Abstract
Background
Lower plasma B-type natriuretic peptide (BNP) concentrations in obese individuals (‘natriuretic handicap’) may play a role in the pathogenesis of obesity-related hypertension. Whether this phenomenon may contribute to hypertension in African Americans is unknown. We tested the hypothesis that body mass index (BMI) is inversely related to BNP concentrations in African Americans.
Methods and Results
We examined the relation of plasma BNP to BMI in 3,742 Jackson Heart Study participants (mean age: 55±13, 62% women) without heart failure using multivariable linear and logistic regression, adjusting for clinical and echocardiographic covariates. The multivariable adjusted mean BNP was higher for lean participants compared to obese participants in both normotensive (p<0.0001) and hypertensive (p<0.0012) groups. In sex-specific analyses, the adjusted mean BNP was higher in lean-hypertensive individuals compared to obese-hypertensive individuals for both men (20.5 pg/mL vs. 10.9 pg/mL; p=0.0009) and women (20.0 pg/mL vs. 13.8 pg/mL; p=0.011) respectively. The differences between lean and obese participants were more pronounced in normotensive participants (men, 9.0 pg/mL vs. 4.4 pg/mL; p<0.0001 and women, 12.8 pg/mL vs. 8.4 pg/mL; p=0.0005). For both hypertensive and normotensive individuals in the pooled sample, multivariable adjusted BNP was significantly related to both continuous BMI (p<0.05 and p<0.0001 respectively) and categorical BMI (p for trend <0.006 and <0.0001 respectively).
Conclusion
Our cross-sectional study of a large community-based sample of African-Americans demonstrates that higher BMI is associated with lower circulating BNP concentrations, thereby extending the concept of a ‘natriuretic handicap’ in obese individuals observed in non-Hispanic whites to this high-risk population.
Keywords: Natriuretic peptide, obesity, hypertension
INTRODUCTION
Obesity is associated with a state of fluid overload that is characterized by both sodium retention and increased cardiac output.1 The physiological effects of obesity should cause brain natriuretic peptide (BNP) concentrations to be higher with excess adiposity; yet several reports have underscored lower circulating concentrations of BNP in the presence of excess weight. It has been postulated that lower natriuretic peptide concentrations in obese individuals may contribute to the burden of sodium-retaining conditions such as hypertension in these individuals. In a recent study by Framingham investigators, obese non-Hispanic individuals were found to have circulating natriuretic peptide concentrations that are inappropriately low for the degree of hypertension (Framingham Offspring Study). However, no prior investigation has examined if the concept of natriuretic handicap in obesity extends to African Americans, a group with considerable burden of both obesity and high blood pressure. The availability of routine plasma BNP measurements on more than 4,000 African Americans individuals in the Jackson Heart Study allows for a comprehensive investigation of the relations of body weight and adiposity to circulating BNP concentrations in this high-risk community, and provides an opportunity to asses if hypertension modifies these relations.
METHODS
The Jackson Heart Study is a prospective community-based cohort initiated in 2000 to investigate cardiovascular disease in African Americans.2 Briefly, approximately thirty percent of the Jackson Heart Study participants were former members of the Jackson cohort of the Atherosclerosis Risk in Communities study and had been selected randomly from the driver’s license registry at the time of the initial recruitment.3 Approximately 23% of those remaining participants were recruited from a commercial listing that represents the overall tri-county population and another 23% were part of a constrained volunteer sample. The final 24% of the participants were recruited through the Jackson Heart Study Family Study, as described previously.4
A total of 3,742 JHS participants with plasma BNP measurements were eligible for the present investigation after excluding a total of 451 participants due to missing anthropometric measurements (n=17); renal insufficiency (defined as a serum creatinine >2.0 mg/mL, n=56), morbidly obese (defined as BMI > 45 kg/m2, n=215), (BNP = 0 and BNP >100 pg/mL, n = 32 + 71 = 103 ) and history of heart failure (n=60). BNP > 100 pg/mL was excluded based on evidence indicating that BNP measurements at this concentration suggests heart failure; this “cutoff” has a sensitivity of 90%, a specificity of 76% and a diagnostic accuracy of 81% for diagnosing heart failure in patients presenting to the emergency room with acute dyspnea that is superior to clinical assessment alone.5 All participants underwent a routine physical examination that included a medical history, and also underwent laboratory assessment for cardiovascular disease risk factors (including plasma BNP concentrations), anthropometry, routine electrocardiography and echocardiography. We calculated body mass index (BMI, kg/m2) as weight in kilograms divided by height in meters squared. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive therapy. Diabetes was defined as fasting plasma glucose ≥126 mg/dL or use of insulin or hypoglycemic medications.
As noted above, participants underwent a standardized 2D echocardiographic examination. M-mode left ventricular (LV) mass was calculated using the American Society of Echocardiography corrected formula 0.8[1.04{(LVDD+IVS+PW)3–(LVDD)3}]+0.6 developed by Devereux.6 Left ventricular systolic dysfunction was defined as a fractional shortening <0.29 or a visually estimated ejection fraction <50%. Left atrial dimensions were measured in end-systole.
B-Type natriuretic peptide measurement
Plasma BNP concentrations were measured in the Jackson Heart Study using a chemiluminescent immunoassay performed on the Siemens Advia Centaur. Quality control samples were assayed within each batch of JHS samples. The coefficient of variation (CV) of the assay was measured at three concentrations: Level 1 (mean = 48.47 pg/mL, CV = 4.2%), Level 2 (mean = 472.94 pg/mL, CV = 3.1%) and Level 3 (mean = 1810.03 pg/mL, CV = 3.4%). The minimal detectable concentration of BNP with this assay was 2.0 pg/mL
Statistical Analyses
We examined the relations of plasma BNP concentrations to BMI using multivariable regression analyses. Plasma BNP concentrations at or below assay detection limit (2 pg/mL) were considered ‘low’ (observed in 20% of women and 22% of men), and ‘normal’ otherwise. Similarly, BMI was treated as a continuous and as a ordinal variable using the World Health Organization/National Institutes of Health classification scheme (Normal <25 kg/m2, overweight 25.0 to <30.0 kg/m2, obese ≥30.0 kg/m2).7 In the obese category, we restricted our analysis to the sample with BMI < 45.0 kg/m2 (N = 3742). Similarly, we used waist circumference (WC) to assess the role of central adiposity particularly in women. We defined categorical WC as low (WC<88cm) and high (WC≥88 cm) in women, and the corresponding WC cutoffs in men were <102 cm and ≥102 cm.
We performed multivariable linear regression with natural log-transformed BNP as the dependent variable. Tobit models, implemented using the SAS LIFEREG procedure (SAS 9.2®), were estimated to account for left censoring of the BNP distribution.8 Regression models included the following covariates: BMI plus age, history of myocardial infarction, diabetes mellitus, current smoking, blood pressure stage (systolic blood pressure <140 and diastolic blood pressure <90 mm Hg; systolic blood pressure 140 to 159 or diastolic blood pressure 90 to 99 mm Hg; systolic blood pressure ≥160 or diastolic blood pressure ≥100 mm Hg, or use of antihypertensive therapy), and serum creatinine in model 1. Additionally, left atrial size, left ventricular mass, and left ventricular systolic dysfunction were included for regression model 2. We also tested for interaction between gender and obesity traits (BMI and WC), but were excluded in the final analyses because it was not significant. Because we modeled a log-transformed dependent variable, we exponentiated the β-coefficient for BMI to characterize the multiplicative effects of adiposity on BNP concentrations expressed in original units. To accommodate missing data for LV mass, we used an indicator variable (measured LV mass, no/yes) and assigned the mean value in place of missing values. In additional models, we replaced the continuous BMI and WC variables with BMI and WC categories, excluded echocardiographic traits and repeated the analyses the same analyses in the pooled sample. Sex by BMI (or WC) interaction was fitted in the case of pooled sample. All analyses were stratified by hypertension status.
Additional Analyses
To investigate further the relation between BMI and BNP, we used generalized additive models (GAM) with penalized splines smoothing function to fit a curve that describes the relationship between BNP and BMI without assuming a linear relationship.
RESULTS
Characteristics of the JHS study sample (mean age, 55±13 years, 62% women) are presented in Table 1. Overall, 1263 (34%) participants were overweight and 1912 (51%) obese. Six percent (n = 215) of the participants were determined to be morbidly obese (BMI >= 45 kg/m2) and were subsequently excluded from this analysis. Both the age-adjusted BNP concentration in women and the age- and sex-adjusted BNP concentration for the pooled sample were significantly different across BMI categories (P for trend in both was ≤ 0.001) (Table 1).
Table 1.
Demographics of the Jackson Heart Study Participants
| Normal BMI (n=567) | Overweight (n=1263) |
Obese (n=1912) |
|
|---|---|---|---|
| Age, years | 54±15 | 56±13 | 54±12 |
| Males, % | 47 | 46 | 30 |
| BMI, kg/m2 | 22.7±1.9 | 27.6±1.4 | 35.2±3.8 |
| Waist circumference, cm | 81.8±7.9 | 92.9±8.3 | 110.5±14.3 |
| Left atrial diameter, cm | 3.3±0.5 | 3.5±0.4 | 3.7±0.4 |
| Left ventricular mass, g | 135.7±30.2 | 147.6±10.4 | 153.1±31.4 |
| Left ventricular mass index | 30.7±8.3 | 34.4±8.1 | 38.1±9.3 |
| Creatinine, mg/dL | 1.0±0.2 | 1.1±0.2 | 1.0±0.2 |
| Hypertension, % (JHS) | 48 | 58 | 67 |
| Antihypertensive therapy, % | 33 | 48 | 59 |
| Diabetes, % | 6 | 15 | 23 |
| Current smoker, % | 23 | 13 | 11 |
| Prior myocardial infarction, % | 3.9 | 4.7 | 4.9 |
| Left ventricular systolic dysfunction†, % | 5.0 | 3.9 | 3.5 |
| BNP‡ (n), pg/mL | |||
| - Men (n=1420) | 13.0 (267) | 10.7 (566) | 9.7 (587) |
| - Female*** (n=2322) | 16.0 (300) | 14.0 (676) | 12.7 (1346) |
| - All** (n=3742) | 14.4 (567) | 12.4 (1263) | 11.2 (1912) |
Sex-specific, age-adjusted BNP levels and age- and sex- adjusted BNP levels in women and all participants ( P for trend ≤ 0.0001).
measured as fractional shortening < 0.29 and ejection fraction < 0.50
Influence of Body mass index, Waist Circumference and Hypertension Status on B-type natriuretic peptide levels: multivariable analyses
BMI was inversely associated with plasma BNP concentrations after adjustments made in regression models 1 and 2 in the pooled sample with or without stratification by hypertension status. (Table 2) In the fully adjusted model , each standard deviation increase in BMI (kg/m2) was associated with a statistically significant (p<0.0001) decrease of 13% and 20% in circulating plasma BNP concentrations among hypertensive and normotensive individuals respectively. This is consistent with a 17% decrement for all participants in the pooled sample. Categorical BMI was also noted to be significantly (p<0.0001) associated with BNP concentration. Both hypertensive and normotensive obese participants had lower BNP concentrations compared to hypertensive and normotensive participants with normal BMI (37% and 43% lower concentrations respectively). Excluding echocardiographic traits from the regression model resulted in a similar significant inverse relation between BNP and BMI; however the effects were attenuated substantially. (Table 2)
Table 2.
Relation of log-BNP to Body Mass Index and Waist Circumference in Hypertensive and Normotensive Jackson Heart Study Participants
| Models | Model 1 | p-value | Model 2 | p-value |
|---|---|---|---|---|
| β coefficient (SE) | β coefficient (SE) | |||
| All Participants | ||||
|
| ||||
| Continuous | ||||
| BMI | −0.084 (0.017) | <0.0001 | −0.191 (0.024) | <0.0001 |
| WC | −0.057 (0.017) | 0.0006 | −0.166 (0.023) | <0.0001 |
| BMI categories | ||||
| Normal | Reference | Reference | ||
| Overweight | −0.145 (0.053) | 0.0059 | −0.240 (0.066) | 0.0003 |
| Obese | −0.271 (0.051) | <0.0001 | −0.539 (0.069) | <0.0001 |
| P for Trend | <0.0001 | <0.0001 | ||
| WC Categories | ||||
| Low adiposity | Reference | Reference | ||
| High adiposity | −0.071 (0.019) | 0.0002 | −0.172 (0.026) | <0.0001 |
| P for Trend | 0.0002 | <0.0001 | ||
|
| ||||
|
Hypertensive
Participants |
||||
|
| ||||
| Continuous | ||||
| BMI | −0.055 (0.027) | 0.0228 | −0.140 (0.035) | <0.0001 |
| WC | −0.042 (0.027) | 0.082 | −0.131 (0.034) | <0.0001 |
| BMI categories | ||||
| Normal | Reference | Reference | ||
| Overweight | −0.137 (0.083) | 0.0964 | −0.250 (0.113) | 0.027 |
| Obese | −0.220 (0.079) | 0.0055 | −0.457 (0.115) | <0.0001 |
| P for trend | 0.006 | 0.0004 | ||
| WC Categories | ||||
| Low adiposity | Reference | Reference | ||
| High adiposity | −0.059 (0.028) | 0.0325 | −0.151 (0.039) | 0.0001 |
| P for trend | 0.033 | <0.0001 | ||
|
| ||||
|
Normotensive
Participants |
||||
|
| ||||
| Continuous | ||||
| BMI | −0.111 (0.025) | <0.0001 | −0.219 (0.033) | <0.0001 |
| WC | −0.074 (0.030) | 0.0019 | −0.182 (0.033) | <0.0001 |
| BMI categories | ||||
| Normal | Reference | Reference | ||
| Overweight | −0.137 (0.067) | 0.0416 | −0.184 (0.078) | 0.0215 |
| Obese | −0.316 (0.067) | <0.0001 | −0.569 (0.087) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | ||
| WC Categories | ||||
| Low adiposity | Reference | Reference | ||
| High adiposity | −0.082 (0.026) | 0.0014 | −0.168 (0.034) | <0.0001 |
| P for trend | 0.0014 | <0.0001 | ||
p<0.0001;
p<0.001;
p<0.05
Model 1 = adjustment for BMI plus age, history of myocardial infarction, diabetes mellitus, current smoking, blood pressure stage (systolic blood pressure <140 and diastolic blood pressure <90 mm Hg; systolic blood pressure 140 to 159 or diastolic blood pressure 90 to 99 mm Hg; systolic blood pressure ≥160 or diastolic blood pressure ≥100 mm Hg, or use of antihypertensive therapy), and serum creatinine,
Model 2 = Model 1 + adjustment for echo left atrial size, echo left ventricular mass, and echo left ventricular systolic dysfunction.
Similarly in the pooled sample, WC was inversely associated with plasma BNP concentration in multivariable adjusted models analyzing continuous and categorical measures. Using WC as a continuous measure, each standard deviation increase corresponded to a significant (p<0.0001)12% and 17% decrement in BNP concentrations among hypertensive and normotensive participants, and 15% among non-stratified sample. In the analysis using categorical measures of WC, normotensive and hypertensive participants with high adiposity had a 26% and 29% lower BNP concentration compared to their counterparts with low adiposity (p=0.0001 and p<0.0001 respectively). The effects of WC were lower in model 2 than in model 1, although the direction of relationship was similar. (Table 2)
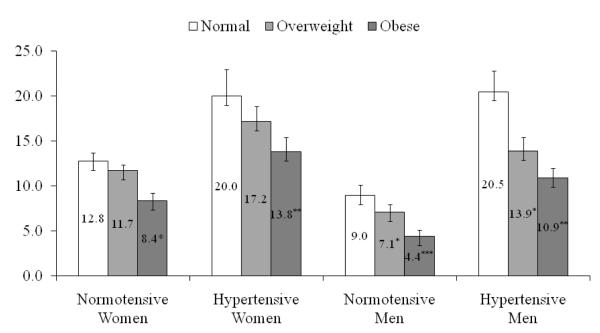
Figure 1 shows sex-specific multivariable adjusted mean BNP concentrations by BMI category and hypertension status. Mean BNP levels for lean, overweight and obese hypertensive participants were 21.9, 18.3 and 14.5 pg/mL for women and 16.6, 11.8 and 9.9 pg/mL for men. Differences in adjusted mean BNP concentration showed that obese participants had significantly (p<0.001) lower adjusted means than lean. Similarly, innormotensive participants, the adjusted mean BNP concentrations for obese men were significantly lower than in lean (8.1 pg/mL) versus 4.0 pg/mL for obese (p<0.0001). The corresponding differences in women were 14.5 pg/mL for lean compared to 9.0 pg/mL for obese (p<0.05). (Figure 1)
Figure 1.
Sex-specific adjusted means and SEs for plasma BNP by BMI and hypertension status
BNP, brain natriuretic peptide; BMI, body mass index
BNP is adjusted for age, history of myocardial infarction, diabetes mellitus, current smoking, blood pressure stage (systolic blood pressure < 140 and diastolic blood pressure < 90 mm Hg; systolic blood pressure 140 to 159 or diastolic blood pressure 90 to 99 mm Hg; systolic blood pressure ≥ 160 or diastolic blood pressure ≥ 100 mm Hg, or use of antihypertensive therapy), serum creatinine, echo left atrial size, echo left ventricular mass, and echo left ventricular systolic dysfunction. Multivariable adjusted means of BNP concentration in lean participants were compared with that of overweight and obese. *p<0.05; **p<0.001; ***p<0.0001.
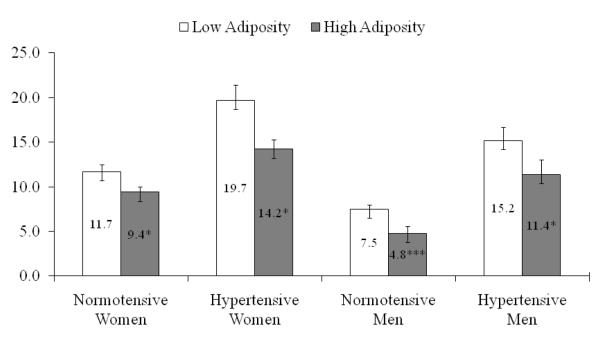
Figure 2 shows multivariable adjusted mean BNP concentrations by high and low adiposity for hypertensive and normotensive participants. Hypertensive women with high adiposity had significantly (p=0.002) lower multivariable adjusted mean BNP levels compared to women with low adiposity category (i.e., 16.9 pg/mL vs. 26.3 pg/mL). Corresponding differences in mean adjusted concentrations for men were 11.3 pg/mL vs 15.9 pg/mL; p = 0.0136. These results were replicated in the normotensives group. (Figure 2) In normotensive women, the adjusted mean BNP concentrations differences between those with low adiposity (14.6 pg/mL) and high adiposity (10.4 pg/mL) were significant (p=0.0153) as was the case in men ( 6.5 and 4.1 pg/mL p<0.0001).
Figure 2.
Sex-specific adjusted means and SEs for plasma BNP by adiposity and hypertension status
BNP, brain natriuretic peptide; BMI, body mass index
BNP is adjusted for age, history of myocardial infarction, diabetes mellitus, current smoking, blood pressure stage (systolic blood pressure < 140 and diastolic blood pressure < 90 mm Hg; systolic blood pressure 140 to 159 or diastolic blood pressure 90 to 99 mm Hg; systolic blood pressure ≥ 160 or diastolic blood pressure ≥ 100 mm Hg, or use of antihypertensive therapy), serum creatinine, echo left atrial size, echo left ventricular mass, and echo left ventricular systolic dysfunction. Multivariable adjusted means of BNP concentration in lean participants were compared with that of overweight and obese. *p<0.05; **p<0.001; ***p<0.0001.
The sex-specific relation of BNP to BMI and WC using models with and without echocardiographic traits are shown in Supplemental Table 1 and SupplementalTable 2 respectively.
Additional Analyses
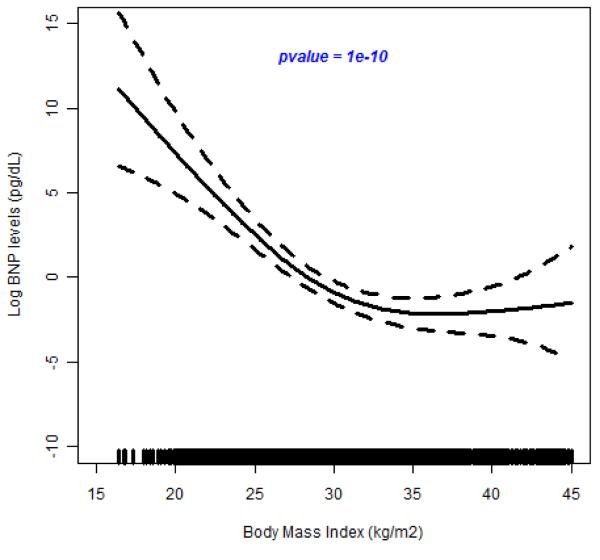
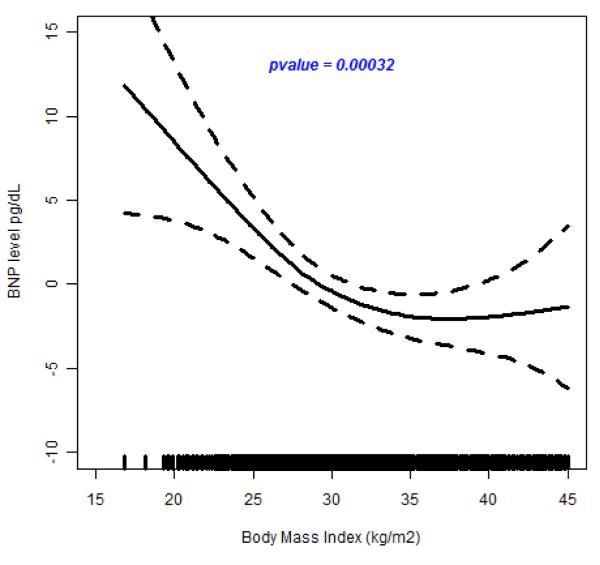
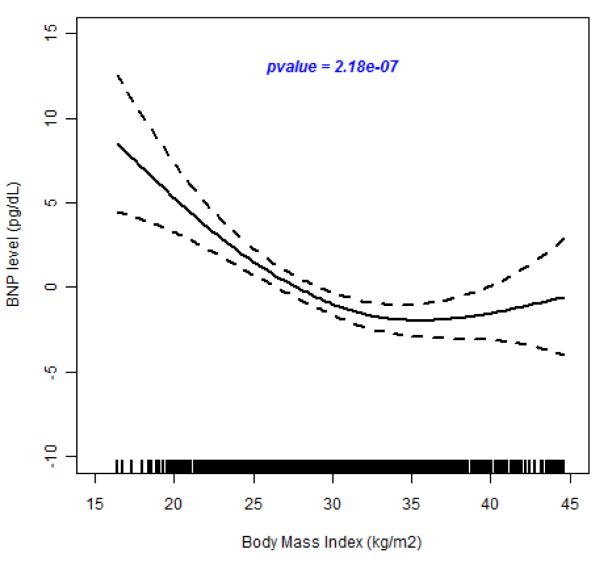
In the test for possible nonlinear relationship between BMI and BNP, we observed a highly significant spline smoothing parameter in all participants (p =6.0×10−10); in hypertensive individuals (p=5.0×10−7) and in normotensive individuals (p=7.2×10−4) (see Figure 3A, 3B and 3C). These results show that the spline for BMI is a strong predictor of plasma BNP concentration. In all cases, BNP decreased with increasing BNP until about 40 kg/m2 when it leveled off. This suggests a nonlinear inverse relation between BMI and circulating BNP concentrations.
Figure 3.
(A-C). Relation of Brain Natriuretic Peptide to Body Mass Index
The results of fiting a penalized cubic spline reveals a significant negative relationship between BNP concentration and BMI in the pooled sample (Figure 3A), in hypertensive individuals (Figure 3B) and in normotensive individuals (Figure3C). The relationship for BMI < 40 kg/m2 is linear but levels off at BMI > 45 kg/m2.
DISCUSSION
Principal Findings
Obese and overweight African American individuals have considerably lower plasma natriuretic peptide concentrations than individuals with a normal BMI, a finding that is not attributable to underlying differences in cardiovascular risk factors or cardiac structure between obese and nonobese subjects, and that extends similar findings in whites. Our findings raise the possibility that augmentation of the natriuretic peptide system may reduce the susceptibility of obese individuals to hypertension. Loss of this protective mechanism may predispose to persistent elevations in blood pressure.
In our study, we found that excluding echocardiographic LV mass and systolic function in the model attenuated the association between BMI (and WC) and BNP concentration. This most likely suggests that echocardographic traits are true confounders with both a strong association of both with BMI (seen in previous analysis of this African American cohort)9-11 and with BNP (established in previously studies).12,13
Previous studies
Our findings support that a higher BMI is associated with a lower BNP concentrations in community-based participants without heart failure, consistent with Framingham findings in non-Hispanic whites.8 The inverse relation may be due to increased expression of NPR-C by adipose tissue resulting in increased clearance of BNP in obese subjects. This explanation would also suggest a potential mechanism of hypertension in obese subjects. We note that though the mean adjusted BNP concentrations were similar between hypertensive participants of Framingham and those of the Jackson Heart Study, normotensive individuals in the Jackson Heart Study had lower mean adjusted BNP concentrations compared to Framingham normotensive participants. Though no direct comparison can be made this finding may be important to African Americans at risk of developing hypertension and cardiovascular complications related to hypertension.
In the Framingham study, N-terminal pro-atrial NP concentrations were also lower in individuals with higher BMI, a finding that suggests that decreased release of NP from the heart, rather than increased clearance, may be responsible for the association between higher BMI and lower natriuretic peptide concentrations. Similarly Dallas Heart Study investigators found a significant association of BMI and NP concentrations.14
A state of reduced NP concentration also exists in obese individuals with heart failure. In one investigation of 318 patients with heart failure, concentrations of BNP were significantly lower in obese than in non-obese subjects (205 ± 22 and 335 ± 39 pg/ml, respectively; p = 0.0007), despite a similar severity of heart failure and cytokine concentrations.15
Mechanisms for reduced natriuretic peptide concentrations in obesity
Obesity and elevated BMI have been associated with decreased circulating concentrations of BNP and N-terminal pro-BNP. Obesity has also been well associated with hypertension, salt retention and increased cardiac output.1 The fact that obesity has been associated with reduced blood concentrations of BNP seems counterintuitive, raising concerns about the diagnostic and prognostic validity of natriuretic peptides in obese patients.16,17
Several potential theories have been proposed in an attempt to explain this paradox. One controversial theory is that in obesity there may be increased expression of NP clearance receptors (NPR-C) that participate in the removal of NP from the circulation.18 Supporting this hypothesis, elevated NPR-C gene expression has been documented in the adipose tissue of humans with obesity 19 and allelic variants of this gene have been associated with lower plasma natriuretic peptide concentrations.20
However, reduced concentrations of N-ANP in obese individuals whose adipose tissue does not carry clearance receptors suggest some other non-clearance mechanism probably plays a more prominent role.8 Supporting a non-clearance mechanism, is the previously established association seen between higher BMI and lower plasma NT-proBNP that is structurally distinct from BNP, and unlikely to be cleared via NPR-C. One explanation could be that impaired synthesis and secretion of natriuretic peptides from the myocardium rather than clearance receptors contribute more to the relation of increased BMI to low circulating NP concentrations.8 Data supporting suppressed synthesis and/or release of NP from cardiomyocytes have been described in recent medical literature.14,21
Finally, NP influence lipid and fatty acid metabolism. Framingham investigators recently found that NP levels in their cohort were inversely associated with all components of the metabolic syndrome except for elevated blood pressure and that several metabolic risk factors (including insulin resistance) were associated with low circulating NP levels, even after adjustment for BMI.22 These findings suggest that perhaps BNP may be more a manifestation of metabolic syndrome rather than related specifically to low BMI.22,23
Joint effects of obesity and hypertension on BNP concentrations in JHS population
Though direct comparison cannot be made, it is true that BNP concentrations were lower in our AA cohort compared to that seen in the Framingham Heart Study in all BMI categories among both normotensive and hypertensive individuals.8 One could theorize that the increased risk for hypertension in this ethnic group may in part be attributed to an attenuated compensatory response compared to their white non-Hispanic counterparts.
BNP has been found to be effective in lowering blood pressure through its effects on natriuresis, sympathetic tone and the renin–angiotensin–aldosterone system. Those with hypertension are thought to have higher BNP concentrations as a compensatory mechanism to the high volume, salt retention state of hypertension. BNP functions to defend against excess salt and water retention, inhibits the production and action of vasoconstrictor peptides, promote vascular relaxation, and inhibits sympathetic outflow.24 However, compared to those who are hypertensive and lean, those who are hypertensive and obese appear to have lower BNP concentrations, as observed in our study.
LIMITATIONS
Given our cross-sectional analysis, we cannot infer any causality about the observed inverse relation between body size and BNP. Also, the Jackson Heart Study is an all African American cohort therefore generalizability of our findings to other ethnic groups is limited. However, given these limitations, the strength of our study includes the large community-based sample of African Americans.
CONCLUSION
In this community-based cohort of African Americans we established that lower BNP concentrations are associated with higher increase BMI categories. The relation of lower BNP concentrations with higher BMI categories was present in both non-hypertensive participants and in hypertensive participants. Our findings extend the concept of ‘natriuretic handicap’ of obesity to African Americans.
Supplementary Material
Acknowledgments
FUNDING SOURCES The Jackson Heart Study is supported by NIH contracts N01-HC-95170, N01-HC-9316885, and N01-HC-95172, HL076784 provided by the National Heart, Lung, and Blood Institute and the National Center for Minority Health and Health Disparities and AG028321 provided by the National Institute of Aging. This study was partially supported by PHS Award UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (NCRR) to one of the authors (A.B.)
Abbreviations
- BNP
- BMI
- WC
- NPR-C
- DHS
- ANP
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Messerli FH, Ventura HO, Reisin E, Dreslinski GR, Dunn FG, MacPhee AA, Frohlich ED. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982;66:55–60. doi: 10.1161/01.cir.66.1.55. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HA, Jr., Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6–17. [PubMed] [Google Scholar]
- 3.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6–29. [PubMed] [Google Scholar]
- 4.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, Steffes MW, Adeyemo A, Zhou J, Taylor HA, Jr., Jaquish C. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15:S6–37. [PubMed] [Google Scholar]
- 5.Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, Wu AH. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 7.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 9.Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, Skelton T. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: The Atherosclerotic Risk In Communities (ARIC) Study. Hypertension. 2004;44:55–60. doi: 10.1161/01.HYP.0000132373.26489.58. [DOI] [PubMed] [Google Scholar]
- 10.Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, Myerson M. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–244. doi: 10.1016/j.ahj.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Fox ER, Sarpong DF, Cook JC, Samdarshi TE, Nagarajarao HS, Liebson PR, Sims M, Howard G, Garrison R, Taylor HA., Jr The relation of diabetes, impaired fasting blood glucose, and insulin resistance to left ventricular structure and function in African Americans: the Jackson Heart Study. Diabetes Care. 2011;34:507–509. doi: 10.2337/dc10-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K, Burnett JC, Jr., Jougasaki M, Nishimura RA, Bailey KR, Saito Y, Nakao K, Redfield MM. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–994. doi: 10.1161/01.hyp.28.6.988. [DOI] [PubMed] [Google Scholar]
- 13.Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 14.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH, Jr., de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JA, Christenson RH, Rao K, Jorge M, Gottlieb SS. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. Am Heart J. 2006;152:1071–1076. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, Steg PG, Omland T, Knudsen CW, Sandberg KR, McCullough PA. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med. 2004;164:2247–2252. doi: 10.1001/archinte.164.20.2247. [DOI] [PubMed] [Google Scholar]
- 18.Sarzani R, Paci VM, ssi-Fulgheri P, Espinosa E, Rappelli A. Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J Hypertens Suppl. 1993;11:S214–S215. [PubMed] [Google Scholar]
- 19.Sarzani R, Paci VM, Zingaretti CM, Pierleoni C, Cinti S, Cola G, Rappelli A, ssi-Fulgheri P. Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. J Hypertens. 1995;13:1241–1246. doi: 10.1097/00004872-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Sarzani R, ssi-Fulgheri P, Salvi F, Serenelli M, Spagnolo D, Cola G, Pupita M, Giantomassi L, Rappelli A. A novel promoter variant of the natriuretic peptide clearance receptor gene is associated with lower atrial natriuretic peptide and higher blood pressure in obese hypertensives. J Hypertens. 1999;17:1301–1305. doi: 10.1097/00004872-199917090-00010. [DOI] [PubMed] [Google Scholar]
- 21.Bayes-Genis A, DeFilippi C, Januzzi JL., Jr Understanding amino-terminal pro-B-type natriuretic peptide in obesity. Am J Cardiol. 2008;101:89–94. doi: 10.1016/j.amjcard.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 23.Tekes S, Cikim AS. The association of brain natriuretic peptide and insulin resistance in obesity-related hypertension. J Hum Hypertens. 2007;21:546–550. doi: 10.1038/sj.jhh.1002194. [DOI] [PubMed] [Google Scholar]
- 24.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.