Abstract
Previous work has indicated an association between seizures early in life and increased risk of psychiatric disorders, including schizophrenia. However, because early life seizures are commonly treated with antiepileptic drugs (AEDs) such as phenobarbital, the possibility that drug treatment may affect later-life psychiatric outcomes needs to be evaluated. We therefore tested the hypothesis that phenobarbital exposure in the neonatal rat increases the risk of schizophrenia-like behavioral abnormalities in adulthood. Thus, in this study, we examined the effects of a single acute neonatal exposure to phenobarbital on adult behavioral outcomes in the rat neonatal ventral hippocampal (nVH) lesion model of schizophrenia. We compared these outcomes to those in rats a) without nVH lesions and b) with nVH lesions, without phenobarbital. The tasks used for behavioral evaluation were: amphetamine-induced locomotion, prepulse inhibition, elevated plus-maze, and novel object recognition task. We found that neonatal phenobarbital treatment (in the absence of nVH lesions) was sufficient to disrupt sensorimotor gating (as tested by prepulse inhibition) in adulthood to an extent equivalent to nVH lesions. Additionally, neonatal phenobarbital exposure enhanced the locomotor response to amphetamine in adult animals with and without nVH lesions. Our findings suggest that neonatal exposure to phenobarbital can predispose to schizophrenia-like behavioral abnormalities. Our findings underscore the importance of examining AED exposure early in life as a potential risk factor for later-life neuropsychiatric abnormalities in clinical populations.
Keywords: Phenobarbital, neonatal brain, rat model, schizophrenia, adult behavior, antiepileptic drug, neuronal apoptosis, prepulse inhibition, amphetamine-induced locomotion
1. INTRODUCTION
Epilepsy and seizures in infancy and/or early childhood is associated with increased risk for a variety of psychiatric disorders including schizophrenia and schizophrenia-like psychoses (Bredkjaer et al. 1998; Caplan et al. 1991, 1997, 1998; Jalava and Sillanpaa 1996; Qin et al. 2005; Sachdev 1998; Schwartz and Marsh 2000; Vestergaard et al. 2005). Furthermore, psychiatric comorbidity in children with epilepsy is significantly more prevalent than in children with other chronic health conditions (Caplan et al. 2008; Jones et al. 2008; for a review see Plioplys et al. 2007).
In an analysis of large clinical cohort, Vestergaard et al reported that a history of either febrile seizures (Vestergaard et al. 2005) or epilepsy (Qin et al. 2005) was associated with a significantly increased risk of schizophrenia. However, Vestergaard and colleagues noted that they “cannot rule out that the association between febrile seizures and schizophrenia is caused by the exposure to antiepileptic drugs rather than the febrile seizures” (Vestergaard et al. 2005). This concern is heightened by the fact that AEDs, such as phenobarbital, when given to neonatal rats, trigger enhanced neuronal apoptosis (ENA) (Bittigau et al., 2001; Forcelli et al., 2011a; Katz et al, 2007; Kim et al, 2007), cause long term alterations in the cortical proteome (Kaindl et al., 2008), and impair neurogenesis (Stefovska et al., 2008). Furthermore, these changes may lead to alterations in behavior (Stefovska et al., 2008, Forcelli et al., 2010, 2011c) and increased seizure susceptibility later in life (Forcelli et al., 2011b). The neurotoxicity of AEDs, and other sedative drugs (i.e., anesthetics, ethanol) have been demonstrated across strains of rats and mice, as well as in non-human primates (Olney et al., 2002; Bittigau et al., 2003; Kim et al., 2007; Brambrink et al., 2010; Farber et al., 2010).
This AED-induced developmental neurotoxicity occurs during a sensitive period corresponding to the “brain growth spurt”, which centers around P7 in the rat. P7 in the rat corresponds to the late third trimester of gestation through infancy in humans (Dobbing and Sands, 1979). Interestingly, the brain regions vulnerable to AED-induced ENA, such as hippocampus, frontal cortex, and ventral striatum (Bittigau et al. 2002; Forcelli et al. 2011c), are the ones that are consistently implicated in schizophrenia (Fornito et al. 2009). Thus, it is possible that AED exposure during a sensitive perinatal period for brain development may predispose to the development of schizophrenia.
We therefore tested the hypothesis that AED exposure in the neonatal period would increase the risk of developing a schizophrenia-like behavioral phenotype. For this purpose, we selected as a basis of comparison, the neonatal ventral hippocampal (nVH) lesion model of schizophrenia in the rat and examined the effects of acute neonatal exposure to phenobarbital on the long-term schizophrenia-like behavioral outcomes characteristic of this model (Marcotte et al. 2001; Tseng et al. 2009). nVH lesioned rats are normal at pre-pubertal age; however, after postnatal day (P) 56 (post-puberty), the lesioned rats show schizophrenia-like behavioral phenotypes including deficits in sensorimotor gating as measured by prepulse inhibition (PPI) (Grecksch et al. 1999; Lipska et al. 1995), locomotor hyperresponsiveness to amphetamine (Flores et al. 1996a; Lipska et al. 1993; Wan and Corbett 1997), and impaired working memory (Le Pen et al. 2000; Lipska et al. 2002).
Although not defining hallmarks of the disease, deficits in PPI, hyperresponsiveness to amphetamine, and impaired memory are all characteristic of patients with schizophrenia (Braff et al., 1978, 1992, 2007; Cadenhead et al., 2000; Hazlett and Buchsbaum, 2001; Kalkstein et al., 2010; Minassian et al., 2007; Parwani et al., 2000; Perry et al., 2002; Weike et al., 2003; van Kammen et al., 1982; for review see Braff, 2010). PPI is a behavioral paradigm that is widely used across species. A conceptual link has been made between deficits in PPI and the inability of patients with schizophrenia to optimally filter or “gate” irrelevant and intrusive sensory stimuli (Swerdlow et al. 2008). Deficits in this pre-attentional process (the reduced ability to inhibit the motor response to a strong stimulus when preceded by a weak stimulus) is considered an endophenotype of schizophrenia (Turetsky et al. 2007). A number of studies have shown these deficits to be reversible by antipsychotic drugs (Le Pen and Moreau 2002; Mansbach et al. 1988; Swerdlow et al. 1991), indicating the predictive validity of this task.
The increased mesolimbic dopamine tone seen in patients with schizophrenia is believed to be a primary substrate of positive, psychotic symptoms (Laruelle and Abi-Dargham, 1999). Consistent with this, patients with schizophrenia exhibit exacerbation of symptoms in response to psychostimulants, such as amphetamine, as well as exaggerated drug-induced hyperactivity (van Kammen et al., 1982). This is modeled in rats by the locomotor stimulation evoked in response to amphetamine. Thus, an increase in amphetamine-induced locomotor activity may be used as a correlate of positive symptoms in preclinical behavioral models. We also examined anxiety-like behavior in the elevated plus maze. This test was selected because decreased anxiety-like behavior in the elevated plus maze has been reported in the nVH model (Wood et al., 2003; Beninger et al., 2009).
Phenobarbital was selected as the AED treatment for these experiments because it was the drug most commonly used for the treatment of febrile seizures when the Vestergaard et al cohort were infants. Furthermore, it remains the most commonly prescribed treatment for neonatal seizures (Bartha et al. 2007). In addition, phenobarbital is one of the most potent inducers of ENA in the developing brain, with peak toxicity at P7 (Bittigau et al. 2002; Katz et al. 2007; Forcelli et al., 2011a). Thus, in this study, we examined the effects of a single acute neonatal exposure to phenobarbital on adult behavioral outcomes in rats with nVH lesions and compared these outcomes to those in rats without nVH lesions or nVH lesions without phenobarbital. Although acute exposure is expected to be less toxic than chronic exposure, an acute dose was selected because it is sufficient to induce pronounced ENA during a critical period. Furthermore, acute doses are relevant to the use of phenobarbital in infants with febrile seizures.
2. MATERIALS AND METHODS
2.1 Animal care and maintenance
A total of 11 female Sprague-Dawley timed pregnant (gestational day 17-18) rats from Charles River Laboratories (Quebec, Canada) were housed in a temperature, humidity and light cycle controlled environment in the Douglas Mental Health University Institute animal facility throughout all experiments. On P4, litters were culled to 9-10 pups per dam with a maximum of 7 male pups. From the 11 dams, 57 pups underwent surgery (on P7), out of which 41 animals survived. The survival rate did not differ between saline and phenobarbital treated animals. Animals in poor health (n=2) were not included in the study. Verification of lesion post-testing (as described in section 2.4) revealed misplaced lesions in four rats; data from these animals were omitted from the analysis. Pups weighed between 15-17g at the time of surgery. The experiments with animals were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Canadian Council of Animal Care, and were approved by the Georgetown University Animal Care and Use Committee and the McGill University Animal Care Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Animal treatments
On P7, pups were weighed (15-17 g) and treated with an equivalent volume of phenobarbital (75mg/kg, i.p.; Sandoz, Canada) or normal saline. The dose of phenobarbital selected falls within the anticonvulsant range in neonatal rats (Kubova and Mares, 1991), and produces robust induction of ENA on P7 (Bittigau et al., 2002; Katz et al., 2007; Forcelli et al., 2011a). Six hours after injection, pups from both groups were randomly selected for nVH lesion or sham surgery. Lesions were conducted as previously described (Flores et al., 1996a, b). Pups were anesthetized by hypothermia and secured on a modified platform fixed to a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). A 30 gauge needle, connected to a 10 μl Hamilton syringe and an infusion pump, was lowered into the ventral hippocampus (AP -3.0 mm and ML +3.5 mm from bregma, DV -5.0 mm from the surface of the skull). The ‘lesion’ group received bilateral infusions of 0.3 μl ibotenic acid (10μg/μl, Sigma, St. Louis, MO, USA) dissolved in 0.1M phosphate buffered saline (PBS, pH 7.4) over a period of 2 min and the ‘sham’ group received the same volume of PBS. The cannula was left in place for 2 min for diffusion of drug, after which the incision was sutured and the pups were placed on a heating pad until fully recovered. This procedure resulted in four groups of animals: saline-sham (n=8), saline-lesion (n=6), phenobarbital-sham (n=10), and phenobarbital-lesion (n=11). Animals were tagged and returned to their dams, where they remained undisturbed until weaning (P21). Starting from P21, all operated pups were housed separately in groups of 2 animals per cage until testing.
2.3 Behavioral Testing
Behavioral testing was initiated on P60, and was conducting during the light phase of the light:dark cycle. To maximize the use of animals, the same cohort of animals was used for all behavioral tests. Behavioral tests were performed as described below. The tests were performed in order starting with the least stressful conditions followed by increasingly stressful/demanding conditions, in the order presented below. Tests were separated by 48-72 hours.
2.3.1 Novel object recognition memory task (NORT)
The NORT exploits the natural tendency of rodents to preferentially explore novel objects in their surrounding as compared to familiar objects. Testing was conducted as previously described (Mumby et al. 2002) in an open-field chamber (60 × 60 × 60 cm) made of dark plexiglass located in a quiet room. Animals were acclimated to the testing arena for 20 min daily for 3 consecutive days, with different pairs of identical three-dimensional junk objects (toys of various shape) placed at opposite corners of the box. Following each session, the objects and testing arena were cleaned with 30% ethanol. On the test day (day 4), the rats were placed in the arena with two new identical objects and allowed to explore the objects for 5 min (familiarization phase); they were then returned to their home cage for 5 min (retention time). The objects were replaced with two new objects; one identical to the objects used during the familiarization phase and the other of a novel shape. The rats were then reintroduced to the arena and allowed to explore the objects for 3 min (testing phase). Behavior was videotaped and scored by observers blind to the animal status. Object exploration was defined as time when the rat was within 2-3 cm of the object, with its head oriented directly towards the object or if animal had at least one forepaw on the object or was sniffing or licking the object. Object recognition memory was defined as the ratio of exploration time for the novel object (TN) over the total exploration time for the novel and familiar (TF) objects [exploration ratio = TN/(TF + TN)]. A value significantly different from 0.5 (chance level, one sample t-test) indicates memory.
2.3.2 Anxiety-like behavior in Elevated plus-maze (EPM)
Rodents have natural preference for closed and dark places over open and lit, along with a strong innate exploratory drive. The EPM exploits these competing preferences by offering a choice between closed and open arms. The proportion of time spent in the closed arms can be used as a measure of anxiety-like behavior (Pellow et al. 1985). Animals were tested in the EPM as previously described (Wood et al. 2003), with sufficient sensitivity to detect changes in both time spent and number of entries into the open arms of the maze. A standard elevated plus maze made from black plexiglass, with two enclosed and two open arms, as well as a central platform was used for testing. The EPM was located in a sound attenuated room with 50 lux illumination from a ceiling-mounted white fluorescent lamp. Each rat was placed on the central platform and its behavior was video recorded for 5 min. The videos were analyzed by treatment-blind observers for the number of entries into the open and closed arms (defined as the number of head pokes made from open/closed arms or the central platform with at least forepaws into the arms), as well as time spent in the open/closed arms.
2.3.3 Prepulse inhibition (PPI) of the acoustic startle response (ASR)
PPI is a measure of sensorimotor gating where startle responses to strong auditory stimuli are attenuated when the stimuli are preceded by a weaker stimulus (Braff et al. 1992; Parwani et al. 2000; Perry et al. 2002; Weike et al. 2000). As described previously (Kamath et al. 2008), PPI of the ASR was measured using a commercially available system (SR-LAB; San Diego Instruments, San Diego, CA) in two sound-attenuating chambers, each equipped with a cylindrical Plexiglas animal enclosure and a small electric fan, which generated a 70 dB background noise and provided ventilation. Sound pressure levels (dB(A) weighting) were measured at the position of the rats ears. Broadband noise pulses were presented via a speaker positioned directly above the animal. An accelerometer affixed to the animal enclosure frame was used to detect and transduce motion resulting from the animals’ response. Noise pulse parameters were controlled using SR-LAB software, which also recorded responses. Animals were acclimated to the enclosure for 5 min before being tested during 37 discrete trials. On the first two trials, the magnitude of the startle response to a 120 dB white noise pulse was measured. These first two startling pulses were presented to habituate the animals to the testing procedure and thus were omitted from the data analysis; all subsequent trials were included in analysis. On the subsequent 35 trials, the startle pulse was either presented alone or 100 ms after the presentation of a 30 ms prepulse. ASR to the pulse was measured following trials with prepulse intensities of 3, 6, 9, 12 and 15 dB above background noise. Prepulses were varied randomly between trials, and each prepulse was presented five times; animals were randomly presented with the startle pulse alone during the other 10 trials. The average intertrial interval was 15 s (range, 5-30 s). Startle responses were determined automatically by the SRLab analysis suite. Startle magnitude was calculated as the average of the startle responses to the pulse-alone trials. PPI was calculated according the formula: %PPI = 100 − (startle response for prepulse + pulse trials/startle response for pulse alone trials) × 100%.
2.3.4 Locomotor activity
Spontaneous and amphetamine-induced locomotor activity are behavioral measures of mesolimbic dopamine activity. Spontaneous and amphetamine-induced locomotor activity was assessed as previously described (Flores et al. 1996b). Acrylic arenas (AccuScan Instruments, Inc., Columbus, OH, USA) 40 × 40 × 30 cm (l × w × h), equipped with infrared sensors were used to assess locomotion. Data collection was performed using the Versamax Software (version 4.0, 2004; AccuScan Instruments, Inc.). Animals were brought from their home environment to the testing room and immediately placed to the activity boxes where their activity was monitored during three segments: (1) spontaneous activity and habituation in the novel environment during first 60 min, (2) locomotor activity after an i.p. injection of saline for 60 min and (3) Locomotor activity after an injection of d-amphetamine sulphate (1.5 mg/kg, free base dissolved in saline, i.p.) for 180 min. For each animal, total horizontal distance traveled (cm) measured in 10 min intervals was used for analysis.
2.4 Histological examination
Upon completion of behavioral testing animals were euthanized by decapitation and their brains were removed and frozen. Thirty-five μm coronal sections at the level of the hippocampus were mounted on pre-coated microscope slides and stained with cresyl violet staining solution (0.5%) for verification of the lesions. Only animals with bilateral lesions confined to the ventral hippocampus were included in the nVH lesion group. Representative lesions are shown in Supplemental Figure 1.
2.5 Statistical Analysis
EPM data were analyzed using 2-way analysis of variance (ANOVA) with lesion status and drug treatments as independent variables. NORT data were analyzed with a one-sample t-test (0.5 = chance performance). PPI data were analyzed with three-factor repeated-measures ANOVA, with lesion status and drug as between-subjects factors, and prepulse intensity as a repeated measure. Locomotor activity data were analyzed using a three-way, repeated-measures ANOVA with test segment as a within subject factor, and lesion status and drug as between-subject factors. Repeated measures analysis was performed using the Greenhause-Geisser correction for violations of sphericity. Wherever required, group differences were analyzed using either Tukey’s or Bonferonni’s post-hoc test. p values < 0.05 were considered significant.
3. RESULTS
3.1 Prepulse Inhibition (PPI) of Acoustic Startle Response (ASR)
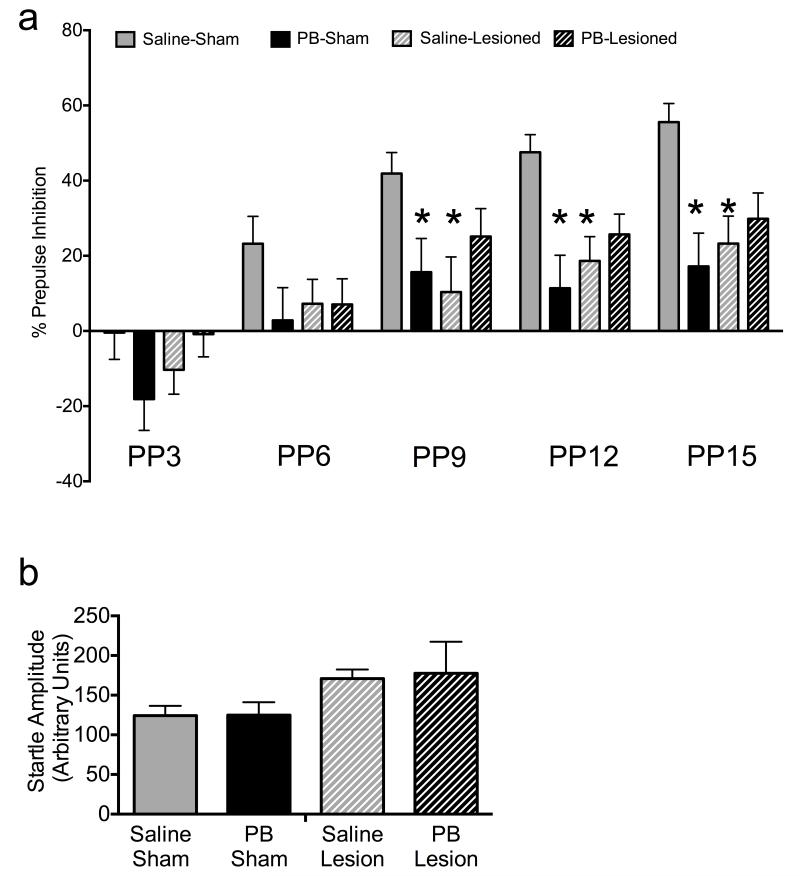
Control (sham-lesioned, saline-treated) animals displayed an increase in PPI with increasing prepulse intensity, as is typical for this paradigm. These data, as well as mean baseline ASR amplitude are shown in Figure 1. nVH-lesioned animals exhibited significant reduction in PPI, as compared to controls (Figure 1a). Phenobarbital-exposed animals without nVH lesions also exhibited significant reduction in PPI, as compared to controls. Phenobarbital-exposed animals with nVH lesions were not significantly different from phenobarbital-exposed animals without nVH lesions (p=0.128). Baseline ASR did not differ between groups (Figure 1b).
Figure 1.
Both neonatal ventral hippocampal (nVH) lesions and neonatal phenobarbital (PB) treatment disrupt PPI. (A) Prepulse inhibition, as a function of prepulse intensity. PP3, 6, 9, 12 and 15 indicate prepulse intensity in dB above background (B) baseline ASR. * - significantly different than control (sham-lesioned, saline-treated), p<0.05
ANOVA of basal acoustic startle response (ASR amplitude) showed neither an effect of lesion (F1,31=2.99, p=0.093), phenobarbital treatment (F1,31=0.016, p=0.89), nor an interaction between lesion status and drug treatment (F1, 31=0.11, p=0.91). A 3-way ANOVA of PPI showed no significant effect of lesion status (F1,31 =1.05, p = 0.31), but a significant main effect of phenobarbital treatment (F1,124 = 2.94, p = 0.096), prepulse intensity (F4,124 = 32.18, p < 0.001) and significant interaction between lesion status and drug treatment (F3,31 = 4.33, p = 0.012). Further analysis indicated non-significant interactions between: lesion status and prepulse intensity (F4,124 = 1.12, p = 0.35), drug treatment and prepulse intensity (F4,124 = 0.90, p = 0.45), and lesion status, drug treatment and prepulse intensity (F4,124 = 1.00, p = 0.44).
Post-hoc analysis (Bonferroni) revealed that nVH-lesioned animals not exposed to phenobarbital had a significant reduction in PPI compared to control animals at prepulse intensities 9, 12 and 15 dB above background (p<0.05). Post-hoc analysis (Bonferroni) also showed that neonatal phenobarbital treatment alone led to a disruption of PPI, as compared to control animals at prepulse intensities 9, 12 and 15 dB above background (p<0.05). Phenobarbital-exposed, nVH-lesioned animals displayed deficits equivalent to those seen in phenobarbital-exposed, sham-lesioned animals.
3.2 Locomotor activity
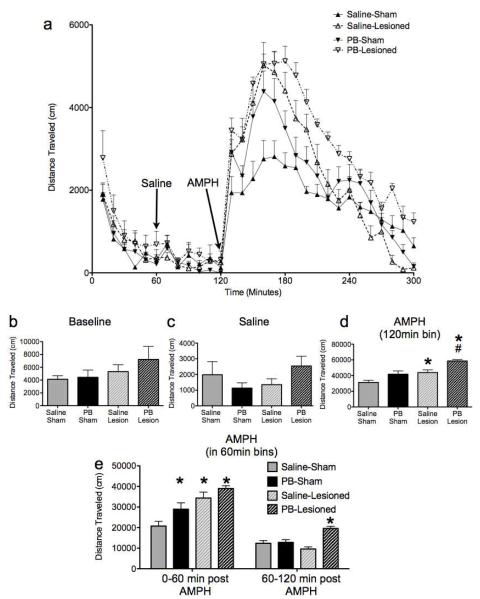
Control (sham-lesioned, saline-treated) animals displayed exploratory activity in the novel locomotor chamber (Figure 2a), which habituated over the course of the first 60 min (Figure 2b). Control animals had no significant response to an injection of saline at 60 min (Figure 2c). As expected based on previous literature (Hall et al. 2008), following injection of amphetamine, control animals displayed a significant (p<0.001) increase in locomotor activity (Figure 2d). This amphetamine-induced response was significantly enhanced in nVH-lesioned animals (p<0.01), without a change in either the initial exploratory response (first 60 min) or the response to saline (second 60 min). Phenobarbital-exposed animals without nVH lesions exhibited a significantly enhanced locomotor response, as compared to controls, during the first 60 min following amphetamine injection (p<0.05; Figure 2e); the response of these animals was not significantly different from controls when comparisons included the full 120 min. Phenobarbital exposed animals with nVH lesions displayed a significantly enhanced response to amphetamine, as compared to both controls and saline-treated nVH-lesioned animals (Bonferroni, p<0.001). This difference was most marked during the recovery phase (60-120 min after amphetamine) when the phenobarbital-exposed nVH-lesioned animals were the only group to exhibit a significant difference from controls (Bonferroni, p<0.0001).
Figure 2.
Both neonatal ventral hippocampal (nVH) lesions and neonatal phenobarbital (PB) exposure increase locomotor response to amphetamine (AMPH). (A) time course of locomotor activity; arrows indicate the time of injection of saline and amphetamine; (B) total distance traveled during the baseline (0-60 min) period; (C) total distance traveled during the saline (60-120 min) period; (D) total distance traveled following amphetamine (120-300 min); (E) 60 min bins following amphetamine. * - significantly different than control (sham-lesioned, saline treated), p<0.05. # - significantly different than saline-exposed, nVH-lesioned group, p<0.05.
When data (horizontal activity) in the locomotor activity were binned by 60-min segment of the test, a three-way ANOVA with test segment as a repeated measure showed a significant main effect of lesion status (F1,31=17.093, p<0.001), drug treatment (F1,31=10.774, p<0.005) and test segment (F3,93=293.611, p<0.001). Additionally, there was a significant three way interaction between test segment, lesion status, and drug treatment (F3,93=3.198, p<0.05), as well as significant interactions between test segment and lesion status (F3,93=12.082, p<0.001), and test segment and drug treatment (F3,93=4.090, p<0.05). Bonferroni corrected pair-wise comparisons were used to assess differences within each test segment. These comparisons revealed no differences between groups during the initial habituation period (0-60min), nor during the post-saline period (60-120min). During the first 60min following amphetamine injection, all three experimental groups displayed significantly more activity than did controls (p<0.05), while in the subsequent 60min, only the phenobarbital-exposed, nVH-lesioned group was significantly different than controls (p<0.001).
When data were analyzed by a 3-way ANOVA with test segment as a repeated measure, but the 120 min following AMPH collapsed into one segment, there was a significant main effect of lesion status (F1,31=17.093, p<0.001), drug treatment (F1,31=10.774, p<0.005) and test segment (F2,62=570.961, p<0.001). Additionally, there was a non-significant three-way interaction between test segment, lesion status, and drug treatment (F2,62=0.699, P=0.591), as well as significant interactions between test segment and lesion status (F2,62=13.800, p<0.001), and test segment and drug treatment (F2,62=10.325, p<0.001). Pair-wise comparisons revealed no group differences during habituation, or following saline injection. Following amphetamine, nVH-lesioned animals displayed a significantly greater response than did controls (p<0.05). Phenobarbital-exposed, nVH-lesioned animals displayed a significantly greater response than phenobarbital-exposed, sham-lesioned animals (p<0.001). Phenobarbital-exposed, sham-lesioned animals did not differ significantly from controls (p=0.056).
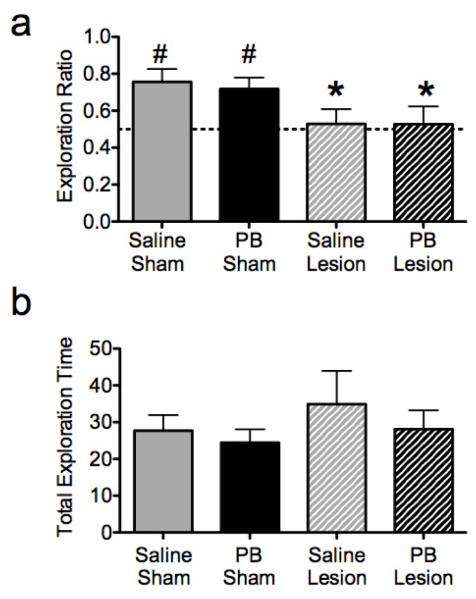
3.3 Novel Object Recognition Task (NORT)
Control (sham-lesioned, saline-treated) animals showed an increased ratio of time spent exploring the novel object to total exploration time (novel + familiar object). This demonstrates memory for the previously explored (familiar) object. Animals with nVH lesions failed to exhibit this increase, showing no difference in exploration time between the novel and familiar objects, resulting in a ratio not significantly different from chance (Figure 3a). The exploration ratio for this group was significantly lower than that for the controls. Phenobarbital exposure did not modify these responses in either lesioned or sham-lesioned animals. Two-way ANOVA revealed a significant main effect of lesion (F1,24=7.14, p=0.013), but neither a significant main effect of drug treatment (F1,24=0.066, p=0.80) nor an interaction between drug treatment and lesion status (F1,24=0.058, p=0.81). An analysis of the exploration ratio using one-sample t-test showed that both saline-sham (t = 10.81, p = <0.001) and phenobarbital-sham (t = 11.65, p = < 0.001) animals had a significant preference for exploring the novel object after the 5 min delay, whereas novel objet exploration of lesioned animals, with or without phenobarbital, did not differ significantly from chance level. The total time exploring both objects did not differ between groups: lesion (F1,24=0.96, p=0.33), drug treatment (F1,24=0.82, p=0.37), interaction between lesion status and drug treatment (F1,24=0.10, p=0.75) (Figure 3b).
Figure 3.
Neonatal ventral hippocampal (nVH) lesions, but not neonatal phenobarbital (PB) treatment disrupts novel object recognition in rats. (A) exploration ratio (time exploring novel object/total time exploring either object; a higher ratio indicates a preference for the novel object); (B) total time spent exploring both objects during the test session. * - significantly different than control (sham-lesioned, saline-treated), p<0.05. # - performance significantly above chance (indicated by the horizontal dotted line), p<0.05.
3.4 Elevated Plus Maze (EPM)
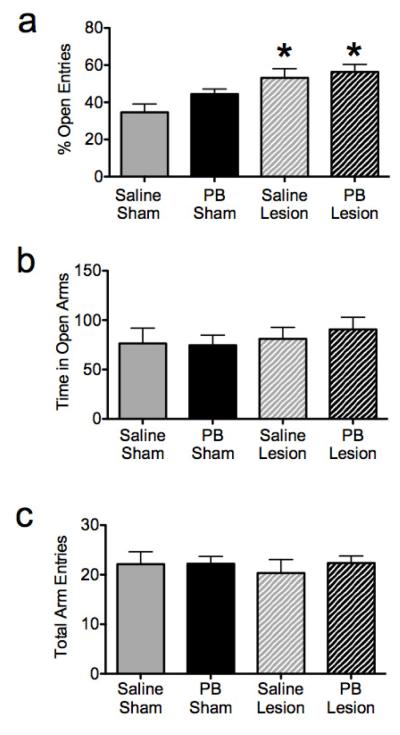
Control (sham-lesioned, saline-treated) animals had a mean time spent in open arms of 76 s, made a mean of 22 total arm entries, with 35% of the entries into the open arms. nVH-lesioned animals displayed a significant increase in the percent of entries into the open arms as compared to controls (p<0.01, Figure 4a) with no difference in total arm entries (Figure 4c) or time spent in open arms (Figure 4b). This increase in percent open arm entries in saline + nVH-lesioned animals is consistent with a decrease in anxiety-like behavior. Phenobarbital exposure did not modify these responses in either lesioned or sham-lesioned animals. A 2-way ANOVA of the percent of entries into the open arm showed a significant main effect of lesion status (F1,31=13.87, p=0.0008), but no effect of phenobarbital treatment (F1,31=2.51, p=0.1227), nor an interaction between lesion status and drug treatment (F1,31=0.6529, p=0.4252). Data on the total number of arm entries showed no significant main effect of either lesion status (F1,31=0.1709, p=0.6822), or phenobarbital treatment (F1,31=0.2858, p=0.5968) nor did it show an interaction between lesion status and drug treatment (F1,31=0.2465, p=0.6231).
Figure 4.
Neonatal ventral hippocampal (nVH) lesions, but not neonatal phenobarbital (PB) exposure decrease anxiety-like behavior in the elevated plus maze. (A) percent open arm entries (B) time in open arms (C) total arm entries.* - significantly different than control (sham-lesioned, saline-treated), p<0.05.
4. DISCUSSION
Here, we demonstrated that neonatal phenobarbital treatment per se leads to disruption in sensorimotor gating function (PPI) in adulthood. Furthermore, neonatal phenobarbital exposure enhanced the adult locomotor response to amphetamine in animals with or without nVH lesions. These results support our hypothesis that AED exposure during a sensitive period in development can predispose to certain schizophrenia-like abnormalities and may worsen behavioral outcomes caused by other predisposing insults.
nVH-lesioned animals that did not receive phenobarbital exhibited behaviors consistent with those in previous reports (for review see Marcotte et al. 2001; Tseng et al. 2009). In particular, these animals exhibited impairments in NORT (Bhardwaj et al. 2005), decreased anxiety like behavior (Wood et al. 2003), deficits in sensorimotor gating function (Le Pen et al. 2003; Le Pen and Moreau 2002; Risterucci et al. 2005), and hyperactivity to acute amphetamine administration (Flores et al. 1996b; Lipska et al. 1993). It is noteworthy that this model is generated by making lesions at an age (P7) of peak vulnerability to ENA induced by AEDs (Bittigau et al. 2002; Lipska et al. 1995). Thus, overlapping mechanisms may be responsible for the later behavioral abnormalities triggered by neonatal AED exposure and hippocampal lesions.
Our data indicate that both nVH lesions and phenobarbital treatment (in sham-lesioned animals) lead to comparable impairments in PPI in adulthood. The neural circuits regulating PPI include the hippocampus, nucleus accumbens, frontal cortex, and amygdala (Braff 2010; Swerdlow et al. 2001). Given the fact that these areas exhibit ENA following a single dose of phenobarbital on P7 (Forcelli et al., 2011a), and that nVH lesions also cause long term changes in several of these areas (Negrete-Díaz et al. 2010; Kamath et al. 2008; Powell et al. 2006), it is not surprising that both treatments lead to disruptions in PPI. The fact that we did not see a worsening of the lesion effect in phenobarbital-exposed animals may be due to the severity of the lesion effect by itself. Despite the parametric nature of PPI, given the profound impairment in PPI caused by the nVH lesions alone, it is difficult to detect additional impairment in this task.
PPI deficits in adult animals also can be induced by neonatal exposure to NMDA receptor antagonists [PCP (Wang et al. 2003) or MK-801 (Harris et al. 2003)]. Although phenobarbital and NMDA receptor antagonists have distinct mechanisms of action, they both induce ENA in the developing brain. This provides further evidence that drugs that induce ENA can disrupt the corticolimbic circuits necessary for normal sensorimotor gating.
Corticolimbic dysfunction has also been suggested to underlie the enhanced locomotor hyperactivity in response to amphetamine seen in nVH-lesioned animals, likely due to an increase in mesolimbic dopamine activity (Le Moal and Simon 1991; Lodge and Grace 2007; Marcotte et al. 2001; Tseng et al. 2009). Our data with amphetamine responses in nVH-lesioned rats confirm previous observations in these animals. However, our data show a significant enhancement of amphetamine-induced locomotion following phenobarbital exposure in the absence of the nVH lesions. Furthermore, the combination of phenobarbital exposure and the nVH lesions results in an even greater enhancement of the amphetamine-induced locomotion than either treatment alone. The increase in amphetamine-induced locomotion by neonatal phenobarbital and its exacerbation of nVH lesion-induced behavior suggest that phenobarbital may disrupt circuits that are either the same or parallel to those disrupted following nVH lesions.
We found that phenobarbital neither induced nor exacerbated nVH lesion-evoked deficits in memory as measured in the NORT, or anxiety-like behavior as measured in the elevated plus maze. These tasks are thus dissociated from PPI and amphetamine-induced locomotion, suggesting that the latter may be more dependent upon neural substrates which are disrupted by phenobarbital-induced ENA. However, it would be premature to conclude that circuitry for memory and anxiety are not disrupted by neonatal phenobarbital exposure, since we have only examined the effects of a single acute phenobarbital treatment. In fact, other studies have reported spatial memory impairment in adult animals following chronic exposure to phenobarbital during the neonatal period (McBride et al. 1985; Pick and Yanai 1984, 1985; Rogel-Fuchs et al. 1992; Stefovska et al. 2008). However, the type of memory assessed in the current study (non-spatial, long term memory) differs from spatial memory previously assessed. Assessment of spatial memory in the nVH lesioned, phenobarbital exposed animals might reveal memory deficits not seen in the present paradigm. Furthermore, analysis of short-term memory, rather than the longer-term memory in the present study may also be useful. With respect to anxiety-like behavior, we have observed decreased anxiety-like behavior in adult rats in the elevated plus maze following phenobarbital exposure from P7 to P14 (Forcelli et al. 2010); these changes are comparable to those obtained in nVH-lesioned animals. This suggests that the effects seen with a single drug exposure may represent only the “tip of the iceberg” with additional deficits appearing with chronic treatment.
Our findings with neonatal exposure to phenobarbital support the hypothesis that AED drug exposure in infancy can predispose to some behavioral abnormalities resembling those that are seen in patients with schizophrenia. While we initially expected that early postnatal exposure to phenobarbital might exacerbate behavioral outcomes associated with the nVH lesion model in the rat, our data indicate that neonatal phenobarbital exposure itself can lead to abnormal behavioral outcomes in the absence of lesions or other insults. The fact that these results were obtained with just a single acute drug exposure suggests that our findings are relevant to acute clinical interventions in addition to chronic therapies. The possibility that chronic drug exposure might lead to more extensive deficits is suggested by recent behavioral data (Forcelli et al., 2011c) that also suggest that newer AEDs may cause less toxicity than phenobarbital (Forcelli et al., 2011c, Katz et al., 2007; Kim et al., 2007). Furthermore, because ketamine and other anesthetic agents also induce ENA (Jevtovic-Todorovic et al., 2003; Ikonomidou et al., 1999), early-life exposure to these medications should be examined as a potential risk factor for neuropsychiatric sequelae.
It is important to note that we did not detect impairment in memory, nor did we assess other cognitive measures that are characteristic of schizophrenia. Such measures would be worthwhile examining in future studies, as the cognitive abnormalities present in patients with schizophrenia are some of the most debilitating. At the same time the behavioral deficits that we have observed are not specific to schizophrenia, but are associated with other neuropsychiatric disorders (for a review Braff et al., 2001), suggesting that the relevance of our findings may extend beyond schizophrenia.
Our findings emphasize the importance of examining drug treatment during development as a potential risk factor for neuropsychiatric abnormalities in adolescence and adulthood. For example, the influence of drug exposure during brain development has yet to be examined as a factor contributing to the schizophrenia-like psychosis and thought disorders that have been observed in children and adolescents with epilepsy (Caplan et al. 1991, 1997, 1998). Finally, our findings support the concern raised by Vestergaard et al. (2005) that AED treatment (especially phenobarbital) may have contributed to the association they reported between febrile seizures and schizophrenia.
Supplementary Material
Supplementary Figure 1. Representative photomicrographs of the ventral hippocampal (VH) from (A) saline-sham and (B) saline-lesioned animals. Black arrow in panel B indicates the position of the nVH lesion.
Adult behavior in rats was studied after acute neonatal phenobarbital (PB) exposure
Effects of PB were compared to those of neonatal ventral hippocampal lesions (nVH)
Neonatal PB and nVH lesions impaired adult prepulse inhibition equivalently
Both neonatal PB and nVH lesions exacerbated adult response to amphetamine
Neonatal PB recapitulated some features of the nVH model of schizophrenia
Acknowledgements
This study was supported by an NIMH grant to AK (MH079991) and a CIHR grant to LKS (MOP-68922) and an NINDS predoctoral fellowship to PAF (NS066822). Dr. Joseph Rochford’s (DMHUI) advice with statistical analyses is gratefully acknowledged.
Abbreviations
- AED
antiepileptic drug
- nVH
neonatal ventral hippocampal
- PPI
prepulse inhibition
- AMPH
amphetamine
- PB
phenobarbital
- P
postnatal day
- NORT
novel object recognition task
- EPM
elevated plus-maze
- ASR
acoustic startle response
- ENA
enhanced neuronal apoptosis
- NMDA
N-Methyl-D-aspartate
- i.p.
intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero D, Ment LR, Silverstein F. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Tuerke KJ, Forsyth JK, Giles A, Xue L, Boegman RJ, Jhamandas K. Neonatal ventral hippocampal lesions in male and female rats: effects on water maze, locomotor activity, plus-maze and prefrontal cortical GABA and glutamate release in adulthood. Behav. Brain Res. 2009;202:198–209. doi: 10.1016/j.bbr.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S, Palardy M, Quirion R, Srivastava L. Impairments in object recognition memory in the neonatal hippocampus lesion model of schizophrenia. Society for Neuroscience; Annual Meeting; 2005.2005. [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Stone C, Callaway E, Geyer MA, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl.) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci. 2010;4:349–371. doi: 10.1007/7854_2010_61. [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredkjaer SR, Mortensen PB, Parnas J. Epilepsy and non-organic non-affective psychosis. National epidemiologic study. Br J Psychiatry. 1998;172:235–238. doi: 10.1192/bjp.172.3.235. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Caplan R, Shields WD, Mori L, Yudovin S. Middle childhood onset of interictal psychosis. J Am Acad Child Adolesc Psychiatry. 1991;30:893–896. doi: 10.1097/00004583-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Caplan R, Arbelle S, Guthrie D, Komo S, Shields WD, Hansen R, Chayasirisobhon S. Formal thought disorder and psychopathology in pediatric primary generalized and complex partial epilepsy. J Am Acad Child Adolesc Psychiatry. 1997;36:1286–1294. doi: 10.1097/00004583-199709000-00022. [DOI] [PubMed] [Google Scholar]
- Caplan R, Arbelle S, Magharious W, Guthrie D, Komo S, Shields WD, Chayasirisobhon S, Hansen R. Psychopathology in pediatric complex partial and primary generalized epilepsy. Dev Med Child Neurol. 1998;40:805–811. doi: 10.1111/j.1469-8749.1998.tb12357.x. [DOI] [PubMed] [Google Scholar]
- Caplan R, Levitt J, Siddarth P, Taylor J, Daley M, Wu KN, Gurbani S, Shields WD, Sankar R. Thought disorder and frontotemporal volumes in pediatric epilepsy. Epilepsy Behav. 2008;13:593–599. doi: 10.1016/j.yebeh.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW. Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol. Dis. 2010;40:200–206. doi: 10.1016/j.nbd.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Barbeau D, Quirion R, Srivastava LK. Decreased binding of dopamine D3 receptors in limbic subregions after neonatal bilateral lesion of rat hippocampus. J. Neurosci. 1996a;16:2020–2026. doi: 10.1523/JNEUROSCI.16-06-02020.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK. Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J. Neurosci. 1996b;16:7366–7375. doi: 10.1523/JNEUROSCI.16-22-07366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Janssen MJ, Stamps LA, Sweeney C, Vicini S, Gale K. Therapeutic strategies to avoid long-term adverse outcomes of neonatal antiepileptic drug exposure. Epilepsia. 2010;51(Suppl 3):18–23. doi: 10.1111/j.1528-1167.2010.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli P, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011a;52:e207–211. doi: 10.1111/j.1528-1167.2011.03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011b;52:e20–22. doi: 10.1111/j.1528-1167.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. The Journal of Pharmacology and Experimental Therapeutics. 2011c doi: 10.1124/jpet.111.188862. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Pantelis C. Reconciling neuroimaging and neuropathological findings in schizophrenia and bipolar disorder. Curr Opin Psychiatry. 2009;22:312–319. doi: 10.1097/YCO.0b013e32832a1353. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bernstein HG, Becker A, Höllt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20:525–532. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Marquez Avila H, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology (Berl.) 2008;195:469–478. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DNC, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur. J. Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS. Sensorimotor gating deficits and hypofrontality in schizophrenia. Front. Biosci. 2001;6:D1069–1072. doi: 10.2741/hazlett. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jalava M, Sillanpää M. Concurrent illnesses in adults with childhood-onset epilepsy: a population-based 35-year follow-up study. Epilepsia. 1996;37:1155–1163. doi: 10.1111/j.1528-1157.1996.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Austin JK, Caplan R, Dunn D, Plioplys S, Salpekar JA. Psychiatric disorders in children and adolescents who have epilepsy. Pediatr Rev. 2008;29:e9–14. doi: 10.1542/pir.29-2-e9. [DOI] [PubMed] [Google Scholar]
- Kaindl AM, Koppelstaetter A, Nebrich G, Stuwe J, Sifringer M, Zabel C, Klose J, Ikonomidou C. Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol Cell Proteomics. 2008;7:2293–310. doi: 10.1074/mcp.M800030-MCP200. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kamath A, Al-Khairi I, Bhardwaj S, Srivastava LK. Enhanced alpha1 adrenergic sensitivity in sensorimotor gating deficits in neonatal ventral hippocampus-lesioned rats. Int J Neuropsychopharmacol. 2008;11:1085–96. doi: 10.1017/S1461145708008845. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Bunney WE, Jr, Docherty JP, Marder SR, Ebert MH, Rosenblatt JE, Rayner JN. d-Amphetamine-induced heterogeneous changes in psychotic behavior in schizophrenia. Am J Psychiatry. 1982;139:991–997. doi: 10.1176/ajp.139.8.991. [DOI] [PubMed] [Google Scholar]
- Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J. Pharmacol. Exp. Ther. 2007;322:494–500. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J. Pharmacol. Exp. Ther. 2007;323:165–173. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- Kubova H, Mares P. Anticonvulsant effects of phenobarbital and primidone during ontogenesis in rats. Epilepsy Res. 1991;10:148–155. doi: 10.1016/0920-1211(91)90007-3. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J. Psychopharmacol. (Oxford) 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl.) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl.) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Marcotte ER, Pearson DM, Srivastava LK. Animal models of schizophrenia: a critical review. J Psychiatry Neurosci. 2001;26:395–410. [PMC free article] [PubMed] [Google Scholar]
- McBride MC, Rosman NP, Davidson SJ, Oppenheimer EY. Long-term behavioral effects of phenobarbital in suckling rats. Exp Neurol. 1985;89:59–70. doi: 10.1016/0014-4886(85)90265-1. [DOI] [PubMed] [Google Scholar]
- Minassian A, Feifel D, Perry W. The relationship between sensorimotor gating and clinical improvement in acutely ill schizophrenia patients. Schizophr. Res. 2007;89:225–231. doi: 10.1016/j.schres.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Díaz JV, Baltazar-Gaytán E, Bringas ME, Vazquez-Roque RA, Newton S, Aguilar-Alonso P, León-Chávez BA, Flores G. Neonatal ventral hippocampus lesion induces increase in nitric oxide [NO] levels which is attenuated by subchronic haloperidol treatment. Synapse. 2010;64:941–947. doi: 10.1002/syn.20835. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin Y-Q, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res. Dev. Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol. Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Martin JR, Jenck F, Moreau JL. Spatial and associative learning deficits induced by neonatal excitotoxic hippocampal damage in rats: further evaluation of an animal model of schizophrenia. Behav Pharmacol. 2000;11:257–268. doi: 10.1097/00008877-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau J-L. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Kew J, Alberati D, Borroni E, Heitz MP, Moreau J-L. Prepulse inhibition deficits of the startle reflex in neonatal ventral hippocampal-lesioned rats: reversal by glycine and a glycine transporter inhibitor. Biol. Psychiatry. 2003;54:1162–1170. doi: 10.1016/s0006-3223(03)00374-3. [DOI] [PubMed] [Google Scholar]
- Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. Am J Psychiatry. 2002;159:1375–1381. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- Pick C, Yanai J. Long-term reduction in spontaneous alternations after early exposure to phenobarbital. Int J Dev Neurosci. 1984;2:223–228. doi: 10.1016/0736-5748(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Pick C, Yanai J. Long term reduction in eight arm maze performance after early exposure to phenobarbital. Int J Dev Neurosci. 1985;3:223–227. doi: 10.1016/0736-5748(85)90027-9. [DOI] [PubMed] [Google Scholar]
- Plioplys S, Dunn DW, Caplan R. 10-year research update review: psychiatric problems in children with epilepsy. J Am Acad Child Adolesc Psychiatry. 2007;46:1389–1402. doi: 10.1097/chi.0b013e31815597fc. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Binder TL, Hori S, Nakabeppu Y, Weinberger DR, Lipska BK, Robertson GS. Neonatal ventral hippocampal lesions produce an elevation of DeltaFosB-like protein(s) in the rodent neocortex. Neuropsychopharmacology. 2006;31:700–711. doi: 10.1038/sj.npp.1300883. [DOI] [PubMed] [Google Scholar]
- Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risterucci C, Jeanneau K, Schöpenthau S, Bielser T, Künnecke B, von Kienlin M, Moreau J-L. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology (Berl.) 2005;180:724–734. doi: 10.1007/s00213-005-2204-8. [DOI] [PubMed] [Google Scholar]
- Rogel-Fuchs Y, Newman ME, Trombka D, Zahalka EA, Yanai J. Hippocampal cholinergic alterations and related behavioral deficits after early exposure to phenobarbital. Brain Res Bull. 1992;29:1–6. doi: 10.1016/0361-9230(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Sachdev P. Schizophrenia-like psychosis and epilepsy: the status of the association. Am J Psychiatry. 1998;155:325–336. doi: 10.1176/ajp.155.3.325. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Marsh L. The psychiatric perspectives of epilepsy. Psychosomatics. 2000;41:31–38. doi: 10.1016/S0033-3182(00)71171-6. [DOI] [PubMed] [Google Scholar]
- Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–45. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J. Pharmacol. Exp. Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl.) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav. Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M, Pedersen C, Christensen J, Madsen K, Olsen J, Mortensen P. Febrile seizures and risk of schizophrenia. Schizophrenia Research. 2005;73:343–349. doi: 10.1016/j.schres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wan RQ, Corbett R. Enhancement of postsynaptic sensitivity to dopaminergic agonists induced by neonatal hippocampal lesions. Neuropsychopharmacology. 1997;16:259–268. doi: 10.1016/S0893-133X(96)00217-5. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D, Johnson KM. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J. Pharmacol. Exp. Ther. 2003;304:266–271. doi: 10.1124/jpet.102.041798. [DOI] [PubMed] [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol. Psychiatry. 2000;47:61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Wood GK, Quirion R, Srivastava LK. Early environment contributes to developmental disruption of MPFC after neonatal ventral hippocampal lesions in rats. Synapse. 2003;50:223–232. doi: 10.1002/syn.10265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative photomicrographs of the ventral hippocampal (VH) from (A) saline-sham and (B) saline-lesioned animals. Black arrow in panel B indicates the position of the nVH lesion.