Abstract
Despite the initial effectiveness of oncogene-directed cancer therapeutics, acquired drug resistance remains the ultimate “Achilles’ heel” for long-term durable remission in cancer patients. Acquisition of drug resistance is not more evident elsewhere than in the use of tyrosine kinase inhibitors (TKIs), Imatinib and Dasatinib, for patients with Chronic Myelogenous Leukemia (CML). Hence, while Imatinib initially produces remission in the chronic phase, ultimately these therapeutics fail via emergence of drug resistance, where CML can inevitably progress to a terminal blast phase culminating in a fatal outcome. Technically, it is challenging to predict the onset of drug resistance in a small number of oncogene-transformed cells, making the decision of when and how to employ second generation tyrosine kinase inhibitors, or employ novel compounds that would be of benefit in treating drug resistant Bcr-Abl mutants mainly retrospective. Here, we characterize a rapid and sensitive real-time Fluorescent Resonance Energy Transfer (FRET) based assay that is able to detect the in vivo activity of Bcr-Abl and its inhibition by small molecule compounds. Due to its real-time and in vivo nature, such an approach has the potential to monitor a drug-resistant phenotype, as well as to identify pharmaceutical agents that inhibit drug-resistant Bcr-Abl oncoproteins in vivo.
Keywords: FRET, CML, Bcr-Abl, Picchu, Imatinib, Crk, Dasatinib
Introduction
Chronic Myelogenous Leukemia (CML) is a multi-phasic myeloproliferative disorder composing ~20% of adult leukemia and represents the prototype of a human cancer responsive to oncogene-targeted therapeutics (1). CML is usually diagnosed during a routine medical visit while in an initial chronic phase, which is cytogenetically characterized by clonal expansion of granulocytes and is well managed with currently available tyrosine kinase inhibitors (2). However, the mean duration of the chronic phase is 3–5 years and is followed by a less well-defined accelerated phase that ultimately leads to the terminal blast phase, in which there is an apparent reversion to earlier lymphoblast and myeloblast progenitors (2). Unlike the chronic phase, the blast phase is often refractory to treatment and can be fatal within 3–6 months (3). It is believed that the accumulation of secondary mutations gives rise to the switch from the chronic phase to the blast phase (4), although the epigenetic factors that drive blast crisis are not completely understood.
The driving force behind CML is the fusion protein Bcr-Abl produced by the Philadelphia Chromosome (2). This results from a translocation between chromosome 22, which harbors the Breakpoint cluster (Bcr) gene, and chromosome 9 containing the c-Abl gene (5). The translocation produces a constitutively active version of the c-Abl tyrosine kinase in the form of Bcr-Abl (5). Inhibition of Bcr-Abl activity is currently the main form of therapy, which employs tyrosine kinase inhibitors such as Imatinib Mesylate (GleevecTM) (6–8) and the more recent Dasatinib (BMS-354825TM) (9, 10). Although such approaches to the treatment of CML are efficacious in the earlier stages of the ailment, patients eventually become refractory to drug therapy and succumb to the disease, often due to mutations in the kinase domain, although some patients, harboring more detrimental mutations such as T315I, exhibit primary resistance to all currently available forms of therapy (11, 12).
Clinical problems associated with acquired resistance to Imatinib have prompted the development of novel ATP competitive small molecule tyrosine kinase inhibitors with activity towards clinically relevant Bcr-Abl mutants (13–17). Given the remarkable progress in the development of second generation TKIs for Imatinib-resistant CML, such as Dasatinib (9,10) and Nilotinib (18), there is an emerging need for in vivo technology that can rapidly and sensitively monitor Bcr-Abl tyrosine kinase activity and guide clinicians into decisions as to when and how to switch therapeutic modalities and evaluate resistance when it occurs in vivo. With this background, we set out to develop a FRET-based assay using the Picchu biosensor (19) capable of detecting in vivo Bcr-Abl activity and inhibition by tyrosine kinase inhibitors. We show here that Picchu is remarkably labile and responsive to Bcr-Abl activity in vivo, becoming inhibited within minutes of TKI administration and hence highly adaptable for real time kinetics. Moreover, Picchu is highly sensitive to both the therapeutic inhibition of wild type Bcr-Abl as well as the drug resistance of clinically relevant Bcr-Abl mutants. Therefore, we posit the Picchu FRET biosensor will have two major utilities - the first for pharmaceutical use in the screening of novel compounds that have high intracellular activity in suppressing clinically-relevant Bcr-Abl mutations, and secondly, as an important clinical tool for diagnosing relapse determination of a drug-resistant phenotype since, unlike currently available diagnostic methods like FISH which detect the Bcr-Abl fusion oncogene, Picchu FRET detects active intracellular Bcr-Abl, an important distinction since the Bcr-Abl gene has been detected in healthy individuals (20). Finally, because of the robustness in the assay in terms of sensitivity, lability, and durability we suggest that a pTyr-SH2 FRET cassette can serve as a general platform for technology to monitor drug resistance for other tyrosine kinases implicated in human cancer.
Materials and Methods
Materials, Drugs, and Antibodies
Imatinib Mesylate was kindly provided by Novartis and Dasatinib was from Bristol-Myers Squibb (BMS) with appropriate MTAs. Immunoblotting was performed using standard procedures with Phospho-Crk II Y221 Antibody (Cell Signaling Technology), total Crk II Antibody (Sigma), Phospho-Abl 245 Antibody (Cell Signaling Technology), and total c-Abl Antibody (Calbiochem). Transfections were performed with Fugene 6 (Roche) according to manufacturer’s instructions.
Plasmids
The DNA for the Picchu FRET probe and for the membrane targeted CAAX tagged Picchu (Picchu-CAAX) have been previously described (19).
Cell Culture and Transfections
32D cells expressing WT Bcr-Abl and T315I Bcr-Abl were kindly provided by Dr. Michael Deininger (Oregon State Health Science Center). HEK 293T cells were obtained from the laboratory stock and CosE37 cells were provided by Dr. Michiyuki Matsuda. Authentication was not performed on 32D, 293T, and CosE37 cells by the authors. 293T cells were maintained in 1X Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 2 mM L-Glutamine in 37°C and 5% CO2. Cells were plated onto either 6 cm or 12-well plates and, the next day, were transfected with 1 or 0.5 µg Bcr-Abl. Twenty-four hours later, Imatinib or Dasatinib treatment was applied followed two hours later by transfection with 1 or 0.5 µg Picchu. Twenty-four to forty-eight hours later, cells were collected and FRET measurements were performed on 50 to 60 µg of total protein. Western Blot analysis was performed on 15 to 20 µg total protein as described above. CosE37 cells were maintained in DMEM supplemented with 10% FBS and 2mM L-glutamine in 37°C and 5% CO2. Cells were plated onto collagen coated glass bottom 35 mm dishes and, the following day, were transfected with 0.25 µg Picchu-CAAX and 2.5 µg Bcr-Abl. Twenty-four hours later cells were either analyzed via flow cytometry or real time FRET microscopy.
Plate reader FRET assay
FRET emissions were measured using a 96-well Bio-Tek Synergy HT plate reader equipped with 440/30, 485/20, and 528/20 nm filters. Excitation of the probe was performed at 440 nm and FRET emissions were read at 528 nm.
Flow Cytometry
Twenty-four hours following transfection, CosE37 cells were trypsinized and analyzed on a Flicyme-320 flow cytometer (Mitsui Engineering & Shipbuilding Co.) equipped with a 408 nm laser diode, a 540/30 YFP filter, and a 494/41 CFP filter. Data was analyzed on the Flicyme Data Station (Mitsui Engineering & Shipbuilding Co.). Where appropriate, cells were treated with 5 µM Imatinib for 10 minutes in suspension prior to the FACS analysis.
Real-time FRET microscopy
CosE37 media was replaced with 2 ml DMEM F-12 media (Gibco) and overlayed with 1.5 ml mineral oil. Real-time FRET microscopy was performed on an IX81 inverted microscope (Olympus) equipped with a laser based autofocusing system (ZDC), a 440AF21 excitation filter (Omega), a 455DRLP Dichroic mirror (Omega), a 535AF26 emission filter (Omega), an automatic XY stage controller (Sigma Koki), a 60x/1.4 oil immersion objective lens (PlanApo), and a CoolSNAP HQ CCD camera (Roper Scientific).
Statistical Analysis
Analysis of a minimum of five cells is recommended when performing time-lapse FRET imaging (21). In order to attain a more accurate measurement of FRET emissions, however, we opted to select 13 – 16 cells. Though, due to the inherent variability among cells in culture, the varying efficiencies of transfection and gene expression, and the unequal exposure of the cells to administered therapeutic agents, the observed responses to tyrosine kinase application differed from cell to cell. In this paper we show data from representative cells of each treatment group, with aggregate inhibition profiles available as supplemental data.
FRET inhibition rate constants
Rate constants for the inhibition of FRET following Imatinib or Dasatinib treatment were calculated as the decrease in FRET over a particular time interval divided by the length of the time interval (r=ΔFRET/ΔT)
Image analysis
Quantitation of immunoblots was performed with GeneTools software (Syngene) while real-time FRET imaging was performed using MetaMorph Software (Roper Scientific).
Results
Application of Picchu FRET for analysis of drug resistance to Bcr-Abl in CML
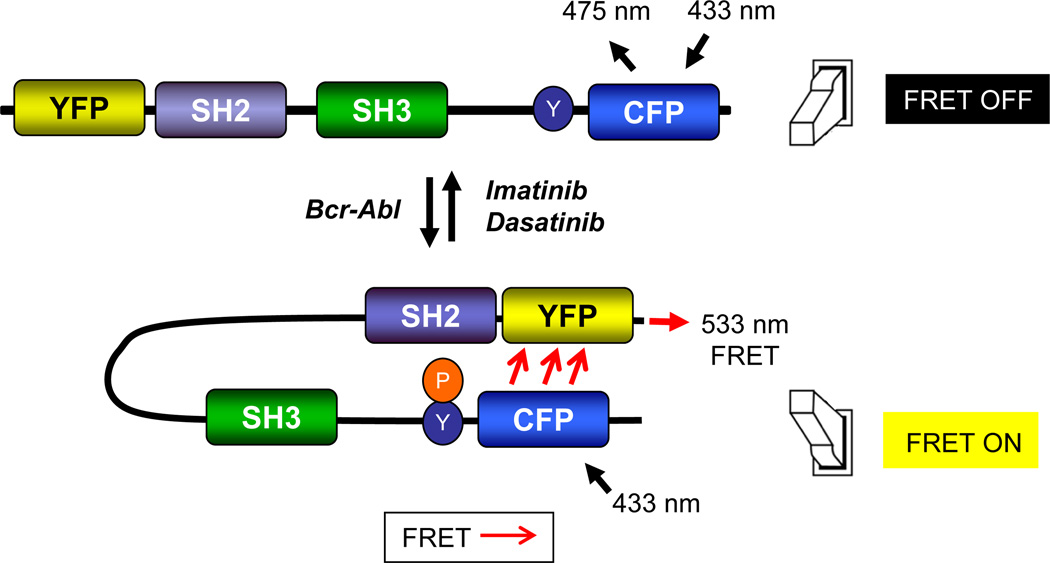
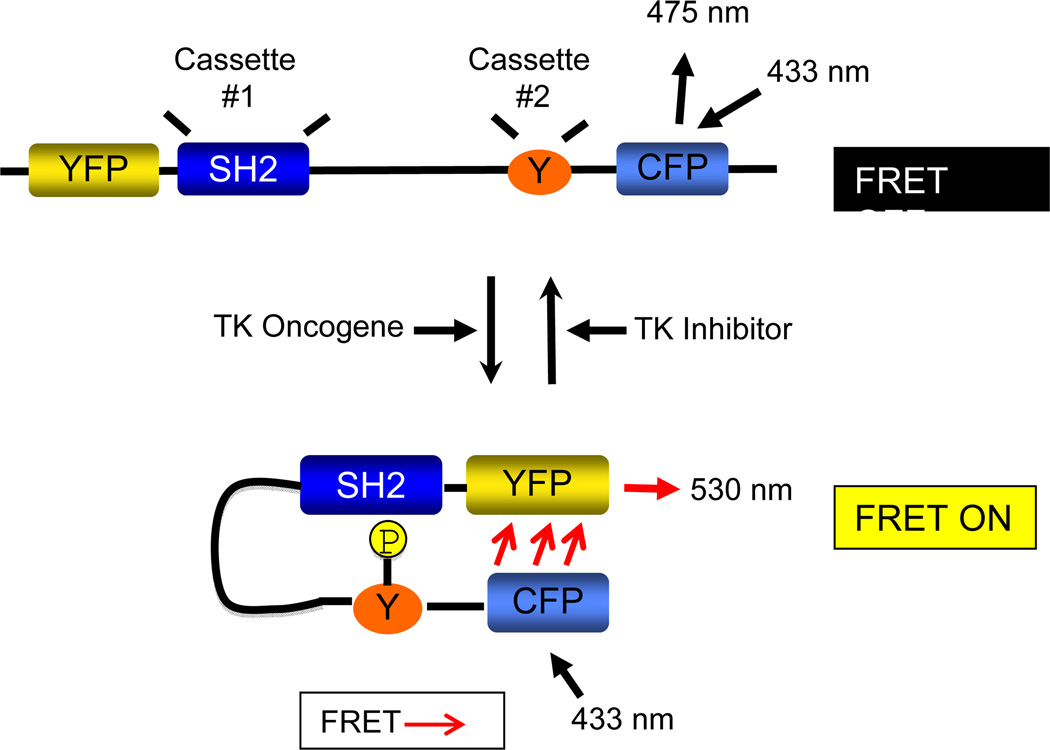
The FRET assay described here exploits the interaction between the Abl tyrosine kinase and the Crk II adaptor protein (22, 23). Following an interaction between Crk and Abl or Bcr-Abl, Crk II is phosphorylated on Tyr221 (Fig. 1), and in so, adopts a closed conformation bringing the N and C-terminal ends together (24), a conformation that has been validated by structural analysis (24, 25). The Picchu probe is similar to the Crk II protein with the addition of an N-terminal YFP (yellow mutant of green fluorescent protein) and a C-terminal CFP (cyan mutant of green fluorescent protein). However, unlike Crk II, Picchu lacks the SH3C following Tyr221 (19), which deletes most of the linker region and SH3C domain and therefore makes the Picchu fusion protein more compact, increasing the FRET efficiency following tyrosine phosphorylation (19). Because phosphorylation of Tyr221 in Picchu by Bcr-Abl also causes an SH2-pTyr intramolecular interaction, allowing the molecule to adopt a closed conformation similar to Crk II (bringing the YFP and CFP within close enough proximity for FRET energy transfer), we reckoned it is well-suited to serve as an in vivo probe to analyze Bcr-Abl activity and inhibition in real time (Fig. 1).
Figure 1. Design of an in vivo FRET assay to monitor Bcr-Abl activity in CML.
The assay is based on the in vivo phosphorylation of the Crk II-derived Picchu biosensor on Tyr(Y)221 by Bcr-Abl. Schematic representation shows that Picchu Y221 phosphorylation by Bcr-Abl induces an intramolecular association between the SH2 domain and pTyr221, bringing the N-terminal and C-terminal ends of the molecule together in a conformation change and yielding an energy transfer from CFP to YFP.
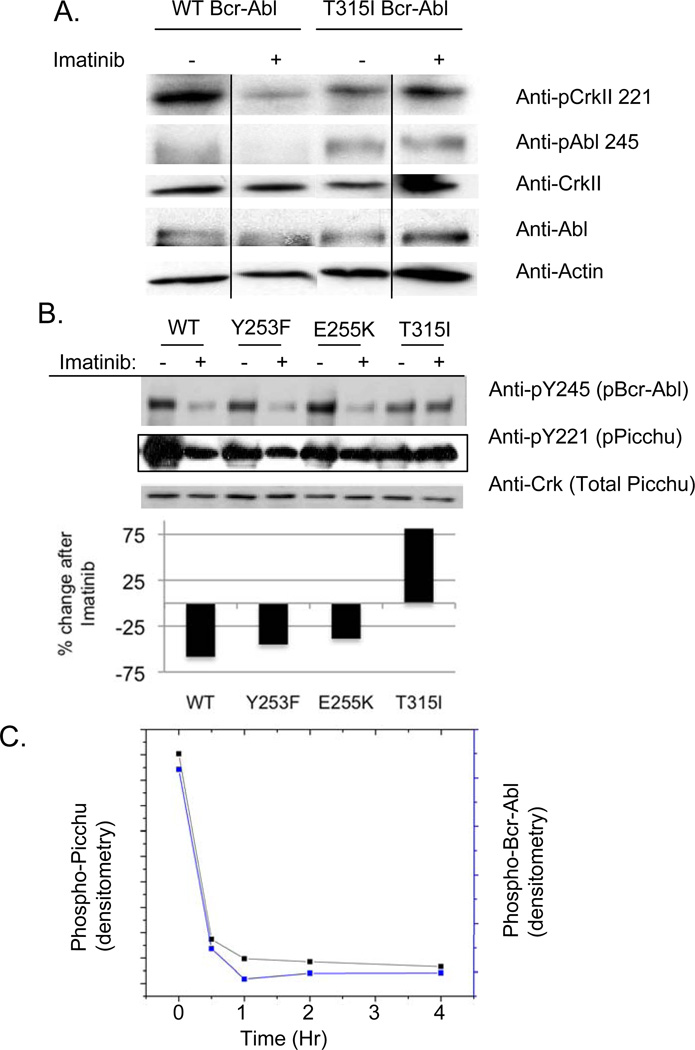
To verify that Crk Tyr221 phosphorylation is representative of Bcr-Abl activity, we analyzed Crk phosphorylation in Bcr-Abl-expressing 32D cells in the presence or absence of Imatinib (Fig. 2A). Tyr221 was highly sensitive to Imatinib in WT Bcr-Abl 32D cells, whereas 32D cells expressing a T315I Imatinib-resistant Bcr-Abl mutant were largely insensitive (lanes 2 versus 4 in Fig. 2A). Similar results were observed in HEK 293T cells expressing clinically relevant Bcr-Abl mutants Y253F, E255K, and T315I and transfected with Picchu (Fig. 2B). Patients with Bcr-Abl T315 mutants fail Imatinib treatment because this substitution near the P-loop structure of the kinase domain blocks drug binding (4). Analogous to the case with Crk, Picchu phosphorylation mirrored the inhibition of the aforementioned mutants as well as the resistance to inhibition of T315I Bcr-Abl (Fig 2B). To extrapolate this effect into a biological assay, a time course assay was performed whereby Picchu and Bcr-Abl were co-expressed in 293T cells, after which, Imatinib was added for 0.5, 1, 2, and 4 hours (Fig. 2C). As shown, Bcr-Abl was inhibited (dephosphorylated on Tyr245) in a time dependent manner, which correlated well with Picchu Tyr221 dephosphorylation. Interestingly, both proteins are dephosphorylated at the earliest time point investigated (30 minutes). This suggests that the effects of tyrosine kinase inhibitors are rapid, which is a pre-requisite for the development of a real-time FRET assay for Bcr-Abl activity. Indeed, as shown below, Picchu-FRET occurs within minutes after Imatinib, suggesting it is well suited for in vivo manipulation. Based on these facts, we posit that Picchu Tyr221phosphorylation is highly labile to dephosphorylation by tyrosine kinase inhibitors and acts as a surrogate marker for Bcr-Abl activity following TKI administration.
Figure 2. Effect of Y221 phosphorylation by Bcr-Abl and lability to Imatinib.
(A) Crk Y221 becomes tyrosine phosphorylated in wild type Bcr-Abl expressing 32D cells (lanes 1 and 2) and T315I Bcr-Abl 32D cells (lanes 3 and 4). Note that Y221 Picchu and Y245 Abl phosphorylation is labile to Imatinib in WT Bcr-Abl cells, but resistant in T315I Bcr-Abl cells (lanes 2 versus 4). As indicated by the vertical lines, lanes 1 and 4 were located on the same gel as lanes 2 and 3 but were not immediately adjacent. (B) Effect of Bcr-Abl tyrosine kinase mutations on Picchu phosphorylation. Detergent lysates were prepared as in panel A, after which equivalent amounts of lysate were immunoblotted with either anti-phosphoY221 Crk (for phosphorylated Picchu in middle panel) or anti-phosphoY245 Abl (top panel). Results demonstrate level of inhibition by 5 µM Imatinib of WT Bcr-Abl and the Y253F, E255K, and T315I mutants. Bars show percent change in Picchu phosphorylation, normalized to expression, following Imatinib. (C) Effect of Imatinib on the tyrosine phosphorylation of Picchu (left axis) and Bcr-Abl (right axis). HEK 293T cells were transiently transfected with Bcr-Abl and Picchu, and after 48 hrs, cells were treated with 5 µM Imatinib for the indicated times. After immunoblotting, gels were scanned and presented as percent of Imatinib untreated.
FRET emission from Picchu reflects activation status of Bcr-Abl and response towards TKIs
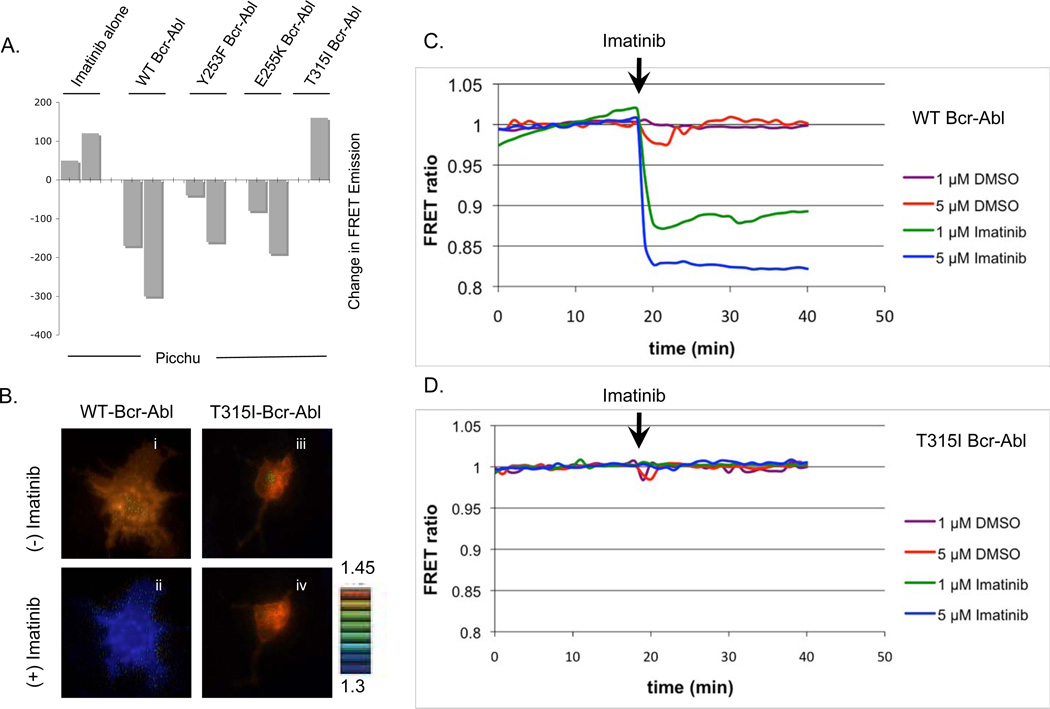
Having established that Bcr-Abl activity correlates with Picchu Tyr221 phosphorylation using immunoblot analysis, we analyzed 530 nm FRET emission from the Picchu probe using a Bio-Tek Synergy HT plate reader to observe if FRET correlated with phosphorylation. As shown in Fig. 3A, total Picchu FRET emission shared a consistent pattern of dephosphorylation after Imatinib treatment. More importantly, when measured against the change in FRET emission with and without Imatinib, we found Bcr-Abl and those mutants responsive to Imatinib showed a significantly decreased FRET (WT, Y253F and E255K), while T315I showed resistance to inhibition in the FRET analysis (Fig. 3A), demonstrating the potential of Picchu-FRET assay to monitor acquisition of drug resistance in vivo.
Figure 3. (A) Effect of Bcr-Abl tyrosine kinase mutations on Picchu FRET.
Phosphorylation of the Picchu probe by Bcr-Abl and the corresponding changes in FRET emissions after Imatinib treatment. Bars correspond to duplicate samples, only one sample is shown for T315I mutant in this experiment. (B) Real time FRET microscopy demonstrating pseudocolor visualization of the change in FRET emission after the addition of 5 µM Imatinib for WT Bcr-Abl (panels i and ii) and the T315I mutant (panels iii and iv). See supplemental real player movie SFig 1 and SFig 2 for real-time kinetics as well as the accompanying supplemental graph, SFig3, depicting FRET tracking for each cell in SFig2. (C and D) Real-time microscopic “tracking” of FRET emissions in CosE37 cells expressing wild type Bcr-Abl (C) and T315I Bcr-Abl (D). DMSO (as control) or Imatinib was added at minute 19. Multi-cell FRET tracking can be found as supplemental data, SFig4.
We next employed FRET microscopy to track FRET emissions in real-time (Fig. 3B–D). Application of Imatinib resulted in a rapid and dose-dependent decrease in the FRET ratio (YFP/CFP) in CosE37 cells expressing WT Bcr-Abl (Fig 3C, SFig4), but not in CosE37 cells expressing T315I Bcr-Abl (Fig. 3D, SFig4). Fig. 3B shows the utility of Picchu FRET in real-time microscopy. Note that addition of Imatinib suppressed WT Bcr-Abl activity (panel i versus ii), but was resistant in cells expressing T315I Bcr-Abl (panel iii versus iv). Such an approach has the potential for application in rapid and sensitive screening of novel drug candidates that inhibit T315I Bcr-Abl in vivo (see supplemental real player movie for in vivo analysis, SFig1, as well as dose-response data, SFig2. Also see SFig3 for the tracking of FRET emissions for each cell in SFig2).
Flow cytometric demonstration of FRET probe utility
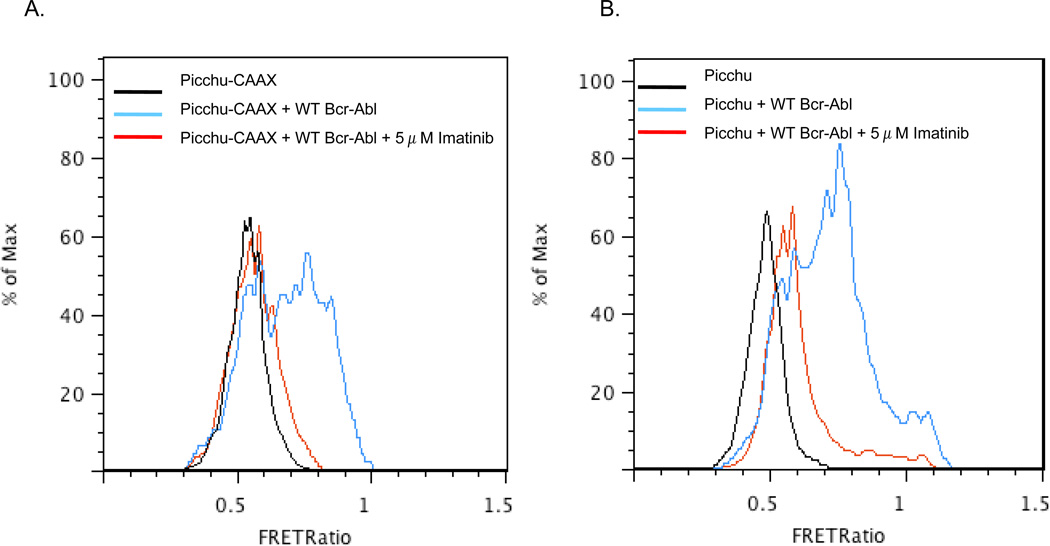
To demonstrate the potential for utilizing Picchu-FRET in analyzing large numbers of cells, for example to monitor drug resistance in a mixed population clinical sample from bone marrow or peripheral blood, we performed flow cytometric studies on CosE37 cells transfected with wild type Bcr-Abl and either a membrane targeted (Picchu-CAAX) or untargeted (Picchu) FRET probe (Fig. 4). Though membrane localization of the probe did appear to produce a more specific response, perhaps due to less background as compared to the widely distributed Picchu probe, with either biosensor both the increased FRET ratio in active Bcr-Abl expressing cells as well as the decrease in the ratio following Imatinib treatment were rapidly detectable, suggesting it should be feasible to collect rare populations of drug-resistant cells prior to complete leukemic relapse.
Figure 4. Analysis of ΔFRET ratios by FACS.
Flow cytometric analysis indicating the FRET YFP/CFP ratio in populations of cells with Picchu-CAAX alone (black), with Picchu-CAAX and Bcr-Abl (blue), and with Picchu-CAAX + Bcr-Abl followed by 5 µM Imatinib treatment (red). In panel B, a soluble form of Picchu was used while in panel A, a membrane targeted CAAX-fused version of Picchu was used. Note that both Picchu isoforms are highly labile to Imatinib.
Pharmacodynamic responses of Picchu FRET to Imatinib and Dasatinib
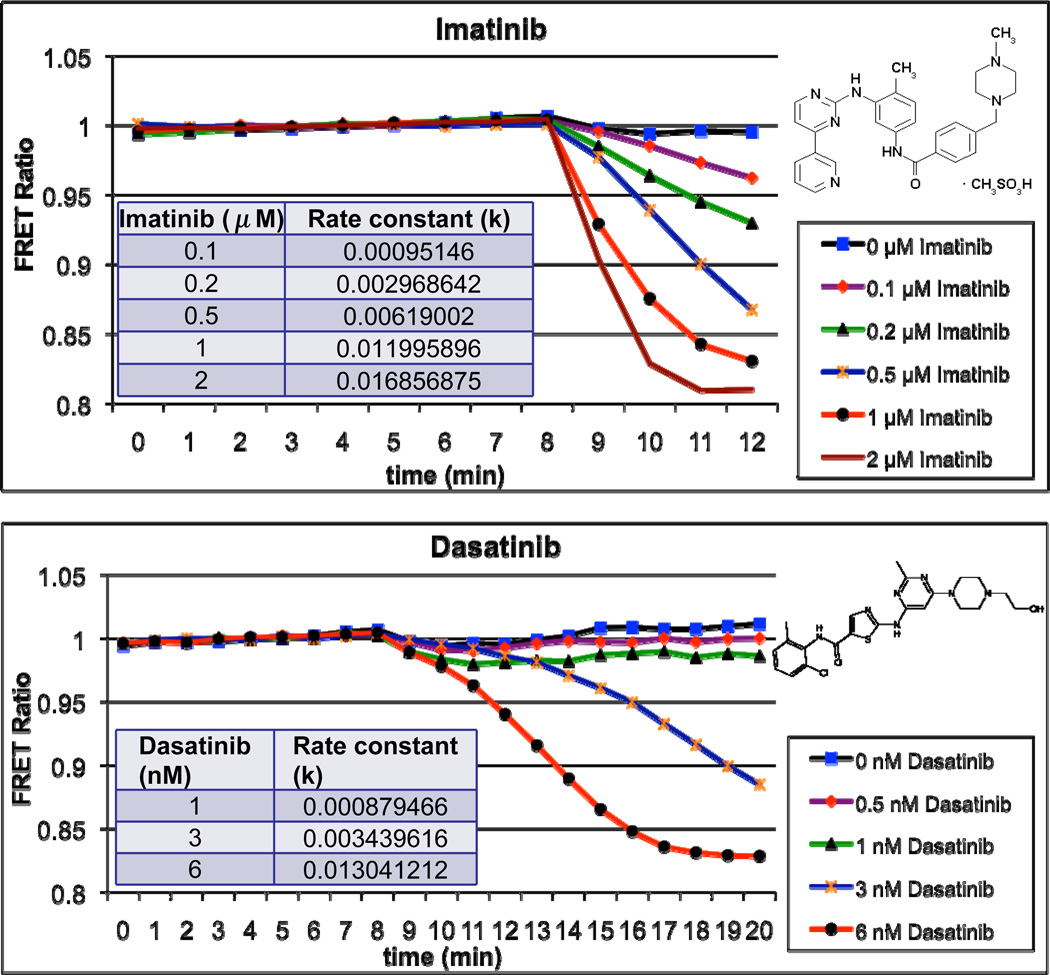
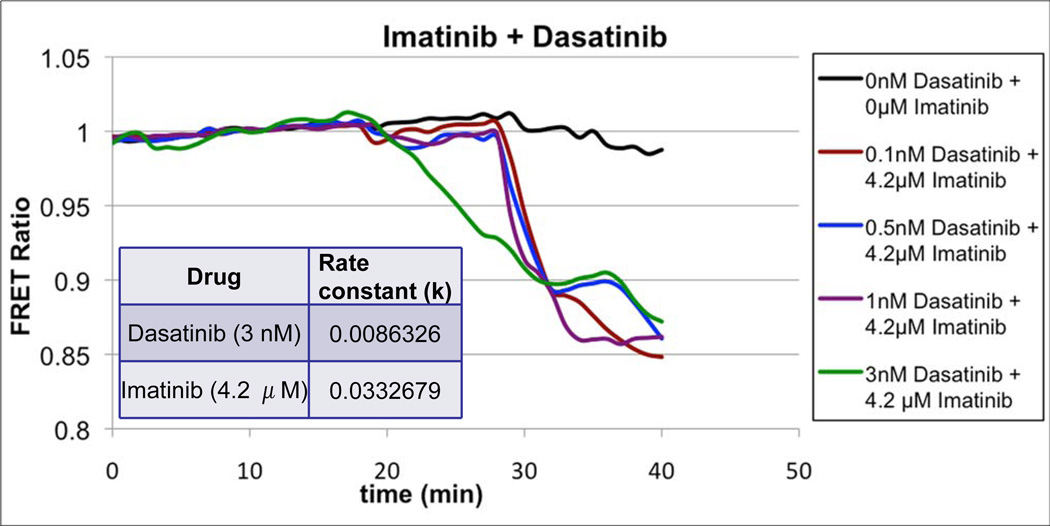
Having established the utility of Picchu as a bioprobe for imaging drug resistance in CML at both the light microscopical level and by FACS staining, we next wanted to evaluate if we could compare sensitivities to different tyrosine kinase inhibitors (TKIs) used clinically, namely Dasatinib a 2-amino-thiazole-5-carboxamide, and Imatinib. Dasatinib and Imatinib have distinct bioavailability in vivo, with Dasatinib being 300-fold more potent than Imatinib in suppressing un-mutated Bcr-Abl, at least partly owing to the fact that Imatinib binds to the inactive configuration of Abl, blocking transition to the active configuration (26, 27), while Dasatinib binds to both the active and inactive P-loop configurations (9). Interestingly, these sensitivity differences were clearly reflected in the responses of cells to TKIs in the Picchu FRET assay (Fig. 5). Hence, not only did FRET depression following Bcr-Abl inhibition by both Imatinib and Dasatinib display striking dose-dependency in vivo (µM for Imatinib and nM for Dasatinib), but the rate constants for inhibition were also characteristically different. This suggests it is possible to identify beneficial synergistic interactions of TKIs with respect to both the level and the duration of kinase inhibition, thus taking advantage of the differences between various drugs in terms of bioavailability and mechanisms of inhibition. Naturally, the corollary to this notion is the utilization of this assay to identify compounds that should not be used in combination, by virtue of their competitive nature. Indeed, after the initial inhibition of Bcr-Abl and concomitant FRET depression following 3 nM Dasatinib treatment in Figure 6, the subsequent application of 4.2 µM Imatinib results in an initial plateau followed by the resumption of kinase suppression, suggesting possible competition between the two compounds, either in binding to Bcr-Abl or in cellular uptake, thus allowing for a momentary reactivation of the kinase.
Figure 5. Real-time microscopic tracking of dose-dependent inhibition of wild type Bcr-Abl by Imatinib (top) and Dasatinib (bottom).
CosE37 cells were transfected to co-express Picchu-CAAX and wild type Bcr-Abl as described and Imatinib (top) or Dasatinib (bottom) was applied at minute 9. Rate constants for each concentration of the kinase inhibitor are provided in the tables.
Figure 6.
Demonstration of the utility of Picchu to detect additive or competitive effects of different therapeutics. CosE37 cells were transfected to co-express Picchu-CAAX and wild type Bcr-Abl as described and Dasatinib, of the indicated concentrations, was added at 19 minutes and Imatinib was added at minute 29.
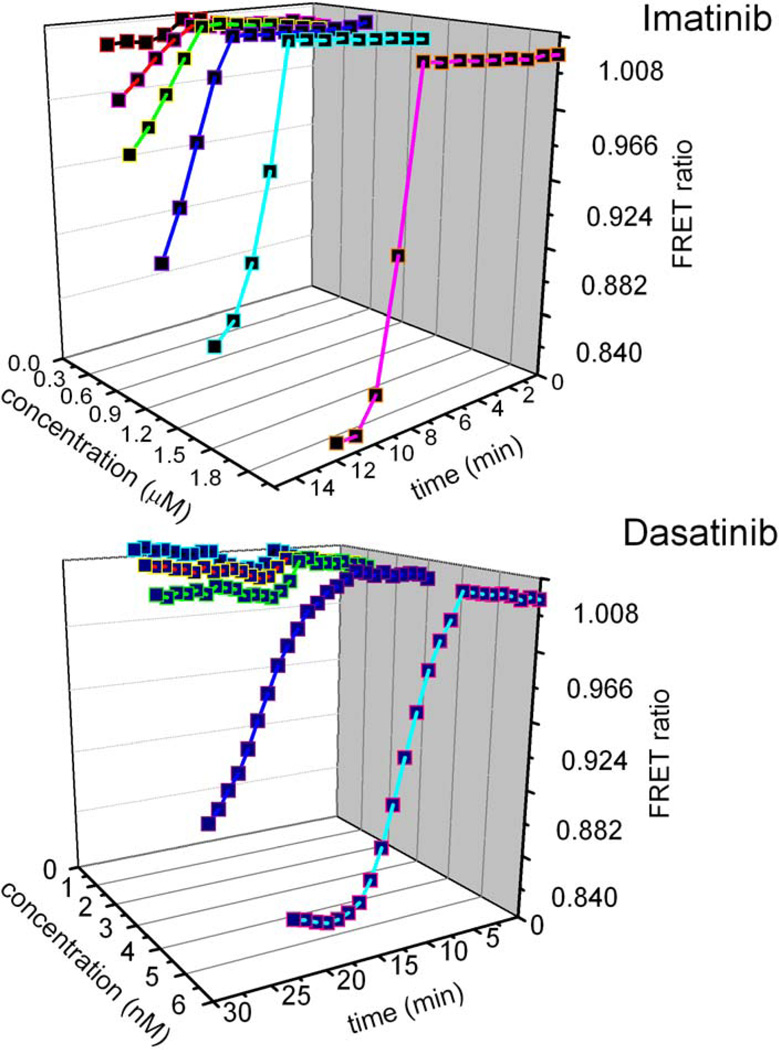
To further validate the Picchu FRET bioprobe as a potential pharmaceutical tool to be used in the development of next generation tyrosine kinase inhibitors, we constructed two examples of three-dimensional Bcr-Abl inhibition profiles (Figure 7). This approach allows for the observation of the dynamic inhibition of Bcr-Abl in a time dependent manner following administration of an array of kinase inhibitors in various concentrations. Such a profile can be created for the inhibition of each clinically relevant Bcr-Abl mutant by every currently available TKI providing the unprecedented ability to rapidly identify the Bcr-Abl mutant(s) driving a patient’s leukemia and administer the kinase inhibitor, or combination of inhibitors, that will push the patient into remission with the greatest potency, longest duration, lowest drug concentration, and fewest side-effects. In this manner, the treatment of CML may be converted from the current “one size fits all” modality to a significantly more tailored management of the patient.
Figure 7. Pharmaceutical profiling of Bcr-Abl inhibition by TKIs.
Two examples of the potential utility of Picchu FRET in pharmaceutical profiling of clinically relevant Bcr-Abl mutations as to their efficient inhibition by therapeutics of varying concentrations. Inhibition is depicted as a function of TKI concentration (left), time (bottom) and ΔFRET ratios (right) for Imatinib (top) and Dasatinib (bottom) to provide a pharmaceutical signature for Bcr-Abl proteins. These data can be extrapolated to select the optimum therapeutic, or combination thereof, for different Bcr-Abl mutations for the selection of TKIs with the most desirable kinase inhibition profiles.
Discussion
The advent of Imatinib mesylateTM over a decade ago was a breakthrough in cancer therapy and resulted in a paradigm shift with respect to molecular targeting of oncogenes. Its remarkable success in clinical trials resulted in fast-track approval by the Food and Drug Administration and Imatinib continues to be frontline therapy today (11). Despite the fact that five-year survival for patients on Imatinib is currently 95 percent, patient relapse and drug resistance continues to make the treatment of CML difficult (12, 28). Often, individuals who have become refractory to a certain tyrosine kinase inhibitor are switched to another and though this approach benefits some patients in the short term, it is not a complete cure, and in particular, those individuals harboring mutations in critical residues such as T315 are untreatable by any means currently available. However, recent investigations have identified potential combinations of kinase inhibitors not only in the case of T315I Bcr-Abl, but also with regard to the resistance inducing efflux of therapeutic compounds by the MDR1 transporter (29, 30). Such findings give credence to the potential for identifying synergistic combinations of therapeutic agents and the utility of the described FRET assay in rapidly and sensitively making such determinations.
The frequent occurrence of drug resistance in oncogene-targeted therapy, in combination with the development of robust second-generation TKI inhibitors, underscores the importance for rapid and selective screening of drug resistance in vivo. Moreover, while Bcr-Abl inhibitors initially induce moderate-term remission, it would be of clinical value to begin second-line TKI therapeutics when resistance first emerges in a few re-populating cells. Because the Picchu FRET assay described here can be adapted for FACS analysis and FACS sorting through a combination of surface marker labeling and detection of Bcr-Abl activity in vivo, the assay can be utilized to identify rare leukemic cell lines, and to indicate their inhibition by or resistance to the applied drug therapy. Such an assay could conceivably be used to analyze patient samples to diagnose CML, confirm Bcr-Abl activity after a diagnosis of CML is suspected, detect relapse in CML patients already receiving treatment, or to determine the therapeutic or combination of therapeutics that would most benefit the patient after their Bcr-Abl mutation has been identified. In this regard, a FRET assay has the added benefit of not only identifying Bcr-Abl but in providing information on its activity – in contrast to currently available diagnostic tools, such as nested PCR, which, although sensitive, simply identify the Bcr-Abl fusion oncogene but provide no useful information with regard to its activation status, a significant shortcoming considering the oncogene has been detected at low levels in healthy individuals (20).
A second utility of Picchu FRET arises from its strong potential as a highly effective pharmaceutical tool that can be used to rapidly screen and identify novel small molecules able to inhibit Bcr-Abl in vivo at lower doses and even candidates that are able to inhibit T315I Bcr-Abl and other such detrimental mutations. Furthermore, this assay may also lend itself to application in the determination of the cell type specificity of a particular CML case through dual labeling for cell surface markers, which may lead to more targeted treatments based on the lineage of the leukemic cells in question. Additionally, the distinct differences in the response of WT Bcr-Abl to Imatinib and Dasatinib as measured by the FRET emissions suggests that Picchu FRET may also be able to reflect the inherent pharmacokinetic variations between therapeutics based on parameters such as mechanism of action, for example dual Src/Abl kinase inhibition by Dasatinib (31), and their differing uptake and efflux profiles. Though numerous uses can be developed for such an assay, it should be stressed that the sensitive and, perhaps more important, in vivo nature of this assay creates certain possibilities and provides a degree of utility that has not been realized with other techniques used to date.
By examining changes in FRET ratios as a function of Imatinib concentration, we estimate that the half-life of Tyr221 phosphorylation was ~3 minutes following Bcr-Abl inhibition. This is quite interesting at a mechanistic level, since it predicts existence of a potent Tyr221 phosphatase that could be identified following a genome-wide shRNA screen against tyrosine phosphatases. As such, we predict that knockdown of a Crk PTPase would significantly delay or prevent the change in FRET ratio. Finally, based on the promising preliminary results of the Picchu probe, we propose such a strategy may have a more generic utility to assess additional tyrosine kinases and their accompanying drug resistance in human cancer. Therefore, it should be feasible to adopt a general strategy to develop a platform of FRET probes that extrapolate the basic paradigm for Picchu. Specifically, the backbone of Picchu would be modified with two cassettes in place of (i) pTyr221 and (ii) the Crk SH2 domain. Accordingly, for each tyrosine kinase to be examined, an optimal phosphorylation motif could be inserted in place of pTyr221 (cassette #2), and an optimal SH2 that associates with the pTyr could be inserted in place of the Crk SH2 domain (cassette #1) (Fig. 8). As an example, the PVPEYINQSVPKRK peptide found in the C-terminus of EGFR containing tyrosine 1068 that is autophosphorylated by the kinase upon EGF stimulation (32) could be substituted for the Tyr221 region of Picchu. Likewise, substitution of the original SH2 domain of Picchu by the SH2 domain of Grb2, which binds to Tyr1068 of EGFR upon its phosphorylation (32), could conceivably produce a dynamic probe for the real-time activity and inhibition of the EGFR kinase, which has been implicated in numerous cancers.
Figure 8. Schematic representation of a generic FRET based biosensor for tyrosine kinases and their inhibitors in human cancers.
Adapted from Picchu, a platform of tyrosine kinase specific FRET cassettes can be generated by replacing pTyr221 and Crk SH2 domain as indicated.
With the recent advancements in rapid profiling of disease states both at the genomic and proteomic levels coupled with the prospective widespread genetic blueprinting of patients and their risk factors, modern medicine is undergoing a paradigm shift from the conventional generalized approach of treatment based on stratification by disease states, to a more custom-fitted and individual-centered modus operandi. In the same vein, with the introduction of oncogene-directed cancer therapy by the advent of Imatinib mesylate, and the extensive list of resistance-conferring Bcr-Abl kinase mutations, we are beginning to realize the possibility of tailor-made therapeutics in the treatment of chronic myelogenous leukemia. The real-time FRET assay, as presented in this report, may be an invaluable factor during this transition and through its versatility and multi-faceted utility as both a clinical tool and as an instrument of rapid real-time therapeutic development, could provide the necessary technology to push through the event horizon into an age of patient-centered medicine.
Supplementary Material
Acknowledgements
We gratefully thank Dr. Etsuko Kiyokawa for assistance with real-time FRET and Yuka Kumagai for instruction in performing the Flicyme flow cytometry experiments. This work was in part supported by NIH grant RO1GM080308, as well as NJCCR pre-doctoral fellowship 08-1093-CCR-EO and by a Kyoto University Global Centers of Excellence international travel grant to AT.
Funding: This work was supported in part by NIH grant RO1GM080308, as well as NJCCR pre-doctoral fellowship 08-1093-CCR-EO and by a Kyoto University Global Centers of Excellence international travel grant to AT.
Abbreviations
- FRET
Fluorescent Resonance Energy Transfer
- CML
Chronic Myelogenous Leukemia
- Picchu
Phosphorylation indicator of Crk II chimeric unit
- TKI
Tyrosine kinase inhibitor
- PCR
Polymerase chain reaction
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. 2009 [Google Scholar]
- 2.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005 Mar;5(3):172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 3.Besa Emmanuel C UW. Chronic Myelogenous Leukemia. 2009 Feb 6; [cited; Available from: http://emedicine.medscape.com/article/199425-overview. [Google Scholar]
- 4.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002 Aug;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 5.Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med. 2003 May 20;138(10):819–830. doi: 10.7326/0003-4819-138-10-200305200-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006 Jul 1;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maekawa T, Ashihara E, Kimura S. The Bcr-Abl tyrosine kinase inhibitor imatinib and promising new agents against Philadelphia chromosome-positive leukemias. Int J Clin Oncol. 2007 Oct;12(5):327–340. doi: 10.1007/s10147-007-0699-1. [DOI] [PubMed] [Google Scholar]
- 8.Mauro MJ, Druker BJ. STI571: a gene product-targeted therapy for leukemia. Curr Oncol Rep. 2001 May;3(3):223–227. doi: 10.1007/s11912-001-0054-z. [DOI] [PubMed] [Google Scholar]
- 9.Kimura S, Ashihara E, Maekawa T. New tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Curr Pharm Biotechnol. 2006 Oct;7(5):371–379. doi: 10.2174/138920106778521532. [DOI] [PubMed] [Google Scholar]
- 10.Tokarski JS, Newitt JA, Chang CY, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006 Jun 1;66(11):5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, O'Brien SG, Cortes J, Radich J. Chronic myelogenous leukemia. Hematology Am Soc Hematol Educ Program. 2002:111–135. doi: 10.1182/asheducation-2002.1.111. [DOI] [PubMed] [Google Scholar]
- 12.O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007 Oct 1;110(7):2242–2249. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 13.Azam M, Daley GQ. Anticipating clinical resistance to target-directed agents : the BCR-ABL paradigm. Mol Diagn Ther. 2006;10(2):67–76. doi: 10.1007/BF03256446. [DOI] [PubMed] [Google Scholar]
- 14.Giles FJ, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman SJ. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007 Jan 15;109(2):500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 15.Gontarewicz A, Balabanov S, Keller G, et al. Simultaneous targeting of Aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008 Apr 15;111(8):4355–4364. doi: 10.1182/blood-2007-09-113175. [DOI] [PubMed] [Google Scholar]
- 16.Swords R, Alvarado Y, Giles F. Novel Abl kinase inhibitors in chronic myeloid leukemia in blastic phase and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Lymphoma Myeloma. 2007 Mar;7 Suppl 3:S113–S119. doi: 10.3816/clm.2007.s.011. [DOI] [PubMed] [Google Scholar]
- 17.Tauchi T, Ohyashiki K. The second generation of BCR-ABL tyrosine kinase inhibitors. Int J Hematol. 2006 May;83(4):294–300. doi: 10.1532/IJH97.06025. [DOI] [PubMed] [Google Scholar]
- 18.Jarkowski A, Sweeney RP. Nilotinib: a new tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Pharmacotherapy. 2008 Nov;28(11):1374–1382. doi: 10.1592/phco.28.11.1374. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa K, Mochizuki N, Ohba Y, Mizuno H, Miyawaki A, Matsuda M. A pair of fluorescent resonance energy transfer-based probes for tyrosine phosphorylation of the CrkII adaptor protein in vivo. J Biol Chem. 2001 Aug 17;276(33):31305–31310. doi: 10.1074/jbc.M104341200. [DOI] [PubMed] [Google Scholar]
- 20.Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995 Oct 15;86(8):3118–3122. [PubMed] [Google Scholar]
- 21.Aoki K, Matsuda M. Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat Protoc. 2009;4(11):1623–1631. doi: 10.1038/nprot.2009.175. [DOI] [PubMed] [Google Scholar]
- 22.Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994 May 15;13(10):2341–2351. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren R, Ye ZS, Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994 Apr 1;8(7):783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- 24.Rosen MK, Yamazaki T, Gish GD, Kay CM, Pawson T, Kay LE. Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature. 1995 Mar 30;374(6521):477–479. doi: 10.1038/374477a0. [DOI] [PubMed] [Google Scholar]
- 25.Kobashigawa Y, Sakai M, Naito M, et al. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007 Jun;14(6):503–510. doi: 10.1038/nsmb1241. [DOI] [PubMed] [Google Scholar]
- 26.Nagar B, Bornmann WG, Pellicena P, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002 Aug 1;62(15):4236–4243. [PubMed] [Google Scholar]
- 27.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000 Sep 15;289(5486):1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 28.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006 Dec 7;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg E, Deng X, Choi HG, et al. Beneficial effects of combining a type II ATP competitive inhibitor with an allosteric competitive inhibitor of BCR-ABL for the treatment of imatinib-sensitive and imatinib-resistant CML. Leukemia. Jul;24(7):1375–1378. doi: 10.1038/leu.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiwase DK, White D, Zrim S, Saunders V, Melo JV, Hughes TP. Nilotinib-mediated inhibition of ABCB1 increases intracellular concentration of dasatinib in CML cells: implications for combination TKI therapy. Leukemia. Mar;24(3):658–660. doi: 10.1038/leu.2009.242. [DOI] [PubMed] [Google Scholar]
- 31.Schenone S, Brullo C, Musumeci F, Botta M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin Investig Drugs. Aug;19(8):931–945. doi: 10.1517/13543784.2010.499898. [DOI] [PubMed] [Google Scholar]
- 32.Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994 Aug;14(8):5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.