Abstract
The ventromedial prefrontal cortex (vmPFC) comprises a set of interconnected regions that integrate information from affective sensory and social cues, long-term memory, and representations of the ‘self’. Though the vmPFC is implicated in a variety of seemingly disparate processes, these processes are organized around a common theme. The vmPFC is not necessary for affective responses per se, but is critical when affective responses are shaped by conceptual information about specific outcomes. The vmPFC thus functions as a hub that links concepts with brainstem systems capable of coordinating organism-wide emotional behavior, a process we describe in terms of the generation of affective meaning, and which could explain the common role played by the vmPFC in a range of experimental paradigms.
Ventromedial prefrontal cortical involvement across psychological domains
Over the past decade, neuroimaging studies have consistently identified the human ventromedial prefrontal cortex (vmPFC) as a key region for numerous and often seemingly disparate functions, including autonomic and endocrine regulation [1], emotion [2], emotion regulation [3], fear conditioning and extinction [4], episodic and semantic memory [5,6], prospection [5], economic valuation [7], self-directed cognition [8], and mentalization about others [9] (Figure 1). Conversely, dysfunction of the vmPFC is thought to be critical in a number of brain disorders, most notably post-traumatic stress disorder (PTSD) [10], depression [11] and dysregulation related to chronic stress [12] and pain [13].
Figure 1.
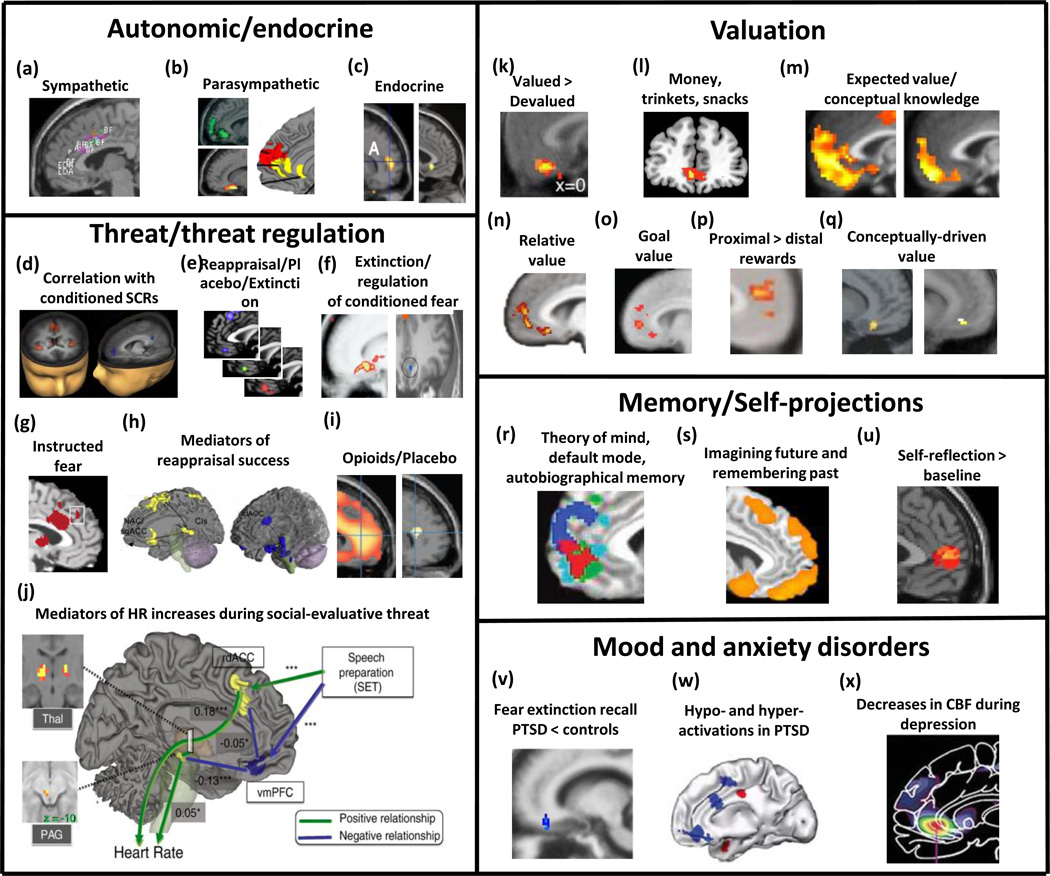
Convergence across fields: VMPFC across social, cognitive, affective, and clinical domains. (a) Dorsal anterior/mid cingulate activations re sympathetic activity during emotional/cognitive tasks and exercise [1]. (b) pgACC, sgACC and rostral MPFC activity correlations with parasympathetic/anti-sympathetic activity; top-left: negative correlation with increases in heart rate during a working memory task [90]; bottom-left: decreases in skin conductance during relaxation [91]; right: results from a meta analysis on brain activations associated with high-frequency heart rate variability during emotional and cognitive tasks (red: emotional>cognitive; yellow: emotional = cognitive) [92] (c) left: Stress-related correlates of natural killer (NK) cells increase [93]; right: grief-evoking words correlates with IL-6 [94]. (d) vmPFC and rdACC activity negatively and positively correlates with conditioned SCR during fear conditioning and revearsal [4]; (e) vmPFC activation in meta-analyses of fear extinction (red), placebo (green) and reappraisal (blue). (f) left: vmPFC/sgACC activity during extinction recall [74]; right: vmPFC/sgACC activity (blue) during reappraisal of conditioned fear [52]. (g) rdACC activity in instructed fear paradigms [50]. (h) Positive (left: sgACC/Nacc) and negative (right: rdACC/amygdala) mediators of successful reappraisal of negative emotions [54] (i) left: opioids activate MPFC [95]; right: placebo activates a common sub-region in rACC [95]. (j) Positive (rdACC, thalamus, PAG) and negative (vmPFC) mediators of heart rate increases during social evaluative threat [65]. (k) vmPFC/mOFC activation for valued vs devalued food [46]. (l) Common coding of value of money, trinkets and snacks [67]. (m) left: integration of conceptual knowledge and value representations during decision-making; right: more rostral activity specific to explicit conceptual knowledge [78]. (n) Relative coding of value in the vmPFC [49]. (o) Goal value: healthiness of food for successful dieters or taste of food for unsuccessful dieters [73]. (p) temporal discounting of subjective value [72]. (q) left: same odor labeled as “cheddar cheese” vs “sweat” [68]; right: same wine labeled as “cheap” vs “pricey” [70]. (r) Convergence (red) of activations related to autobiographical memory (light blue), theory of mind (dark blue) and default-mode network (green) [96]. (s) Activity related to imagining past or future events or remembering past events [97]. (t) Activity related to self-reflection [98]. (u) Reduced activation during recall of extinction in PTSD patients vs controls [38]. (v) Hypo- (blue) and hyper- (red) activations in response to aversive stimuli in PTSD patients vs controls [10]. (w) Reduced cerebral blood flow (CBF) in depressed patients vs controls [79]. sgACC: subgenual anterior cingulate cortex, pgACC: perigenual anterior cingulate cortex, rACC: rostral ACC, rdACC: rostro-dorsal anterior cingulate cortex, MPFC: medial prefrontal cortex, vmPFC: ventromedial prefrontal cortex, mOFC: medial orbitofrontal cortex, Nacc: nucleus accumbens, PAG: periaqueductal gray matter SCR: skin conductance response, IL-6: interleukin -6.
Although this convergence is often briefly acknowledged, it remains unclear why these different functions should overlap in the vmPFC, and what its broad, underlying roles might be in the coordination of adaptive behavior. It is tempting to attribute this functional diversity to the heterogeneity of the vmPFC itself, which comprises several distinct cytoarchitectonic areas spanning from the anterior cingulate cortex to the frontal pole. However, the major subdivisions of the vmPFC are interconnected with each other and with subcortical nuclei in several interlocked functional systems [11], prompting a number of researchers to characterize the region’s broad functional roles with terms such as ‘affect’ [14], ‘regulation’ [3], ‘valuation’ [7,15], ‘self-projection’ [5], ‘self-reference’ [8], ‘mentalizing’ [9], ‘somatic markers’ [16], ‘visceromotor’ [11], and ‘default-mode’ because of its high resting metabolism [17]. Although each of these characterizations is extremely useful in providing a common explanation to a broad range of vmPFC-related phenomena, none of them seems to account for the range of processes that involve the vmPFC, and many of these broad views are difficult to reconcile with animal studies on the functional impairments with vmPFC damage or inactivation.
Here, we argue that the functional role of vmPFC is not reducible to any one of these functional categories. Rather, it serves as a hub that connects systems involved in episodic memory, representation of the affective qualities of sensory events, social cognition, interoceptive signals, and evolutionarily conserved affective physiological and behavioral responses. As such, it plays a unique role in representing conceptual information relevant for survival and in transducing concepts into affective behavioral and physiological responses. To conceptualize the organism in context is to conceive the meaning of a situation (a particular constellation of environmental and internal cues) for one’s physical and social well being and future prospects. We argue that the vmPFC is essential for this class of processes. Affective meaning is closely related to concepts such as ‘affective appraisal’ [18], ‘situated conceptualization’ [19], ‘valuation’ [7,15], and ‘goal-driven’ value learning [20]. However, we envision the construction of affective meaning as involving a unique set of ingredients that are not necessarily involved in these other concepts, including a) constructing representations of a ‘situation’ (or ‘schema’) from precise configurations of cues; b) recalling similar past situations and abstracting essential features to guide prospection about potential future outcomes; c) evaluating potential outcomes for benefit and harm to the organism (‘self’); and d) triggering appropriate physiological and emotional responses, or modifying ongoing ones. Thus, a meaning-centered view of vmPFC predicts that vmPFC and its subcortical connections are not essential for simple forms of affect, valuation, and affective learning, but are essential when conceptual information drives affective physiological and behavioral responses.
A system of systems: vmPFC bridges conceptual and affective processes
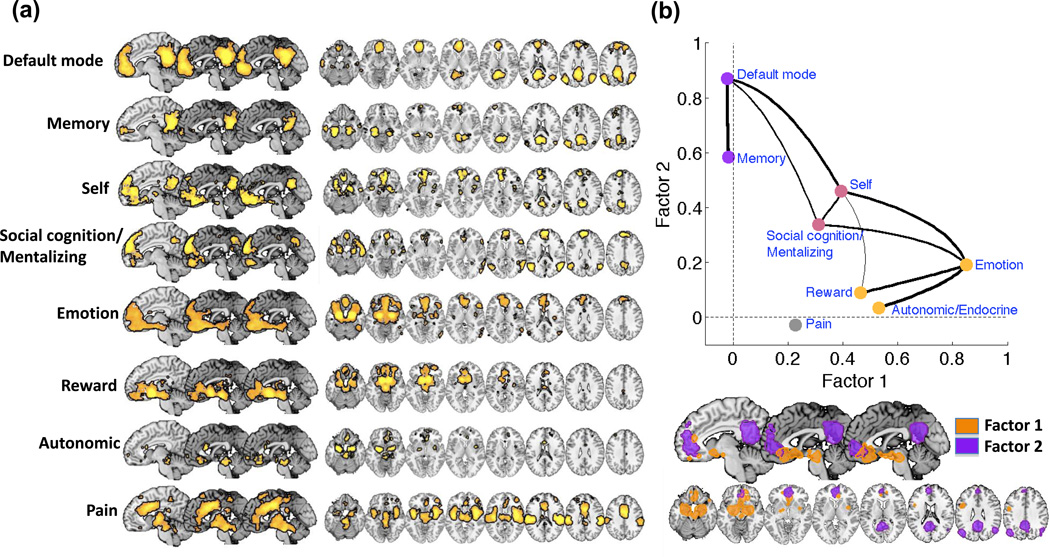
In the last few years, it has become possible to integrate human neuroimaging results across thousands of studies [21], which now allows us to link broad classes of psychological processes with the functional brain networks that underlie them. A recent database of nearly 4,400 studies, called Neurosynth (www.neurosynth.org; [21]), permits the construction of meta-analytic maps of consistent activations across studies based on terms frequently used in articles. One interesting feature of this approach is that it allows identifying activations specifically associated with particular psychological domains, as specified by a set of terms. For example, studies of ‘mentalizing,’ a type of social cognition involving thinking about others’ minds and intentions, can be defined based on the use of the words empathy or empathizing, ‘theory of mind’, mentalizing or mentalization, and related terms. We used neurosynth to obtain maps of brain networks specifically associated with functional tasks related to the ingredients of affective meaning. These included maps of studies related to memory and ‘default mode’ function, self-reflection, social cognition/mentalizing, emotion, reward, and autonomic and endocrine changes (Figure 2; see Supplementary Online Material for details). We assessed the relationships between these maps and the brain regions that are commonly involved in all of these processes, thus assessing which brain regions are likely to be essential for integrating information across systems. We also compared these maps with a map of experimental pain, which is affective but not conceptually driven per se (that is, pain does not always require the conceptual construction of meaning to induce affect).
Figure 2.
A meta-analytic view: Convergence of meaning-related processes in vmPFC. (a) Results of an automated reverse inference meta-analysis using Neurosynth (neurosynth.org). Color maps display the probability terms related to vmPFC’s functions given observed activation (P term|activation), (b) Results of a factor analysis with two factors on the meta-analytic reverse inference patterns of activation for the terms “default mode”, “memory”, “self”, “social cognition/mentalizing”, “reward”, “autonomic/endocrine”, “emotion” and “pain”. Top panel: Factor loadings associated with each term. “Emotion”, “autonomic/endocrine” and “reward” strongly loaded on factor 1, “memory” and “default mode” strongly loaded on factor 2, “self” and “social cognition/mentalizing” loaded on both factors, “pain” did not substantially load on any factor. Bottom panel: Spatial extent of the regions associated with the two factors (including voxels with loadings in the top 1% of values across the brain). Factor 1 comprised a large ventro-caudal portion of the vmPFC, subcortical structures (amygdala, striatum and midbrain), right insula and left lateral prefrontal cortex. Factor 2 comprised a more rostral and dorsal portion of the vmPFC, the posterior cingulate cortex (PCC) and bilateral intraparietal sulcii (IPS). The specific searches that defined each category were as follows. In the list below, |, &, and ~ indicate logical OR, AND, and NOT, respectively. * indicates a wildcard including all words beginning with the stem preceding the star. Default mode: “default | resting state | dmn | default mode”. Memory: Average of “episod*” “autobiograph*” “retriev* | recollect*”. Self: “(self | subjective)”. Social cognition/mentalizing: “empath* | theory.of.mind | tom | mentaliz* | trait | (inference & others)”. Emotion: “emotion* | mood | valence | arousal | affective”. Reward: “(reward* | monet* | gain | cocaine | eating | reinforc* | incent* | love | joy | (positive & hedonic) | (positive & emotion) | (positive & affect)) &~ (negative & emotion) ”. Autonomic/endocrine: “autonomic* | scr | hr | cortisol | conductance | heart”. Pain: “pain* | noxious | nocicept*”. Additional details can be found in the Online Supplementary Materials.
This comparison reveals striking overlap in vmPFC across all of the ‘meaning-related’ process domains (Figure 2a), as well as functional specialization in different parts of the MPFC and associated regions. For instance, in addition to vmPFC, the ‘default mode’ and memory maps include the posterior cingulate cortex (PCC), hippocampus and nearby medial temporal regions, and the inferior parietal lobule. The emotion, reward, and autonomic maps include ventral striatum/pallidum, amygdala, ventral tegmental area, periaqueductal gray (PAG), and parts of the insula and lateral prefrontal cortex. The self and social cognition maps include both cortical features of the memory maps and subcortical features of the emotion and reward maps, as well as dmPFC regions linked specifically with social cognition [9]. The pain map is largely distinct from the others, and includes multiple parts of the ventromedial and cingulate cortices, including vmPFC, rostro-dorsal anterior cingulate cortex (rdACC), and the dorsal anterior cingulate (dACC), insula, and somatosensory cortices.
Identifying the boundaries of these zones and understanding their differential roles in affective processes across species is an important and ongoing effort (Box 1). In contrast to vmPFC, dACC has strong, direct anatomical connections with motor areas and spinal motor neurons [22] and is reliably engaged in cognitive control processes, response selection, and affect and autonomic function linked to demands on skeletomotor action (Box 1; for reviews see [23,24]). Based on connectivity with other brain areas, the vmPFC and dACC are distinct regions connected to different posterior and subcortical networks, and the rdACC is a ‘transition zone’ with connectivity to both networks (Box 1), perhaps giving it a special role in translating between affective meaning and action, including functions such as error and outcome monitoring [25] and motivated action. This could in part explain why rdACC is more frequently activated by aversive stimuli, such as pain and ‘fear’ cues, which are usually associated with a strong desire to avoid or terminate an unpleasant experience. Interestingly, though ‘pain’ as a category is associated with activity in all three zones, stimulus intensity is most strongly linked to dACC [26], pain experience to rdACC [27] and expectations about pain to vmPFC [27].
Box 1.
Subdivisions of ventromedial prefrontal and anterior cingulate cortex
At a broad level, mPFC may be divided into ventromedial (vmPFC), rostral dorsal cingulate (rdACC), and dorsal cingulate (dACC) zones (Figure Ia)— corresponding to anterior cingulate, anterior midcingulate, and posterior midcingulate zones in Vogt and colleagues’ four-region model [85]—and the dorsomedial prefrontal cortex. Neuroimaging is just beginning to be able to weigh in on the precise boundaries of these regions and their functional homologies across species.
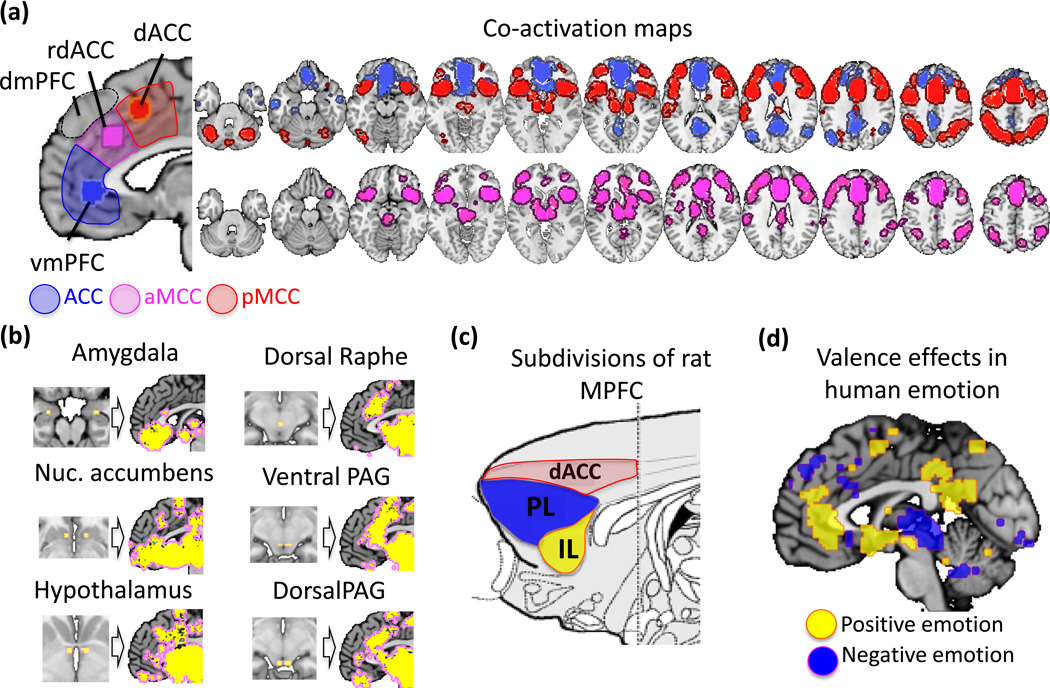
The dACC and vmPFC are strongly dissociable in human studies, based on both activation and connectivity with other brain regions. This is illustrated in Figure I by co-activation maps across the 1,669 studies included in this review (see Supplementary Online Materials for details). dACC activation typically co-occurs with activation in a lateral fronto-parietal network, whereas vmPFC activations co-occur with activation in a network consisting of medial and temporal cortex [17]. The rdACC is connected to both networks with about equal strength, implying that it may serve as a bridge between the vmPFC representation of constructed ‘meaning’ and dorsal systems for directing action and attention. In addition, rdACC is particularly strongly co-activated with a number of subcortical ‘affective’ structures (Figure IB), including the basolateral amygdala, nucleus accumbens, hypothalamus, periaqueductal gray (PAG), and dorsal raphe nucleus. This connectivity suggests that, like vmPFC, rdACC is integral in shaping subcortical responses and may participate in the construction and/or deployment of ‘meaning.’
Though we consider vmPFC and rdACC to be part of the same functional zone at the broadest level, they are also clearly dissociable. RDACC and vmPFC have different functional profiles, paralleling a distinction between prelimbic (PL) and infralimbic (IL) cortices in rats (Figure Ic). PL is associated with the maintenance and recall of responses to aversive stimuli [86]—particularly when contextual information is important—and IL is associated principally with the extinction of aversive responses. For example, PL stimulation impairs fear extinction, and PL activity after extinction predicts reduced extinction recall [87], whereas IL activity has the opposite effects [87]. This reciprocal engagement of the PL and IL in contextual representations of threat and safety appears to be paralleled by similar findings in humans showing that the rdACC and vmPFC show a preference for negative and positive emotion, respectively [88] (Figure Ie), and for sympathetic vs. parasympathetic autonomic activity [1] (Figure 1a).
It is possible that this distinction is due to specialization by content (positive vs. negative affect), process (response selection vs. value updating), or something else. Recent evidence linking PL and rdACC to appetitive, drug seeking behavior [80] argues in favor of a process account. One view is that rdACC is involved in translating between ‘meaning’ and action systems—including selecting meaning-guided actions under conditions of uncertainty [23,24] and monitoring the consequences of actions [25]—whereas ventral vmPFC are primarily involved in updating representations of the internal state of the organism.
A factor analysis of the eight domain maps that included consistent vmPFC activity shows two distinct subsystems, which also overlap only in the vmPFC (Figure 2B). The first subsystem encompasses the ventro-caudal vmPFC and subcortical structures associated with affect, valuation, and autonomic/endocrine control. This system might be called the ‘affect generation’ system, as the reward, autonomic, and emotion maps load uniquely on this factor. The second subsystem comprises the anterior and dorsal portion of the vmPFC and the PCC. This might be called the ‘simulation’ subsystem, as the memory and default-mode maps load uniquely on this factor, and as this system appears to be involved in constructing internal models of the world based on mnemonic information, and using them to imagine projected future scenarios [5]. The self and social cognition/mentalizing maps were similar to one another and loaded equally on both factors, suggesting that these processes share features of both sub-systems. In this sense, the vmPFC could be considered a ‘system of systems’, binding together large-scale networks involved in memory and projection, self-perception, social cognition, emotion, reward, and autonomic and endocrine function.
The anatomical connectivity of the vmPFC parallels these functional findings, and reinforces the view of vmPFC as an integrative center. As has been reviewed elsewhere [28,29], vmPFC is unique among cortical regions in that it projects directly to nuclei involved in affect and peripheral regulation at all levels of the subcortical neuraxis, including hypothalamus, amygdala, ventral striatum/pallidum, periaqueductal gray (PAG), parabrachial complex, parapontine reticular formation, raphe nuclei, nucleus tractus solitarius, and spinal autonomic ganglia. These vmPFC-subcortical pathways are thought to constitute a ‘visceromotor’ system whose functions are to coordinate several aspects of autonomic activity [11]. The vmPFC is also directly connected with memory-related circuits in hippocampus and medial temporal lobe [11] that can subserve both memory and future projection[5]. It is also monosynaptically connected with cortical structures associated with sensory hedonics and expectancies for specific rewards [15] (OFC), mentalizing and theory of mind [9] (dmPFC), and goal formation and maintenance (dlPFC and frontopolar cortex). Thus, the functional regions that are co-activated with vmPFC in large-scale meta-analyses are also largely monosynaptically connected with vmPFC, and the pattern of connectivity suggests a unique integration of information in systems involved in higher-level cognition with those involved in the most basic forms of affective experience and physiological regulation.
Several psychological constructs can be thought of as emergent properties of such a system for integrating conceptual and affective information, including ‘valuation’[7,15], ‘goal-directed behavior’ [20] and ‘emotion’ [19,30]. Indeed, peripheral changes in response to environmental threats and opportunities are central to what it means to have an ‘emotional response’ [31]. However, the functional properties of the systems connected to vmPFC suggest that its function may not be reducible to these concepts. ‘Valuation’, for instance, implies evaluation on a single dimension of valence (good/bad or approach/avoid) that serves as the basis for the construction of preferences. But while affective value can be conceptually informed, it need not be: certain types of value do not necessarily draw on memories and explicit representations of the future. Conversely, value can be purely ‘cognitive’, without requiring direct links to peripheral output systems or the ‘organism-wide’ behavioral changes associated with strong emotional responses. We argue that vmPFC’s function is not to reduce complex situations to a single, actionable ‘value’ dimension, but rather to link situational appraisals to specific patterns of behavioral and autonomic responses afforded by the particular context. That is, vmPFC’s role may not be to determine how threatening or appetitive an object is, but rather to determine the specific response (fight, flee, consume, protect, nurture, rest) that is most adaptive given the particular situation. The topography of vmPFC-PAG and vmPFC-hypothalamus connections, which are organized along the lines of such adaptive behavioral responses, supports this view of vmPFC’s function [29]. One prediction that follows from this view is that without vmPFC, value is still estimated and motivated behavior occurs, but value and emotion are more ‘reflexive’ and not situation-appropriate, as occurs with ‘utilization behavior’ [32] and other reports of orbital/ventromedial prefrontal damage [33].
A specific role for the vmPFC in learning and choice: insights from animal lesion studies
The human neuroimaging results reviewed above reveal the broad involvement of vmPFC across studies involving memory, self-reference, emotion, affective value, and peripheral regulation. Studies in animals complement this data by revealing a selective pattern of effects following vmPFC lesions. These studies suggest that vmPFC is not necessary for basic forms of affective learning and valuation, but is necessary to optimally guide behavior when the meaning of events has to be inferred from particular configurations of situational cues.
Fear extinction recall
One paradigm that involves anticipatory valuation is fear conditioning, in which sensory cues (CS+) come to predict an aversive stimulus such as painful shock (UCS). The vmPFC is not required for the learning or expression of anticipatory conditioned responses (CRs), including ‘fear’ behavior [34]. For such responses, a largely subcortical circuit linking sensory cortex, thalamus, amygdala, PAG, and brainstem is sufficient [35]. Neither is vmPFC generally required for fear extinction (but see [36]), in which cues are presented without reinforcement and the CR diminishes over time [34]. VMPFC is critical, however, for more complex forms of learning. Lesions of the ventral part of rat vmPFC, the infralimbic cortex (IL), cause enhanced recovery of fear a day after extinction training [34]. These effects have typically been interpreted in terms of a deficit in extinction retrieval. Here, we propose that retrieval of extinction memories requires inferring the meaning of the predictive cue from a mental model of the task. During the ‘extinction recall’ test, the rat must determine which of the two outcomes the CS+ currently predicts—threat during initial learning, and safety during extinction—with impoverished input as to which environmental cues are associated with which context.. This could entail a search through memories rapidly formed in the hippocampus during previous phases for cues informative on which context is now relevant [37]. One prediction that follows from this is that overtraining on extinction should eliminate the need for an intact vmPFC during retrieval.
Findings in animals are paralleled by human studies of fear extinction and reversal, which show vmPFC and hippocampal co-activation during the recall of extinction [38], suggesting parallel function in animals and humans and perhaps the involvement of explicit memory systems. In another recent human study [39], vmPFC responded more strongly to a safety cue (CS−) that was previously a threat cue (CS+) than it did to the “naive,” pre-reversal CS−. This effect is consistent with greater demand on conceptual systems, which must rapidly and flexibly determine the current cue value post-reversal.
Stressor controllability
In this paradigm, one group of rats experiences a series of shocks that can be terminated with an instrumental response. Another group of rats experiences an identical series of shocks, determined by the behavior of the first group, so that the external events are matched but only the first group experiences control. Control speeds subsequent escape learning [40] and slows subsequent fear conditioning [41], reduces threat-related serotonin responses in the dorsal raphe to novel contexts [40], and buffers the animals against later adverse effects of inescapable shock [42]. vmPFC inactivation abolishes all these benefits of control without affecting the learning of escape responses per se. Thus, vmPFC is not necessary for representing the aversive value of shocks or learning to escape them, but it is crucial for integrating information about the external environment and the efficacy of one’s actions—or, more simply, conceiving of the meaning of the shocks in context.
Appetitive learning
A large literature on appetitive learning also suggests that the MPFC-OFC system is critical for representing the subjective value of the specific outcomes associated with certain cues or actions [15]. Lesions of this system do not result in impairments in responding to cues that are predictive of food rewards, nor do they impair avoidance of a food that has been paired with illness or satiated. However, the system is critical for reinforcer devaluation, a test in which two cues (Pavlovian devaluation) or two actions (instrumental devaluation) are associated with two foods, one of which is subsequently devalued by satiation or pairing with illness. OFC/MPFC lesions abolish the abrupt decrease in Pavlovian or instrumental responses associated with the now-devalued food typically observed in normal animals.
One interpretation of these effects is that the devalued cue or action is associated with two values: a positive value learned pre-devaluation, and an aversive value that is not specifically linked to the action through training and must be inferred using conceptual/episodic systems [15]. This type of behavior is often cast in terms of “model-based” (Pavlovian or instrumental devaluation) or “goal-directed” behavior (instrumental devaluation only) [20], which implies the explicit representation of conceptual information and is generally contrasted with the more habitual and incremental “model-free” decision making [43]. Following devaluation, the value of the action as represented by the model-free, incremental value-learning system remains high (the action was associated with tasty rewards), but the value of the action represented by the model-based system has changed.
Converging effects in a number of related paradigms has led to the idea that the OFC-vmPFC system represents expectations about specific outcomes [15,20], which perhaps provides clues as to the roots of conceptual, prospective thought. While there may be important differences between lateral OFC and vmPFC based on their anatomical status as ‘sensory association’ and ‘visceromotor’ regions [11], the two systems are interconnected and function similarly in many respects. Lateral OFC appears to be important for the assignment of value to specific cues [15,44], whereas vmPFC is more important for the action value of those items [45], whether behavioral, 'emotional,' or visceromotor.
In humans, both regions track the diminished value of outcomes following devaluation [46] (figure 1k). Moreover, vmPFC lesions in humans and monkeys have been shown to interfere with decision-making when the value of alternative outcomes is close together [45,47], or reverses rapidly [48], and is sensitive to the values of all available choices, not only the best ones [45,49] (Figure 1n). One explanation for these complex properties is that vmPFC is involved in integrating conceptual, explicit representations of the potential outcomes into the construction of value. Such conceptual information, which we predict draws on hippocampal memory systems, is particularly necessary when the values assigned to stimuli and actions change rapidly or suddenly, making slower habit-learning systems inadequate for optimal behavior.
VMPFC involvement in conceptually driven affect in humans
Given the findings discussed above, the vmPFC, and adjacent rdACC, should be particularly important for affective responses driven by conceptual appraisals. More specifically, rdACC should be more active when there is greater demand for immediate motivated behavior or action monitoring, whereas vmPFC should be more active when it is necessary to update representations of the situational context itself—though the action vs. context updating dichotomy is confounded with valence in the literature, leading to ambiguity on the functional dissociations between these regions (see Box 1).
The effects of conceptual information are difficult to isolate in animal studies, but can be readily manipulated in humans through language. For example, in instructed fear, participants are informed through language that a cue will be followed by a strong shock, which is sufficient to produce a well-characterized pattern of increases in the amygdala and rdACC and decreases in vmPFC (both of which may be part of the broad ‘meaning construction’ response; see Box 1) [50,51] (Figure 1g). Conversely, instructions to conceptualize the CS+ in an emotionally positive manner (e.g., think of a blue square as a calming, peaceful ocean) increases vmPFC activity and decreases amygdala and skin conductance responses [52] (figure 1f).
The vmPFC is important in several other paradigms involving conceptually driven affect in humans. In reappraisal paradigms, participants are trained through language to generate positive (or negative) conceptual frames for aversive events. This conceptually-driven form of emotion regulation was recently shown to consistently activate the vmPFC in a recent meta-analysis of emotion regulation studies [53] (figure 1e). Moreover, these effects appear to be mediated through vmPFC’s extensive connections with subcortical structures. In one recent study, positive reappraisal reduced amygdala responses to aversive visual images and increases activity in the ventral striatum, which in turn predict reappraisal effects on emotional experience [54] (figure 1h). In this study, vmPFC activity was associated with increased reappraisal success in a manner mediated by changes in the ventral striatum, though other dorsomedial and lateral prefrontal regions are likely to be important as well [54,55]. One difficulty with reappraisal paradigms is that they require cognitive effort and external attention [56,57], which produces large de-activations in vmPFC across many paradigms [17]. The level of vmPFC activity in fMRI studies may depend on the balance between demand on meaning construction and stimulus-focused processes [55]. That notwithstanding, a prediction of the meaning-centered view is that reappraisal will be impaired with vmPFC lesions. A second prediction is that reappraisal-related fMRI activity will emerge when controlling for activity in lateral cortical ‘attention systems’ (partially done in [54]; see Supplementary Online Materials for additional discussion).
Another example of the direct effects of conceptual knowledge on the processing of affective stimuli is placebo analgesia: The modification of pain by belief in a treatment. Studies of placebo analgesia show reliable increases in vmPFC [53] (Figure 1e) and reliable connections between vmPFC and PAG [58]. Some forms of placebo analgesia require the presence of endogenous opioids, and correlated increases in endogenous opioid activity are found in both structures under placebo treatment [59] (Figure 1i). While placebo studies often involve conditioning procedures, these may often serve primarily to alter expectations and the meaning of pain in context [60,61], and a recent study found that vmPFC activity was correlated with the strength of both expectations of analgesia and pain relief [62].
Finally, social evaluative threat provides converging evidence for vmPFC involvement in meaning-driven emotion. In this paradigm, participants prepare a speech on a personally relevant topic to be given in front of a panel of ‘expert’ judges. The ‘active ingredients’ in this challenge are conceptual—social feedback is provided that threatens participants’ social and intellectual competence—and they are powerful. Such procedures have elicited reliable autonomic and endocrine responses in hundreds of studies [63]. In a pair of studies [64,65], speech preparation elicited activity increases in dorsal vmPFC/dACC and decreases in ventral vmPFC (figure 1j). Both effects tracked the individual time course of autonomic changes during the challenge, and the relationships between vmPFC and autonomic changes were mediated in part by activity in PAG. In another recent study, vmPFC damage increased perceived threat and negative affect and altered endocrine responses to this type of challenge [66].
These studies complement human studies of basic economic value [67] (figure 1l), which mix conceptual information with other forms of learned value. An emerging view is that activity in vmPFC, particularly the ventral/medial orbitofrontal portion deactivated in stress studies, predicts value after the integration of diverse kinds of conceptual information. For example, the same odor produces larger vmPFC activations when paired with the words “cheddar cheese,” than when paired with the word “sweat” [68] (figure 1q). Greater vmPFC activity is elicited in comparisons between flavors labeled as “rich and delicious” vs. “boiled vegetable water” [69], wines labeled as expensive vs. cheap [70], and skin creams presented as “rich and moisturizing” vs. “basic” [71]. In addition, vmPFC responds more strongly to more highly valued immediate rewards than delayed ones [72] (Figure 1p). Finally, vmPFC activity can also track the value of outcomes after integrating information about long-term goals, such as the health value of tasty, but diet-incompatible, foods [73] (figure 1o). Altogether, these results clearly show that representations in the vmPFC are strongly influenced by various forms of conceptual information, and that vmPFC mediates processes that extend beyond preferences, including physiological responses related to well being.
Ventromedial prefrontal cortex and meaning
The convergence of data presented here indicates a role for the vmPFC in a class of functions whose boundaries cut across traditional categories (e.g. value, affect), but which is in certain respects more specific than previous characterizations: These functions involve the infusion of conceptual information into the generation of affective responses. The critical process is one of combining elemental units of information—from sensory systems, interoceptive cues, long-term memory—into a gestalt representation of how an organism is situated in its environment, which then drives predictions about future events. A straightforward term for such processes is affective meaning, a sense of the significance of events for an organism’s well being and future prospects.
The value for the organism of constructing meaning is that it allows mental representations to be flexible, constructive, and future-oriented, and to depend on the anticipated transaction between the organism and the environment rather than superficial cues. A principal challenge in navigating the world is that the relationships between sensory cues and the transactions they imply are often complex. A smile is pleasant coming from a friend, but may signal danger coming from a competitor. The words “great job” can mean “signal pride, jealousy, or disdain, depending on the context in which they are uttered. We are constantly faced with the need to make inferences by generalizing from past situations—and, as there are seldom second chances to avoid major threats or capitalize on unique opportunities, to do so without the benefit of trial-and-error learning.
A ‘meaning-centered’ view of vmPFC function is closely aligned with recent views of the orbital-medial prefrontal system as involved in ‘goal-directed’ or ‘model-based’ learning [43], representing expectancies about specific outcomes [15], retrieving fear inhibitory memories [74], or the generation of subjective value and emotion based on conceptual information [19]. We do not intend for ‘meaning’ to replace these other terms as a label for this system’s function, but rather complement them and provide a broader framework that integrates findings across animal and human studies. Thinking of vmPFC in terms of meaning rather than more basic concepts like affect, value, memory, or visceromotor control also help explain its role in more complex processes not reviewed in depth here, such as ‘courage’ [75], ‘closeness’ [76], ‘social standing’ [77] and ‘the emergence of concepts’ [78] (figure 1m). This view also affords some new predictions. VMPFC should be critical for emotion to the degree that it is elicited by conceptual information; critical for learning to the degree that ‘model-based’ learning is required [43]; critical for autonomic and endocrine responses driven by abstract information and verbal communication; critical for decision-making when the space of options is under-constrained and strong priors are advantageous; and critical for value derived from knowledge about the underlying properties of valued items.
This view also has implications for disorders that involve vmPFC, which may (not coincidentally) involve a breakdown in flexible meaning generation. Dysfunction of the vmPFC is thought to be critical in anxiety disorders such as PTSD [10] (Figure 1vw), depression [79] (figure 1x), counterproductive motivation in drug addiction [80], and dysregulation of autonomic and endocrine systems related to chronic stress [12] and pain [13]. The structural or functional abnormalities observed in these conditions are typically accompanied by hyper-reactivity in subcortical circuits triggering threat responses [10] (figure 1w), suggesting that these disorders might be exacerbated by a reduced capacity to appropriately assign affective meaning to sensory and internal cues. Depression, for example, is characterized by biases towards negative information and rumination [81] and PTSD by over-generalization of threat cues [82]. Cognitive-behavioral therapy and other forms of meaning-centered therapy [83] target these pathological interpretations of the self and the world and break them down, and are thought to be effective for all these disorders.
Conclusions
To summarize, building on extensive data regarding the role of vmPFC in subjective value, affect, memory and visceromotor control, we propose that meaning-guided affect as represented in vmPFC is a) generative and can be easily transferred to new situations or configurations of informational elements; b) shows sensitive dependence to the precise configuration of elements, but not to incidental variations in sensory appearance; and c) can change rapidly during learning. This view of affective meaning is closely aligned with the idea that ‘affective appraisal’ and ‘situated conceptualization’ [84] are critical emotion-generating processes, and with other dual-process views that suggest that ‘model-based’ or ‘goal-directed’ systems operate alongside simpler habit learning systems to guide reward-driven learning in animals [20,43]. Unlike simpler forms of learning and valuation, affective meaning arises from the fast recombination of conceptual information extracted from long-term memory into predictive models of the self in context, which drive both decision-making and physiological affective responses. This highly integrative process appears to rely on the vmPFC, which binds together large-scale networks involved in several functions that are necessary to construct affective meaning: memory and future projection, self-perception, social cognition, emotion, reward, and autonomic and endocrine function. Although much remains to be learned about how these different functions combine and interact in the vmPFC, we believe that considering these different functions in the broader context of affective meaning will help further our understanding of vmPFC’s role in each of these separate functions.
Box 2.
Questions and Future directions
The inherently constructive nature of vmPFC’s processes seems to rely on its extensive connections with a number of cortical and sub-cortical structures. However, “meaning” is likely to reflect a class of processes rather than a unitary process. Are there different kinds of “meaning” and are they associated with different patterns of connectivity? Future brain imaging research should explore the specific patterns of vmPFC’s connectivity associated with different types of tasks that bear on “meaning.”
The effects of vmPFC activations on subcortical circuits appear to be predominantly inhibitory. Is the inhibition of subcortical responses intrinsically tied to the higher adaptive value of affective meaning, which automatically competes with responses generated at subcortical levels, or can vmPFC sometimes prime and act synergistically with subcortical structures?
Activity in the vmPFC is inversely correlated with activity in rdACC across a variety of emotional and decision-making tasks. What drives this inverse correlation? Is it valence (content) or demand on action and attention systems (process) [80]? Are both regions reciprocally inhibitory or does activity in one of the two regions has precedence over the other?
Psychiatric disorders characterized by emotional dysregulation are consistently associated with structural and functional abnormalities in the vmPFC. These disorders are also often associated with several neurocognitive deficits and are frequently comorbid with other clinical and personality disorders. How can these comorbidities be explained by the broader integrative role of cognitive, emotional and self-related processes of the vmPFC and can it help improve our psychiatric nosologies?
How is the developmental trajectory of vmPFC’s structure and connections related to the development of the capacity to construct affective meaning [89], and how is it influenced by favorable and unfavorable early life experiences? What are the effects of prefrontal morphological and functional changes in aging on flexible meaning generation in older adults?
Supplementary Material
Figure I.
Subdivisions and connectivity of the medial prefrontal cortex. (a) Four functionally distinct zones include ventromedial prefrontal cortex (vmPFC), rostral dorsal anterior cingulate (rdACC), dorsal anterior cingulate (dACC), and dorsomedial prefrontal cortex (dmPFC). We envision meaning construction as occurring principally in vmPFC and rdACC, with substantial contributions from dmPFC. To illustrate the differential connectivity of the vmPFC and dACC zones, ‘seed’ regions were selected based on the peak activation frequencies across the 1,669 studies and 8 domain areas summarized in this review. Areas significantly co-activated with each seed region are shown at the right (see Supplementary Online Materials for details). The dACC (red) and vmPFC (blue) regions co-activated with distinct brain networks. Additional subcortical connectivity with vmPFC was apparent below the stringent thresholds used here (P < .05 corrected). rdACC (purple) showed co-activation with both networks, and particularly strong co-activation with periaqueductal gray (PAG) and other subcortical areas. (b) Further exploration of medial prefrontal co-activation with subcortical regions. For each of the six areas shown, small ‘seed’ regions were placed in the subcortical area, and co-activated areas in the medial prefrontal cortex are shown (P < .0001). rdACC is co-activated with each area, with particularly strong co-activation with dorsal PAG and raphe nuclei associated with active responses to threat, suggesting a possible homology with prelimbic cortex in rats. (c) Location of prelimbic and infralimbic cortices in rats, adapted from [99]. (d) A meta-analytic summary of 138 PET and fMRI studies of positive and negative emotional experience (374 experimental contrasts, selected from a database of 234 studies of emotion described in [88]). Colored regions indicate areas with significantly greater density of activations related to positive (yellow) and negative (blue) emotional experience. Positive emotions more consistently activate the vmPFC, posterior cingulate cortex, ventral striatum and supplementary motor areas, and pre-supplementary motor area. Negative emotions more consistently activate the PAG, rdACC, dmPFC and deep cerebellar areas.
Acknowledgements
We would like to thank Lisa Feldman Barrett, Richard Lane, Steve Maier, Antonio Rangel, and Geoff Schoenbaum for helpful comments and discussion of this manuscript, and Tal Yarkoni for assistance with the Neurosynth database. This work was funded by NIDA 1RC1DA028608 (T.D.W.), R01 MH076136 (T.D.W.), and R01DA027794 (T.D.W and D.S.), and by a post-doctoral scholarship from the Canadian Institutes of Health Research grant (CIHR) to M.R. Matlab code implementing all analyses is available at: http://wagerlab.colorado.ed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Critchley HD, et al. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton. Neurosci. Basic Clin. 2011;161:34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Wager T, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of Emotions. 3rd edn. The Guilford Press; 2008. pp. 249–271. [Google Scholar]
- 3.Etkin A, et al. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn. Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Binder JR, et al. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 10.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price JL, Drevets WC. Neurocircuitry of Mood Disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane RD, Wager TD. The new field of Brain–Body Medicine: What have we learned and where are we headed? NeuroImage. 2009;47:1135–1140. doi: 10.1016/j.neuroimage.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Apkarian AV, et al. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush G, et al. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 15.Schoenbaum G, et al. Does the orbitofrontal cortex signal value? Ann. N. Y. Acad. Sci. 1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damasio A. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 17.Raichle M, et al. Proc. Natl. Acad. Sci. U. S. A. Vol. 98. 2001. A default mode of brain function; pp. 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer KR. Appraisal considered as a process of multi-level sequential checking. In: Scherer KR, et al., editors. Appraisal processes in emotion: Theory methods, research. Oxford University Press; 2001. pp. 92–120. [Google Scholar]
- 19.Barrett LF. Solving the Emotion Paradox: Categorization and the Experience of Emotion. Pers. Soc. Psychol. Rev. 2006;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- 20.Balleine BW, O'Doherty JP. Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarkoni T, et al. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picard N, Strick P. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 23.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Snellenberg J, Wager TD. Cognitive and motivational functions of the human prefrontal cortex. In: Christensen AL, Bougakov D, Goldberg E, editors. Luria's Legacy in the 21st century. Oxford University Press; 2009. pp. 30–61. [Google Scholar]
- 25.Rushworth MFS, et al. Frontal Cortex and Reward-Guided Learning and Decision-Making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Coghill R, et al. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 27.Atlas LY, et al. Brain Mediators of Predictive Cue Effects on Perceived Pain. J. Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price JL. Definition of the Orbital Cortex in Relation to Specific Connections with Limbic and Visceral Structures and Other Cortical Regions. Ann. N.Y. Acad. Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 29.Bandler R, et al. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 30.Cacioppo J, Gardner W. Emotion. Ann. Rev. Psychol. 1999;50:191–214. doi: 10.1146/annurev.psych.50.1.191. [DOI] [PubMed] [Google Scholar]
- 31.Cacioppo J, et al. Relationship between facial expressiveness and sympathetic activation in emotion: A critical review, with emphasis on modeling underlying mechanisms and individual differences. J Pers. Soc. Psychol. 1992;62:110–124. doi: 10.1037//0022-3514.62.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Lhermite F. “Utilization behaviour” and its relation to lesions of the frontal lobes. Brain. 1983;106:237–255. doi: 10.1093/brain/106.2.237. [DOI] [PubMed] [Google Scholar]
- 33.Beer J, et al. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J. Cogn. Neurosci. 18:871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 34.Quirk GJ, et al. The Role of Ventromedial Prefrontal Cortex in the Recovery of Extinguished Fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeDoux J, et al. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sierra-Mercado D, et al. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology. 2010;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milad MR, et al. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol. Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiller D, et al. From Fear to Safety and Back: Reversal of Fear in the Human Brain. J. Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 41.Baratta MV, et al. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daw ND, et al. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 44.Walton ME, et al. Separable Learning Systems in the Macaque Brain and the Role of Orbitofrontal Cortex in Contingent Learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentin VV, et al. Determining the Neural Substrates of Goal-Directed Learning in the Human Brain. J. Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchida A, et al. Beyond Reversal: A Critical Role for Human Orbitofrontal Cortex in Flexible Learning from Probabilistic Feedback. J. Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fellows L, Farah M. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 49.Boorman ED, et al. How Green Is the Grass on the Other Side? Frontopolar Cortex and the Evidence in Favor of Alternative Courses of Action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Mechias M-L, et al. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 51.Phelps EA, et al. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 52.Delgado MR, et al. Neural Circuitry Underlying the Regulation of Conditioned Fear and Its Relation to Extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diekhof EK, et al. Fear is only as deep as the mind allows. NeuroImage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 54.Wager TD, et al. Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochsner KN, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neuro. 2004;16:1–27. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 56.Urry HL, et al. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Reekum CM, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 58.Meissner K, et al. The Placebo Effect: Advances from Different Methodological Approaches. J. Neurosci. 2011;31:16117–16124. doi: 10.1523/JNEUROSCI.4099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wager TD, et al. Proc. Natl. Acad. Sci. U. S. A. Vol. 104. 2007. Placebo effects on human µ-opioid activity during painin; pp. 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benedetti F, et al. Conscious expectation and unconscious Conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montgomery GH, Kirsch I, editors. Pain. Vol. 72. 1997. Classical conditioning and the placebo effect; pp. 107–113. [DOI] [PubMed] [Google Scholar]
- 62.Wager TD, et al. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickerson S, et al. When the social self is threatened: Shame, physiology, and health. J Pers. 2004;72:1191–1126. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 64.Wager TD, et al. Brain mediators of cardiovascular responses to social threat. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchanan TW, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chib VS, et al. Evidence for a Common Representation of Decision Values for Dissimilar Goods in Human Ventromedial Prefrontal Cortex. J. Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Araujo IE, et al. Cognitive Modulation of Olfactory Processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Grabenhorst F, et al. How Cognition Modulates Affective Responses to Taste and Flavor: Top-down Influences on the Orbitofrontal and Pregenual Cingulate Cortices. Cereb Cortex. 2007;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 70.Plassmann H, et al. Marketing actions can modulate neural representations of experienced pleasantness. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCabe C, et al. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc. Cogn. Affect. Neurosci. 2008;3:97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClure SM. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 73.Hare TA, et al. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 74.Milad MR, et al. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Nili U, et al. Fear Thou Not: Activity of Frontal and Temporal Circuits in Moments of Real-Life Courage. Neuron. 2010;66:949–962. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Krienen FM, et al. Clan Mentality: Evidence That the Medial Prefrontal Cortex Responds to Close Others. J. Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gianaros PJ, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cogn. Affect. Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumaran D, et al. Tracking the Emergence of Conceptual Knowledge during Human Decision Making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drevets W, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 80.Peters J, et al. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLaughlin KA, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behav. Res. Ther. 2011;49:186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lissek S, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Ellis A, Joffe Ellis D, editors. Rational emotive behavior therapy. American Psychological Association; 2011. [Google Scholar]
- 84.Barsalou LW. Grounded Cognition. Annu. Rev. Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 85.Palomero-Gallagher N, et al. Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Hum. Brain. Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oswald BB, et al. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: A study based on trace eyeblink conditioning in the rabbit. Neurobiol. Learn. Mem. 2010;93:37–45. doi: 10.1016/j.nlm.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindquist K, et al. Behav. Brain Sci. The brain basis of emotion: a meta-analytic review. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blakemore S-J. NeuroImage. Imaging brain development: The adolescent brain. (in press) [DOI] [PubMed] [Google Scholar]
- 90.Gianaros PJ, et al. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagai Y, et al. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “defalut mode” of brain function. NeuroImage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 92.Thayer JF, et al. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Ohira H, et al. Regulation of natural killer cell redistribution by prefrontal cortex during stochastic learning. NeuroImage. 2009;47:897–907. doi: 10.1016/j.neuroimage.2009.04.088. [DOI] [PubMed] [Google Scholar]
- 94.O'Connor M-F, et al. When grief heats up: Pro-inflammatory cytokines predict regional brain activation. NeuroImage. 2009;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrovic P. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 96.Spreng RN, et al. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2008;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 97.Addis DR, et al. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 98.van der Meer L, et al. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Gabbott PLA, et al. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:1–33. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.