Abstract
Background
Infections with Streptococcus pneumoniae (pneumococcus) are a cause of significant child mortality in the world. Pneumococcal glycoconjugate vaccines are expensive and provide limited serotype coverage. The 23-valent pneumococcal polysaccharide vaccine (Pneumovax®) may provide wider serotype coverage but is reported to be weakly immunogenic in children under 2 years of age. We have previously reported that Pneumovax® administered to healthy 12 month old Fijian infants elicits significant serotype-specific IgG responses. However, the functional capacity of these responses in 12 month old infants is not known.
Objective
To assess the functional, serotype-specific immune response of 12 month old infants following immunization with Pneumovax®.
Methods
Functional responses of 12 month old infants were assessed using the opsonophagocytic and antibody avidity assay against 8 serotypes and 23 serotypes, respectively.
Results
71% of infants produced strong opsonophagocytic activity against 4 of 8 serotypes and 30% produced high avidity serotype-specific IgG antibodies to 10 of 23 serotypes at 2 weeks post- Pneumovax®. Responses were protective for most serotypes that cause disease in western countries while responses to most of the epidemiologically relevant serotypes for developing countries were low.
Conclusion
This is the first comprehensive study evaluating the functional antibody response to Pneumovax® in 12-month old infants. Pneumovax® induced functional antibody responses to several serotypes causing disease in Western countries but induced poorer responses to serotypes that are responsible for the majority of disease in developing countries. Pneumovax® may be of benefit in some populations but further studies are required before this can be recommended in developing countries.
Keywords: pneumococcal polysaccharide vaccine, antibody, opsonophagocytosis, avidity, function, serotype, Pneumovax®, 23vPPV
Introduction
Streptococcus pneumoniae is the most common cause of bacterial pneumonia, non-epidemic meningitis, bacteraemia and otitis media in children. In developing countries an estimated one million deaths per year in children under 5 years of age are attributable to pneumococcal disease1. More than 90 serotypes have been described based on the capsular polysaccharide structure of pneumococcus2.
Pneumovax®, a 23-valent pneumococcal polysaccharide vaccine (23vPPV), contains the 23 pneumococcal serotypes that cause up to 90% of invasive pneumococcal disease (IPD) in unvaccinated children less than 5 years of age in the USA3 and 83% of IPD in children under 5 years of age in Fiji4. Immunization with 23vPPV induces production of anti-capsular IgG antibodies by T-cell independent mechanisms. Protection against S. pneumoniae is mediated by opsonophagocytosis of the organism in the presence of complement and serotype-specific antibody5, 6. Current opinion suggests that whilst 23vPPV provides immune protection in children greater than 2 years of age, the polysaccharide-specific T-independent response is poorly developed in younger children due to immaturity of the infant immune system characterized by the lack of a functional splenic marginal zone7. Consequently, the WHO and national health authorities do not advocate the use of 23vPPV in children under 2 years of age. Nevertheless, Indigenous Australian children receive 23vPPV at 18 months following a 3 dose pneumococcal conjugate vaccine (PCV7; Prevenar®) primary series and the use of 23vPPV following a primary series with PCV7 has been shown to boost the response to Prevenar® serotypes and be immunogenic for non-PCV7 serotypes8–10. However the capacity of infants <2 years of age to produce functional antibody responses to purified polysaccharide antigens remains uncertain.
The serotype-specific IgG response to 23vPPV is one of the main tests used to investigate children with suspected immune deficiency. However, data on the ‘normal’ immune response to 23vPPV are limited11, 12. Expert guidelines for the interpretation of an adequate response to 23vPPV in the context of evaluation of immunocompetence and Specific Antibody Deficiency (SAD)13 are available. In children aged 2–5 years, it has been suggested that an adequate response to the 23vPPV be defined as a post- immunization titer ≥1.3 µg/ml and/or a four-fold or greater increase from the pre-immunization titer for ≥50% of serotypes tested13, 14. Importantly, however, these criteria were derived from small study cohorts, selected patient populations, or studies examining IgG responses to a limited number of serotypes using an older generation ELISA. We have recently characterised the serotype-specific IgG response following 23vPPV in 12 month old children15. We found that 95% of infants generated an ‘adequate antibody response’ to polysaccharide antigens (as defined by current expert guidelines13), although some serotypes were poorly immunogenic in the majority of infants with less than 30% of infants mounting adequate IgG responses to serotypes 6B, 14 and 23F following immunization15. This was the first detailed evaluation of serotype-specific IgG responses to polysaccharide antigens in infants less than 2 years of age, and moreover, was the only study to assess infant antibody responses using the 3rd generation WHO ELISA16 which is known to offer higher specificity and correlate more closely with the functional opsonophagocytic assay (OPA)17, 18.
Nevertheless, the functional activity of serotype-specific IgG produced by infants less than 2 years of age has not been fully established, and confirmation of an adequate immune response to polysaccharide antigens in infants requires more detailed characterization of the functional capacity of serotype-specific IgG.
In this study, we evaluated the functional antibody response to a single dose of 23vPPV in the absence of prior pneumococcal immunization in 12 month old infants by measurement of serotype-specific antibody avidity to all 23 serotypes in Pneumovax® and serotype-specific opsonophagocytic activity against 8 serotypes in pre- and post-immunization sera. This is the first comprehensive evaluation of functional antibody responses to all 23 serotypes in Pneumovax® in a cohort of healthy infants.
Methods
Study population
The Fiji Pneumococcal Project (FiPP) was a single-blind open-label randomized phase II study assessing the safety, immunogenicity and impact on S. pneumoniae nasopharyngeal carriage of 0, 1, 2 or 3 doses of PCV7 followed by a booster of 23vPPV. Fijian infants were recruited at 6 weeks of age and stratified by ethnicity at randomization. Fijians are Pacific Islander people comprising 57% Indigenous Fijians and 38% Indo-Fijians (of Indian ethnicity)4. The ethnic composition of this study reflected the composition of Fiji (Table SI). Sixty-three infants were randomized to receive a full dose of 23vPPV at 12 months of age without prior PCV7. This study was approved by the Fiji National Research Ethics Review Committee and the University of Melbourne Human Research Ethics Committee, and written informed consent was obtained from study participants’ parents. Blood samples were collected immediately prior to 23vPPV and at 2-weeks and 5-months post-23vPPV.
Opsonophagocytic assay
OPA to serotypes 1, 4, 5, 6B, 9V, 14, 18C and 23F were performed at the University of Alabama at Birmingham (Alabama, USA) using standard multiplexed methods measuring the killing of pneumococci by differentiated HL-60 cells19. Results were expressed as an opsonization index (OI) representing the serum interpolated dilution that kills 50% of bacteria. The lower limit of detection was OI=4 and samples with an OI reading below the limit of detection were assigned an OI value of 2. An adequate functional response for each serotype was defined as a serotype-specific OI ≥ 819. Antibody potency for each serotype was calculated by dividing the opsonization index by the IgG antibody concentration for each serotype. Concentrations of serotype-specific IgG to the 23 serotypes in 23vPPV were measured by modified 3rd generation WHO ELISA15, 20 and have been reported previously15.
Antibody avidity
The avidity of serotype-specific IgG for each serotype in 23vPPV was determined using a previously published method21. The assay is based on the dissociation of low avidity antigen-antibody complexes by the chaotropic agent, sodium thiocyanate (NaSCN) in the modified 3rd generation WHO ELISA validated in our laboratory22, 23. A 0.5M NaSCN was used for all serotypes except serotype 19F (0.65M) and serotype 14 (0.8M). Avidity indices (AI) were calculated as the percent of antibody that remained bound following NaSCN elution, using an interpolation optical density (OD) value of 0.5 Units (Interpolation valueNaSCN/ Interpolation valuePBS/FCS) × 100. The avidity of serotype-specific IgG was arbitrarily assigned as low (AI<40%), intermediate (AI=40–49%) or high (AI≥50%).
Statistical analysis
Analysis was performed using GraphPad Prism version 4.03 (GraphPad Software Inc, USA). For avidity analyses, the median AI (MAI) and interquartile ranges (IQR) were calculated and statistical significance was assessed by Wilcoxon matched pairs test. The geometric mean for opsonic indices (GMOI) and antibody potency (GMP) and 95% confidence intervals (95% CI) were compared using the Student’s t-test. The proportions of infants with OI ≥ 8 and AI ≥ 50 were analysed using the McNemar’s test. A p-value < 0.01 was considered statistically significant to account for the multiple comparisons.
Results
Sixty-three infants were randomized at 6-weeks of age to receive 23vPPV as their primary pneumococcal immunization at 12-months of age. Fifty-six infants received the 12-month 23vPPV. Information on infant characteristics and withdrawals from the study are available in the online repository (Tables SI and SII) and has been reported previously20.
Proportions of infants with positive serotype-specific OPA and avidity responses
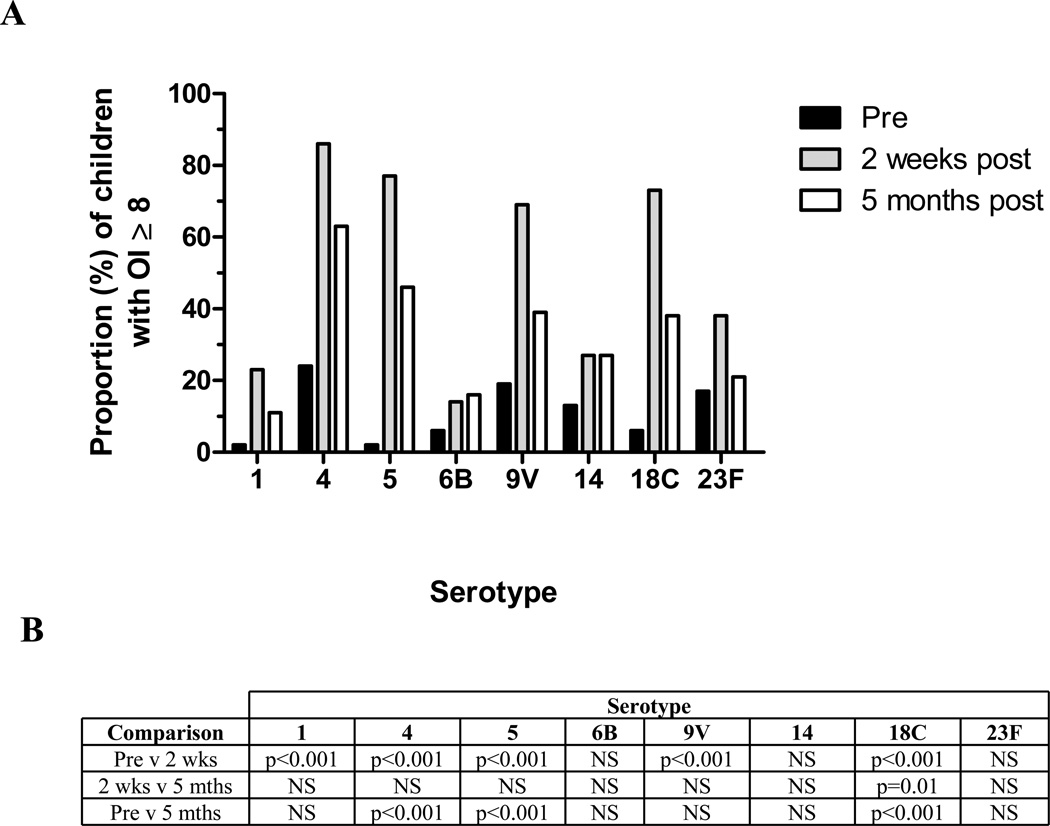
In this study, 71% of infants produced an OI ≥ 8 to at least 4 of the 8 serotypes tested at 2 weeks post-immunization (Table IA). At 5 months post-immunization, 53% of infants still exhibited an OI ≥ 8 to 3 of these 8 serotypes. However, the proportion of infants with an OI ≥ 8 for epidemiologically relevant serotypes (1, 5, 6B, 14, 19F, 23F) in this age group increased significantly as compared to the proportion at pre-immunization only for serotypes 1 (p<0.001) and 5 (p<0.001) at 2 weeks post-23vPPV and only serotype 5 (p<0.001) at 5 months post-23vPPV (Table IB).
Table I.
| A: The proportion of infants with an adequate functional response following 23vPPV immunization | ||||
|---|---|---|---|---|
| OPAa | AIb | |||
| Number of serotypes |
2 weeks | 5 months | 2 weeks | 5 months |
| 0 | 100% | 100% | 100% | |
| 1 | 98% | 91% | 98% | |
| 2 | 89% | 76% | 100% | 91% |
| 3 | 84% | 53% | 94% | 78% |
| 4 | 71% | 20% | 89% | 70% |
| 5 | 35% | 7% | 83% | 65% |
| 6 | 22% | 5% | 69% | 50% |
| 7 | 11% | 5% | 63% | 43% |
| 8 | 2% | 4% | 52% | 35% |
| 9 | 35% | 20% | ||
| 10 | 31% | 19% | ||
| 11 | 24% | 17% | ||
| 12 | 19% | 15% | ||
| 13 | 13% | 15% | ||
| 14 | 13% | 13% | ||
| 15 | 13% | 11% | ||
| 16 | 7% | 9% | ||
| 17 | 6% | 7% | ||
| 21 | 4% | 4% | ||
| 22 | 2% | 2% | ||
| B: The proportion of infants with an adequate functional response to epidemiologically relevant serotypes following 23vPPV immunizationa | ||||
|---|---|---|---|---|
| OPA response (%)b | Avidity response (%)c | |||
| Serotype | 2 weeks post | 5 months post | 2 weeks post | 5 months post |
| 1 | 29* | 11 | 11 | 9 |
| 5 | 77* | 46* | 15 | 11 |
| 6B | 14 | 16 | 21 | 13 |
| 14 | 27 | 27 | 21 | 18 |
| 19F | NTd | NT | 25 | 22 |
| 23F | 38 | 21 | 19 | 13 |
OPA - adequate response is defined as an OPA titre ≥ 8 (n=55) to 8 serotypes tested.
AI - adequate response defined as AI ≥ 50% (n=53) to 23 serotypes tested
based on data published by PneumoADIP;
defined as OI ≥ 8;
defined as MAI ≥ 50%;
NT = not tested;
p<0.01 compared to pre-immunization (n=56)
At 2 weeks post-immunization, 63% of infants had an AI ≥ 50% to at least 7 of the 23 serotypes examined, however this fell to 43% by 5 months. Fifteen infants responded with AI≥50% at both 2 weeks and 5 months post-immunization. There were 18 infants with AI≥50% at 2 weeks but failed to respond by 5 months post-immunization while another eight infants with AI≥0% at 5 months post-immunization but not at 2 weeks post-immunization. At both time points, one infant had an AI ≥ 50% to 22 of 23 serotypes whereas one infant had an AI < 50% to all 23 serotypes (this infant also had an undetectable OI to all 8 serotypes at 2-weeks post-23vPPV immunization). However, there were no significant increases in the MAI or the proportion of infants with MAI ≥ 50% for those serotypes causing most cases of IPD in this age group (1, 5, 6B, 14, 19F and 23F) at 2 weeks or 5 months post-immunization (Table IB).
Opsonophagocytic activity of the serotype-specific pneumococcal antibody response following 23vPPV immunization
Prior to 23vPPV immunization, the GMOI was less than 8 for all serotypes examined (Table II). Serotype-specific GMOI increased significantly 2 weeks after immunization for all serotypes except 6B and 14. The greatest rise in GMOI was observed for serotypes 4 (GMOI=246; p<0.0001), 5 (GMOI=28; p<0.0001), 9V (GMOI=87; p<0.0001) and 18C (GMOI=67; p<0.0001) compared with pre-immunization sera (Table II). At 5 months post-23vPPV, the GMOI were undetectable for all serotypes apart from serotypes 4 (GMOI=16) and 9V (GMOI=10). However, the GMOI at 5 months post-immunization were significantly lower than at 2 weeks post-immunization for all serotypes except 6B and 14. Low OPA responses to serotypes 6B, 14 and 23F were observed in the majority of children at both 2 weeks and 5 months post-immunization, with OIs ≤ 16 (Table II) consistent with the low serotype-specific IgG titers for these serotypes reported previously15.
Table II.
Antibody concentration, OPA index and antibody potency in 12 month old infants at 2 weeks and 5 months post 23vPPV immunization
| IgG (µg/mL) | OPA | Antibody Potency | |||||
|---|---|---|---|---|---|---|---|
| Serotype | Time | GMCa (95% CId) |
sig | GMOIb (95% CI) |
sig | GMPc (95% CI) |
sig |
| 1 | Pree 2 wksf 5 mthsg |
0.2 (0.1–0.2) 2.0 (1.5–2.8) 0.8 (0.6–1.0) |
p<0.0001 p<0.0001 p<0.0001 |
2 (2–3) 5 (3–7) 3 (2–3) |
p<0.001 p<0.001 NS |
3 (2–3) 4 (3–5) |
NS |
| 4 | Pre 2 wks 5 mths |
0.1 (0.1-0.1) 2.4 (1.8–3.2) 0.7 (0.5–0.9) |
p<0.0001 p<0.0001 p<0.0001 |
6 (4–11) 246 (127–479) 16 (10–30) |
p<0.0001 p<0.0001 p<0.01 |
103 (65–166) 22 (16–38) |
p<0.0001* |
| 5 | Pre 2 wks 5 mths |
0.3 (0.2–0.3) 2.8 (2.1–3.6) 0.9 (0.7–1.1) |
p<0.0001 p<0.0001 p<0.0001 |
2 (2–2) 28 (18–43) 7 (5–10) |
p<0.0001 p<0.0001 p<0.0001 |
10 (8–14) 8 (6–10) |
NS |
| 6B | Pre 2 wks 5 mths |
0.1 (0.1–0.2) 0.3 (0.2–0.4) 0.2 (0.2–0.3) |
p<0.0001 p<0.01 p<0.01 |
3 (2–4) 5 (3–8) 4 (3–7) |
NS NS NS |
17 (9–26) 20 (13–32) |
NS |
| 9V | Pre 2 wks 5 mths |
0.1 (0.1-0.1) 1.2 (0.9–1.6) 0.4 (0.3–0.5) |
p<0.0001 p<0.0001 p<0.0001 |
5 (3–9) 87 (39–101) 10 (6–18) |
p<0.0001 p<0.0001 NS |
73 (35–153) 25 (14–48) |
NS |
| 14 | Pre 2 wks 5 mths |
0.2 (0.2-0.2) 0.4 (0.3–0.6) 0.7 (0.5–1.0) |
p<0.0001 p<0.01 p<0.0001 |
4 (2–6) 10 (5–21) 7 (4–14) |
NS NS NS |
25 (12–45) 10 (6–17) |
NS |
| 18C | Pre 2 wks 5 mths |
0.1 (0.1-0.1) 1.2 (0.9–1.7) 0.5 (0.4–0.7) |
p<0.0001 p<0.0001 p<0.0001 |
3 (2–4) 67 (33–133) 6 (4–10) |
p<0.0001 p<0.0001 p<0.01 |
56 (31–94) 12 (8–20) |
p<0.0001 |
| 23F | Pre 2 wks 5 mths |
0.2 (0.1–0.3) 0.4 (0.3–0.6) 0.3 (0.2–0.7) |
p<0.0001 p<0.01 NSh |
5 (3–8) 16 (7–33) 5 (3–9) |
p<0.001 p<0.001 NS |
40 (20–70) 17 (12–32) |
NS |
GMC = Geometric mean concentration;
GMOI = Geometric mean opsonization index;
GMP = Geometric mean potency;
CI = Confidence interval;
significance between response at pre and 2 weeks post-immunization;
significance between response at 2 weeks and 5 months post-immunization;
significance between response at pre and 5 months post-immunization;
NS = Not significant;
one outlier was removed from this analysis as this infant had a very low IgG concentration of 0.37µg/mL but a very high OI (1736).
When antibody potency was assessed as an additional measure of the quality of the functional antibody response at both time points following 23vPPV immunization, the GMP was generally higher at 2 weeks compared to 5 months post-immunization for all serotypes except 1 and 6B (Table II). A significantly higher GMP was only achieved for serotypes 4 (GMP=103; p<0.0001) and 18C (GMP=56; p<0.0001) at 2 weeks compared to 5 months post-23vPPV reflecting an almost 5-fold rise in potency. Comparisons of post- and pre-immunization GMPs were not undertaken due to the low serotype-specific IgG levels detected in pre-immunization sera.
Prior to immunization, the majority of infants had undetectable serotype-specific OI (reported as OI=2). At 2 weeks post-immunization, most infants had functional OI (ie. OI ≥ 8; Figure 1): 86% (serotype 4), 77% (serotype 5), 73% (serotype 18C) and 69% (serotype 9V). A significantly higher proportion of infants demonstrated functional OI at 2 weeks post-23vPPV compared to pre-immunization for all serotypes (p<0.001), except 6B, 14 and 23F. However, there was no difference in the proportions of infants with OI ≥ 8 between 2 weeks and 5 months post-immunization for all serotypes except 18C (p=0.01). Compared to pre-immunization, serotype 4 was the only serotype where a majority of infants maintained an OI ≥ 8 at both time points (84% at 2 weeks; 63% at 5 months; Figure 2). Nevertheless, the proportion of infants with functional OI at 5 months post-immunization remained significantly higher than at pre-immunization for serotypes 4 (p<0.001), 5 (p<0.001) and 18C (p<0.001) (Figure 1).
Figure 1.
A. Proportion of infants with an opsonization titer (OI) ≥ 8 to the eight serotypes tested by OPA following immunization with 23vPPV (n=55). Black bars indicate prior to immunization, grey bars indicate 2 weeks post-immunization and white bars indicate 5 months post-immunization. B. The level of significance between the proportions of infants with OI ≥ 8 between each time point.
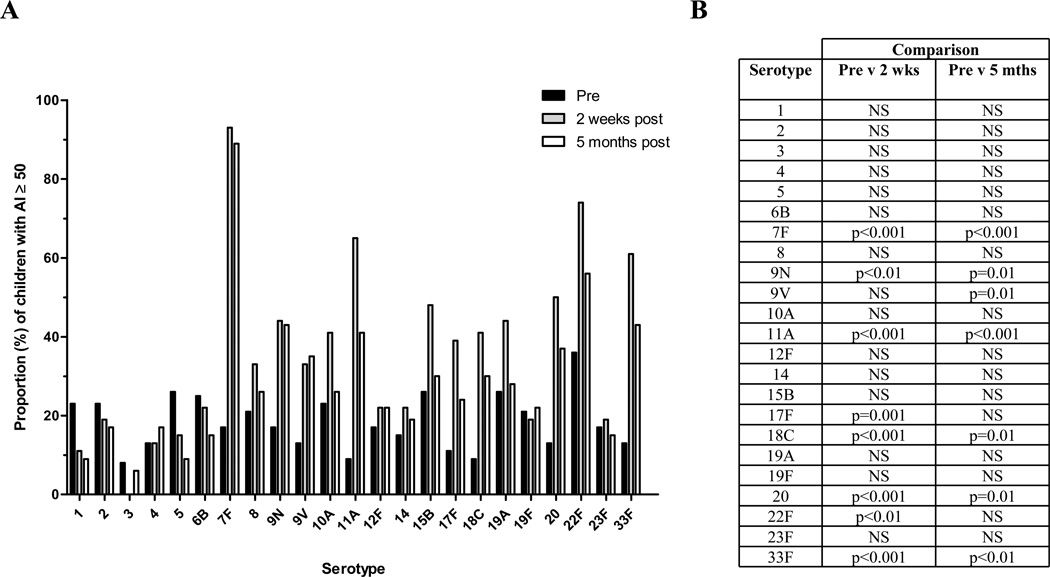
Figure 2.
A. Proportion of infants with an Avidity Index ≥ 50% to all vaccine serotypes following immunization with 23vPPV (n=53). Black bars indicate prior to immunization, grey bars indicate 2 weeks post-immunization and white bars indicate 5 months post-immunization. B. The level of significance between the proportions of infants with AI ≥ 50% between each time point.
Avidity of serotype-specific IgG following immunization with 23vPPV
The functional serotype-specific IgG response to 23vPPV in 12 month old infants was further assessed by measuring antibody avidity. Prior to 23vPPV immunization, the MAI was 0% for all serotypes except 22F (MAI=38%; Table III). At 2 weeks post-immunization, there was a significantly higher MAI than at baseline for 12 of the 23 serotypes assessed, with high MAI (≥50%) for 4 serotypes: 7F (MAI=73%; p<0.0001), 11A (MAI=58%; p<0.0001), 22F (MAI=68%; p<0.0001) and 33F (MAI=59%; p<0.0001); and intermediate MAI (40–49%) for 8 serotypes: 8 (MAI=43%; p<0.0001), 9N (MAI=47%; p<0.0001), 9V (MAI=45%; p<0.0001), 10A (MAI=43%; p<0.001), 15B (MAI=49%; p<0.0001), 18C (MAI=45%; p<0.0001), 19A (MAI=43%; p<0.0001) and 20 (MAI=49%; p<0.0001) (Table III). At 5 months post-immunization, the MAI decreased significantly compared to 2 weeks post-immunization for 14 of 23 serotypes, with the greatest reductions (to an MAI < 40%) for serotypes 10A, 11A, 17F, 18C, 19A and 33F (all p<0.001). In contrast, the MAI for serotype 3 increased over this time but was not significant. At 5 months post-immunization, antibody avidity remained significantly above pre-immunization measurements for only 2 serotypes: 7F (MAI=70%; p<0.0001) and 22F (MAI=54%; p<0.0001) while serotypes 8 (MAI=40%; p<0.0001), 9N (MAI=45%; p<0.0001) and 33F (MAI=43%; p<0.0001) fell to intermediate levels (MAI = 40–50%; Table III).
Table III.
Median avidity index (MAI) and interquartile range (IQR) of 12-month old infants before and after immunization with 23vPPV.
| MAI (IQRa) | |||
|---|---|---|---|
| Serotype | Pre-immunizationb | 2-weeks post-23vPPVc | 5-months post-23vPPVd |
| 1 | 0 (0–4.5) | 19 (7–31) | 18 (0–36) |
| 2 | 0 (0–48) | 31 (19–47) | 30 (20–43) |
| 3 | 0 (0–4) ** | 13 (10–18) | 16 (11–25) *** |
| 4 | 0 (0-0) ** | 23 (16–30) | 17 (0–34) |
| 5 | 0 (0–51) | 31 (16–45) | 22 (5–36) |
| 6B | 0 (0–50) | 0 (0–45) ** | 0 (0-0) |
| 7F | 0 (0-0) *** | 73 (63–81) | 70 (61–77) *** |
| 8 | 0 (0–48) *** | 43 (33–52) | 40 (34–50) *** |
| 9N | 0 (0–38) *** | 47 (34–59) | 45 (28–55) *** |
| 9V | 0 (0-0) *** | 45 (35–59) | 36 (0–55) *** |
| 10A | 0 (0–47) ** | 43 (0–61) ** | 0 (0–52) |
| 11A | 0 (0-0) *** | 58 (45–71) *** | 39 (0–65) *** |
| 12F | 0 (0-0) ** | 31 (0–47) | 0 (0–49) |
| 14 | 0 (0-0) | 0 (0–48) | 0 (0–44) |
| 15B | 0 (0–54) *** | 49 (26–60) | 35 (0–53) |
| 17F | 0 (0-0) *** | 39 (29–58) ** | 25 (0–50) *** |
| 18C | 0 (0-0) *** | 45 (29–59) ** | 32 (0–54) *** |
| 19A | 0 (0–52) *** | 43 (0–65) ** | 20 (0–55) |
| 19F | 0 (0–40) | 25 (0–41) | 22 (0–41) |
| 20 | 0 (0-0) *** | 49 (0–69) | 0 (0–67) ** |
| 22F | 38 (0–70) *** | 68 (44–75) | 54 (38–69) ** |
| 23F | 0 (0–20) | 0 (0–40) | 0 (0–14) |
| 33F | 0 (0-0) *** | 59 (40–66) ** | 43 (0–63) *** |
IQR = interquartile range;
MAI comparison between pre-immunization and 2-weeks post-immunization;
MAI comparison between 2-weeks and 5-months post-immunization:
MAI comparison between pre-immunization and 5-months post-immunization;
p< 0.001
p< 0.0001 (n=53)
Prior to immunization, the proportion of infants with an AI ≥ 50% was highest for serotype 22F (Figure 2). High avidity (≥50%) responses were detected in more than half the infants at 2 weeks post-immunization and were significantly higher than pre-immunization for serotypes 7F (93% of infants; p<0.001), 11A (65%; p<0.001), 20 (50%; p<0.001), 22F (74%; p<0.01) and 33F (61%; p<0.001). Other serotypes induced poor avidity responses with less than 20% of infants achieving an AI ≥ 50% for serotypes 1, 2, 3, 4, 5, 19F and 23F at 2 weeks. Serotype 3 was associated with the lowest avidity response, with no infant having an AI ≥ 50% (range: 0 to 49%) despite having adequate serotype-specific IgG. Similarly, the proportion of infants with an AI ≥ 50% at 5 months post-immunization was significantly higher than at pre-immunization for serotypes 7F (89%; p<0.001), 11A (42%; p<0.001) and 33F (42%; p<0.01; Figure 2). Moreover, the proportion of infants with AI ≥ 50 remained unchanged between 2 weeks and 5 months post-immunization for all 23 serotypes, suggesting the persistence of avidity responses for up to 5 months post-immunization.
Discussion
This is the first study to comprehensively evaluate the functional antibody response to 23vPPV in a large cohort of healthy infants. In this study, 71% of infants produced functional antibody responses to at least 4 of the 8 serotypes assessed by opsonophagocytosis which persisted for several serotypes (4, 5, 9V and 18C). However, only 2 serotypes (1 and 5) are prominent causes of disease in this age group globally.
The polysaccharide capsule is the major virulence factor by which pneumococci cause invasive disease. Capsular polysaccharides are thymus independent type II (TI-2) antigens and directly stimulate B-lymphocytes to produce antigen-specific opsonic antibody without immunological memory or affinity maturation. Polysaccharide antigens, with bound complement C3d, bind directly to the B-lymphocyte through CD21 which then associates with CD19 to enhance and prolong antigen signalling24. Based on a limited number of studies, this response is considered absent in the neonate and poor in children <2 years of age. This may be due to lower CD21 expression on neonatal and infant B cells25 and/or the immaturity of the splenic marginal zone26. The ontogeny of the immune response to TI-2 antigens in early life has not been formally assessed in larger groups of children. Previous studies suggest that infants 6 to 12-months of age can generate immune responses to some antigens in 23vPPV11, 12 however these studies were small, measured responses to a few serotypes and used old generation assay methods with poor specificity. A recent report demonstrating that, for all serotypes, serotype-specific IgG responses to 23vPPV increases with age and was most apparent during the first 11 months of life, further supports our data27.
We have recently reported that 23vPPV is immunogenic in this cohort of 12 month old healthy infants15. Serotypes 2, 3, 8 and 22F were the most immunogenic serotypes, while the least immunogenic serotypes were 6B, 14 and 23F, consistent with previous reports11, 12, 28. In addition, the fact that IgG levels were detected 2 weeks post-immunization adds further support to the early responsiveness to Pneumovax®. In this study, we further characterized the antibody response to polysaccharide vaccine by measuring the functional antibody response in this same cohort of infants. Although serum levels of serotype-specific IgG antibody (measured with the 3rd generation WHO ELISA) have been shown to correlate closely with functional antibody activity, particularly in post-immunization sera29, 30, OPA is still considered the gold-standard measurement of functional anti-pneumococcal antibody. Measurement of antibody avidity also offers information on antibody function31, 32. Using both the OPA and avidity assays, we showed an increase in functional antibody levels in the majority of infants. OPA responses were generally strongest at 2 weeks post-immunization and diminished by 5 months, whereas antibody avidity increased at 2 weeks post-immunization and remained stable (or increased slightly) over this time. This highlights that OPA and avidity assays provide different insight into functional activity of serotype-specific IgG.
Importantly, serotypes that induced the poorest responses by OPA were 1, 6B and 14, which account for approximately 30% of IPD globally in children under 5 years33. This suggests that 23vPPV may be ineffective at inducing functional immunity to these critical IPD-causing serotypes. Nevertheless, 23vPPV did induce a functional response to serotype 5 at both time points. Serotype 5 is an important disease causing serotype in children <5 years of age in developing countries (10–12% of global IPD) and is ranked among the top 3 IPD serotypes in young children both in GAVI-eligible and non-GAVI eligible countries3 (countries with a Gross National Income <US $1,500 per capita are eligible for GAVI support to purchase vaccines). In contrast, 23vPPV induced significant OPA and avidity responses to serotypes 4, 9V and 18C post-immunization, which are prevalent causes of IPD in North America and Oceania where the burden of disease is much lower.
Studies comparing both OPA and avidity measurements of functional pneumococcal antibody responses have yielded inconsistent results. While previous reports have shown poor correlation between these two assays post-23vPPV5, 34, 35, a high degree of correlation was found when OPA was compared with the amount of functional IgG (avid IgG) following combined PCV/23vPPV schedules36. Although our present findings support the immunogenicity of 23vPPV in inducing functional IgG against selected serotypes in infants, several issues concerning its use in children <2 years require further clarification. When a micro-dose of 23vPPV was given to these infants at 17 months to mimic natural infection, immune hyporesponsiveness was observed only for infants immunized with the full-dose 23vPPV at 12 months, as evidenced by no serotype-specific IgG, avidity or OPA response for all serotypes tested21, 37, 38. The clinical impact of 23vPPV is also not clear; while 23vPPV was immunogenic, there was no effect on nasopharyngeal carriage for any serotypes39. Moreover, although 23vPPV induced strong responses to most serotypes relevant to Western countries, responses were poor to the majority of serotypes that cause disease in developing countries. Therefore, further studies evaluating the clinical impact of 23vPPV in developing countries are required before the use of 23vPPV can be recommended in this setting. In a recent cohort study, pneumococcal vaccination was associated with a significantly increased risk of hospitalizations for non-viral acute lower respiratory tract infections. This was associated with the receipt of 23vPPV in indigenous Australians 5–23 months of age (hazard ratio = 1.39; p=0.002)40. Long term follow up of nasopharyngeal colonisation in our infant cohort will be important to clarify these issues with this vaccine.
In summary, this study demonstrates that 12 month old infants are capable of producing functional serotype-specific IgG responses for many of the serotypes following 23vPPV immunization. Moreover, this response was substantial in some infants and persisted for up to 5 months post-immunization. However, the functional activity for the serotypes responsible for most IPD worldwide was low.
Clinical Implications.
Pneumococcal polysaccharide vaccine (Pneumovax®) induces functional antibody responses to several clinically-relevant serotypes in infants at 12 months of age.
Acknowledgements
The authors wish to sincerely thank all the FiPP staff and families participating in the study, and the many other people who contributed to the study including: Amanda O’Brien, Kathryn Bright, Jane Nelson, Samantha Colquhoun, Bin Amy Chen, Timothy Gemetzis, Amy Auge, Katherine Gilbert, Evan Willis, Philip Greenwood, David Klein, Elizabeth Horigan, and Farukh Khambaty. The University of Alabama at Birmingham has IP rights on some reagents and MHN, RLB and JL are employees of the university.
Sources of Funding:
Funding was provided by grants from the National Institute of Allergy and Infectious Diseases (NIAID), USA (2 U01 AI052337-05), the Australian National Health and Medical Research Council (NHMRC grant number 251648) and was supported by the Victorian Government's Operational Infrastructure Support Program. Pneumovax® was kindly donated by CSL Biotherapies, Australia. MHN is supported by NIH contract AI-30021.
Abbreviations
- 23vPPV
23-valent pneumococcal polysaccharide vaccine (Pneumovax®)
- PCV7
7-valent pneumococcal conjugate vaccine (Prevenar®)
- AI
Avidity Index
- CFU
Colony-forming unit
- CPS
Cell wall polysaccharide
- ELISA
Enzyme linked immunosorbent assay
- FCS
Foetal Calf Serum
- FiPP
Fiji Pneumococcal Project
- GAVI
Global Alliance for Vaccines and Immunisation
- GMC
Geometric Mean Concentration (IgG)
- GMOI
Geometric Mean Opsonization Index
- GMP
Geometric Mean Potency
- IPD
Invasive pneumococcal disease
- IQR
Interquartile range
- MAI
Mean avidity Index
- MOPA
Multiplexed opsonophagocytic assay
- NaSCN
Sodium thiocyanate
- OD
Optical density
- OI
Opsonization index
- OPA
Opsonophagocytic assay
- PBS
Phosphate buffered saline
- TI-2
T independent type II antigens
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wardlaw T, Johansson E, Hodge M. UNICEF/WHO; 2006. Pneumonia: The forgotten killer of children. [Google Scholar]
- 2.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS medicine. 2010:7. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell FM, Carapetis JR, Tikoduadua L, Paeds D, Chandra R, Seduadua A, et al. Invasive pneumococcal disease in Fiji: clinical syndromes, epidemiology, and the potential impact of pneumococcal conjugate vaccine. The Pediatric infectious disease journal. 2010;29:870–872. doi: 10.1097/INF.0b013e3181ec7ae2. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrom N, Vakevainen M, Verho J, Kilpi T, Kayhty H. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2007;75:1794–1800. doi: 10.1128/IAI.01673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melin M, Jarva H, Siira L, Meri S, Kayhty H, Vakevainen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infection and immunity. 2009;77:676–684. doi: 10.1128/IAI.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taillardet M, Haffar G, Mondiere P, Asensio MJ, Pleau-Pison T, Burdin N, et al. Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. The Journal of infectious diseases. 2010;202:470–479. doi: 10.1086/653739. [DOI] [PubMed] [Google Scholar]
- 8.Leach AJ, Morris PS, Mackenzie G, McDonnell J, Balloch A, Carapetis J, et al. Immunogenicity for 16 serotypes of a unique schedule of pneumococcal vaccines in a high-risk population. Vaccine. 2008;26:3885–3891. doi: 10.1016/j.vaccine.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25:312–319. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 10.Russell FM, Licciardi PV, Balloch A, Biaukula V, Tikoduadua L, Carapetis JR, et al. Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy. Vaccine. 2010;28:3086–3094. doi: 10.1016/j.vaccine.2010.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas RM, Paton JC, Duncan SJ, Hansman DJ. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen RU, Leiva LE, Javier FC, 3rd, Sacerdote DM, Bradford N, Butler B, et al. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol. 1998;102:215–221. doi: 10.1016/s0091-6749(98)70089-2. [DOI] [PubMed] [Google Scholar]
- 13.Paris K, Sorensen RU. Assessment and clinical interpretation of polysaccharide antibody responses. Ann Allergy Asthma Immunol. 2007;99:462–464. doi: 10.1016/S1081-1206(10)60572-8. [DOI] [PubMed] [Google Scholar]
- 14.Hare ND, Smith BJ, Ballas ZK. Antibody response to pneumococcal vaccination as a function of preimmunization titer. The Journal of allergy and clinical immunology. 2009;123:195–200. doi: 10.1016/j.jaci.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balloch A, Licciardi PV, Russell FM, Mulholland EK, Tang ML. Infants aged 12 months can mount adequate serotype-specific IgG responses to pneumococcal polysaccharide vaccine. J Allergy Clin Immunol. 2010;126:395–397. doi: 10.1016/j.jaci.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–3826. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin SA. Correlates of protection induced by vaccination. Clinical and vaccine immunology : CVI. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblatt D, Southern J, Ashton L, Andrews N, Woodgate S, Burbidge P, et al. Immunogenicity of a reduced schedule of pneumococcal conjugate vaccine in healthy infants and correlates of protection for serotype 6B in the United Kingdom. The Pediatric infectious disease journal. 2010;29:401–405. doi: 10.1097/INF.0b013e3181c67f04. [DOI] [PubMed] [Google Scholar]
- 19.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–1009. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell FM, Balloch A, Tang ML, Carapetis JR, Licciardi P, Nelson J, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009;27:5685–5691. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell FM, Balloch A, Licciardi PV, Carapetis JR, Tikoduadua L, Waqatakirewa L, et al. Serotype-specific avidity is achieved following a single dose of the 7-valent pneumococcal conjugate vaccine, and is enhanced by 23-valent pneumococcal polysaccharide booster at 12 months. Vaccine. 2011;29:4499–4506. doi: 10.1016/j.vaccine.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien KL, Moisi J, Romero-Steiner S, Holder P, Carlone GM, Reid R, et al. Pneumococcal antibodies in a child with type 14 pneumococcal conjugate vaccine failure. Vaccine. 2009;27:1863–1868. doi: 10.1016/j.vaccine.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 23.Balloch A, Licciardi PV, Leach A, Nurkka A, Tang ML. Results from an inter-laboratory comparison of pneumococcal serotype-specific IgG measurement and critical parameters that affect assay performance. Vaccine. 2010;28:1333–1340. doi: 10.1016/j.vaccine.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Breukels MA, Zandvoort A, Rijkers GT, Lodewijk ME, Klok PA, Harms G, et al. Complement dependency of splenic localization of pneumococcal polysaccharide and conjugate vaccines. Scand J Immunol. 2005;61:322–328. doi: 10.1111/j.1365-3083.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- 25.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clinical and experimental immunology. 2010;162:271–279. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. The Journal of experimental medicine. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laferriere C. The Immunogenicity of pneumococcal polysaccharides in infants and children: A meta-regression. Vaccine. 2011;29:6838–6847. doi: 10.1016/j.vaccine.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 28.Kayhty H, Ahman H, Eriksson K, Sorberg M, Nilsson L. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr Infect Dis J. 2005;24:108–114. doi: 10.1097/01.inf.0000151022.92222.be. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Licciardi PV, Balloch A, Russell FM, Nahm MH, Mulholland K, Tang ML. Pneumococcal glycoconjugate vaccines produce antibody responses that strongly correlate with function. Nature reviews. Drug discovery. 2011;10:393. doi: 10.1038/nrd3012-c1. [DOI] [PubMed] [Google Scholar]
- 31.Ekstrom N, Ahman H, Verho J, Jokinen J, Vakevainen M, Kilpi T, et al. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2005;73:369–377. doi: 10.1128/IAI.73.1.369-377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licciardi PV, Balloch A, Russell FM, Mulholland EK, Tang ML. Antibodies to serotype 9V exhibit novel serogroup cross-reactivity following infant pneumococcal immunization. Vaccine. 2010;28:3793–3800. doi: 10.1016/j.vaccine.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 33.PneumoADIP. Pneumococcal Regional Serotype Distribution for Pneumococcal TPP. [Accessed 16 June 2011];2008 Available: http://vaccineamc.org/files/TPP_Codebook.pdf. [Google Scholar]
- 34.Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. doi: 10.1186/1742-4933-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine. 2007;25:2518–2527. doi: 10.1016/j.vaccine.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Balloch A, Licciardi PV, Russell FM, Burton R, Nahm M, Mulholland K, et al. Does serotype-specific IgG and avidity to Streptococcus pneumoniae following infant immunisation correlate with functional opsonophagocytic activity?. 6th International Symposium on Pneumococci and Pneumococcal Diseases; Reykjavik, Iceland. 2008. [Google Scholar]
- 37.Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months, of, age, a randomized controlled trial. Vaccine. 2010;28:3341–3349. doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell FM, Carapetis JR, Burton RL, Lin J, Licciardi PV, Balloch A, et al. Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age. Vaccine. 2011;29:535–544. doi: 10.1016/j.vaccine.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell FM, Carapetis JR, Satzke C, Tikoduadua L, Waqatakirewa L, Chandra R, et al. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clinical and vaccine immunology : CVI. 2010;17:1970–1976. doi: 10.1128/CVI.00117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Grady KA, Lee KJ, Carlin JB, Torzillo PJ, Chang AB, Mulholland EK, et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian indigenous infants 5–23 months of age following pneumococcal vaccination: a cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:970–978. doi: 10.1086/651079. [DOI] [PubMed] [Google Scholar]