Abstract
We demonstrate that potassium 1-13C-phosphoenolpyruvate becomes hyperpolarized potassium 1-13C –phospholactate with 13C T1 = 36 s after molecular hydrogenation by PASADENA (Parahydrogen and Synthesis Allows Dramatically Enhanced Nuclear Alignment). This proof-of-principle study was conducted with a fully protonated molecular precursor. 13C was polarized to a level of 1 %, corresponding to nearly 4,000 fold sensitivity enhancement at 3 T. The relevant homo- and heteronuclear spin-spin couplings are reported.
Keywords: Parahydrogen, NMR, PASADENA, hyperpolarization, 13C, phospholactate, phosphoenolpyruvate, PEP
Hyperpolarized magnetic resonance (MR) of 13C and 15N exogenous metabolic contrast agents is a rapidly developing field, which progressed from proof-of-principle studies in the early 2000s1,2 to the first clinical trial in men.3 The underlying driving force stems in part from the demonstrated capability to detect abnormal metabolic pathways in cancer4–7 and other diseases with impaired metabolism.8 Hyperpolarization of long-lived nuclear spin states increases sensitivity by 4–6 orders of magnitude2,9 with nuclear spin polarization approaching the order of unity. Hyperpolarized metabolic agents such as 1-13C-pyruvate can report on metabolic status of tumors similarly to positron emission tomography (PET) tracers.
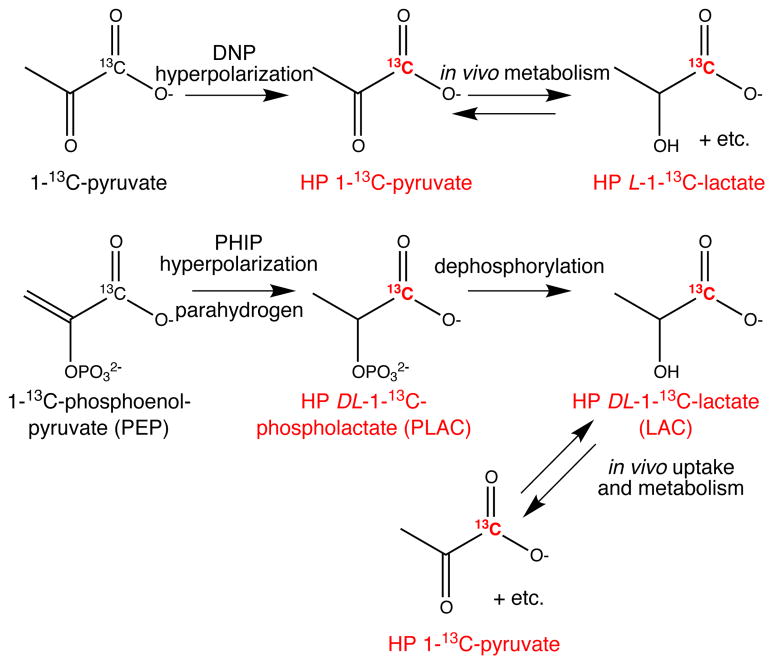
One of the main bottlenecks for preclinical and clinical application of hyperpolarized MR is the development and validation of relevant compounds that can probe biochemical pathways in vivo. Dynamic nuclear polarization (DNP)10 has been most widely used to date with the main drawback of long (~ 1 h) polarization cycles. Parahydrogen induced polarization (PHIP) offers significantly faster preparations with hyperpolarization cycles as short as 1 minute. However, it is limited by the availability of the required unsaturated molecular precursors that are necessary for molecular addition of parahydrogen11,12, which acts as a source of spin order. An additional requirement for increased relaxation time of the hyperpolarized 13C or 15N site is the absence of directly attached protons allowing for in vivo spin lattice relaxation time T1 as long as 43 seconds13 and 2 minutes14 respectively. As a result, the smallest PHIP moiety consists of an unsaturated C=C or C≡C bond adjacent to a labeled 13C or 15N site. For 13C hyperpolarized compounds, this represents a three carbon limitation successfully exemplified by acrylate moiety by a number of groups.15,16 However, hydrogenation of acrylate results in propionate, which turns out to be useful only for angiographic applications.1,15 In contrast, a leading DNP hyperpolarized metabolic agent 1-13C-pyruvate is also a three-carbon molecule carrying an extra oxygen atom in addition to the three-carbon skeleton of propionate, Fig. 1. Hyperpolarized 1-13C-pyruvate is not amenable by PHIP.
Figure 1.
The molecular diagrams of PHIP and DNP hyperpolarization of 1-13C-pyruvate and 1-13C-phospholactate (PLAC) and their metabolism for application as metabolic hyperpolarized (HP) agents.
Here, we describe hyperpolarization of 1-13C-phospholactate by PHIP. While both 1-13C-propionate and 1-13C-phospholactate are carboxylic acids with three-carbon backbones, 1-13C-phospholactate has an extra phosphate protected oxygen. The latter makes it similar to pyruvate, as it can be potentially metabolized, Fig. 1. This is a proof-of-principle study demonstrating (i) the feasibility of molecular addition of parahydrogen with established and commercially available Rh-based water-soluble catalyst and resulting in 13C hyperpolarization, (ii) sufficiently long life time of this potentially new metabolic contrast agent, and (iii) complete characterization of homo- and heteronuclear spin-spin couplings. The latter are necessary for optimization of PHIP polarization efficiency especially for perdeuterated unsaturated precursors, which represent a three spin system consisting of two nascent parahydrogen protons and a 13C or 15N nucleus. In addition, perdeuterated precursors may result in hyperpolarized compounds with longer relaxation time.17 Deuteration minimizes the number of nearby protons, which have significantly greater magnetic moment compared to that of deuterons. As a result, 13C T1 decrease due to dipolar relaxation mechanism may be somewhat minimized.17
The double C=C bond is stable with phosphate moiety in the second position, Fig. 1, allowing for molecular hydrogenation and PHIP. The unsaturated precursor is non-toxic 1-13C-phosphoenolpyruvate (PEP, Sigma-Aldrich #589,462), which becomes 13C hyperpolarized racemic 1-13C-phospholacate (PLAC) with D- and L- forms. The protection by phosphate group allows stabilizing the carbon-carbon double bond adjacent to the oxygen atom. While other protecting groups can potentially be used, the phosphate moiety could be quickly removed enzymatically18 by a suitable choice of phosphatase to yield hyperpolarized 1-13C-lactate (LAC), Fig. 1. Moreover, phosphate ions are biologically compatible and therefore do not require additional purification steps other than removal of phosphatase prior to in vivo administration.
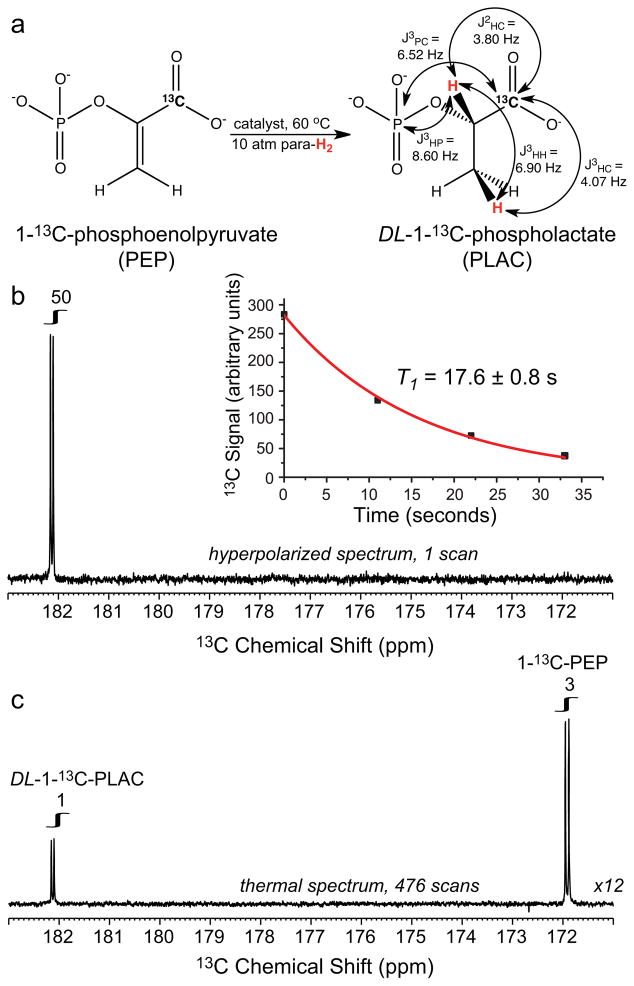
Experimental polarization procedures and equipment were identical to those described earlier.9,19 13C PHIP polarization of PLAC, Fig. 2a, was conducted in a 0.0475 T polarizer9 with 2.5 mM water soluble Rh-based catalyst19,20 using the Gold-man polarization transfer sequence.16 99.8% D2O (Sigma-Aldrich-Isotec # 617,385) was used as a medium, pH = 11 using 18 mM phosphate buffer, for 4 second long hydrogenation of 1.8 mM PEP for high resolution 13C studies using an 11.7 T Bruker NMR spectrometer equipped with a 5 mm X-H RF probe, Fig 2. Following the PHIP procedure, hyperpolarized solution was injected in a 5 mm NMR tube. The NMR tube was inserted into the NMR spectrometer and the solution degassed during an ~ 30 second delay. The degassing step is necessary to eliminate residual parahydrogen-nitrogen bubbles and their associated susceptibility gradients and broad lines. 1H decoupled 13C spectra, Fig. 2b, were acquired approximately 60 seconds or 3×T1 after PHIP hyperpolarization resulting in hyperpolarization decay by 20 fold. Nevertheless, a 50 fold signal enhancement of PLAC was observed, Fig. 2b, when compared to the 13C signal recorded from the same sample at Boltzmann levels of 13C polarization, Fig. 2c. This 50-fold enhancement corresponds to a 13C polarization of 0.05% at 11.7 T and 298 K. Taking into account 20-fold polarization loss during ~ 60 s delivery and degassing procedure, polarization of 1% was produced in the polarizer. The conversion of PEP (13C δ = 171.9 ppm) to PLAC (13C δ = 182.1 ppm) was only 25% due to non-optimal catalyst preparation. The Rh-based catalyst was further optimized for low field in situ NMR studies at 0.0475 T by titration of Bis(norbornadiene)rhodium(I) tetrafluoroborate (Strem # 45-0230, Newburyport, MA) and 3, 3′-(1,4-butanediylbis(phenylphosphinidene))bispropanesulfonic, disodium salt (Sigma-Aldrich-Isotec #Q36333-SPEC), Shchepin, R.V., unpublished results.
Figure 2.
13C PASADENA hyperpolarization of potassium 1-13C-phospholactate; a) the diagram of molecular hydrogenation of potassium 1-13C-phosphoenolpyruvate (PEP) yielding 1-13C-phospholactate (PLAC), b) proton decoupled single scan 13C spectrum of hyperpolarized DL-1-13C-PLAC; the insert shows T1 decay of 13C hyperpolarized signal of PLAC measured with 15° excitation RF pulses. All 13C spectra are acquired on a 500 MHz Bruker spectrometer equipped with X-H dual tuned RF coil and identical acquisition parameters, c) proton decoupled 13C spectrum of Boltzmann polarized mixture of DL-1-13C-PLAC and unreacted 1-13C-PEP using the same sample as in spectrum 2b except for nuclear spin polarization; note the doublet peak appearance due to J3PC ~ 6.5 Hz, a signature of carbon-phosphorous spin-spin couplings in PLAC and PEP. The conversion of PEP (13C δ = 171.9 ppm) to PLAC (13C δ = 182.1 ppm) was only 25% due to non-optimal catalyst preparation.
Low field in situ detection of 13C hyperpolarization was conducted with 2.0 mM PEP in aqueous medium with double de-ionized water, pH = 9.8 using 22 mM phosphate buffer resulting in 99+% chemical conversion to PLAC, pH = 8.8. While excellent PEP chemical conversion is achieved, in vivo use of the hyperpolarized contrast agent would require additional pH neutralization and catalyst removal. It should also be noted that D-isomers may not be metabolized in vivo and may be toxic. The signal intensity of a single scan 13C hyperpolarized spectrum, 0.007 millimoles of PLAC, Fig. 3b, was compared to that of a 13C spectrum acquired using 170 millimoles of sodium 1-13C-acetate at Boltzmann polarization at 0.0475 T and 308 K, Fig. 3a.9 1.0 % polarization was achieved corresponding to 13C signal enhancement ε = 240,000 fold at 0.0475 T and 308 K. It is in good agreement with our high field studies. 13C T1 was measured by monitoring 13C hyperpolarization decay using a time series of 13C spectra acquired every 20 seconds with 30° excitation pulses, Fig. 3c. The T1 of 1-13C at 0.0475 T was 36 ± 2 seconds, which is twice as long as the T1 measured at 11.7 T in D2O. The T1 decay was simulated as an exponential decay taking into account the effect of RF pulses on 13C magnetization. A likely explanation for longer T1 at low magnetic field is a reduced chemical shift anisotropy, which is expected to dominate T1 relaxation at high magnetic fields.21 Similar field effect was reported for the 13C T1 in 2-hydroxyethyl-1-13C-propionate-2,3,3-d3.9
Figure 3.
13C NMR spectroscopy conducted at 0.0475 T. a) 13C reference spectrum of 170 millimoles (14 g in 50 mL D2O) of sodium 1-13C-acetate, Boltzmann polarization at 308 K, 32 averages, b) 13C spectrum of 0.007 millimoles, 1.5 mg in 3.6 mL H2O at pH = 9.0, hyperpolarized potassium DL-1-13C-PLAC, polarization P = 1.0% corresponding to signal enhancement by 240,000 fold measured by comparing 13C signal intensity of hyperpolarized 0.007 millimoles of PLAC with that of 170 millimoles of sodium 1-13C-acetate at Boltzmann polarization at 0.0475 T and 308 K, Fig. 3a.9 c) decay of 13C hyperpolarized PLAC signal measured with 30° excitation pulses. The T1 decay was simulated (red trace) as an exponential decay taking into account the effect of RF pulses on 13C magnetization.
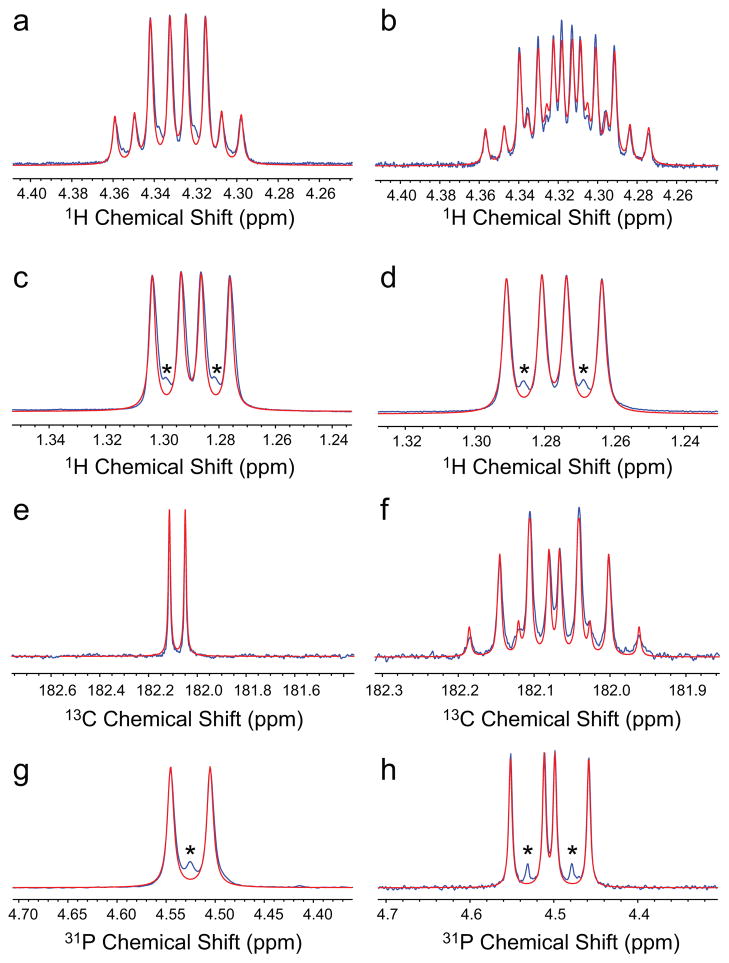
The solution used in PHIP low field studies was reduced in volume using a rotary evaporator, dissolved in 99.8% D2O and used for high-resolution studies of PLAC spin-spin couplings, Fig. 4. The combination of heteronuclear spectra, Fig. 4, over-determines the problem of complex multiplets and allows for accurate measurements of the spin-spin coupling magnitudes shown in Fig. 2a.: J3HH = 6.90 Hz, J3HC = 4.07 Hz, J2HC = 3.80 Hz, J3PC = 6.52 Hz, J3HP = 8.60 Hz. The knowledge of spin-spin couplings is critical for optimal performance of PHIP polarization transfer sequences that convert spin order of nascent parahydrogens to detectable 13C magnetization.
Figure 4.
Multinuclear high-resolution 11.7 T NMR spectroscopy (blue trace) and simulations (red trace) of PLAC at pH = 8.8: 1H spectroscopy with 31P decoupling, (a) – methine peak and (c) – methyl peak and without 31P decoupling, (b) – methine peak and (d) – methyl peak, 13C spectra with 1H decoupling (e) and without 1H decoupling (f), 31P spectra with 1H decoupling (g) and without 1H decoupling (h). The resonances marked with * correspond to peaks from natural abundance PLAC without 1-13C enrichment.
1-13C-phospholactate is suitable for measurement of spin-spin couplings due to 13C enrichment. The PHIP product of perdeuterated 1-13C-PEP, PLAC-d2, could lengthen 13C T1 somewhat and yield higher polarization, with the caveat that the extra deuterons in PLAC would pose synthetic challenges and complicate the spectra of 1H, 13C and 31P, making spin-spin coupling measurements more arduous. An alternative would be to use natural abundance PEP similarly to the strategy utilized for 13C hyperpolarized succinate,17 but this would require recording 13C spectra at natural abundance levels of 13C resulting in many hours of acquisition time. If PEP is perdeuterated, the spin system participating in polarization transfer dynamics would be reduced to three spins: 13C and two nascent parahydrogens in PLAC. Since Goldman’s polarization transfer16 is designed for three-spin systems and is not expected to perform well for systems with more than 3 spins, it is likely to result in a significantly greater hyperpolarization using perdeuterated PEP compared to the 5-spin system used here.17,22
While the metabolic relevance of PLAC itself for biomedical imaging is yet to be seen, the corresponding relevance of LAC as an imaging agent is certainly well-established23. One possibility to produce LAC from PLAC would be to enzymatically cleave the phosphate moiety.18 It is also possible that phosphatases of blood could cleave phosphate moiety after intravenous injection of PLAC. Fast hyperpolarization of LAC by PHIP method would present a clear advantage over DNP method and would make this promising contrast agent available to a broader range of biomedical scientists for cancer and cardiovascular research.7 Moreover, if successful, this strategy of using phosphate protected −C=C-O- motifs and subsequent cleavage could be extended to hyperpolarization of other metabolic contrast agents including 15N-choline.
Acknowledgments
We thank for funding support NIH ICMIC 5P50 CA128323-03, 5R00 CA134749-03, R25 CA136440, 3R00CA134749-02S1.
References
- 1.Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Magn Reson Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 2.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigneron DB, Nelson S, Harzstark A, Bok R, Kurhanewicz J. NCI Translational Science Meeting 2011: From Molecular Information to Cancer Medicine. Washington, DC: 2011. [Google Scholar]
- 4.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, Kohler SJ, Tropp J, Hurd RE, Yen YF, Nelson SJ, Vigneron DB, Kurhanewicz J. Cancer Res. 2008;68:8607–8615. doi: 10.1158/0008-5472.CAN-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Nat Med. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, in’t Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Nature. 2008;453:940–U73. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 7.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merritt ME, Harrison C, Storey C, Sherry AD, Malloy CR. Magn Reson Med. 2008;60:1029–1036. doi: 10.1002/mrm.21760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waddell KW, Coffey AM, Chekmenev EY. J Am Chem Soc. 2011;133:97–101. doi: 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abragam A, Goldman M. Rep Prog Phys. 1978;41:395–467. [Google Scholar]
- 11.Bowers CR, Weitekamp DP. Phys Rev Lett. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- 12.Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- 13.Chekmenev EY, Norton VA, Weitekamp DP, Bhattacharya P. J Am Chem Soc. 2009;131:3164–3165. doi: 10.1021/ja809634u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudalbu C, Comment A, Kurdzesau F, van Heeswijk RB, Uffmann K, Jannin S, Denisov V, Kirik D, Gruetter R. Phys Chem Chem Phys. 2010;12:5818–5823. doi: 10.1039/c002309b. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Magn Reson Mat Phys Biol Med. 2005;18:245–256. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 16.Goldman M, Johannesson H. C R Physique. 2005;6:575–581. [Google Scholar]
- 17.Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. J Am Chem Soc. 2008;130:4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murai S, Saito H, Shirato P, Kawaguchi T. J Pharmacol Toxicol Methods. 2001;46:103–109. doi: 10.1016/s1056-8719(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 19.Coffey AM, Shchepin RV, Wilkens K, Waddell KW, Chekmenev EY. J Magn Reson. doi: 10.1016/j.jmr.2012.04.012. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gridnev ID, Higashi N, Asakura K, Imamoto T. J Am Chem Soc. 2000;122:7183–7194. [Google Scholar]
- 21.Levy GC, Edlund U. J Am Chem Soc. 1975;97:5031–5032. [Google Scholar]
- 22.Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. J Magn Reson. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen AP, Kurhanewicz J, Bok R, Xua D, Joun D, Zhang V, Nelson SJ, Hurd RE, Vigneron DB. Magn Reson Imaging. 2008;26:721–726. doi: 10.1016/j.mri.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]