markers associated with vesicular membranes, synaptic cytoplasm and vesicular fusion, in 111 brains of patients with and without of dementia and wide range of ages from 70 to 103 years. We hypothesized that—in contrast to our own(Haroutunian et al., 2008; Prohovnik et al., 2006) and others’(Head et al., 2007; Savva et al., 2009; Ubhi et al., 2010) studies suggesting a decrease of the association of AD neuropathological hallmark markers and dementia with increasing age—dementia at all ages would be characterized by a general decrease in synaptic proteins suggestive of broad scale synaptic dysfunction rather than a failure of specific aspects of the neurotransmitter secretory process.
MATERIAL AND METHODS
Ethics Statement
Diagnostic and dementia assessment consent procedures were approved by the institutional review boards of Mount Sinai Medical Center, Jewish Home and Hospital and the JJ Peters VA Medical Center. Consents for brain donation were obtained in writing from the legal next of kin of all donors.
Sample description
Postmortem brains subjects participating in studies of aging and early dementia, were received over a period of 20 years by the Mount Sinai School of Medicine Department of Psychiatry Brain Bank. One hundred and eleven study brains were selected from over 1,600 potential specimens. The brains selected were free of any discernable neuropathology or met CERAD neuropathologic criteria(Mirra et al., 1991) for definite, probable or possible AD with no other significant neuropathologic, neurological or psychiatric comorbidities. Exclusions included significant cerebrovascular disease, Lewy body disease, Frontotemporal dementia, Parkinson’s disease, schizophrenia or any other condition known to affect cognition (such as hepatic encephalopathy, severe kidney disease or chemotherapy close to death) as previously described(Haroutunian et al., 2000; Serby et al., 2003).
All subjects died of natural causes with no history of licit or illicit drug abuse or neurological disease. Cognitively intact subjects with no evidence of neurological or neuropsychiatric diseases were matched with dementia subjects by age, postmortem interval (PMI) and brain pH(Butterworth and Tennant, 1989). The predominant causes of death were cardiovascular disease and myocardial infarction, cancer, septicemia and bronchopneumonia. Brain specimens from subjects who were comatose for more than 6 hours prior to death, or had seizures or fever (>39°C) during the 24 hours prior to death, were excluded from the current study. All subjects were evaluated in detail for cognitive status during the last 6 months of life with a postmortem CDR (see below) and the neuropathological assessment procedures were as previously described(Haroutunian et al., 1998; Haroutunian et al., 1999).
The Clinical Dementia Rating (CDR) scale assesses cognitive and functional impairments associated with dementia and provides specific severity criteria for classifying subjects as non-demented (CDR= 0), questionably demented (CDR= 0.5), or increasing levels of severity of dementia from CDR=1 (mild dementia) to CDR=5 (terminal dementia)(Morris et al., 1997). A previously described(Haroutunian et al., 1998; Haroutunian et al., 1999) multi-step consensus approach was applied to the postmortem assignment of CDR scores based on cognitive and functional status during the last 6 months of life, evaluated through medical charts, antemortem neuropsychological assessments (when available), family members, and institutional staff.
Preparation of Brain Specimens and Total RNA
The precise tissue handling procedures have been described in detail(Davis et al., 1999; Haroutunian et al., 1998; Haroutunian et al., 2006; Haroutunian et al., 1999). Grey matter (approximately 0.8 - 1 cm3) from the superior temporal gyrus (STG, Broadmann area - BA22) were dissected from flash frozen coronal sections, pulverized at −80°C, and aliquoted. The STG was chosen because of prior studies demonstrating its involvement in synaptic loss during the dementia process(Brown et al., 1998; Heffernan et al., 1998) and because of its vulnerability to AD associated deficits(Haroutunian et al., 1998; Haroutunian et al., 1999; McDonald et al., 2009). Aliquots (50 mg) from the STG tissue samples were used for qPCR and enzyme-linked immunoadsorbent assays (ELISAs). Brain tissue aliquots were homogenized in 600 μl of lysis buffer with zirconium beads (~2mm diameter) at 4000 rpm for 1 minute using a bead-based cell disrupter/micro homogenizing system (MS-100, Tomy Digital Biology Co, Tokyo, Japan). Total RNA isolation was performed using a Maxwell 16 Nucleic Acid/Protein Isolation system and Total RNA Purification Kits (both are from Promega Corp., Madison, WI). cDNA was generated from 2μg of total RNA using High-Capacity cDNA synthesis kits (Applied Biosystems, Foster City, CA) according to the manufacturer protocol.
RT-qPCR
The mRNA levels of seven synaptic markers were measured by qPCR using TaqMan® MGB probes and primer sets (See Table 1) using an ABI Prism® 7900HT Sequence Detection System (all from Applied Biosystems). For relative quantification of mRNA expression, relative values of examined genes were calculated using the standard curve method, and were further normalized to the geometric means of endogenous control-genes as described previously(Dracheva et al., 2005). Four housekeeping genes (B2M, PGK1, GUSB and PPIA) were used as the endogenous references following testing for their expression stability using geNorm (http://medgen.ugent.be/~jvdesomp/ genorm/).
Table 1.
Description of TaqMan assays used in the study
| Target | (Assay ID) | NCBI Ref. | Exon target | Isoform specificity |
|---|---|---|---|---|
| CPLX1 | Hs00362510_m1 | NM_006651.3 | 3 | 4 major isoforms |
| CPLX2 | Hs01895806_sH | NM_001008220.1 | 5 | 3 major isoforms |
| Septin 5 | Hs00160237_m1 | NM_002688.4 | 9 | - |
| SNAP-25 | Hs00938962_m1 | NM_130811.1 | 7 | 2 major isoforms |
| STX1A | Hs00270282_m1 | NM_004603.2 | 3 | 2 isoforms |
| SYP | Hs00300531_m1 | NM_003179.2 | 2 | 2 major isoforms |
| Synaptobrevin | Hs00249914_m1 | NM_016830.2 | 4 | All isoforms |
| B2M | 4333766T | NM_004048.2 | 2 / 3 | |
| GUSB | 4333767F | NM_000181.1 | 11/ 12 | |
| PGK1 | 4333765F | NM_000291.3 | 4/5 | |
| PPIA | 4333763F | NM_021130.3 | - |
Synaptic antibodies
Monoclonal antibodies from hybridoma tissue culture supernatants were used to detect SNAP-25 (clone SP12, IgG1 subclass), syntaxin (STX, clone SP6, IgG1 subclass), synaptobrevin - VAMP (clone SP10, IgM subclass) , synaptophysin (SYP, clone EP10, IgG1 subclass), CDCrel-1/septin 5 (SEPT5, clone SP18, IgG1 subclass), complexin I (CPLXI, clone SP33, IgG1 subclass) and complexin II (CPLXII, clone LP27, IgG1 subclass). Secondary antibodies used were: peroxidase-conjugated anti-mouse IgG and IgM (Jackson Immunoresearch Laboratories Inc., West Grove PA). Antibody specificity was confirmed by Western blotting, and additional previously reported studies of fusion proteins and immunocytochemistry (Barakauskas et al., 2010; Barr et al., 2004; Sawada et al., 2002).
Synaptic proteins ELISA
Tissue specimens (50 mg) were homogenized in Tris/Triton solution: 250 mM sucrose, 50 mM Tris–HCl (pH 7.4), 1 mM EDTA, 2 mM EGTA, 1% Triton X100 containing 1mM PMSF and supplemented with complete cocktails of proteinase/phosphatase inhibitors (Pierce Biotech Inc, Rockford, IL). Total protein concentration in the tissue homogenates was determined with a CBQCA Quantitation Kit (Molecular Probes Inc, Eugene, OR). Samples were diluted to a standard protein concentration (60 ug/ml) for the ELISA, and then duplicate samples were diluted over a 64-fold range in a 384-well plate (Nalgen Nunc International, Rochester NY). Assays were performed using previously published protocols(Barakauskas et al., 2010) (Honer et al., 2002). Synaptic proteins were detected by incubating with antibodies diluted in 5% milk/TBS (1:10 v/v dilution). Immunoreactivity was compared between samples as the amount of protein required to give a chosen optical density within this range. To compare immunoreactivities between plates, samples were normalized to a reference-generic sample representing the complete set of samples, which was run on each of the 6 plates. The intra-run coefficient of variation (CV), calculated using the reference sample was <10% for all runs.
Statistical Analyses
Dementia was dichotomized based on the CDR score (non-demented: CDR=0 or CDR=0.5; demented: CDR≥1). Ages were categorized by tertiles as young-old (70-81 years old), middle-old (82-88), and oldest-old (89-103). Characteristics of the sample were compared using analyses of variance and Pearson’s χ2. There were no clear relationships between the gene and protein expression measures and the common potential confounders such as PMI and pH, however, since PMI differed between the age groups for both demented and non-demented cases (see Table 2), PMI was employed as a covariate. Analyses of covariance evaluated associations of the seven synaptic markers (SNAP-25, STX, synaptobrevin, SYP, SEPT5, CPLXI, and CPLXII) with age tertile and dementia status. The age by dementia status interaction term examined whether the associations of dementia status with synaptic markers gene and protein expression differed by age.
Table 2. Characteristics of the sample.
YO= young-old; MO= middle-old; OO= oldest-old; F=female; PMI=postmortem interval
| Non Demented | Demented | Total | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YO | MO | OO | Total | p-value | YO | MO | OO | Total | p-value | |||
| N | 21 | 18 | 18 | 57 | 15 | 19 | 20 | 54 | 111 | |||
|
Age (mean, SD) |
74.7 (3.5) |
85.2 (2.2) |
93.2 (3.3) |
83.8 (8.3) |
<0.005 | 76.9 (3.1) |
85.4 (2.0) |
95.9 (4.1) |
86.9 (8.3) |
<0.005 | 85.3 (8.4) |
0.053 |
| Sex (%F) | 52.4 | 61.1 | 72.2 | 61.4 | 0.45 | 80.0 | 78.9 | 85.0 | 81.5 | 0.88 | 71.2 | 0.02 |
|
PMI (minutes: means, SD) |
879 (585) |
637 (603) |
444 (367) |
665 (554) |
0.046 | 878 (768) |
298 (277) |
365 (286) |
484 (522) |
0.001 | 577 (544) |
0.08 |
| PH | 6.4 (.34) |
6.3 (.32) |
6.4 (.26) |
6.4 (.31) |
0.57 | 6.3 (.22) |
6.3 (.28) |
6.3 (.29) |
6.3 (.26) |
0.78 | 6.3 (.29) |
0.07 |
RESULTS
Table 2 presents the characteristics of the sample. PMI differed by age, with the youngest tertile having longer PMI than the other age tertiles in both non-demented (p=0.046) and demented (p=0.001) subjects. There were no significant age differences among non demented or demented subjects in pH. Although the majority of the sample were women (71.2%), a significantly higher proportion of demented subjects were women (p=0.02). There were no significant sex differences by age with dementia status.
Synaptic protein levels were significantly lower with dementia for all synaptic proteins (p values range from 0.03 [9% loss of STX] to < 0.0005 [22% loss of CPLXI) except SEPT5 (p=0.26) (Table 3 and Figure1). Similarly, the mRNA expression levels of STX (p = 0.01), SYP (p = 0.001), and SNAP-25 (p = 0.01), and on a trend level CPLXI (p=0.06), were lower in the superior temporal gyrus of persons with dementia relative to the non-demented controls. Importantly, synaptic protein expression levels were not associated with age. For gene expression, only STX levels were associated with age (p = 0.02) with the youngest group having higher gene expression.
Table 3.
Analyses of covariance results (controlling for PMI)
| Synaptic Marker | Dementia main effect | Age main effect | Interaction |
|---|---|---|---|
| Gene expression | |||
| CPLX1 | F (1, 90)=3.76; p=0.06 | F (2, 90)=1.18; p=0.31 | F (2, 90)=2.09; p=0.13 |
| CPLX2 | F (1, 91)=0.04; p=0.85 | F (2, 91)=0.44; p=0.65 | F (2, 91)=0.56; p=0.57 |
| SEPT5 | F (1, 91)=2.74; p=0.10 | F (2, 91)=1.23; p=0.30 | F (2, 91)=0.70; p=0.50 |
| SNAP-25 | F (1, 90)=6.26; p=0.01 | F (2, 90)=1.65; p=0.20 | F (2, 90)=0.26; p=0.77 |
| Synaptobrevin | F (1, 90)=2.33; p=0.13 | F (2, 90)=0.71; p=0.50 | F (2,90)=0.80; p=0.45 |
| STX | F (1, 91)=6.75; p=0.01 | F (2, 91)=4.29; p=0.02 | F (2, 91)=1.52; p=0.23 |
| SYP | F (1, 91)=11.31; p=0.001 | F (2, 91)=0.51; p=0.60 | F (2, 91)=1.17; p=0.32 |
| Protein expression | |||
| CPLX1 | F (1, 104)=36.55; p<0.0005 | F (2, 104)=0.17; p=0.85 | F (2,104)= 1.36; p=0.29 |
| CPLX2 | F (1, 103)=7.36; p=0.008 | F (2, 103)= 0.07; p=0.93 | F (2, 103)= 0.27; p=0.77 |
| SEPT5 | F (1, 104)=1.31; p=0.26 | F (2, 104)=1.82; p=0.17 | F (2, 104)=1.03; p=0.36 |
| SNAP-25 | F (1, 101)=15.58; p<0.0005 | F (2, 101)= 0.62; p=0.54 | F (2, 101)=1.78; p=0.17 |
| Synaptobrevin | F (1, 103)=4.76; p=0.03 | F (2, 103)=0.15; p=0.86 | F (2, 103)=2.23; p=0.11 |
| STX | F (1, 98)=6.06; p=0.02 | F (2, 98)=1.40; p=0.25 | F (2, 98)=1.78; p=0.17 |
| SYP | F (1, 92)=12.34; p=0.001 | F (2, 92)=0.48; p=0.62 | F (2, 92)=3.24; p=0.04 |
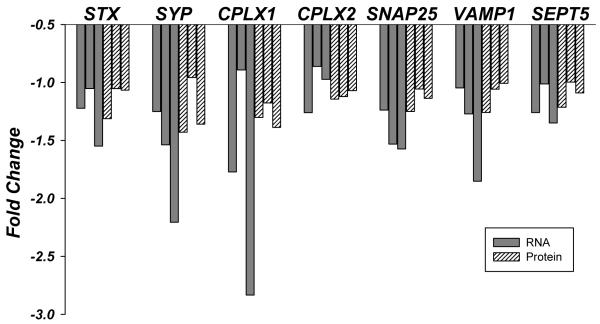
Figure 1.
Changes of mRNA (gray bars) and protein (pattern bars) levels of seven synaptic markers in the superior temporal gyrus of demented subjects as a function of three age tertiles: from left to right young-old, middle old and oldest old. Fold change represents ratio of the means of mRNA or protein levels between non-demented and demented subjects. Corresponding p-values for dementia, age, or the interaction of dementia by age are shown in the Table 3.
The associations of protein expression levels with dementia did not change as a function of age (p values > 0.11), with the exception of SYP (p = 0.04), for which the young-old and oldest-old ages had lower protein expression levels with dementia but there was no difference for the middle-age group. The associations of gene expression levels with dementia also did not change as a function of age (p values > 0.13). All results were essentially unchanged with the inclusion or exclusion of PMI in the statistical model.
To examine the association of each protein expression level with dementia beyond the contribution of the other genes, in secondary analyses, we performed logistic regression controlling for PMI. The overall test for all seven protein expression levels was strongly significant (χ2=33.85; df=7; p<0.0005), but none of the protein expression levels remained significant controlling for all of the others. In a similar analysis, the overall test for all seven mRNA expression levels was significant (χ2=16.61; df=7; p=0.020), but none of the mRNA expression levels remained significant controlling for all of the others. In additional secondary analyses, we performed linear multiple regression for age as a continuous variable controlling for PMI, to examine the contribution of each gene or protein expression level beyond the contribution of the others. None of the gene or protein expression levels were significant controlling for the others, and the overall models were also not significant.
DISCUSSION
Gene and protein expression of the broad spectrum of synaptic markers studied were associated with dementia even in the oldest-old. This observation suggests that AD-associated dementia is characterized by a general failure of synaptic function which remains constant with increasing age, even when neuropathologic hallmarks of Alzheimer’s disease become less prominent in the oldest-old(Haroutunian et al., 2008). This general observation suggests a dissociation of synaptic dysfunction from neuropathologic indices such as the deposition of NPs and development of NFTs. Perhaps as importantly, the gene and protein levels of synaptic protein did not change as a function of the 40 year age-span of this study, suggesting that the contribution of synaptic loss to the dementing process is not a function of aging per se.
The current study indicates that proteins involved in vesicular membranes, vesicular secretory processes, fusion, and exocytosis were all affected in persons with dementia (with particularly large effect size for CPLX-1 protein expression). This implies that synaptic deficit is a general process affecting synaptic integrity rather that the failure of a specific component of the processes involved in neurotransmitter packaging and release. This interpretation is reinforced by the results of the regression analyses, where the overall tests were statistically significant but none of the gene or protein expression levels association with dementia remained significant beyond the contribution of the others. One could speculate that the broad deficits in synaptic proteins associated with dementia imply synaptic pruning or cell loss, rather the failure of specific features of neurotransmitter packaging, fusion or exocytosis.
Although some studies suggest that, like NPs and NFTs, synaptic function deficits are not causal substrates of dementia but rather represent the end consequences of underlying “toxic/pathogenic” factors or neurobiological processes(Selkoe, 2008; Shankar et al., 2008), the present study distinguishes synaptic markers from NPs and NFTs because they are associated with dementia even in extremely old ages. Aβ oligomers—the toxic amyloid precursor protein metabolites which progressively accumulate in the brains of AD patients—have been associated with reductions of synaptic proteins(Pham et al., 2010), and implicated in the impairment of synaptic plasticity(Selkoe, 2008; Shankar et al., 2008). Accumulation of oligomeric Aβ or abnormalities in presenilins(Zhang et al., 2009) in the brain of young-old and oldest-old persons could contribute to synaptic dysfunction without necessarily involving NPs and NFTs. Oxidative-stress-induced DNA damage to genes encoding for synaptic proteins has been suggested as another underlying mechanism for synaptic dysfunction in AD(Forero et al., 2006). Mitochondria may also play an important role in the regulation of synaptic function because of their ability to regulate calcium levels as well as the production of reactive oxygen species(Mattson and Liu, 2002). These mechanisms are consistent with synaptic loss being an intervening causal stage of the neurodegenerative cascade or, alternatively, an NP- or NFT-independent consequence of these processes.
Expression of synaptic proteins in the hippocampal synaptoproteome of old Fischer 344 X Brown Norway rats was altered compared to young rats(VanGuilder et al., 2010). Similarly, age related decreases in SYP were found in rhesus macaques(Haley et al., 2010). In a sample of individuals ranging from 16 to 98 years of age, those older than 60 had an average 20% decrease in synapse density compared with younger individuals(Masliah et al., 1993). In contrast, our study focused only on the elderly, who are at risk for dementia, with the youngest subject being 70 years old. Synaptic protein loss may be a feature of early stages of aging, but it appears to be a non-linear process that plateaus by the time cognitively normal individuals reach middle- and oldest-old ages. Consistent with this view, subjects under 60 had a correlation of age with presynaptic terminal counts of −0.52 while subjects over 60 had a correlation of +0.22(Masliah et al., 1993).
These findings are consistent with an earlier study that showed decreased levels of SYP in demented compared to non-demented nonagenarians(Head et al., 2007). SYP was more strongly associated with dementia severity as age increased in another study(Ubhi et al., 2010). These results contrast with consistent recent findings, including from our group, showing that the hallmark features of AD, NPs and NFTs, correlate less strongly with dementia as age increases(Haroutunian et al., 2008; Head et al., 2007; Prohovnik et al., 2006; Savva et al., 2009; Ubhi et al., 2010). There is evidence suggesting that presynaptic dysfunction may be an early event in the pathogenesis of dementia(Zhang et al., 2009), that compensatory postsynaptic changes may occur in response to presynaptic deficits(Dekosky and Scheff, 1990), that synaptic loss precedes amyloid deposition and plaque formation(Mucke et al., 2000; Scheff et al., 2007) and that plaque formation might start with synaptic alterations(Masliah et al., 2006). The very elderly have less brain reserve(Stern et al., 2008) than younger elderly, and thus the first steps of the neurodegenerative process might be sufficient to promote dementia, while, at the other end, high mortality might preclude the development of full-fledged plaques. Similarly, greater cellular resistance to the deleterious effects of synaptic loss in brains of young elderly could promote more plaque formation and deposition than in older elderly. The validity of these hypotheses cannot be ascertained from the current or similar postmortem studies, but it can be addressed by in vitro and animal model experiments.
The association of synaptic protein expression with dementia was stronger than that of gene expression. The correlations between gene and protein expression of the synaptic markers examined were either of small magnitude (e.g. SYP; r=0.29, p=0.006) or non-existent (e.g. SEPT5; r=−0.02; p=0.82). This dissociation might be due to the cellular localization of synaptic proteins and their mRNAs. Since mRNAs are most frequently localized to the soma, mRNA measures reflect the levels of transcripts in neurons whose cell bodies are located within the superior temporal gurus. Synaptic proteins, on the other hand, are by definition localized to the presynaptic compartment and reflect levels of all neurons with terminals in the superior temporal gyrus irrespective of whether their cell bodies are within the gyrus or at distal loci projecting to the superior temporal gyrus such as the visual and association cortices(Seltzer and Pandya, 1978). That protein levels were more closely associated with dementia than mRNA levels may reflect broad, pan-cortical, deficits in synaptic function rather than deficits localized to the superior temporal gyrus alone.
Strengths of this study include the broad panel of synaptic markers, the use of both the coding mRNA and of the protein levels, the breadth of ages of the elderly subjects, and the exclusion of neuropathology other than AD, to avoid interpretational and mechanistic confounds introduced by neuropathologies such as stroke, Lewy body dementia and other non-AD associated neuropathologies. Of course this sampling selectivity came at the cost of reduced generalizability of the findings to the greater population of the elderly with dementia. Since the majority of the subjects were from nursing home and assisted living facilities, generalization of the results to all elderly persons with AD-associated dementia must be drawn with caution, however, it is noteworthy that in the US, approximately 40% of the oldest-old live in institutions(He W et al., 2005) and these results are consistent with those of nonagenarians living in the community(Head et al., 2007). While PMIs were generally low for all groups, they did differ statistically between the different age groups, but inclusion or exclusion of PMI as a covariate from the statistical tests of significance led to essentially identical results. Although inspection of the data did not reveal differences between cases with CDR 0 and CDR 0.5, which were grouped together as non-demented, the sample sizes were too small for meaningful comparisons. To make it feasible to study a broad range of synaptic proteins, we focused primarily on severely demented subjects and compare them to non-demented. Thus, there was not enough variation of CDR among demented cases (81% had CDR=5) to examine associations of a full distribution of the dementia spectrum with synaptic RNA and protein losses. Finally, we did not examine synaptic proteins representing post-synaptic structures despite the well known loss of dendritic spines in AD(Akram et al., 2008). However, in nonagenarians, post-synaptic density 95 (PSD-95) was not associated with dementia severity(Head et al., 2007).
Acknowledgement
supported by NIA grants K01 AG023515-01 and R01 AG034087, P01 AG02219, P50 AG05138, as well as by Ira T. Hirschl, CIHR MOP-14037, the Graubard Fund and the Berkman Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akram A, Christoffel D, Rocher AB, Bouras C, Kovari E, Perl DP, Morrison JH, Herrmann FR, Haroutunian V, Giannakopoulos P, Hof PR. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiol.Aging. 2008;29:1296–1307. doi: 10.1016/j.neurobiolaging.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakauskas VE, Beasley CL, Barr AM, Ypsilanti AR, Li HY, Thornton AE, Wong H, Rosokilja G, Mann JJ, Mancevski B, Jakovski Z, Davceva N, Ilievski B, Dwork AJ, Falkai P, Honer WG. A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology. 2010;35:1226–1238. doi: 10.1038/npp.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Young CE, Sawada K, Trimble WS, Phillips AG, Honer WG. Abnormalities of presynaptic protein CDCrel-1 in striatum of rats reared in social isolation: relevance to neural connectivity in schizophrenia. Eur.J.Neurosci. 2004;20:303–307. doi: 10.1111/j.0953-816X.2004.03457.x. [DOI] [PubMed] [Google Scholar]
- Brown DF, Risser RC, Bigio EH, Tripp P, Stiegler A, Welch E, Eagan KP, Hladik CL, White CL., III Neocortical synapse density and Braak stage in the Lewy body variant of Alzheimer disease: a comparison with classic Alzheimer disease and normal aging. J.Neuropathol.Exp.Neurol. 1998;57:955–960. doi: 10.1097/00005072-199810000-00007. [DOI] [PubMed] [Google Scholar]
- Butterworth J, Tennant MC. Postmortem human brain pH and lactate in sudden infant death syndrome. J.Neurochem. 1989;53:1494–1499. doi: 10.1111/j.1471-4159.1989.tb08543.x. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann.Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–1406. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- Dekosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann.Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J.Neurosci.Res. 2005;79:868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch.Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Forero DA, Casadesus G, Perry G, Arboleda H. Synaptic dysfunction and oxidative stress in Alzheimer’s disease: emerging mechanisms. J.Cell Mol.Med. 2006;10:796–805. doi: 10.1111/j.1582-4934.2006.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley GE, Kohama SG, Urbanski HF, Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Dordr.) 2010;32:283–296. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Davis KL. The human homolog of the QKI gene affected in the severe dysmyelination “quaking” mouse phenotype: downregulated in multiple brain regions in schizophrenia. Am.J.Psychiatry. 2006;163:1834–1837. doi: 10.1176/ajp.2006.163.10.1834. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch.Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch.Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, Libow LS, Lesser GT, Maroukian M, Grossman HT. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch.Neurol. 2008;65:1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Serby M, Purohit DP, Perl DP, Marin D, Lantz M, Mohs RC, Davis KL. Contribution of Lewy body inclusions to dementia in patients with and without Alzheimer disease neuropathological conditions. Arch.Neurol. 2000;57:1145–1150. doi: 10.1001/archneur.57.8.1145. [DOI] [PubMed] [Google Scholar]
- He W, Sengupta M, Velkoff VA, Debarros JA. 65+ in the United States: Current population reports, special studies. Department of Health Services and Department of Commerce; 2005. pp. 23–209. [Google Scholar]
- Head E, Corrada MM, Kahle-Wrobleski K, Kim RC, Sarsoza F, Goodus M, Kawas CH. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol.Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan JM, Eastwood SL, Nagy Z, Sanders MW, McDonald B, Harrison PJ. Temporal cortex synaptophysin mRNA is reduced in Alzheimer’s disease and is negatively correlated with the severity of dementia. Exp.Neurol. 1998;150:235–239. doi: 10.1006/exnr.1997.6772. [DOI] [PubMed] [Google Scholar]
- Honer WG. Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiol.Aging. 2003;24:1047–1062. doi: 10.1016/j.neurobiolaging.2003.04.005. [DOI] [PubMed] [Google Scholar]
- Honer WG, Falkai P, Bayer TA, Xie J, Hu L, Li HY, Arango V, Mann JJ, Dwork AJ, Trimble WS. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb.Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- Katsel P, Tan W, Haroutunian V. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS.One. 2009;4:e7642. doi: 10.1371/journal.pone.0007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer’s disease. J.Alzheimers.Dis. 2006;9:91–99. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular.Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr., Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van BG, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J.Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat.Struct.Mol.Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N.Engl.J.Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, Mann JJ, Dwork AJ, Falkai P, Phillips AG, Honer WG. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol.Psychiatry. 2002;7:484–492. doi: 10.1038/sj.mp.4000978. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Dekosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Schnaider BM, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63:1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav.Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Serby M, Brickman AM, Haroutunian V, Purohit DP, Marin D, Lantz M, Mohs RC, Davis KL. Cognitive burden and excess Lewy-body pathology in the Lewy-body variant of Alzheimer disease. Am.J.Geriatr.Psychiatry. 2003;11:371–374. [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat.Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Beeri MS, Schmeidler J, Rosendorff C, Angelo G, Mavris RS, Grossman HT, Elder GA, Carrion-Baralt J, West R. C-reactive protein and memory function suggest antagonistic pleiotropy in very old nondemented subjects. Age Ageing. 2009;38:237–241. doi: 10.1093/ageing/afn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Flynn J, Steffener J, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb.Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.Census Bureau Percent Distribution of the Projected Population by Selected Age Groups and Sex for the United States: 2010 to 2050. 2008 http://www.census.gov/population/www/projections/summarytables.html.
- Ubhi K, Peng K, Lessig S, Estrella J, Adame A, Galasko D, Salmon DP, Hansen LA, Kawas CH, Masliah E. Neuropathology of dementia with Lewy bodies in advanced age: a comparison with Alzheimer disease. Neurosci.Lett. 2010;485:222–227. doi: 10.1016/j.neulet.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J.Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, Rosendorff C, Silverman JM. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am.J.Geriatr.Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]