Abstract
OBJECTIVES
Evaluate the efficacy of a physical activity program (Seattle Protocol for Activity: SPA) for low-exercising older adults, compared to educational health promotion program (HP), combination treatment (SPA+HP), and routine medical care control conditions (RMC).
DESIGN
Single-blinded, randomized controlled trial with 2 × 2 factorial design. SETTING: November 2001 to September 2004, in community centers in King County, Washington.
PARTICIPANTS
273 community-residing, cognitively intact older adults (mean age, 79.2 y; 62% women).
INTERVENTIONS
SPA (in-class exercises with assistance setting weekly home exercise goals), and HP (information about age-appropriate topics relevant to enhancing health), with randomization to four conditions: SPA only (n = 69), HP only (n = 73), SPA+HP (n = 67), and RMC control (n = 64). Active treatment participants attended nine group classes over three months, followed by five booster sessions over one year.
MAIN OUTCOME MEASURES
Self-rated health (SF-36) and depression (GDS). Secondary ratings of physical performance, treatment adherence, and self-rated health and affective function were also collected.
RESULTS
At 3-months, participants in SPA exercised more and had significantly better self-reported health, strength, and general well-being (p<.05) than participants in HP or RMC. Over 18 months, SPA participants maintained health and physical function benefits, and had continued to exercise more than non-SPA participants. SPA+HP was not significantly better than SPA alone. Better adherence was associated with better outcomes.
CONCLUSION
Older adults participating in low levels of regular exercise can establish and maintain a home-based exercise program that yields immediate and long-term physical and affective benefits.
Keywords: exercise, health promotion, aging, older adults
INTRODUCTION
Exercise may well be the single most important thing we can do to keep ourselves healthy as we age. Clinical and population-based studies have consistently demonstrated that regular physical activity increases strength and stamina, reduces risk for developing many common disabling age-related illnesses, maintains functional independence despite chronic illness, and reduces all-cause mortality in older adults.1–3
Recent randomized trials of home-based exercise programs for older adults have shown that such programs can significantly increase activity and positively impact physical health.4–7 Studies suggest that older exercisers are less anxious,8 sleep better,9, 10 and report better quality of life.9, 11 Regular exercise has also been reported to have mood-enhancing effects often comparable or superior to antidepressant medications, with benefits sustained over time.12–14 Finally, evidence suggests physical activity may reduce the risk of cognitive decline15, 16 and delay the onset of dementia in older adults.17–20
Between the years 2006–2008 in the US, however, only 26% of older adults age 65–74 engaged in regular leisure-time physical activity; for those age 75–84, only 20% did so, and among those 85 and over, the number dropped to 10.5%.21 Recent exercise intervention studies, although often successful when targeting individuals with particular disorders,22–25 have consistently reported a lack of long-term adherence, especially for older adults.26–29 Although motivating individuals with age-related disabilities to begin and sustain an exercise program is challenging, there are well-accepted guidelines for the development of such programs.30, 31 Key practices for promoting physical activity in older adult populations indicate that effective exercise programs should include social support, strategies to increase self-efficacy, activity choices, health contracts, assurances of safety, and positive reinforcement.30–33
This study investigated an easy-to-follow exercise program specifically designed to build upon these guidelines and utilize behavior change principles that have been shown effective with older adults with a range of physical limitations.34, 35 We were particularly interested in developing, implementing, and evaluating a program with potential to be exported into the community. It needed to be inexpensive, requiring no cumbersome, specialized or costly equipment, safe for persons with a variety of comorbid health conditions, and easy to implement and follow. It also needed to be home-based, to enable older adults residing in a variety of domiciles – homes, apartments, and independent retirement centers – to participate. Finally, it needed to be systematic and standardized, yet allow enough flexibility to capture the interest and ability of individuals with varying degrees of physical limitations.
The primary aim of the current study was to develop a physical activity program that would meet these needs, and to test its efficacy compared to health promotion education and routine medical care. SPA (Seattle Protocol for Activity) is a nine-session, home-based program in which participants learn a range of balance and flexibility, strengthening, and aerobic physical activity skills. SPA was investigated alone and in combination with a Health Promotion program (HP) that was designed to further reinforce healthy behaviors and provide mood-enhancing benefits above and beyond those directly related to exercise alone. It was hypothesized that: 1) SPA participants would improve significantly on measures of self-reported health and mood, whereas HP participants would show improvements in mood, compared with participants receiving routine medical care; and 2) participants in the combination SPA+HP program would show greater benefits than those in SPA or HP alone.
METHODS
Participants
Participants were 273 older adults recruited from a Group Health Cooperative/University of Washington cohort of persons without cognitive impairment,36 community mailings, and local independent-living retirement centers. The study was approved by Institutional Review Boards of both the University of Washington and Group Health. Eligibility requirements included: living independently in the community, ambulatory, English-speaking, and not participating in regular exercise (≤ 150 minutes in the past week and not already attending a structured exercise program). Subjects were screened to rule out dementia using the Blessed Telephone Information-Memory-Concentration test.37 Participants with stable chronic illnesses were not excluded from the study. Primary physicians for all participants were asked if there was any health reason the participant should not be enrolled in an exercise program, but no persons were excluded based on physician feedback. See Table 1 for baseline participant characteristics.
Table 2.
Session topics for each active treatment condition.
| Seattle Protocol for Activity (SPA) | Health Promotion (HP) |
|---|---|
| Introduction to aerobic/endurance exercises | Identifying and setting personal healthy habit goals, maintaining motivation |
| Introduction to upper & lower body strength (shoulder flexion, shoulder extension, quadriceps, hamstring curls) & flexibility (triceps stretch, neck stretch, hamstring stretch, quadriceps stretch) exercises | Rationale for increasing pleasant events to enhance mood, overcoming obstacles to regular meaningful activity |
| More upper and lower body strength (biceps curls, lateral pull down, hip flexion, hip extension) & flexibility (top and inside forearm stretch, hip flexor stretch, ankle/calf stretch) exercises | Progressive relaxation training and practice, development of a daily relaxation practice plan |
| Balance & coordination exercises | Nutrition for healthy aging |
| Achieving and maintaining exercise goals | Personal nutritional choices |
| Measuring your exercise progress | Maximizing memory in daily life |
| Maintaining your momentum | Life long learning |
| Making exercise fun | Review and looking ahead |
| Progress in exercise and looking ahead | Communication |
| Maintenance & healthy living | Advance planning |
| Home exercise equipment | Safety: Home, driving, medication |
| Community resources | Enhancing personal resources |
| Exercise videos | Review of year’s goals & termination |
| Wrap up and one year celebration | Introduction to active goal setting |
Procedures
Participants were randomized (in blocks of 8–10 consecutive subjects) into four treatment conditions: Seattle Protocol for Activity (SPA), Health Promotion (HP), SPA plus Health Promotion (SPA+HP), or Routine Medical Care (RMC), This ensured an even flow through the four conditions and balanced time trends, such as seasonal patterns. Assessments were conducted at baseline, 3 months (post-treatment), and 6, 12, and 18-months (follow up) by interviewers blind to treatment assignment. Assessments and treatment sessions were conducted in community senior centers and retirement residences.
Treatment Conditions
Participants in SPA and HP conditions met in small groups for 9 weekly 60 minute sessions followed by 2 bi-weekly 60 minute sessions over 3 months, followed by three monthly and two quarterly booster sessions, for a total of 14 sessions over one year. Master’s-level trainers experienced in conducting exercise groups with older adults led all groups. Standardized treatment manuals included detailed instructions to group trainers, participant assignments and handouts, and forms for monitoring subject adherence. Trainers tracked exercises and content discussed in each session, and met weekly with supervisors to monitor treatment adherence. An outline of treatment sessions is provided in Table 2 and treatment manuals are available from the senior author.
Table 3.
Significant SPA effects* at post-test (3-months), with baseline values imputed for missing post-tests.
| Measure | Mean difference (95% CI) | p-value† |
|---|---|---|
| Primary Outcomes | ||
|
| ||
| SF-36 Health Status Survey - General Health Perceptions | 2.5 (0.4,4.6) | .018 |
|
| ||
| Secondary Outcomes | ||
|
| ||
| Self-Rated Health and Health Behaviors | ||
| Exercise minutes, past week | 39.3 (0.2,78.4) | .049 |
| Physical Activity Scale for the Elderly – Muscle strength, endurance (scaled score) | 0.13 (0.01,0.24) | .027 |
| Affective Function | ||
| Perceived Quality of Life | 2.3 (0.2,4.4) | .030 |
| Psychological General Well-Being Index (PGWB) | 3.3 (0.3,6.2) | .030 |
| PGWB – Self-Control | 1.0 (0.2,1.7) | .009 |
| PGWB – Vitality | 0.8 (0.0,1.5) | .040 |
Main exercise effects from the 2 × 2 design, indicating participants in SPA and SPA+HP were significantly better than HP and RMC subjects on outcomes shown.
Mean differences are the coefficients of SPA in this model, and represent the effect of the SPA intervention, controlling for HP and the baseline value of the outcome.
SPA classes
The SPA curriculum had three main goals: 1) to provide a safe and effective home-based physical activity program, 2) to enhance short- and long-term adherence to activity goals using group social support, activity contracts, and positive reinforcement, and 3) to maximize short- and long-term treatment benefits. SPA classes included instruction in warm up and cool down exercises, and progressive resistance training using elastic tubing to increase participant strength, with exercises for each major muscle group (see Table 2). Balance and flexibility training was also provided to reduce risk for falls.38, 39 Participants completed 8 – 12 repetitions of each exercise in class and had instructions to repeat strength training exercises on two additional nonconsecutive days between class, and to complete 3 to 5 days a week of aerobic training (primarily walking at a moderate level of intensity increasing in duration as participants were able, to at least 30 minutes per day) on their own, outside of class. Balance and coordination exercises were encouraged for use as cool-down after walking.
SPA classes emphasized exercise safety, finding ways to make exercise enjoyable, and the long-term benefits of a sustained physical activity program. All exercises were linked to practical activities of daily living that are important for maintaining independence (e.g., ability to get up from a chair without assistance or carrying groceries). Each session included strategies for overcoming obstacles to increasing activity, such as scheduling challenges or physical limitations. Participants used checklists and pedometers to monitor their daily exercise outside of class, and these were reviewed at each session. Participants were encouraged to join community-based exercise programs when the SPA classes ended.
HP classes
The goal of the HP curriculum focused on encouraging participants to maintain a healthy lifestyle, and to engage in regular activities designed improve mood and reduce stress. Content for the classes were drawn from health promotion and problem-solving treatments for depression that were developed and tested in community-based primary care settings. 40, 41 Participants in the health promotion groups discussed a variety of age-appropriate topics in weekly classes including strategies to enhance motivation to engage in healthy behaviors, increase participation in pleasant events, and develop a daily relaxation practice (see Table 2). Sessions encouraged group discussion, using questions and examples from participants’ personal experiences. Written handouts and goal setting assignments accompanied each topic. Participants set individualized personal health promotion goals and used checklists to monitor their progress that were reviewed at each session. No exercise routines were introduced, practiced, or recommended.
SPA+HP classes
The SPA+HP curriculum covered each of the topics in SPA and HP. Participants in this condition attended consecutive SPA and HP classes, and completed assignments and checklists for both exercise and health promotion goals.
RMC
Participants in RMC received routine care from their health care providers, including advice and support from their primary physicians and community support services. They were not provided with structured exercise recommendations, nor were they given health promotion information as part of this study. Outcome Measures
Primary Outcomes
Self-rated health and mood were rated using the Physical function, Role Physical, and General Health Perceptions subscales of the SF-36 Health Status Survey,42 a widely used measure for physical and emotional health status;43 and the Geriatric Depression Scale (GDS).44
Secondary Outcomes
Four categories of secondary outcomes were collected.
Physical Performance was measured using the 6-Minute Walk Test,45 which assessed aerobic endurance by measuring the number of steps walked in 6 minutes. Grip Strength was measured in both hands using a hand-held Jamar dynamometer (Patterson Medical Products, Bollingbrook, IL). A total of 2 attempts in maximal effort were performed, and the average value in kilograms in the dominant was reported.
Self-Rated Health and Health Behaviors were assessed with the Physical Activity Scale for the Elderly (PASE),46, 47 which rated frequency of participation in leisure, household, and work-related or volunteer activities during the previous week; the Physician-based Assessment and Counseling for Exercise (PACE),48 that categorized participants into stages of exercise activity readiness, and self-reported Exercise Minutes (“During the past week, how much total time did you spend walking for exercise or doing other aerobic activity?”).
Affective Function was assessed with: a) the Psychological General Well-Being Index (PGWB);49, 50 b) the Perceived Quality of Life Scale (PQOL),51 c) the Penn State Worry Questionnaire (PSWQ),52 and e) the Chronic Disease Self-Efficacy scales.53.
Treatment Adherence was measured by monitoring class attendance, ratings of homework completion (not attempted, attempted, completed), and participant homework logs.
Adverse events
Participants completed an adverse symptom checklist at post-test and follow-up visits. All health status changes (illness, fall, hospitalization, or death) were reviewed by a data safety committee for determination of whether they were attributable to study participation.
Statistical Methods
The study was designed to have 80% power (alpha = 0.05, two-sided) to detect effect sizes of 0.4 SD for the SPA and HP treatment effects, assuming a 20% drop-out. Recruitment exceeded expectations, so there was greater than 90% power for this effect size at post-test and longitudinally.
Between-group comparisons of baseline covariates were conducted using Fisher’s exact tests, t-tests, or non-parametric Kruskal-Wallis tests, as appropriate. Cox proportional hazards survival analyses were used to determine whether baseline characteristics predicted subject attrition.
Primary outcome analyses compared the four conditions using a 2 × 2 factorial design, with indicator variables for SPA and HP. The SPA effect was tested by comparing the mean difference between SPA and SPA+HP versus the HP alone and RMC. The HP effect was tested in an analogous manner (SPA+HP and HP alone versus SPA alone and RMC). This design assumed that the effect of the combined intervention would be equal to the sum of the effects of SPA and HP alone.
For the pre-post analyses, the outcome at the 3-month visit was regressed on the treatment conditions, controlling for baseline values:
These analyses were based on intent to treat (ITT), using all randomized participants. Baseline values were carried forward for participants missing the post-test. Longitudinal analysis used a repeated measures design, employing four post-treatment visits (3, 6, 12, and 18 months) and time, controlling for baseline values. Mixed models were used, with random effects for intercept and time, and an autoregressive correlation structure, which assumed that consecutive visits were more highly correlated than nonconsecutive visits:
Time-by-condition interactions were assessed with the same model structure.
The primary longitudinal analyses used all available data for each subject.
A series of supplemental analyses were conducted to assess the robustness of randomization and model assumptions. Pre-post analyses were repeated without imputation for missing post-tests, and potential outcome confounders (baseline exercise, BMI, depression, age, gender, and marital status) were evaluated by entering baseline values one at a time as covariates in the appropriate models, and noting changes in the estimated treatment effect. Next, “dose” variables for SPA and HP based on attendance and homework completion were used in place of the SPA and HP effects. Finally, an SPA−HP interaction term was introduced into the primary models to test the validity of the additive assumptions underlying the SPA+HP intervention. A similar series of secondary analyses were conducted for the longitudinal analyses, including analyses only on participants with 18 months of follow-up.
RESULTS
Preliminary analyses revealed no significant pretreatment group differences on subject demographics except for marital status (Table 1), which did not affect the significance of any outcomes.
Baseline health status of participants
At baseline, 25% of participants had fallen or nearly fallen during the past 2 weeks, 33% complained of moderate to very severe pain, 28% had body mass index levels in the obese range (BMI ≥ 30), 66% had systolic blood pressure in the hypertensive range (> 140), 21% had elevated total cholesterol (> 200), and 14% had elevated hemoglobinA1c levels (> 6). Thirty percent were mildly to moderately depressed (Geriatric Depression Scale > 11). Twenty-five percent of participants reported no exercise during the prior week and 35% exercised less than 1 hour/week.
Participant study adherence
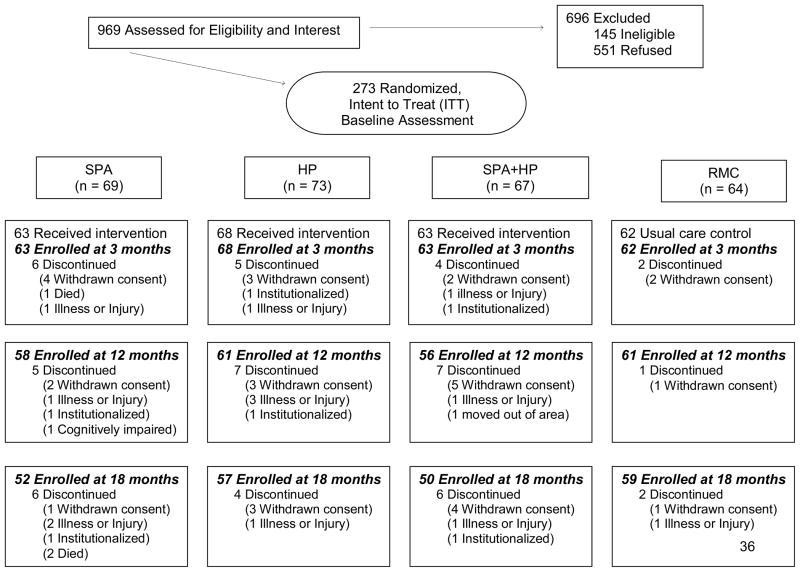
Of 273 participants who began the study, 258 (95%) completed the 3-month assessment, 239 (88%) completed the 12-month assessment, and 218 (80%) competed the 18-month assessment (Figure 1). There were no significant differences in rates of attrition between the treatment conditions. The only significant predictor of attrition was that subjects with post-high school education were less likely to withdraw.
Figure 1.
Flow of participants through the trial.
At 12 months, adherence to all three active treatment conditions was excellent. Participants attended 72% of classes during the active 9 session intervention period, and 63% of the five monthly or quarterly booster sessions. Eighty percent of all exercise-related homework was turned in during the active treatment period, and 51% of participants continued to complete activity forms on their own, throughout the entire follow-up period. Seventy-five percent of all health promotion homework was turned in throughout the treatment period. There were no significant differences in adherence based upon gender or age.
3-month ITT analyses
Primary outcomes
At the 3-month assessment (Table 3), participants in SPA and SPA+HP reported significantly better general health perceptions on the SF-36 (p<.05) and a trend for improvement in physical function (SF-36; p=.054) than subjects not assigned to an exercise condition (HP or RMC participants). There were no significant differences on the GDS for any treatment condition.
Table 4.
Significant longitudinal SPA effects.*
| Measure | Mean Difference (95% CI) | p-value† |
|---|---|---|
| Primary Outcomes | ||
|
| ||
| SF-36 Health Status Survey - Physical Functioning | 2.9 (0.9, 4.9) | 0.005 |
|
| ||
| SF-36 Health Status Survey - General Health Perceptions | 3.0 (1.4, 4.7) | < 0.001 |
|
| ||
| Secondary Outcomes | ||
|
| ||
| Physical Function | ||
| 6-minute walk (steps) | 28.4 (6.7, 50.1) | 0.011 |
| Self-Rated Health and Health Behaviors | ||
| Exercise minutes, past week | 31.4 (4.7, 58.1) | 0.022 |
| Physical Activity Scale for the Elderly (PASE)–Walking (scaled score) | 0.08 (0.01, 0.15) | 0.029 |
| Physician-based Assessment and Counseling for Exercise (PACE) | 0.4 (0.1, 0.7) | 0.003 |
| Affective Function | ||
| Perceived Quality of Life (PQOL) | 2.3 (1.0, 3.7) | 0.001 |
| Penn State Worry Questionnaire (PSWQ) | −1.6 (−2.7, −0.5) | 0.004 |
| Psychological General Well-Being Index (PGWB) | ||
| Total Score | 3.2 (1.0, 5.5) | 0.004‡ |
| General Health | 0.5 (0.1, 0.9) | 0.017‡ |
| Self-Control | 0.5 (0.1, 1.0) | < 0.001‡ |
| Depression | 0.4 (0.1, 0.7) | 0.020 |
| Positive Well-Being | 0.6 (0.1, 1.1) | 0.016 |
| Vitality | 0.7 (0.1, 1.2) | 0.019 |
Main exercise effects from the 2 × 2 design, indicating participants in SPA and SPA+HP were significantly better than HP and RMC subjects on outcomes shown.
Mean differences are the coefficients of SPA in this model, and represent the average effect of the SPA intervention, controlling for HP and the baseline value of the measure.
The mean differences in the table represent the average effect of the SPA intervention, which was larger at initial visits and declined over time.
Secondary outcomes
Participants in SPA and SPA+HP also reported significantly more exercise minutes (p<.05), better muscle strength and endurance (PASE; p<.05), and improvements in quality of life (PQOL; p<.05), general wellbeing (PGWB; p<.05), perceived self-control (PGWB; p<.01), and vitality (PGWB; p<.05) (Table 3). There was a trend for improvement in emotional well-being (PGWB; p=.055). Participants who received health promotion education (SPA+HP or HP alone) reported worrying significantly less than those in SPA alone or RMC (PSWQ 1.7 points lower (3.3, 0.1); p<.05).
3-month supplemental analyses
When analyses were repeated without imputation (i.e., including only participants with post-test data), all findings were stronger than the primary ITT results. No covariate changed the significance of outcome variables in the ITT analyses, nor were there differences in affective outcomes for subjects with higher levels of baseline depression (GDS > 8). Participants in the SPA and SPA+HP conditions who had better attendance and rates of homework completion had better scores on anxiety, general and emotional health, and performed significantly better on the 6-minute walk.
18-month longitudinal outcomes
Primary outcomes
At 18 months, participants in SPA and SPA+HP maintained improvements in general health perceptions (SF-36; p<.001), and reported significantly better physical function on the SF-36 (p<.01) (Table 4). No significant differences on the GDS were obtained.
Appendix 1.
Means (SD) for SPA outcome data statistically significant at post test or longitudinally.
| Group | Baseline Mean (± SD) |
3 month Mean (± SD) |
12 month Mean (± SD) |
18 month Mean (± SD) |
|
|---|---|---|---|---|---|
| N | No EX† | 137 | 130 | 122 | 116 |
| EX | 136 | 126 | 114 | 102 | |
|
| |||||
| Exercise minutes, past week | No EX | 96.9 (90.5) | 147.3 (162.7) | 141.5 (134.1) | 152.1 (167.4) |
| EX | 113.5 (111.8) | 198.0 (202.1) | 178.1 (209.2) | 149.7 (162.4) | |
|
| |||||
| 6-minute walk, steps | No EX | 706.6 (132.4) | 701.5 (147.1) | 691.9 (163.3) | 682.5 (154.3) |
| EX | 702.1 (157.1) | 730.7 (156.1) | 745.6 (113.3) | 730.3 (97.6) | |
|
| |||||
| Patient-centered Assessment and | No EX | 4.1 (1.9) | 4.3 (1.9) | 4.2 (2.1) | 4.1 (2.0) |
| Counseling for Exercise (PACE) | EX | 4.0 (1.8) | 4.6 (1.8) | 4.5 (2.0) | 4.4 (2.3) |
|
| |||||
| Physical Activity Scale (PASE), | No EX | 0.10 (0.21) | 0.14 (0.40) | 0.31 (0.45) | 0.40 (0.54) |
| Muscle Strength, Endurance | EX | 0.11 (0.29) | 0.27 (0.57) | 0.43 (0.57) | 0.42 (0.60) |
|
| |||||
| Perceived Quality of Life Scale | No EX | 78.9 (15.0) | 79.5 (15.7) | 79.8 (16.1) | 79.6 (14.9) |
| EX | 79.7 (15.1) | 82.1 (13.9) | 80.9 (13.0) | 80.7 (13.5) | |
|
| |||||
| Penn State Worry Questionnaire | No EX | 34.8 (11.0) | 34.4 (12.1) | 34.4 (12.5) | 34.0 (11.9) |
| (PSWQ) | EX | 33.9 (9.6) | 33.2 (10.6) | 32.3 (10.5) | 32.0 (9.4) |
|
| |||||
| Psychological General | No EX | 68.7 (16.0) | 72.6 (16.7) | 84.1 (14.9) | 82.9 (14.9) |
| Well-Being (PGWB) | EX | 70.3 (15.3) | 77.6 (17.6) | 84.6 (13.8) | 83.8 (15.8) |
|
| |||||
| PGWB Depression | No EX | 11.3 (2.3) | 11.9 (2.2) | 13.2 (2.2) | 13.4 (1.9) |
| EX | 11.2 (2.4) | 12.3 (2.4) | 13.4 (1.8) | 13.3 (2.2) | |
|
| |||||
| PGWB Self-Control | No EX | 8.7 (3.8) | 9.5 (4.0) | 12.7 (2.2) | 12.9 (2.0) |
| EX | 9.1 (4.2) | 10.9 (4.0) | 12.8 (2.1) | 12.9 (2.2) | |
|
| |||||
| PGWB Vitality | No EX | 10.5 (3.8) | 11.3 (4.0) | 13.5 (3.7) | 13.4 (3.4) |
| EX | 10.6 (3.8) | 12.2 (4.1) | 13.8 (3.3) | 13.4 (3.7) | |
|
| |||||
| PGWB General Health | No EX | 8.4 (3.0) | 9.0 (2.8) | 10.4 (2.8) | 10.0 (3.0) |
| EX | 8.8 (2.7) | 9.8 (3.0) | 10.6 (2.7) | 10.3 (3.2) | |
|
| |||||
| PGWB Positive Well-Being | No EX | 11.8 (2.8) | 12.2 (3.2) | 13.9 (3.5) | 13.4 (3.7) |
| EX | 11.8 (3.0) | 12.9 (3.7) | 13.8 (3.6) | 13.7 (3.4) | |
|
| |||||
| SF-36 General Health | No EX | 70.2 (14.6) | 69.4 (16.9) | 69.2 (16.3) | 68.1 (16.9) |
| Perceptions | EX | 73.8 (15.8) | 75.4 (14.2) | 74.2 (14.9) | 73.1 (15.4) |
|
| |||||
| SF-36 Physical Functioning | No EX | 67.8 (23.2) | 66.7 (23.8) | 67.4 (24.1) | 65.0 (24.9) |
| EX | 68.1 (22.8) | 69.9 (22.8) | 70.4 (25.4) | 69.9 (24.9) | |
*Data shown are for outcomes in the 2 × 2 factorial design analyses, testing the difference between subjects receiving exercise (SPA and SPA+HP) compared to the non-exercising conditions (HP and RMC).
Secondary outcomes
Over 18 months, participants in the two SPA conditions continued to report more weekly exercise minutes (p<.05) than those in HP or RMC (Table 4). Furthermore, participants in SPA and SPA+HP maintained improvements in quality of life (PQOL; p<.001), general wellbeing (PGWB; p<.01), perceived self-control (PGWB; p<.01), and vitality (PGWB; p<.05), compared to HP and RMC participants. In addition, several new significant differences between SPA and SPA+HP compared to HP and RMC emerged. SPA and SPA+HP participants walked significantly more (PASE; p<.05), took significantly more steps on the 6-minute walk (p<.05), and reported greater exercise willingness (PACE; p<.01) than HP or RMC participants. Additional affective benefits included significantly lower worry scores (PSWQ; p<.01), and less depression and increased positive well being on the PGWB (p<.05) for SPA and SPA+HP than for HP and RMC. The effect of HP in reducing worrying was not maintained in longitudinal analyses. Appendix 1 (online table) shows the means and standard deviations for all outcome variables included in Tables 3 and 4 at all four sampling points.
18-month supplemental analyses
Supplemental analyses confirmed the primary results. When longitudinal analyses were repeated for the 218 participants who completed 18 months of follow-up, no baseline covariate changed the significance of outcome variables. When the dose of the interventions was evaluated, participants in the SPA and SPA+HP conditions with higher rates of adherence showed better scores on SF-36 physical and mental health, PASE muscle strength and endurance, lower anxiety, and higher self Efficacy (depression and social/recreational subscales) than HP and RMC participants.
In the final set of supplemental analyses, a SPA−HP interaction term was introduced into the primary models to test the validity of the additive assumptions underlying the SPA+ HP intervention. The additive assumptions were not supported.
Adverse Events
Only one minor adverse event was attributed to the intervention (an allergic reaction to the elastic tubing). There were three deaths unrelated to treatment reported (2 deaths from lung cancer and colon cancer after the 1-year followup, and 1 death from unknown causes at post test).
DISCUSSION
This study investigated comparative efficacy of an exercise and health promotion intervention to improve physical and emotional functioning in sedentary older adults. We sought to develop a safe, easy-to-follow, exercise program that would help older adults with chronic illnesses to gradually increase their amount physical activity to recommended levels that could be maintained. Study results confirmed that participants actively participated in exercise training, attended classes, and complied with exercise directives. Study attrition at 18 months was only 20%, a rate considerably lower than some other community-based exercise interventions for older adults.54 Furthermore, following conclusion of active treatment, they continued exercising at significantly higher rates than control subjects.
Our first hypothesis was that SPA participants would improve significantly on measures of self-reported health and mood, whereas HP participants would show improvements in mood, compared with participants receiving routine medical care. The first part of this hypothesis was confirmed. Participants in SPA or SPA+HP exercised more, reported better muscle strength and endurance, better general health and wellbeing, greater vitality, greater self control, and higher quality of life at 3-months than participants in HP or RMC, with most improvements maintained over 18 months. The second part of this hypothesis, that HP participants would demonstrate improved affect, was only partially supported. Over 18 months of follow up, participants in SPA and SPA+HP worried less, and had greater positive well being. However, the only significant difference that could be attributed to HP alone was less worry in the HP and SPA+HP conditions at the 3-month assessment. This difference was not maintained over 18 months.
Contrary to our hypotheses, there were no significant differences in depression for any treatment condition as measured by the GDS. This finding may reflect the fact that subjects did not have unduly high levels of depression at study entry (mean GDS = 10.9). Subjects did, however, report reduced depression symptoms on the PGWB over 18-months of follow-up.
Our second study question was whether outcomes would be enhanced by combining exercise with education in health promotion. We hypothesized participants receiving SPA+HP would experience significantly better outcomes than those receiving SPA only or HP only. This hypothesis was not supported. The addition of health promotion strategies did not enhance outcomes. SPA and SPA+HP were not significantly different, and each yielded significant gains compared to HP alone or routine medical care. Thus, it appears that the SPA treatment protocol was the “active ingredient” responsible for improvements seen in this investigation.
There are limitations to this study. Older adults with severe mobility limitations and those who were cognitively impaired or institutionalized were ineligible. Our sample also represented a fairly highly educated population. Consequently, results cannot be generalized to non-ambulatory, cognitively impaired, or less educated older adults. Further study with these vulnerable populations is needed.
Second, our exercise intervention was conducted by trained interventionists, motivated and committed to helping participants succeed. Given the simplicity of our program, we believe that other trainers will be equally successful, but that remains to be shown.
Third, because assessments were conducted in community settings, including senior centers and retirement homes, testing conditions for the 6-minute walk varied widely from site to site. In light of this variability, we determined that the most constant way to report outcome data for this variable was number of steps rather than reporting distance walked. We recognize that this makes clinical interpretation of the 6-minute walk results more difficult.
Fourth, we acknowledge that all outcome measures were tested at p=0.05, which does increase our risk of multiple comparison type-I error. If the alpha level had been divided among the four primary outcomes, the primary longitudinal findings would still have been statistically significant, although the post-test results for the SF-36 General Health perceptions would not have been. As always, it will be important to confirm our findings in future investigations.
Last, of 969 potential participants who were invited to participate in the study, 551 declined. We have no way of knowing if those declining would have been eligible for the study, however, as in all studies of this nature, it is likely that those most needing such programs are least likely to enroll. As volunteers, it is also likely that those who did enroll were more highly motivated to participate in exercise than those who declined.
With these limitations in mind, this study indicates that relatively sedentary and/or chronically ill older adults benefited from and continued the SPA exercise program for 3 months of active treatment and for at least 15 months after formal treatment concluded (a total of 18 months). Recent reviews of the literature have called for exercise intervention trials that include older participants with chronic disease, assess health-related quality of life outcomes, and provide home-based programs.5, 12, 55, 56 In the SPA study, we addressed each of these issues. We also avoided methodological weaknesses identified in earlier studies by developing and testing a systematic and structured approach to treatment that is sufficiently detailed to enable replication. Furthermore, we systematically evaluated the level of attendance and exercise adherence for 18 months, during and after active treatment.
Our goal was to determine whether a structured, systematic home-based program of simple-to-follow exercises and training in overcoming obstacles to exercise initiation and maintenance would be successfully implemented with relatively sedentary older adults, and if so, whether it would be successful in improving their physical health status when compared to routine health care. The results of this study support the efficacy of SPA with this at-risk elderly population. These results, coupled with program’s strengths – it is inexpensive, requires no specialized or costly equipment, is easy to implement, and was well received – make SPA a viable and potentially powerful evidence-based intervention to be implemented in community settings.
Table 1.
Baseline characteristics of participants.*
| SPA† (n=69) | HP (n=73) | SPA+HP (n=67) | RMC (n=64) | TOTAL (n=273) | |
|---|---|---|---|---|---|
| Age (mean (± SD)) | 80.3 (6.1) | 79.6 (4.6) | 78.3 (5.3) | 78.3 (4.2) | 79.2 (5.2) |
| Gender | |||||
|
| |||||
| Male | 39.1% | 34.3% | 43.3% | 35.9% | 38.1% |
| Female | 64.1% | 65.7% | 56.7% | 64.1% | 61.9% |
|
| |||||
| Education | |||||
|
| |||||
| Less than high school | 4.3% | 5.5% | 6.1% | 3.1% | 4.8% |
| High school | 13.0% | 19.2% | 18.2% | 17.2% | 16.9% |
| Post high school | 82.6% | 73.5% | 75.8% | 79.7% | 78.3% |
|
| |||||
| Marital status | |||||
|
| |||||
| Married | 30.4% | 41.1% | 59.7% | 43.8% | 43.6% |
| Widowed | 40.6% | 43.8% | 28.4% | 39.1% | 38.1% |
| Divorced, separated or never married | 29.0% | 15.1% | 11.9% | 17.2% | 18.3% |
|
| |||||
| Ethnicity | |||||
|
| |||||
| White, not Hispanic | 89.9% | 89.0% | 91.0% | 92.2% | 90.5% |
| Asian/Pacific Islander | 5.8% | 2.7% | 1.5% | 3.1% | 3.3% |
| Black, not Hispanic | 4.4% | 5.5% | 6.0% | 3.1% | 4.8% |
| Hispanic | - | - | 1.5% | - | 0.4% |
| Native American | - | 1.4% | - | 1.6% | 0.7% |
| Multiracial | - | 1.4% | - | 0.4% | |
Values are expressed as percentage unless otherwise indicated. All group comparisons p>.05 except for marital status, significantly different at p<.007 for marital status (percent married).
SPA=Seattle Protocol for Activity; HP=Health Promotion; SPA+HP=SPA plus Health Promotion; RMC=Routine Medical Care
Acknowledgments
Funding/Support: This study was supported by grants AG14777, AG06781, and AG05136 from the National Institute on Aging.
Role of the sponsor: The National Institute on Aging was not involved in study design, implementation, or analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency or of the Centers for Disease Control and Prevention.
We gratefully acknowledge the contributions of our project staff, research coordinators, group trainers, and interviewers, including Amy Moore, MS, June van Leynseele, MS, Lyn Holt, MS, Raymond Houle, Alicia Korkowski, MS, Katie Carter, BA, Julie Cleveland, BA, Jenee Hoard, BA, Adam Templeton, BS, and Joanne Webb, BA. We also would like to thank members of the Data Safety and Monitoring Board, including Marilynn Albert, MSN, ARNP, and James LoGerfo, MD.
Footnotes
Trial Registration. clinicaltrials.gov Identifier: NCT00097643
Author Contributions: Dr. Teri, as principal investigator, had full access to all study data and takes responsibility for all aspects of the study, including the integrity of the data and the accuracy of the data analysis. Drs. McCurry, Logsdon, and Larson assisted with acquisition of the data. Drs. Teri, Logsdon and McCurry supervised the study, Drs. Gibbons and Buchner provided statistical expertise, Drs. Teri, McCurry, Logsdon, and Gibbons assisted with the writing of the manuscript, and all authors contributed to study concept, design, interpretation of data, and critical review of the manuscript.
References
- 1.Löllgen H, Böckenhoff A, Knapp G. Physical activity and all-cause mortality: An updated meta-analysis with different intensity categories. Int J Sports Med. 2008;30:213–224. doi: 10.1055/s-0028-1128150. [DOI] [PubMed] [Google Scholar]
- 2.Ueshima K, Ishikawa-Takata K, Yorifuji T, et al. Physical activity and mortality risk in the Japanese elderly: a cohort study. Am J Prev Med. 2010;38(4):410–418. doi: 10.1016/j.amepre.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 3.Buchner DM, Beresford SAA, Larson EB, et al. Effects of physical activity on health status in older adults II: Intervention studies. Annu Rev Public Health. 1992;13:469–488. doi: 10.1146/annurev.pu.13.050192.002345. [DOI] [PubMed] [Google Scholar]
- 4.Pahor M, Blair SN, et al. LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 5.Rejeski WJ, Marsh AP, Chmelo E, et al. The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci. 2009;64(4):462–467. doi: 10.1093/gerona/gln041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King AC, Baumann K, O’Sullivan P, et al. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: A randomized controlled trial. J Gerontol Med Sci. 2002;57A(1):M26–M36. doi: 10.1093/gerona/57.1.m26. [DOI] [PubMed] [Google Scholar]
- 7.Nelson ME, Layne JE, Bernstein MJ, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol Med Sci. 2004;59A(2):154–160. doi: 10.1093/gerona/59.2.m154. [DOI] [PubMed] [Google Scholar]
- 8.De Moor MHM, Beem AL, Stubbe JH, et al. Regular exercise, anxiety, depression and personality: A population based study. Prev Med. 2006;42:273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Chen KM, Chen MH, Chao HC, et al. Sleep quality, depression state, and health status of older adults after silver yoga exercises: cluster randomized trial. Int J Nurs Stud. 2009;46(2):154–163. doi: 10.1016/j.ijnurstu.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.King AC, Pruitt LA, Woo S, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elavsky S, McAuley E, Motl RW, et al. Physical activity enhances long-term quality of life in older adults: Efficacy, esteem and affective influences. Ann Behav Med. 2005;30(2):138–145. doi: 10.1207/s15324796abm3002_6. [DOI] [PubMed] [Google Scholar]
- 12.Blumenthal JA, Babyak MA, Doraiswamy M, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather AS, Rodriguez C, Guthrie MF, et al. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: Randomized controlled trial. Br J Psychiatry. 2002;180:411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- 14.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: A randomized, controlled trial. J Gerontol: Med Sci. 2001;56(8):M497–M504. doi: 10.1093/gerona/56.8.m497. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 18.Barnes DE, Yaffe K, Satariano WA, et al. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 19.Kramer AF, Colcombe SJ, McAuley E, et al. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26S:S124–S127. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Accessed April 21, 2010];National Center for Health Statistics. Health Data Interactive [online] Available at http://www.cdc.gov/nchs/hdi.htm.
- 22.Ashworth NL, Chad KE, Harrison EO, et al. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004017. doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Focht BC, Rejeski WJ, Ambrosius WT, et al. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;53(5):659–665. doi: 10.1002/art.21466. [DOI] [PubMed] [Google Scholar]
- 24.Mangione KK, Craik RL, Tomlinson SS, et al. Can elderly patients who have had a hip fracture perform moderate- to high-intensity exercise at home? Phys Ther. 2005;85(8):727–739. [PubMed] [Google Scholar]
- 25.Villareal DT, Banks M, Sinacore DR, et al. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166(8):860–868. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 26.Chao D, Foy CG, Farmer D. Exercise adherence among older adults: Challenges and strategies. Control Clin Trials. 2000;21:212S–217S. doi: 10.1016/s0197-2456(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 27.Dunstan DW, Daly RM, Owen N, et al. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28(1):3–9. doi: 10.2337/diacare.28.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health. 2005;27(1):25–32. doi: 10.1093/pubmed/fdh197. [DOI] [PubMed] [Google Scholar]
- 29.van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults: A review. Am J Prev Med. 2002;22(2):120–133. doi: 10.1016/s0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 30.ACSM. Physical activity programs and behavior counseling in older adult populations. Med Sci Sports Exerc. 2004;36(11):1997–2003. doi: 10.1249/01.mss.0000145451.08166.97. [DOI] [PubMed] [Google Scholar]
- 31.Cress ME, Buchner DM, Prohaska T, et al. Best practices for physical activity programs and behavior counseling in older adults populations. J Aging Phys Act. 2005;13(1):61–74. doi: 10.1123/japa.13.1.61. [DOI] [PubMed] [Google Scholar]
- 32.Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: The challenges for changing behavior. Am J Prev Med. 2003;25(3 Suppl 2):172–183. doi: 10.1016/s0749-3797(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 33.Rejeski WJ, Brawley LR. Functional health: Innovations in research on physical activity with older adults. Med Sci Sports Exerc. 2006;38(1):93–99. doi: 10.1249/01.mss.0000183191.65316.0a. [DOI] [PubMed] [Google Scholar]
- 34.Hellman EA. Use of the stages of change in exercise adherence model among older adults with a cardiac diagnosis. J Cardiopulm Rehabi. 1997;17(3):145–155. doi: 10.1097/00008483-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavior management in patients with Alzheimer disease: A randomized controlled trial. JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 36.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 37.Kawas C, Karagiozis H, Resau L, et al. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995;8(4):238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 38.da Silva RB, Costa-Paiva L, Morais SS, et al. Predictors of falls in women with and without osteoporosis. J Orthop Sports Phys Ther. 2010;40(9):582–588. doi: 10.2519/jospt.2010.3239. [DOI] [PubMed] [Google Scholar]
- 39.Schmid AA, Van Puymbroeck M, Koceja DM. Effect of a 12-week yoga intervention on fear of falling and balance in older adults: a pilot study. Arch Phys Med Rehabil. 2010;91(4):576–583. doi: 10.1016/j.apmr.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Wallace JI, Buchner DM, LG, et al. Implementation and effectiveness of a community-based health promotion program for older adults. J Gerontol A Biol Sci Med Sci. 1998;53(4):M301–M306. doi: 10.1093/gerona/53a.4.m301. [DOI] [PubMed] [Google Scholar]
- 41.Williams JW, Jr, Barrett J, Oxman T, et al. Treatment of dysthymia and minor depression in primary care: A randomized controlled trial in older adults. JAMA. 2000;284(12):1519–1526. doi: 10.1001/jama.284.12.1519. [DOI] [PubMed] [Google Scholar]
- 42.Ware JE, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 43.Dexter PR, Stump TE, Tierney WM, et al. The psychometric properties of the SF-36 Health Survey among older adults in a clinical setting. J Clin Geropsychol. 1996;2:225–237. [Google Scholar]
- 44.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a Geriatric Depression Screening Scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 45.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 46.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 47.Martin KA, Rejenski WJ, Miller ME, et al. Validation of the PASE in older adults with knee pain and physical disability. Med Sci Sports Exerc. 1998;31(5):627–633. doi: 10.1097/00005768-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Heath GW. The quantity and quality of physical activity for health and fitness: A behavioral approach to exercise prescription. In: Frontera WR, Dawson DM, Slovik DM, editors. Exercise in rehabilitation medicine. Champaign, IL: Human Kinetics Publishers; 1999. p. 484. [Google Scholar]
- 49.Dupuy HJ. The Psychological General Well-Being (PGWB) Index. In: Wenger NK, Mattson ME, Furberg CD, Elinson J, editors. Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. 1984. pp. 170–183.pp. 353–356. [DOI] [PubMed] [Google Scholar]
- 50.Ware JE, Johnston SA, Davies AR, Brook RH. Conceptualization and measurement of health for adults in the Health Insurance Study. Santa Monica, CA: The RAND Corporation; 1979. Publication No. R-1987/3-HEW. [Google Scholar]
- 51.Patrick DL, Danis M, Southerland LI, et al. Quality of life following intensive care. J Gen Intern Med. 1988;3:218–223. doi: 10.1007/BF02596335. [DOI] [PubMed] [Google Scholar]
- 52.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 53.Lorig K, Stewart A, Ritter P, et al. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 54.Schmidt JA, Gruman C, King MB, et al. Attrition in an exercise intervention: a comparison of early and later dropouts. J Am Geriatr Soc. 2000;48(8):952–960. doi: 10.1111/j.1532-5415.2000.tb06894.x. [DOI] [PubMed] [Google Scholar]
- 55.Cyarto EV, Moorhead GE, Brown WJ. Updating the evidence relating to physical activity intervention studies in older people. J Sci Med Sport. 2004;7 (1 Suppl):30–38. doi: 10.1016/s1440-2440(04)80275-5. [DOI] [PubMed] [Google Scholar]
- 56.King AC. Interventions to promote physical activity by older adults. J Gerontol Biol Sci Med Sci. 2001;56A (Special Issue II):36–46. doi: 10.1093/gerona/56.suppl_2.36. [DOI] [PubMed] [Google Scholar]