Abstract
Larvae of the sawfly Tenthredo zonula are specialized on Hypericum. Whether the sawfly is able to sequester plant metabolites was unknown. Aerial materials of Hypericum perforatum and H. hirsutum, as well as dissected larvae and prepupae of T. zonula, were analyzed by HPLC to determine the presence and content of flavonoid glycosides (rutin, hyperoside, isoquercitrin, and quercitrin) and naphthodianthrones (pseudohypericin and hypericin). All flavonoid glycosides were detected in both Hypericum species, with hyperoside as major compound in H. perforatum (ca. 1.7 μmol/g fresh weight, FW) and isoquercitrin in H. hirsutum (0.7 μmol/g FW). Naphthodianthrones were present at low concentrations (0.02 μmol/g FW) in the former, and almost undetected in the latter species. In the body parts (i.e., hemolymph, digestive tract, salivary glands, or miscellaneous organs) of T. zonula, the surveyed compounds were detected more frequently in prepupae than in larvae. The compounds were not present in every sample, and flavonoid glycosides especially occurred in highly variable amounts, with maximal concentrations of 41 μg rutin/prepupa in salivary glands, 8 μg hyperoside/prepupa in hemolymph (= 0.36 μmol/g FW), 32 μg isoquercitrin/prepupa in salivary glands, and 63 μg quercitrin/larva in miscellaneous organs (mainly composed of the integument). We conclude that flavonoid glycosides are sequestered since they were detected in organs other than the digestive tract of larvae, and because prepupae are a non-feeding stage. The naphthodianthrone pseudohypericin, but not hypericin, occurred generally in the digestive tract (up to 0.25 μg/larva). Both naphthodianthrones and related unidentified compounds, but not flavonoid glycosides, were found in the larval excrement. The highly variable distributions of flavonoid glycosides and naphthodianthrones in T. zonula larvae and prepupae make it difficult to determine the ecological significance of these metabolites.
Keywords: Hypericum, St. John’s wort, Tenthredo zonula, Sawfly larva, Hemolymph, Flavonoid glycosides, Naphthodianthrones, Hypericin, Sequestration, Hymenoptera, Symphyta, Phytotoxicity, Chemical defense
Introduction
Sequestration is a physiological adaptation that enables ingested secondary metabolites to be selectively stored and accumulated in the body (Duffey, 1980; Opitz and Müller, 2009). It occurs in most insect groups although it is considered a relatively rare phenomenon (Whitman et al., 1990; Rowell-Rahier and Pasteels, 1992). Most families of sawflies (Hymenoptera, Symphyta) contain species with larvae that are able to sequester plant secondary compounds. Larvae belonging to the families Diprionidae and Pergidae sequester the terpenes from resinous tree needles and Eucalyptus leaves, respectively, in an esophageal diverticulum (Eisner et al., 1974; Schmidt et al., 2010). In the Tenthredinidae, sequestration is known for all studied species of the genus Athalia (Müller et al., 2001; Opitz et al., 2010) and species of the tribe Phymatocerini (Schaffner et al., 1994; Boevé and Schaffner, 2003; Prieto et al., 2007). All sawflies sequestering plant secondary metabolites are considered specialists in that they feed on a narrow set of plant species belonging to one genus or family. A restricted diet is also typical for sawflies taken as a whole, with the exception of members of the subfamily Tenthredininae (Tenthredinidae), which includes both specialists and generalists (Taeger et al., 1998; Lacourt, 1999). Little is known about the sequestration ability of Tenthredininae larvae. The iridoid glycoside catalpol was detected in total extracts of larvae, prepupae and exuviae of Tenthredo grandis (Tenthredininae), a specialist on Chelone glabra (Scrophulariaceae) (Bowers et al., 1993). In the current study, we analyzed several organs from immature stages of Tenthredo zonula Klug, a specialist on common St. John’s wort Hypericum perforatum L. (section Hypericum, Hypericaceae). A field population of T. zonula was found on common H. perforatum, but also on hairy St. John’s wort (Hypericum hirsutum L., section Taeniocarpium). To our knowledge, this represents the first observation that the latter species also serves as a host plant for T. zonula.
The contemporary use of H. perforatum is as an alternative herbal treatment for mild to moderate depression in North America and Europe. This has resulted in its increased cultivation in several countries (e.g., Canada, the United States, Germany, Poland, France) and collection from the wild in others (e.g., Romania, Serbia, Hungary). The naphthodianthrones hypericin and pseudohypericin, which have been isolated only from certain species of Hypericum, along with flavonoid glycosides such as rutin, hyperoside, isoquercitrin, and quercitrin, may contribute to the bioactivity of the extracts (Nahrstedt and Butterweck, 2010). Flavonoid glycosides are found in both leaf and floral tissues of Hypericum species, and make up 2-4% of the dry weight (Hölzl and Petersen, 2003). In Hypericum, exudate-containing glands appear as lines or dots, and are of two types: translucent (pale yellow to amber in color) and dark (red to nearly black), found variously on the stems, leaves, sepals, petals, and the anther connective (Robson, 2002). Anatomical and histochemical studies of H. perforatum have shown that the translucent glands are actually subepidermal cavities, lined with two layers of flattened, thin-walled secretory cells (Ciccarelli et al., 2001a). Evidence suggests that essential oil components and acylphloroglucinol derivatives (i.e., hyperforin) are biosynthesized in these secretory cells and exuded into the cavities (Soelberg et al., 2007). Dark glands (more correctly called “nodules”), meanwhile, are specialized clusters of cells containing wax and the intensely red naphthodianthrone pigments hypericin and pseudohypericin (Ciccarelli et al., 2001b). The concentrations of the naphthodianthrones vary according to the species, but generally range from 0.1-0.3% of the dry tissue weight (Hölzl and Petersen, 2003). Hypericum perforatum plants have black nodules scattered on the leaf, petal, and sepal surface, within the leaf, petal and sometimes sepal margin, and on the stamen connective tissue (Robson, 2002). On H. hirsutum plants, reddish nodules appear occasionally on the stems; and black nodules are scattered on the leaf and petal surface, as well as within the sepal and petal margin. Those on the sepal margin in this species can be either sessile or on cilia, while in H. perforatum, they are always sessile (Robson, 2002, 2010).
The localization of the naphthodianthrones in tissues of Hypericum is of interest because these compounds can cause phototoxic effects in grazing insects and mammals that feed on the plants (Knox and Dodge, 1985a). These compounds are photoreactive and when activated by light they catalyze the formation of singlet oxygen, which has damaging effects on enzymes (i.e., inactivation), cell membranes (i.e., disruption through lipid peroxidation) and DNA tertiary structure (Knox and Dodge, 1985b; Berenbaum, 1987). Insects such as leaf beetles and moth caterpillars that feed on Hypericum possess behavioral and physiological adaptations that prevent these damaging effects (Larson, 1986; Sandberg and Berenbaum, 1989; Aucoin et al., 1990, 1991, 1995; Duffey and Pasteels, 1993; Guillet et al., 2000).
We observed larvae of T. zonula feeding on H. perforatum and H. hirsutum, actively consuming leaf tissues containing both translucent glands and dark nodules. We were interested in exploring whether this species sequesters the bioactive metabolites from its host-plant, and if so, in which organs and what amounts. Furthermore, we proposed to compare the physiology and ecology of T. zonula with that of other insects that feed on Hypericum. We determined the amounts of specific flavonoid glycosides (rutin, hyperoside, isoquercitrin, and quercitrin), as well as the naphthodianthrones hypericin and pseudohypericin, in H. perforatum and H. hirsutum, and analyzed the distribution of these compounds in several organs of larvae and prepupae of T. zonula.
Methods and Materials
Plant and Insect Material
A population of T. zonula was found in the field (Dinant, Belgium; July 2007) feeding on sympatric H. perforatum and H. hirsutum plants, which grew in monospecific or mixed clusters on the logged edge of a forest. All plant and insect samples used were collected in this location and were identified by using the taxonomic keys published in Robson (2002, 2010) and Lorenz and Kraus (1957), respectively.
Ten aerial branches of H. perforatum and six of H. hirsutum were collected in the field (on July 05, 2007), and individually marked. The cut branches were placed in water, while one additional plant of each species was transplanted to a pot. All plants were brought to the laboratory and maintained at 10°C and exposed to natural daylight conditions. For chemical analysis, 5–10 cm long twigs (comprising leaves and flowers) were taken from each of the 10 H. perforatum and 6 H. hirsutum branches on three dates (July 6th, 13th, and 20th) as well as from the 2 potted plants. Twigs were dried in the dark to a moisture content of less than 2% and stored at room temperature until extraction was conducted. We established that 100 mg of dried plant material were equivalent to 1 g of fresh plant material (1:10 w/w). Voucher specimens of the plants are deposited at the Institute for Pharmaceutical Sciences (Karl-Franzens-University, Graz, Austria).
Tenthredo zonula larvae (Fig. 1a) were collected in the field by gently beating plant clusters above a square net placed horizontally on the ground. Larvae were approximately four times more numerous on H. perforatum than on H. hirstutum. The density of the larvae on the plants was variable: ca. 50-70% of plant clusters had 1–3 larvae, while the others had none. A few prepupae were collected as well, which indicates that this stage (Fig. 1b) remains without feeding on the plant for a period of time, before pupation in the soil. Most prepupae used in this study, however, were obtained by rearing the field-collected larvae (Fig. 1c). The general feeding behavior of the sawflies was observed while larvae were reared for 2–6 d on leaves taken from plant material from both cut aerial branches and potted plants (see above) until the last larval instar, or prepupal stage. For chemical analyses, 4 to 7 individuals were pooled when they reached either the last or penultimate larval instar, or prepupal stage, whereas excrement from more numerous larvae, at several instars, was combined as one sample. Hemolymph droplets were collected in glass capillaries by gently piercing the integument with forceps, and were deposited in vials after measuring the volumes. Average amounts of hemolymph per individual were 8 μl for larvae and 7 μl for prepupae, but the maximal amount collected from an individual (i.e., 13 μl and 11 μl, respectively) was used to calculate their amounts in μg/individual; values in μmol/g fresh weight (FW) were obtained by considering 1 μl hemolymph as equivalent to 1 mg FW. The individuals were frozen prior to the dissection, at 4°C, of several organs: the digestive tract (its anterior and median parts); the salivary glands; and miscellaneous organs (i.e., integument, muscles, and fat body). The salivary glands (Fig. 1d) were sometimes absent or poorly developed and were discarded. The prepupa is a non-feeding stage, so its digestive tract is empty and was not collected. All samples were immediately stored in the dark, at −20 or −30°C until chemical analysis. Voucher specimens of sawfly immature stages are deposited at the Royal Belgian Institute of Natural Sciences (Brussels, Belgium).
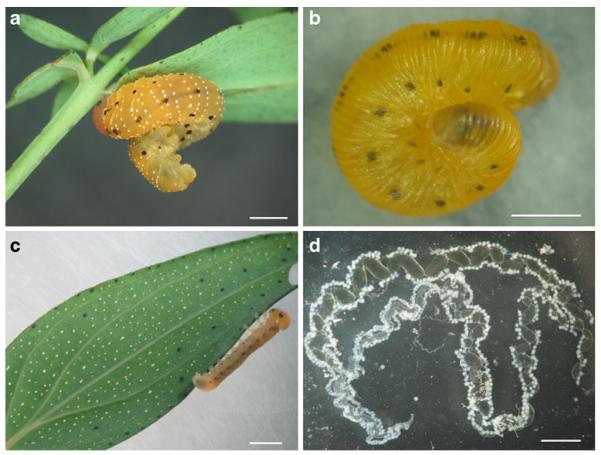
Fig. 1.
Sawfly Tenthredo zonula. a Last instar larva attached to the underside of a leaf of Hypericum perforatum; when not feeding, the larva maintains its abdomen typically curled. b Prepupal stage; a prepupa never feeds. c Young larva on a leaf of H. perforatum where translucent and dark glands are visible on its underside. d Dissected salivary glands from a prepupa. Bar=2 mm. Photographed by J-L Boevé
HPLC Analysis
Ammonium acetate and glacial acetic acid, as well as acetonitrile (ACN) and methanol (MeOH), were of HPLC grade and purchased from Carl Roth GmbH (Karlsruhe, Germany). Dried plant material was ground to a fine powder and 250 mg of the powder were extracted four times with 2.5 ml aliquots of MeOH with sonication for 10 min. After 5 min centrifugation at 792 × g, the extracts were combined in a 10-ml volumetric flask and filled up to the final volume with MeOH. A 1 ml subsample of this solution was filtered through a 0.45 μm PTFE filter (Carl Roth GmbH), dried under nitrogen, and stored in an amber glass vial at −20°C until analysis, at which time each sample was re-dissolved in 1 ml MeOH. Each insect sample (hemolymph, digestive tubes, salivary glands, and miscellaneous organs) was extracted with 1 ml MeOH with sonication for 10 min and centrifuged for 5 min at 792 × g. The clear supernatants were pipetted into 1-ml volumetric flasks and filled to the final volume with MeOH, filtered through a 0.45 μm PTFE filter (Carl Roth GmbH), and directly analyzed. Each sample solution was injected in triplicate.
The identity and purity (>95%) of rutin, hyperoside, isoquercitrin, quercitrin, pseudohypericin, and hypericin standards (Fig. 2) (Chromadex, Inc., Irvine, CA, USA) were confirmed by chromatographic (high pressure liquid chromatography-diode array detection, HPLC-DAD), spectral (1D- and 2D-nuclear magnetic resonance, NMR), and electrospray ionization mass spectrometry (ESIMS) methods. Stock solutions of each standard were prepared at concentrations of 1.0 mg/ml in MeOH and serial dilutions were made with MeOH. Within the range of concentrations injected (0.025-100 μg/ml), the detector response was linear (correlation coefficient R2=0.998).
Fig. 2.
Flavonoid glycosides and naphthodianthrones found in the plants Hypericum perforatum and H. hirsutum, and the sawfly Tenthredo zonula
All data were recorded and processed using Agilent 1100 Chemstation software (Agilent Technologies, Santa Clara, CA, USA). HPLC analysis was performed on an Agilent 1100 Separations Module equipped with a DAD (Agilent Technologies), using a Synergi MAX-RP 80Å column (150 × 2.1 mm, 4 μm particle size) from Phenomenex (Torrance, CA, USA) at a flow rate of 300 μl min−1, 5 μl injection per sample (Ganzera et al., 2002). Briefly, the mobile phases consisted of 10 mM ammonium acetate buffer adjusted to pH 5.0 with glacial acetic acid (A) and a 9:1 mixture of ACN and MeOH (B). Gradient elution was performed as follows: linear gradient from 13% B to 17% B over the first 10 min, followed by a gradient to 100% B over the next 25 min, followed by re-equilibration for 7 min. All separations were performed at 40°C. Peaks were assigned by spiking selected samples with standard compounds, and comparison of the UV spectra and retention times. HPLC–DAD/ESIMS (positive and negative modes) was performed on a Thermo Finnigan Surveyor liquid chromatography instrument with Thermo Quest Surveyor photodiode array detector, autosampler, and MS pump, and a Thermo Finnigan LCQ-XP mass detector equipped with an ESI source run by Xcaliber software (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectra were detected and recorded in a scan range of m/z=100 to 1,000, using a transfer capillary temperature of 350°C, a spray voltage of 5.00 kV and a sheath gas flow of 70 units.
Results
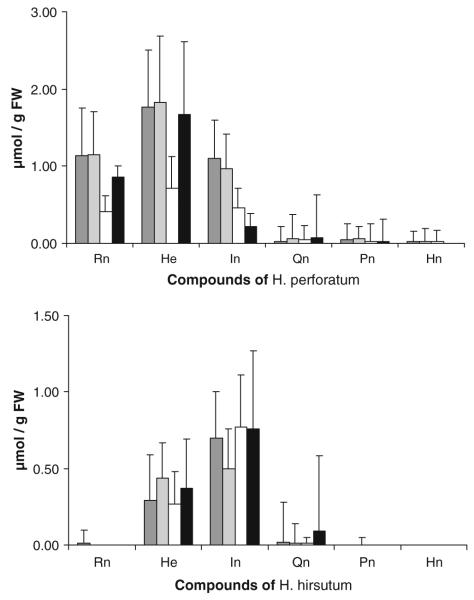
The amounts of flavonoid glycosides (rutin, hyperoside, isoquercitrin, and quercitrin) and naphthodianthrones (pseudohypericin and hypericin) measured in H. perforatum and H. hirsutum are given in Fig. 3. All compounds were found in both species, although not in every sample of each species. Two samples of H. perforatum differed from the other samples of this species in that they lacked rutin. The major flavonoid glycoside detected was hyperoside in H. perforatum (ca. 1.7 μmol/g FW) and isoquercitrin in H. hirsutum (ca. 0.7 μmol/g FW). Overlapping peaks were observed in many samples of H. hirsutum in the chromatographic region where quercitrin would have appeared, preventing quantification of this compound in some cases. Rutin in H. hirsutum and naphthodianthrones in both plant species were infrequently detected, and when detected, they generally were present as minor constituents (0.01 to 0.05 μmol/g FW).
Fig. 3.
Concentrations of flavonoid glycosides and naphthodianthrones in Hypericum samples. The compounds are: rutin (Rn), hyperoside (He), isoquercitrin (In), quercitrin (Qn), pseudohypericin (Pn), and hypericin (Hn). Values are given in μmol/g FW and as means±SD, N=3 technical replicates for each plant sample. From 10 H. perforatum and 6 H. hirsutum branches having been cut in the field and maintained at the laboratory, leaves and inflorescences were taken from them after 1, 8, and 15 d (i.e., dark grey, light grey, and white bars, respectively, for each compound), and from one potted plant of each species (black bar)
Amounts of the six plant metabolites detected in extracts of specific body parts (hemolymph; digestive tract; salivary glands; pooled miscellaneous organs) of dissected T. zonula larvae and prepupae are presented in Table 1. All compounds were detected at some level in the sawflies, although not in all samples or in all body parts. In particular, the concentrations of the flavonoid glycosides were highly variable. The detection of the six compounds was as frequent for larvae reared on one of the cut branches (24 out of 96 potential detections) as for those reared on a potted plant (8 out of 48) (P=0.29, Fisher exact probability test), but their detection was associated with the sawfly stage, since a lower frequency of detections was observed in larvae (32 out of 144) than in prepupae (11 out of 18) (P=0.001; individuals reared on H. perforatum; Table 1).
Table 1.
Concentration of flavonoid glycosides and naphthodianthrones in dissected Tenthredo zonula immature stages
| Sawfly stage/Plant | Qtt | Organ | Rn | He | In | Qn | Pn | Hn |
|---|---|---|---|---|---|---|---|---|
| Larva/ Hypericum perforatum, cut |
39 μl | Hem. Hem.a |
– | – | – | – | – | 0.01±0.15 [TR] |
| 4 | Dig. tr. | – | – | – | – | 0.02±0.14 | – | |
| 3 | Sal. gl. | – | – | – | – | – | – | |
| 4 | Misc. org. | – | – | – | 54.72±13.61 | TR | 0.01±0.17 | |
| Larva/ H. perforatum, cut |
44 μl | Hem. Hem.a |
– | – | – | – | TR [TR] |
0.02±0.19 [TR] |
| 5 | Dig. tr. | – | – | – | 4.10±0.69 | – | – | |
| 2 | Sal. gl. | 2.07±0.45 | – | – | – | TR | – | |
| 5 | Misc. org. | – | – | – | – | – | – | |
| Larva/ H. perforatum, cut |
40 μl | Hem. Hem.a |
– | – | – | OV [OV] |
– | – |
| 5 | Dig. tr. | – | TR | 1.84±0.67 | – | TR | – | |
| 4 | Sal. gl. | – | – | – | – | – | – | |
| 5 | Misc. org. | – | – | – | – | TR | – | |
| Larva/ H. perforatum, cut |
35 μl | Hem. Hem.a |
– | 3.25±1.71 [0.20±0.21] |
– | OV [OV] |
– | – |
| 4 | Dig. tr. | – | 1.48±0.47 | – | – | 0.25±0.17 | – | |
| 3 | Sal. gl. | 0.96±0.33 | – | 10.46±4.23 | – | – | – | |
| 4 | Misc. org. | – | – | 7.96±1.19 | 62.48±16.72 | TR | – | |
| Larva/ H. perforatum, potted |
46 μl | Hem. Hem.a |
– | – | – | OV [OV] |
– | – |
| 6 | Dig. tr. | – | 2.60±0.69 | 6.20±1.09 | – | 0.02±0.19 | ||
| 2.5 | Sal. gl. | – | – | – | – | – | – | |
| 6 | Misc. org. | TR | – | – | – | – | – | |
| Larva/ H. perforatum, potted |
40 μl | Hem. Hem.a |
– | – | – | – | – | – |
| 7 | Dig. tr. | – | – | 0.86±0.31 | – | 0.06±0.22 | ||
| 3.5 | Sal. gl. | – | – | – | – | – | – | |
| 7 | Misc. org. | 0.86±0.24 | – | – | – | – | – | |
| Prepupa/ H. perforatum, cut |
48 μl | Hem. Hem.a |
4.97±1.17 [0.15±0.23] |
8.08±1.68 [0.36±0.19] |
10.03±2.12 [0.45±0.31] |
– | – | 0.04±0.16 [TR] |
| 6 | Sal. gl. | 41.19±11.63 | – | 32.33±12.06 | – | – | 0.06±0.13 | |
| 6 | Misc. org. | 4.59±1.14 | 2.38±0.96 | – | – | TR | 0.01±0.09 | |
| Prepupa/ H. hirsutum, potted |
24 μl | Hem. Hem.a |
– | – | – | – | – | – |
| 4 | Sal. gl. | – | – | – | – | – | – | |
| 4 | Misc. org. | – | – | – | – | – | – | |
| Excrement | 0.11±<0.01 | 0.12±0.01 |
For eight batches of 4–7 individuals, hemolymph (Hem.) was collected prior to dissection of digestive tract (Dig. tr.), salivary glands (Sal. gl.), or remaining, miscellaneous organs (Misc. org.). The quantity in μl, or number of organs, is indicated in the column headed Qtt. Data are given as mean±SD of three technical replicates, in μg/individual. Values of hemolymph are also given in μmol/g FW (Hem.a, in brackets). If the compound was present at <0.005 of the given concentration unit, it is recorded as TR, and if it was not detected it is indicated with a dash (–). OV indicates overlapping peaks, which made quantification impossible. The compounds are: rutin (Rn), hyperoside (He), isoquercitrin (In), quercitrin (Qn), pseudohypericin (Pn), and hypericin (Hn)
The highest levels of flavonoid glycosides in the hemolymph were detected in prepupae that had fed during the larval stage on H. perforatum (as high as an absolute value of 10 μg/individual=0.45 μmol/g FW of isoquercitrin). In contrast, the four flavonoid glycosides generally were not detected in the hemolymph of larvae although the results for quercitrin were unclear due to overlapping peaks. Flavonoid glycosides also were detected infrequently in the digestive tracts of the larvae. However, the salivary glands of prepupae that had fed as larvae on H. perforatum had high concentrations of rutin (41 μg/individual) and isoquercitrin (32 μg/individual). The highest amounts of quercitrin were detected in several of the samples of miscellaneous organs from larvae that had fed upon H. perforatum. The naphthodianthrones pseudohypericin and hypericin were detected more often in larvae and prepupae reared on cut branches (15 out of 38 potential detections) than on those reared on potted plants (2 out of 22) (P=0.01, Fisher exact probability test). Pseudohypericin was detected most frequently in the digestive tract and/or miscellaneous organs with a maximum for the digestive tract of 0.25 μg/individual. Hypericin was found only in sawflies that had fed upon cut branches of H. perforatum.
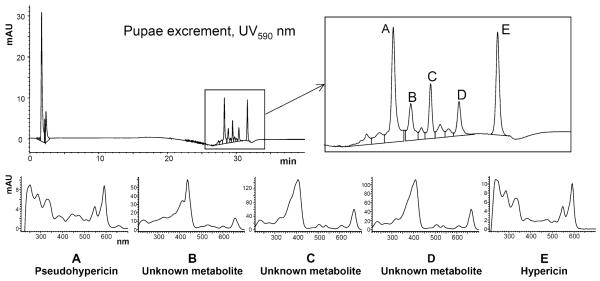
Pseudohypericin and hypericin were the only screened metabolites detected in the excrement of larvae reared on H. perforatum, at concentrations of 0.11 and 0.12 μg/mg DW excrement, respectively, which indicates that the major portions of the ingested naphthodianthrones were excreted without undergoing chemical changes. The presence of both of these naphthodianthrones in the excrement was confirmed by further analysis of this extract using HPLC–DAD/ESIMS (negative mode), but this revealed several additional peaks with UV spectra and mass fragmentation patterns that shared some characteristics with hypericin and pseudohypericin (Fig. 4; Table 2).
Fig. 4.
UV–Vis absorbance spectra from peaks corresponding to the known naphthodianthrones, pseudohypericin A and hypericin E, and to as-yet-unidentified, but related, lipophilic compounds B–D
Table 2.
Negative and positive quasi-molecular and collision induced fragment ions of peaks A-E detected in excrement of Tenthredo zonula larvae
| Peak | Identity | m/z (anions) | Ion | m/z (cations) | Ion |
|---|---|---|---|---|---|
| A | Pseudohypericin | 519 | [M-H]− | – | |
| 487 | [M-H-CH3OH]− | – | |||
| 429 | [M-H-CO2-CO-H2O]− | – | |||
| B | Unknown | – | 594 | [M+H]+ | |
| – | 534 | [M+H-CH2=C=O-H2O]+ | |||
| C | Unknown | – | 536 | [M+H]+ | |
| – | 508 | [M+H-CO]+ | |||
| – | 435 | [M+H-3CO-H2O]+ | |||
| D | Unknown | – | 533 | [M+H]+ | |
| – | 489 | [M+H-CO2]+ | |||
| – | 473 | [M+H-CH2=C=O-H2O]+ | |||
| E | Hypericin | 503 | [M-H]− | – | |
| 487 | [M-H-CH4]− | – | |||
| 433 | [M-H-CH2=C=O-CO]− | – |
Not detected (–)
Discussion
Amounts of flavonoid glycosides and naphthodianthrones measured in H. perforatum and H. hirsutum during this study (Fig. 3) are in agreement with those measured during previous studies (Ganzera et al., 2002; Crockett et al., 2005). The primary goal of the current research was to determine whether these secondary metabolites could be detected in larvae and prepupae of T. zonula, and to examine the body distribution of these compounds. While all compounds were observed at some amount in one or more insect samples, our results indicate a patchy distribution in the sawfly body parts. Their occurrence, nonetheless, seems to be influenced by the host-plant’s chemical profile and by the developmental stage at which the sawfly specimens were analyzed.
Our study reveals that T. zonula is able to sequester flavonoid glycosides, since these compounds were detected in body parts other than the digestive tract. For example, the compounds were found in the larval integument, which formed a main part of the dissected ‘miscellaneous organs’, as well as in the prepupal hemolymph and salivary glands. Among the isolated organs, only the hemolymph could be weighed, but these measurements allowed comparisons to be made between compound concentrations in plant and sawfly samples. For instance, the concentration of isoquercitrin was about 1 μmol/g FW in H. perforatum (Fig. 3) and 0.5 μmol/g FW in the prepupal hemolymph (Table 1). The latter concentration is equal to about 10 μg/individual, and the compound occurred at 32 μg/individual in the salivary glands. As compared to concentrations measured in the plant tissue, isoquercitrin is approximately half as concentrated in the hemolymph of prepupae, and 1.5 times more concentrated in the salivary glands. In the case of prepupae, we conclude that sequestration occurs because this non-feeding stage, which always has an empty digestive tract, can only accumulate plant compounds from the earlier (larval) instars.
Tenthredo grandis is the only other Tenthredo species that has been studied previously for the possible sequestration of plant metabolites (Bowers et al., 1993). In that study, total extracts of the prepupae contained the iridoid glycoside catalpol at a level up to 3.86% DW, and this compound reached 1.87% DW in leaves of the host plant, C. glabra. Thus, both Tenthredo species are able to sequester glycosides, at least at the prepupal stage, and we suggest for T. zonula larvae that quercitrin is selectively sequestered in the integument, and rutin and isoquercitrin, in the salivary glands. Larval hemolymph may be another storage site for quercitrin, but a methodological problem of overlapping peaks did not always allow quantification. Furthermore, not every flavonoid glycoside was detected in the analyzed batches of larvae. Thus, one should be cautious when discussing the ecological role of these sequestered metabolites as chemical defenses.
Several fates are known for plant metabolites ingested by sawfly larvae, even within one species. For instance, larvae of Rhadinoceraea nodicornis (Tenthredinidae, Blennocampinae) sequester some steroidal alkaloids from the host plant, Veratrum, in the hemolymph, either directly or after metabolism. However, the larvae excrete or degrade other steroidal alkaloids (Schaffner et al., 1994). In this sawfly species, zygadenine and 3-acetyl-zygadenine are present in the hemolymph at a total concentration of 1.18 μg/mg DW (= 24 μg/individual), whereas 1.77 μg/mg DW of their common precursor, 3-angeloyl-zygadenine, is found in the plant leaves. Larvae of other Blennocampinae species belonging to the genus Monophadnus sequester two furostanol saponins in the hemolymph (1.6 and 17.5 μmol/g FW). These compounds are present in the host plant, Helleborus, at much lower concentrations (0.008 and 0.268 μmol/g FW) (Prieto et al., 2007). Similarly, glucosinolates and iridoid glucosides are sequestered in the hemolymph by members of the Tenthredinidae genus Athalia (Müller et al., 2001; Opitz et al., 2010). Thus, sequestration of plant metabolites occurs repeatedly in Tenthredinidae sawfly larvae. Sequestration renders the hemolymph highly repellent and sometimes toxic to natural enemies such as ants, and provides these species with an efficient chemical defense.
Many classes of secondary metabolites, including naphthodianthrones, can be induced in H. perforatum in response to feeding damage from both generalist and specialist herbivores (Sirvent et al., 2003), but also to mechanical wounding such as cutting the plant stem (Lawton and Lamb, 1987). Both naphthodianthrones were found in T. zonula larvae that had been fed on cut branches as well as potted plant material, but pseudohypericin was detected more often and in higher amounts than hypericin, and only pseudohypericin was detected in the digestive tract (0.02 to 0.25 μg/individual). The excrement collected from larvae contained 0.11 μg/mg of pseudohypericin, and 0.12 μg/mg of hypericin, but no flavonoid glycosides. These results support the hypothesis that naphthodianthrones are specifically excreted from T. zonula larvae. There may be several reasons why only pseudohypericin is detected in the digestive tract. First, the ingested plant material generally contains more pseudohypericin than hypericin. If one assumes a daily consumption of a 36 mm2 piece of H. perforatum leaf tissue (= 18 mg fresh plant material; SLC, personal observation), a larva would ingest about 0.1 – 0.8 μg of hypericin and 0.1 – 1.1 μg of pseudohypericin, which are amounts roughly in the range of those detected in the digestive tract. Second, hypericin may be metabolized to pseudohypericin through the activity of microsomal detoxification enzymes often associated with gut tissues such as cytochrome P450 enzymes, which hydroxylate the substrate (Feyereisen, 1999). Since equivalent amounts of pseudohypericin and hypericin could be identified in the larval excrement, it is likely that hypericin is only partially converted to pseudohypericin in the gut. Third, due to the more apolar nature of pseudohypericin, it does not pass through the gut wall as easily as hypericin (Menn, 1978). However, hypericin as well as pseudohypericin were rarely found in other body parts than the digestive tract, but were clearly present in the excrement.
In the excrement, three additional peaks with similar, but not identical, spectral and mass fragmentation pattern characteristics were seen in the HPLC profiles and were absent from all other insect and plant samples (Fig. 4; Table 2). While the naphthodianthrones hypericin and pseudohypericin exhibit UV maxima at 545 and 590 nm, these compounds displayed intense absorbance peaks between 400–430 nm, and secondary maxima at 660–670 nm. For hypericin, typically observed collision-induced fragment ions of the parent species [M-H]− include [M-H-CH4]− (−16 from parent ion, m/z 487) and [M-H-CH2=C=O-H2O]− (−60 from parent ion, m/z 443); for pseudohypericin, such ions include [M-H-CO2]− (−44 from parent ion, m/z 475), [M-H-CH2=C=O-H2O]− (−60 from parent ion, m/z 459) and [M-H-3CO-H2O]− (−101 from parent ion, m/z 417). The fragmentation patterns observed for peaks B, C, and D showed interesting similarities that could correlate to a loss of [–CH2=C=O-H2O]+ for peak B (−60 from the parent ion, m/z 534); a loss of [−3CO-H2O]+ for peak C (−101 from the parent ion, m/z 435); and a loss of both [−CO2]+ (−44 from the parent ion, m/z 489) and [−CH2=C=O-H2O]+ (−60 from the parent ion, m/z 473) (Table 2). Although further isolation and structural elucidation are needed to fully clarify the structures of these compounds, it is intriguing to note that several fragment ions seen in the molecular ion pool are 2 mass units greater than several seen in the ion pools of hypericin and pseudohypericin, suggesting that a reduction has taken place. Additionally, the mass of peak D (533) could be reached by the addition of two methyl groups to hypericin, and the mass of peak C (536), by the addition of a single methyl group to pseudohypericin. These results could indicate either that partial metabolism of hypericin and/or pseudohypericin has occurred in the gut, in which case we hypothesize that the compounds represented at least by peaks C and D present in the excrement extract are metabolites of these compounds, or that the compounds may be the result of post-excretory degradation. These data taken together suggest that T. zonula larvae have developed physiological mechanisms that enable them to reduce the absorption of the phototoxic naphthodianthrones by an efficient elimination as a waste by-product.
Other insects, in particular the leaf beetles (e.g., Chrysolina brunsvicensis, C. geminata, C. hyperici, and C. varians; Coleoptera, Chrysomelidae), possess similar biochemical and physiological adaptations, and also display a specialized feeding behavior that allow them to ingest plant material containing naphthodianthrones without negative effects (Larson, 1986; Duffey and Pasteels, 1993). Analyses of the body distribution of hypericin in C. brunsvicensis support the hypothesis that this species contains the majority of hypericin within the gut lumen, and then rapidly eliminates it through the feces (Duffey and Pasteels, 1993). Particular cuticular pigments and, to a lesser extent, cuticle thickness also have been demonstrated to confer protection against the phototoxicity of hypericin in adult Chysolina beetles (Berenbaum, 1987; Fields et al., 1990). To block the phototoxic activity of ingested naphthodianthrones, C. hyperici is believed to sequester the natural antioxidant molecule β-carotene, in part responsible for the yellow coloration of Hypericum petals (Aucoin et al., 1990). Furthermore, elevated levels of antioxidant enzymes such as catalase and glutathione peroxidase have been measured in larvae of the H. perforatum specialist Anaitis plagiata (Lepidoptera, Lepidopteridae) when fed leaf discs treated with hypericin or reared strictly on H. perforatum tissue (Aucoin et al., 1991, 1995). Higher constitutive levels of enzymes such as glutathione reductase and glutathione S-transferase have not been demonstrated for the chrysomelid specialists although they have been measured in two generalist orthopterans, Tettigonia viridissima and Conocephalus discolor, which only occasionally feed on H. perforatum (Guillet et al., 2000). Some insects, such as the larvae of Platynota flavedana (Tortricidae), are protected from phototoxic effects through a behavioral adaptation, tying together leaves to form a shaded shelter within which they feed (Sandberg and Berenbaum, 1989). We do not know whether larvae of T. zonula have a particularly thickened cuticle or specific cuticular pigments that would potentially protect them against the phototoxic effects of the naphthodianthrones. The larvae do avoid light, displaying a negative phototropism in response to daylight/windows and the cold light source of a binocular microscope (JLB, personal observation). Moreover, in the field, they often are found settling on the underside of a leaf (Fig. 1a), but this behavior is frequently observed in sawfly larvae, regardless of their host plant species. More studies are necessary to fully understand the etho-physiological adaptations in T. zonula, and to assess the ecological role of flavonoid glycosides and naphthodianthrones as chemical defense metabolites in this species.
Acknowledgments
We thank three anonymous reviewers for their helpful comments. Funding for SLC was provided through a Hertha Firnberg Stipendium (T345-B05) from the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) in Austria.
Contributor Information
Sara L. Crockett, Institute of Pharmaceutical Sciences, Department of Pharmacognosy, Karl-Franzens-University, 8010 Graz, Austria
Jean-Luc Boevé, Department of Entomology, IRSNB-KBIN, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, 1000 Brussels, Belgium.
References
- Aucoin RR, Fields P, Lewis MA, Philogène BJR, Arnason JT. The protective effect of antioxidant to a phototoxin-sensitive insect herbivore, Manduca sexta. J. Chem. Ecol. 1990;16:2913–2924. doi: 10.1007/BF00979483. [DOI] [PubMed] [Google Scholar]
- Aucoin RR, Philogène BJR, Arnason JT. Antioxidant enzymes as biochemical defense against phototoxin-induced oxidative stress in three species of herbivorous lepidoptera. Arch. Insect Biochem. Phys. 1991;16:139–152. [Google Scholar]
- Aucoin RR, Guillet G, Murray C, Philogène BJR, Arnason JT. How do insect herbivores cope with the extreme oxidative stress of phototoxic host plants? Arch. Insect Biochem. Phys. 1995;29:211–226. doi: 10.1002/arch.940290210. [DOI] [PubMed] [Google Scholar]
- Berenbaum MR. Charge of the light brigade: Phototoxicity as a defense against insects. In: Heitz J, Downum KR, editors. Light-Activated Pesticides; ACS Symposium Series 339; American Chemical Society, Washington D.C.. 1987.pp. 206–216. [Google Scholar]
- Boevé J-L, Schaffner U. Why does the larval integument of some sawfly species disrupt so easily? The harmful hemolymph hypothesis. Oecologia. 2003;134:104–111. doi: 10.1007/s00442-002-1092-4. [DOI] [PubMed] [Google Scholar]
- Bowers MD, Boockvar K, Collinge SK. Iridoid glycosides of Chelone glabra (Scrophulariaceae) and their sequestration by larvae of a sawfly, Tenthredo grandis (Tenthredinidae) J. Chem. Ecol. 1993;19:815–823. doi: 10.1007/BF00985011. [DOI] [PubMed] [Google Scholar]
- Ciccarelli D, Andreucci AC, Pagni AM. Translucent glands and secretory canals in Hypericum perforatum L. (Hyperiacaceae): morphological, anatomical and histochemical studies during the course of ontogenesis. Ann. Bot. 2001a;88:637–644. [Google Scholar]
- Ciccarelli D, Andreucci AC, Pagni AM. The “black nodules” of Hypericum perforatum L. subsp. perforatum: morphological, anatomical, and histochemical studies during the course of ontogenesis. Israel J. Plant Sci. 2001b;49:33–40. [Google Scholar]
- Crockett S, Schaneberg B, Khan I. Phytochemical profiling of new and old world Hypericum (St. John’s wort) species. Phytochem. Anal. 2005;16:479–485. doi: 10.1002/pca.875. [DOI] [PubMed] [Google Scholar]
- Duffey SS. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 1980;25:447–477. [Google Scholar]
- Duffey SS, Pasteels JM. Transient uptake of hypericin of chrysomelids is regulated by feeding behavior. Phys. Entomol. 1993;18:119–129. [Google Scholar]
- Eisner T, Johnessee JS, Carrel J, Hendry LB, Meinwald J. Defensive use by an insect of a plant resin. Science. 1974;184:996–999. doi: 10.1126/science.184.4140.996. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Insect P450 enzymes. Annu. Rev. Entomol. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- Fields PG, Arnason JT, Philogène JR. Behavioural and physical adaptations of three insects that feed on the phototoxic plant Hypericum perforatum. Can. J. Zoo1. 1990;68:339–346. [Google Scholar]
- Ganzera M, Zhao J, Khan IA. Hypericum perforatum – Chemical profiling and quantitative results of St. John’s wort products by an improved high-performance liquid chromatography method. J. Pharm. Sci. 2002;91:623–630. doi: 10.1002/jps.10057. [DOI] [PubMed] [Google Scholar]
- Guillet G, Podenszfinski C, Regnault-Roger C, Arnason JT, Philogène BJR. Behavioral and biochemical adaptations of generalist and specialist herbivorous insects feeding on Hypericum perforatum (Guttiferae) Environ. Entomol. 2000;29:135–139. [Google Scholar]
- Hölzl J, Petersen M. Chemical constituents in Hypericum. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 77–93. [Google Scholar]
- Knox JP, Dodge AD. Isolation and activity of the photodynamic pigment hypericin. Plant Cell Environ. 1985a;8:9–25. [Google Scholar]
- Knox JP, Dodge AD. Singlet oxygen and plants. Phytochemistry. 1985b;24:889–896. [Google Scholar]
- Lacourt J. Répertoire des Tenthredinidae ouest-paléarctiques. Mém. SEF. 1999;3:1–432. [Google Scholar]
- Larson RA. Insect defenses against phototoxic plant chemicals. J. Chem. Ecol. 1986;12:859–870. doi: 10.1007/BF01020256. [DOI] [PubMed] [Google Scholar]
- Lawton MA, Lamb CJ. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol. Cell Biol. 1987;7:335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz H, Kraus M. Die Larvalsystematik der Blattwespen (Tenthredinoidea und Megalodontoidea) Akademie-Verlag; Berlin: 1957. [Google Scholar]
- Menn JJ. Comparative aspects of pesticide metabolism in plants and animals. Environ. Health Perspect. 1978;27:113–124. doi: 10.1289/ehp.7827113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C, Agerbirk N, Olsen CE, Boevé J-L, Schaffner U, Brakefield PM. Sequestration of host plant glucosinolates in the defensive hemolymph of the sawfly Athalia rosae. J. Chem. Ecol. 2001;27:2505–2516. doi: 10.1023/a:1013631616141. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A, Butterweck V. Lessons learned from herbal medicinal products: the example of St. John’s wort. J. Nat. Prod. 2010;73:1015–1021. doi: 10.1021/np1000329. [DOI] [PubMed] [Google Scholar]
- Opitz SEW, Müller C. Plant chemistry and insect sequestration. Chemoecology. 2009;19:117–154. [Google Scholar]
- Opitz SEW, Jensen SR, Müller C. Sequestration of glucosinolates and iridoid glucosides in sawfly species of the genus Athalia and their role in defense against ants. J. Chem. Ecol. 2010;36:148–157. doi: 10.1007/s10886-010-9740-3. [DOI] [PubMed] [Google Scholar]
- Prieto JM, Schaffner U, Barker A, Braca A, Siciliano T, Boevé J-L. Sequestration of furostanol saponins by Monophadnus sawfly larvae. J. Chem. Ecol. 2007;33:513–524. doi: 10.1007/s10886-006-9232-7. [DOI] [PubMed] [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae) 4(2). Section 9. Hypericum sensu lato (part 2): subsection 1. Hypericum series 1. Hypericum. Bull. Nat. Hist. Mus. Lond. (Bot.) 2002;32:61–123. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Hypericaceae) 5(2). Sections 17. Hirtella to 19. Coridium. Phytotaxa. 2010;4:127–258. [Google Scholar]
- Rowell-Rahier M, Pasteels JM. Third trophic level influences of plant allelochemicals. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd Edition, Vol. 2, Ecological and Evolutionary Processes. Academic Press Inc.; San Diego, California: 1992. pp. 243–277. [Google Scholar]
- Sandberg SL, Berenbaum MR. Leaf-typing by tortricid larvae as an adaptation for feeding on phototoxic Hypericum perforatum. J. Chem. Ecol. 1989;15:875–885. doi: 10.1007/BF01015183. [DOI] [PubMed] [Google Scholar]
- Schaffner U, Boevé J-L, Gfeller H, Schlunegger UP. Sequestration of Veratrum alkaloids by specialist Rhadinoceraea nodicornis Konow (Hymenoptera, Tenthredinidae) and its ecoethological implications. J. Chem. Ecol. 1994;20:3233–3250. doi: 10.1007/BF02033723. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mckinnon AE, Moore CJ, Walter GH. Chemical detoxification vs mechanical removal of host plant toxins in Eucalyptus feeding sawfly larvae (Hymenoptera: Pergidae) J. Insect Phys. 2010;56:1770–1776. doi: 10.1016/j.jinsphys.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Sirvent T, Krasnoff SB, Gibson DM. Induction of hypericins and hyperforins in Hypericum perforatum in response to damage by herbivores. J. Chem. Ecol. 2003;29:2667–2681. doi: 10.1023/b:joec.0000008011.77213.64. [DOI] [PubMed] [Google Scholar]
- Soelberg J, Jørgensen LB, Jäger AK. Hyperforin accumulates in the translucent glands of Hypericum perforatum. Ann. Bot. 2007;99:1097–1100. doi: 10.1093/aob/mcm057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taeger A, Altenhofer E, Blank SM, Jansen E, Kraus M, Pschorn-Walcher H, Ritzau C. Kommentare zur Biologie, Verbreitung und Gefährdung der Pflanzenwespen Deutschlands (Hymenoptera, Symphyta) In: Taeger A, Blank SM, editors. Pflanzenwespen Deutschlands (Hymenoptera, Symphyta). Kommertierte Bestandsaufnahme. Verlag Goecke & Evers; Keltern: 1998. pp. 49–135. [Google Scholar]
- Whitman DW, Blum MR, Alsop DW. Allomones: chemicals for defense. In: Evans DL, Schmidt JO, editors. Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators. State University of New York Press; Albany, New York: 1990. pp. 289–351. [Google Scholar]