Abstract
Background
A critical issue in devising effective interventions for the treatment of children’s behavioral and emotional problems rests upon identifying genuine family environmental factors that place children at risk. In most twin and family studies, environmental factors are confounded with both direct genetic risk from parents and the indirect effect of genes influencing parents’ ability to provide an optimal rearing environment. The present study was undertaken to determine whether parental psychopathology, specifically parental antisocial behavior (ASP), is a genuine environmental risk factor for juvenile conduct disturbance, depression, and hyperactivity, or whether the association between parental ASP and children’s behavioral and emotional problems can be explained as a secondary consequence of the intergenerational transmission of genetic factors
Methods
An extended Children of Twins design (E-COT) comprised of data collected on 2,674 adult female and male twins, their spouses, and 2,454 of their children was used to test whether genetic and/or family environmental factors best accounted for the association between parental antisocial behavior children’s behavioral problems. An age matched sample of 2,826 juvenile twin pairs from the Virginia Twin Study of Adolescent Behavioral Development (VTSABD) was also included to examine developmental differences in gene expression by partitioning child specific transmissible effects from those effects that persist into adulthood. The fit of alternative models was evaluated using the statistical program Mx
Results
We found distinct patterns of transmission between parental antisocial behavior and juvenile conduct, depression, and hyperactivity. Genetic and family environmental factors accounted for the resemblance between parents’ ASP and children’s conduct disturbance. Family environmental factors alone explained the association between child depression and parental ASP, and the impact of parental ASP on hyperactivity was entirely genetic.
Conclusions
These findings underscore differences in the contribution of genetic and environmental factors on the patterns of association between parental antisocial behavior and juvenile psychopathology, having important clinical implications for the prevention and amelioration of child behavioral and emotional problems.
Keywords: Children of twins, Parental antisocial behavior, Juvenile depression, Conduct disturbance, Hyperactivity, Genetic risk, Family environment
Introduction
Mental health problems in children engender significant hardships on the children, their families, and the larger community. Left untreated, they can have serious implications for later adult development in the form of health services utilization, employment, financial difficulties, and pain to self and others (Knapp, 2008). Because early treatment can significantly reduce risk, an understanding of the underlying developmental processes in children is critical.
It is well known that parental psychopathology and impaired parent child relationships are one of the strongest risk factors affecting children’s behavioral and emotional health. Parents with a history of antisocial behavior show significant impairments in many aspects of parenting (Johnson, Smailes, Cohen, Kasen, & Brook, 2004; Hoeve et al., 2009), including inadequate supervision and monitoring (Loeber & Stouthamer-Loeber, 1986); a coercive, hostile, and inconsistent parenting style (Patterson, 1982; Farrington, 1995); lack of parental warmth (Patterson, 1982; Macoby & Martin, 1983; Barber & Buehler, 1996); parental negativity (Feinberg, Button, Neiderhiser, Reiss, & Hetherington, 2007); and abuse and neglect (Henry, Moffit, Robins, Earls, & Silva, 1993; Eaves, Prom, & Silberg, 2010).
Family studies have consistently shown a high concentration of antisocial behavior within families that also extend to other forms of child psychopathology (Farrington, Gundry, & West, 1975; Rutter, Giller, & Hagell, 1998; Bornovalova, Hicks, Iacono, & McGue, 2010). However, because genetic and family environmental factors are often confounded, how parental and child behaviors are related is not clear. Familial resemblance could reflect the direct environmental impact of the parental behavior, a shared genetic influence, or bidirectional interactions that include the effect of the child on the parental behavior. There may also be indirect genetic influences expressed in the rearing environment that antisocial parents provide. It is likely that multiple processes are involved.
Despite their many strengths, conventional twin and family studies are not able disentangle the role of genetic and family environmental factors in the transmission of risk from parents to their children. The study of the children of twins (COT) is a powerful method for disaggregating the effect of the family environment from any genetic liability that parents and children may share. The informativeness of studying adult twins and their children for resolving genetic and non-genetic transmission was initially described by Nance and Corey (Nance & Corey, 1976), Heath and others (Heath, Kendler, Eaves, & Markell, 1985), and more recently by Keller, Medland, and Duncan (2010). Since then, the COT design has been used for identifying salient aspects of the family that have a direct environmental impact on the child. These include the potential impact of parental conduct disturbance (D'Onofrio et al., 2007a); divorce (D'Onofrio et al., 2007b); alcohol use (Slutske et al., 2008); parental depression (Silberg, Maes, & Eaves, 2010; Singh et al., 2010) and parental anxiety (Narusyte, Neiderhiser, & D'Onofrio B, 2008). The inclusion of data on juvenile twins within an extension of the Children of Twins design (E-COT) allows an estimation of bi-directional transmission between parental and child behavior simultaneously. A study by Narustye and colleagues (Narusyte et al., 2008) showed that the association between maternal overprotectiveness and child anxiety could be entirely accounted for by anxiogenic child behaviors evoking parental overinvolvement. Data on juvenile twins combined with data on the children of twins, as was used in the present study, can also inform how transmissible factors may have a different expression at different phases of development (Silberg et al., 2010).
Several genetically informed studies of parental depression show a direct causal effect of depression on children’s behavior apart from any genetic liability parents and children share. A COT study of the offspring of MZ twins discordant for depression showed depression only in the children of the affected MZ twin. Since MZ twins are genetically identical, any difference between the cousins is strong evidence for an environmental effect (Singh et al., 2010). A similar pattern of results was reported in a recent E-COT study that modeled the genetic and environmental relatedness between family members to estimate the risk of parental depression on children’s conduct disturbance and depression (Silberg et al., 2010). Convincing evidence for the direct environmental impact of parental depression derives from other studies using varying designs: adopted away offspring (Tully, Iacono, & McGue, 2008), an in vitro fertilization study (Harold et al., 2011), and intervention studies reporting an improvement in children’s behavioral and emotional functioning following treatment of mother’s depression (Pilowsky, Wickramaratne, & Talati, 2008).
The case for parental antisociality is less clear. An in vitro study of genetically unrelated and related parents and children reported an absence of a direct genetic effect for parental antisocial behavior (Harold et al., 2011). The intergenerational transmission of parental ASP was shown to be genetic in girls, but environmental in boys (D'Onofrio et al., 2007a). The efficacy of intervention studies (Scott, 2005) implies that the association is environmentally mediated. However, changes in children’s behavior in response to changes in parental treatment do not negate the possibility of genetic influence.
Given the ambiguity surrounding the relative influence of genetic and family environmental factors in parental antisociality, the present study was undertaken to identify genetic and family environmental factors that increase risk to children. Using data on adult MZ and DZ twins, their spouses, and their children along with a matched sample of juvenile twins, we studied the pattern of transmission for three common forms of child psychopathology: conduct disturbance, hyperactivity, and depression. By including juvenile twins in our model, we are able to partition those etiological factors specific to childhood from those having long-term implications in adulthood, as expressed as ASP in the parents. This method allows us to consider that the same underlying genetic and environmental liability may have different phenotypic expressions during childhood and adulthood.
Methods
Ascertainment
The Children of Twins Study
Informed consent for the Extended Children of Twins study (E-COT) was obtained from the Institutional Review Board at Virginia Commonwealth University (IRB #2127). Of the nearly 14,000 twins from the Mid-Atlantic Twin Registry (MATR) that were located and contacted, 3343 met the eligibility criteria of having at least one child that was between the ages of 9 and 17. Of these, 2774 or 83% of twin families agreed to participate in the study. Eighty-two percent of the sample was Caucasian, 16% African American, and 2% Hispanic. Because of the relatively small sample of minorities, the analysis was confined to Caucasian families.
There were 856 complete adult twin pairs of known zygosity having at least one child. Of these, 297 were monozygotic females; 121 were monozygotic males; 146 were dizygotic females; 68 were dizygotic males; and 224 were opposite sex. There was a total of 1290 spouses of twins and maternal ratings on 2454 children. Seventy percent of the families had one child, 28% two children, and the remainder three or more children. The age of the children ranged from 9 to 17 with a mean age of 13.53 and a standard deviation of 2.54. Three hundred and eighty-six families had one or more children on both sides of the kinship (i.e., cousins). The average age difference between the cousin pairs was 2.36 with an S.D. of 1.70. Approximately one-half of the cousin pairs were opposite sex.
The biological parents of seventy-eight percent of the children were married or living together; 21% were from non-intact families through divorce or separation. Less than 1% of the sample of parents was widowed. Between 2005–2007 when the data were collected, 4% percent of the parents had less than a high school education, 22% a high school diploma, 30% an advanced degree, the remainder some college education. The median family income of the COT sample was $50,000–$70,000 per year. The average parental age of the sample was 41.5.
The Virginia Twin Study of Adolescent Behavioral Development (VTSABD)
The VTSABD is a population based, longitudinal, prospective study of juvenile twins and their parents. The recruitment was school based resulting in a participation rate of 74.5% (Eaves et al., 1997; Simonoff et al., 1997). The VTSABD sample comprised 1413 juvenile twin pairs, including 322 monozygotic males, 412 monozygotic females, 185 dizygotic males, 191 dizygotic females, and 303 opposite sex pairs. The children’s ages are slightly younger than the COT sample ranging, from age 8 through 18 with a mean of 11.99 (S.D. of 2.57). Seventy-seven percent of the biological parents were married, 22% separated or divorced, and 1% were widowed. In 1991–1994, 7% had less than a high school diploma, 17% a high school diploma, 40% an advanced degree, and 36% had some college but no advanced degree. The median salary range for the VTSABD families was $50,000–$56,000 per year. The average parental age was 42.3.
Assessment
Children of Twins (COT)
Parental antisociality and children’s psychopathology
Antisocial behavior in adult twins and their spouses was assessed using questions derived from a more extensive interview used to diagnose Antisocial Personality Disorder (Prom-Wormley et al., 2009; Eaves et al., 2010; Foley et al., 2004). These included questions about physical violence, serious police contact or arrest, financial irresponsibility, and erratic employment. Subjects were asked to indicate the lifetime presence of each behavior. The scale has adequate reliability and internal consistency shown by a Cronbach alpha estimate of .61 (Cronbach, 1960). Conduct disturbance and hyperactivity in the children were assessed via maternal report using the Rutter ‘A’ Scale (Rutter, Tizard, & Whitmore, 1970). Mothers were asked whether the specific behavior “certainly applied,” “somewhat applied,” or “did not apply” to each child in the past three months. The test-retest reliability and inter-rater reliability was .64 and .74, respectively. Scale scores significantly correlated with clinical diagnosis (Schachar, Rutter, & Smith, 1981; Hewitt et al., 1997). The study specific Cronbach alpha estimate was .71. Children’s depressive symptoms were assessed via child self-report using a shortened version of the Mood and Feelings Questionnaire (MFQ). The MFQ also showed good reliability indicated by a high Cronbach alpha estimate of 69. The shortened MFQ has a test retest-reliability of .66 and correlates highly with more extensive psychiatric measures of depression (Angold, Erkanli, Silberg, Eaves, & Costello, 2002). For comparability, the children’s assessment was identical to that used in a previous E-COT study of parental depression (Silberg et al., 2010).
The Virginia Twin Study of Adolescent Behavioral Development (VTSABD)
Child psychopathology
The data collected on the VTSABD juvenile twin pairs reflect the “extended” component of the COT model. These data were included in the model to separate any child specific genetic and environmental effects from those transmissible effects specific to parental ASP. To measure child symptoms, the Rutter ‘A’ scale and the shortened child MFQ were used, identical to those used in the Children of Twins Study.
Statistical Analysis
Resolving genetic and environmental transmission in the families of twins
The family relatedness of MZ and DZ twin parents, their cotwins, spouses, and children provide the information for resolving the nature of transmission between parents and their offspring. The nature of the design is as follows: The child is as alike genetically to their biological parents as s/he is to their MZ parent’s cotwin, but is provided a rearing environment only by their parent. As a result, any difference in the association between the child and their MZ parent and his/her aunt/uncle is an estimate of the direct effect of the parental environment. Additionally, any difference in the association between children’s behaviors and antisocial behavior in the aunt/uncles of MZ parents as compared to children and the aunt/uncles of DZ parents is a test of the inter-generational transmission of genetic factors.
For resolving intergenerational transmission, the following familial correlations were estimated (SAS Institute, 2002): 1) MZ and DZ twin correlations for antisocial behavior in the adult COT twins; 2) parental ASP and conduct disturbance, depression, and hyperactivity in their children (phenotypic correlations); 3) juvenile behavior and ASP in the cotwins of the MZ or DZ parents (avuncular correlations); and 4) the MZ and DZ juvenile twin correlations from the VTSABD sample. Only the offspring data from the VTSABD twins were used for estimating juvenile specific genetic and environmental effects on behavior (Silberg et al., 2010).
Structural Equation Modeling
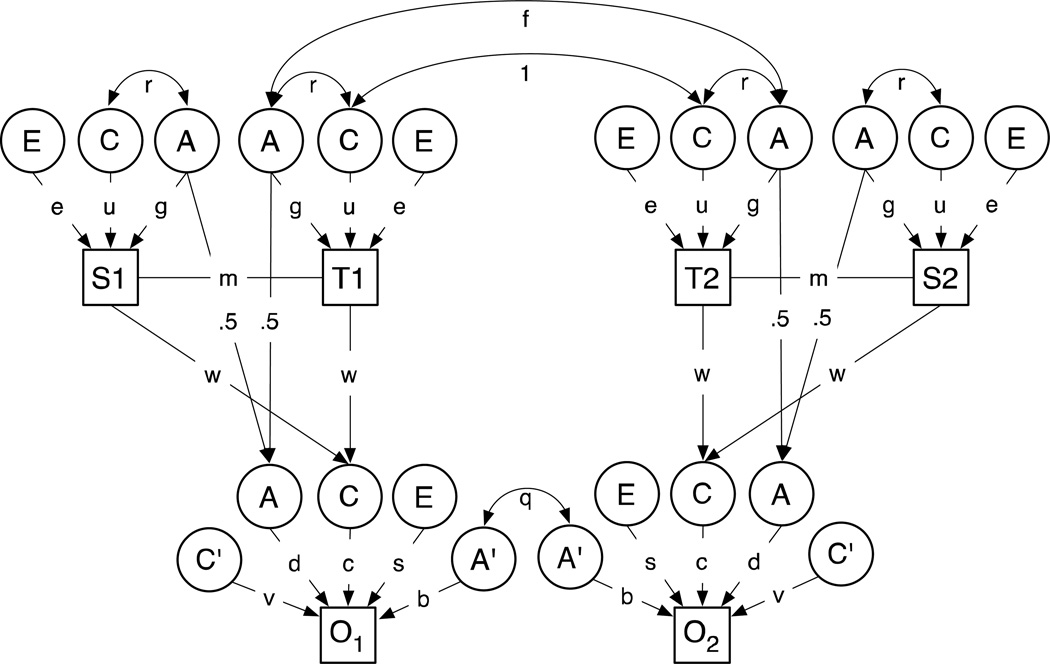
We adopted a more rigorous approach for disaggregating the effect of the shared family environment from any genetic liability transmitted from parent to child using the Children of Twins model shown in Figure 1. The model is based upon the familial association between ASP in MZ and DZ twin pairs, their spouses, and their children. The model includes twins of an adult pair, T1 and T2, their spouses, S1 and S2, and a child from each twin family, offspring 1 (O1) and offspring 2 (O2) (up to 3 children were included). Alternative models were fitted and evaluated using likelihood ratio chi-square tests using the statistical program Mx (Neale, Boker, Xie, & Maes, 2003).
Figure 1. Children of Twins Model (COT).
All latent and measured variables are assumed to be standardized to unit variance to simplify the derivation of expected correlations between relatives. Note that there is assumed to be no residual variance on the parentally derived shared environment (C) because differences are assumed to be explained entirely by regression on parental phenotype. The effects of Mendelian segregation, however, contribute residual variance in life-course persistent genetic effects (A) which explains a proportion 1- ½ (1+g2m) of the total variance in A.
Key to symbols:
T1=Twin 1; T2=Twin 2; S1=Spouse of Twin 1; S2=Spouse of Twin 2; O1=Offspring of Twin 1; O2=Offspring of Twin 2
A: additive genetic effects expressed in both adults and children (“life course persistent”); A’: residual additive genetic effects specific to children (“juvenile limited”); C: shared environmental effects expressed in both adults and children (“life course persistent”); C’: residual, juvenile specific, shared environmental effects in twins and siblings; E: adult unique environmental effect
The basic model incorporates a number of potentially important genetic and environmental sources of variation: additive genetic effects, A; shared or “common” environmental effects, C; and individual-specific within-pair environmental influences, E. However, this basic model is extended in several directions (see Figure 1) to take into account developmental differences between adults and juveniles and to represent alternative theories about the influence of parents on their children.
The full model for the correlations between relatives involves 7 free parameters: three genetic parameters, g, d and b; three “shared environmental” parameters, u,c, and v; and the correlation between spouses, m.
We recognize that different genes may affect the phenotype in adults (adult twins and their spouses) and juveniles (the children of twins). The model allows for genes, represented by the latent variables, A, that have effects that persist over the life course. Although these genetic factors may persist through development, their contribution to the phenotype may be different in adults and children. This difference is captured in the model by specifying separate parameters, g and d, for the path from life-course persistent genetic effects, A, to adult (g) and juvenile (d) measures.
Not all genetic effects are life-course persistent. Some, represented by A’ in Figure 1, are only expressed in juveniles and do not contribute to adult variation. The effect of these juvenile-limited genetic effects is denoted by the path b in the figure. These are estimated using the data on the juvenile twins.
We assume that some of the shared environmental effects (C) persist over the life-course and affect the phenotype of both adults (C) and juveniles (C’). Some, C’, are assumed to be juvenile-specific. A critical feature of the current model and data set is its ability to identify some aspects of the non-genetic effect of parents on the environment of their offspring and to separate these direct environmental effects of parents from any secondary correlation due to the genetic correlation between generations. In this case, we assume that all the life-course persistent effects of the juvenile shared environment may be traced to the parental phenotype. The regression of juvenile C on the measured phenotype of mothers and fathers is represented by the path coefficient w. The regression of the adult phenotype on life-course persistent shared environment is u. The corresponding regression on the life-course persistent shared environment in juveniles, C, is c in the figure. The path from juvenile-specific shared environmental effects, C’, to juvenile phenotype is v. The direct path from parental phenotype to juvenile phenotype (i.e., the environmental effect of each parent on his/her child with the correlated effects of genes and the other parent partialled out is given by the product wc.
Assortative mating, M,, increases the correlation between relatives for transmissible genetic and environmental influences that affect the trait on which assortment is based (Heath & Eaves, 1985; Medland & Keller, 2009). Since parents are assumed to influence the children genetically, through genes showing life-course persistent genetic effects, and environmentally through their effects of their phenotypes on the shared environment of their children, we expect a passive genotype-environment correlation, r, between the life-course persistent genetic effects, A, and the life-course persistent environmental effects C. r may be expressed in terms of other model parameters (see below).
The free and derived parameters of the model are summarized for convenience in Table 1. The derived parameters are estimated from other paths in the model. Parameter definitions in the code follow those in Figure 1. In addition to the tabulated parameters of the structural model, the model includes parameters for the means of adult and juvenile male and female subjects, and for the standard deviations of adults and juveniles. Models are fitted to the raw square root transformed data regressing out the effects of age, gender, and sample origin.
Table 1.
Summary of parameters of structural model for correlations between relatives
| Parameter | Description | Free1 |
|---|---|---|
| M | Correlation between spouses | F |
| G | Path from persistent additive genetic effect to adult phenotype | F |
| D | Path from persistent additive genetic effect to juvenile phenotype | F |
| B | Path from juvenile limited genetic effect to juvenile phenotype | F |
| U | Path from adult shared environment to adult phenotype | F |
| W | Path from parental phenotype to juvenile shared environment | D |
| C | Path from juvenile shared environment to juvenile phenotype | F |
| V | Path from juvenile specific shared environment to phenotype | F |
| R | Correlation between persistent genetic and shared environmental effects | D |
| Wc | Partial regression of juvenile outcome on parental phenotype | D |
| Q | Correlation between juvenile specific additive genetic effects | D |
| F | Correlation between additive genetic effects of siblings/twins | D |
Note1: Parameters are designated as free (F), or derived (D) from other model parameters.
RESULTS
Patterns of Familial Resemblance
The twin correlations for adult antisocial behavior, juvenile depression, conduct disturbance, and hyperactivity, and the parent-child, and aunt/uncle–niece/nephew correlations are shown in Table 2. The correlation for parental antisocial behavior is .35 for MZ twins and .18 for DZ twins. These are consistent with a genetic model with no apparent effect of the shared environment. There are genetic influences on all three child behaviors shown by correlations of .73MZ / .34DZ for conduct, .34MZ / .17DZ for depression, and .54MZ / .01DZ for hyperactivity. The greater similarity between children’s CD and depression and ASP in their parents compared to their aunts/uncles is consistent with an environmental effect of parental ASP. This is particularly the case for juvenile depression shown by a zero correlation between children’s depression and their aunt/uncle’s ASP. In addition to an environmental effect, the comparison of the MZ avuncular correlation of .07 to the DZ avuncular correlation of .04 also implies a genetic influence of parental ASP on children’s CD. The similarity in the child-parent and child-aunt/uncle correlation in MZ twins for child hyperactivity is also illustrative of a genetic model of family resemblance.
Table 2.
Twin, parent-child, avuncular-child, and cousin correlations in MZ and DZ twins for parental antisocial behavior child conduct disturbance, depression, and hyperactivity.
| Adult twin correlations 1 | Parental Antisocial Behavior | ||
|---|---|---|---|
| MZ adult | .35 (n=424) .18 (n=441) |
||
| DZ adult | |||
| Child twin correlations2 | Conduct | Depression | Hyperactivity |
| MZ child (734) | .73 | .34 | .54 |
| DZ child (679) | .34 | .17 | .01 |
| Parent Child Correlations3 | |||
| MZ parent (n=1173) | .14 | .13 | .16 |
| DZ parent (n=1279) | .13 | .09 | .11 |
| Avuncular Child Correlations | |||
| MZ avuncular (n=1011) | .07 | .02 | .14 |
| DZ avuncular (n=956) | .04 | .00 | .01 |
Adult twin correlations, Children of Twins Study (COT)
Juvenile twin correlations, Virginia Twin Study of Adolescent Behavioral Development (VTSABD)
Parent-offspring correlations from COT families
Avuncular-child correlations from COT families
Structural Equation Modeling
Intergenerational transmission of risk from parental ASP to juvenile conduct disturbance
Table 3 presents the model fitting results and associated parameter estimates and confidence intervals for child CD.
Table 3.
Summary of Model-Fitting Results for Adult ASP and Juvenile Conduct Disturbance in Children of Twins
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 |
|---|---|---|---|---|---|---|---|---|---|
| IFAIL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | .25 | .25 | .26 | .21 .25 .27 | .26 | .25 | .25 | .25 | .25 |
| G | .70 | .00! | −.64 | .59 .64 .65 | .70 | .71 | .00! | .72 | .16 |
| D | .28 | .00! | −.42 | .07 .28 .49 | .21 | .43 | .34 | .00! | .76 |
| B | .71 | .00! | .81 | .61 .71 .72 | .86 | .63 | .67 | .75 | .00! |
| U | −.12 | .55 | .00! | .00! | −.11 | −.15 | .55 | −.16 | .50 |
| W | .63 | .63 | .63 | .63 | .63 | .63 | .63 | .63 | .63 |
| C | .10 | .24 | .00! | .01 .12 .22 | .15 | .00! | .22 | .22 | .08 |
| V | .37 | .78 | .00! | .45 .47 .53 | .00! | .49 | .47 | .46 | .48 |
| R | .51 | .00 | .51 | .51 | .51 | .50 | .00 | .50 | .22 |
| Wc | .06 | .15 | .00 | .06 | .09 | .00 | .14 | .14 | .05 |
| A | .64 | .00 | −.64 | .64 | .64 | .63 | .00 | .64 | .28 |
| F | .55 | .50 | .55 | .55 | .55 | .55 | .50 | .55 | .51 |
| −2lnL | 9249.21 | 9486.98 | 9285.76 | 9249.73 | 9277.77 | 9257.63 | 9271.33 | 9256.56 | 9262.10 |
| K | 7 | 4 | 4 | 6 | 6 | 6 | 6 | 6 | 6 |
| χ2 | - | 237.76 | 36.55 | 0.51 | 28.56 | 8.42 | 22.21 | 7.34 | 12.92 |
| d.f. | - | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 |
| p | - | <0.000 | <0.000 | 0.4385 | <0.000 | 0.0285 | <0.000 | 0.003 | <0.003 |
Model 1 constitutes the baseline in the principal genetic and environmental parameters which are estimated without constraint. The results for other reduced models are also summarized. Model 2 excludes all genetic effects from the model (i.e., g=d=b=0), and Model 3 attempts to remove all shared environmental effects (u=c=v=0). Both reduced models show a significantly poorer fit, particularly for omission of genetic effects, yielding χ2(3)=237.77 for the joint effects of genes and χ2(3)=36.59 for those of the shared environment. Models 4–8 attempt other reductions in the attempt to identify the most salient effects. Model 4 removes shared environmental effects for adult depression (u=0). This reduced model does not fit significantly worse than the full model 1, implying that the shared environmental effect on adult ASP can be excluded. Models 5–9 explore the effects of individually setting each of the remaining shared environmental parameters (v, c) and two genetic parameters (g, d, b) to zero. These model-comparison statistics indicate that none of these parameters can be eliminated without worsening the fit significantly. The best fitting model shows a significant environmental impact, c, of parental ASP on children’s CD (χ2(1)=8.42, p<.03), and a significant genetic association, d, between parent ASP and child CD (χ2(1)=7.35, p<.003). As expected, not all genetic variation in juvenile CD can be explained by the same genes that influence adult ASP. The estimate of juvenile-specific genetic effects on conduct disorder, b, is highly significant (χ2(1)=12.88, p<.0003).
A complete understanding of family resemblance for adult ASP and child CD requires both genetic and shared environmental effects. The fact that the correlation between parental ASP and juvenile conduct disorder has both a genetic and environmental component derived from the parents results in a modest contribution of passive genotype-environmental covariance to individual differences in conduct disorder that accounts for 2rdc=3.36% of the total variance. The final, best-fitting model indicates that a proportion g2=41% of the total variation in adult ASP can be attributed to the cumulative additive effects of genetic differences. The remaining 59% is attributable to the unique environmental effects that are uncorrelated between adult twins. Genetic effects that are shared with adult ASP explain d2=8% of variation in juveniles. Juvenile specific genetic effects explain a large proportion b2=50%, with a small but significant contribution of c2=1.4% due to the environmental impact of parental ASP on their children. There are also significant shared environmental effects v=22% that are specific to child CD. The remaining 16% of the variation is assigned to unique environmental influences. Table 3 summarizes these estimates.
Intergenerational transmission of risk from parental ASP to juvenile depression
Table 4 presents the model fitting results, confidence intervals, and associated parameter estimates for child depression.
Table 4.
Summary of Model-Fitting Results for Adult ASP and Parental ratings of Juvenile Depression in Children of Twins.
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| IFAIL | 0 | 0 | 0 | 0 | 0 | 0 | |
| M | .23 | .23 | .23 | .24 | .23 | .24 | .24 |
| G | .64 | .00! | .67 | .67 | .67 | .67 | .67 |
| D | .38 | .000! | .31 | .00! | .00! | .00! | .00! |
| B | .53 | .00! | .90 | .94 | .61 .65 .70 | .00! | .66 |
| U | .49 | .43 | .00! | .00! | .00! | .00! | .00! |
| W | .64 | .64 | .63 | .64 | .64 | .64 | .63 |
| C | .12 | .14 | .00! | .17 | .08 .14 .20 | .14 | .00! |
| V | .68 | .88 | .00! | .00! | .63 .68 .72 | .88 | .70 |
| R | .06 | .00 | .50 | .37 | .37 | .37 | .37 |
| Wc | .077 | .09 | .00 | .12 | .09 | .09 | .00 |
| A | .07 | .63 | .47 | .48 | .47 | .47 | .47 |
| F | .50 | .50 | .53 | .53 | .53 | .53 | .55 |
| −2lnL | 16706.68 | 16893.78 | 16843.18 | 16833.65 | 16709.87 | 16986.76 | 16729.99 |
| K | 7 | 4 | 4 | 4 | 5 | 4 | 4 |
| χ2 | - | 187.1 | 136.5 | 126.97 | 3.19 | 200.08 | 23.31 |
| d.f. | - | 3 | 3 | 3 | 2 | 3 | 3 |
| p | - | <.000 | <.000 | <.000 | 0.0741 | <.000 | <.0000 |
As expected, the estimates of the genetic and environmental components of adult ASP are comparable to those reported for the previous analysis of conduct disturbance (g2 explains 67% of the total variance), with no evidence of an effect from the shared environment. The genetic variation in juvenile depression cannot be explained by the same genes that influence adult ASP (Model 5, χ2(2)=16709.87, d=0), whereas the estimate of juvenile-specific genetic effects on depression is highly significant (Model 6, χ2(3)=16986.79). Any familial resemblance between parental ASP and juvenile depression is accounted for by aspects of the rearing environment provided by the parents – eliminating the environmental impact of parental ASP on juvenile depression, ‘c’ results in deterioration in fit of the model (Model 7). Overall the best model, combining parsimony and goodness and fit appears to be Model 5, which estimates separate genetic influences on adult ASP and juvenile depression, but a significant environmental impact of parental ASP on child depression. There are significant juvenile specific genetic and shared environmental effects not shared with adult ASP. An absence of significant genetic effects transmitted from parents rules out any support for any passive genotype-environmental covariance to explain variation in juvenile depression. Shared environmental effects explained by parental ASP accounts for approximately 2% of the total variation in juvenile depression. Genetic effects that are juvenile-specific account for b2=42%, and the shared environmental factors specific to childhood explain v2=46% of the variation.
Intergenerational transmission of risk from parental ASP to childhood hyperactivity
The model fitting results, confidence intervals, and associated parameter estimates for child hyperactivity are presented in Table 5. A relatively simple model explains variation in childhood hyperactivity. Shared environmental factors, both c and v, can be eliminated, but the omission of both transmissible parental genetic effects associated with adult ASP (Model 4, χ2(1}=30.95) and juvenile specific genetic effects (Model 5, χ2(1}=53.16) results in a markedly poorer model fit. Although parental ASP has an effect on the rearing environment parents provide (w), it does not explain any significant variation in child hyperactivity.
Table 5.
Summary of Model-Fitting Results for Adult ASP and Hyperactivity in Children of Twins.
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| IFAIL | 0 | 0 | 0 | 0 | 0 |
| M | .22 | .23 | .23 | .23 | .21 |
| G | .68 | .00! | .63 | .63 | .54 |
| D | .73 | .00! | .15 .23 .32 | .00! | .56 |
| B | .00 | .00! | .52 .60 .66 | .65 | .00! |
| U | .44 | .54 | .00! | .00! | .00! |
| W | .64 | .64 | .64 | .64 | .64 |
| C | .17 | .12 | .00! | .00! | .00! |
| V | .00 | .34 | .00! | .00! | .00! |
| R | .39 | .00 | .49 | .50 | −.33 |
| Wc | .11 | .08 | .00 | .00 | .00 |
| A | .50 | .00 | .63 | .63 | .43 |
| F | .53 | .50 | .55 | .55 | .52 |
| −2lnL | 9614.513 | 9696.22 | 9618.99 | 9649.94 | 9672.158 |
| K | 7 | 4 | 4 | 3 | 3 |
| χ2 | - | 81.71 | 4.48 | 30.95 | 53.16 |
| d.f. | - | 3 | 3 | 3 | 4 |
| p | - | <.000 | .2141 | <.000 | 0.000 |
DISCUSSION
The present study underscores the deleterious impact of parental antisocial behavior on the behavioral and emotional health of children. We have demonstrated that antisocial behavior in parents is associated with a wide range of child problems that include conduct disturbance, depression, and hyperactivity. Although all are phenotypically related to parental ASP, the intergenerational transmission of parental ASP is not the same across phenotypes. Although antisocial behavior in parents is an environmental risk factor for depression and conduct disturbance, it is a genetic risk factor for conduct disturbance and hyperactivity. Approximately 8% of genetic differences in child CD and 5% of the variation in hyperactivity are shared with the genes related to parental ASP.
The genetic and environmental association between parent and child antisocial behavior is consistent with the aggregation of antisocial behavior in families. Our results are also consistent with a marked genetic influence on childhood ADHD and long-term associations reported between childhood ADHD and adult antisociality (Manuzza & Klein, 2000). The few studies of antisocial behavior and childhood depression impede drawing firm conclusions regarding transmission, but evidence for an association between maternal antisocial behavior and depression in early childhood (Goldstein et al., 1994) coincides with the present findings.
The heritability estimate of 40% for adult ASP is nearly identical to that of other studies (Rhee & Waldman, 2002) and exemplifies how a highly genetic parental trait can have wide ranging environmental effects. The direct phenotypic impact of parental ASP accounted for nearly 40% of the variation in the rearing environment provided to the child, conveying a significant influence on children’s liability to depression and conduct disturbance.
The intergenerational transmission of both genetic and environment factors to children’s CD resulted in a significant passive genotype-environment correlation in which the child receives both direct genetic risk and an impaired parental environment arising from the indirect effect of genes for parental ASP. There was no evidence that passive genotype-environment correlation was a significant mechanism in depression or hyperactivity. For depression, the transmissible effect was solely environmental, and for hyperactivity solely genetic.
The extended component of the COT design using a matched sample of juvenile twins enabled us to separate genetically and environmentally influenced behaviors specific to childhood from those persisting into adulthood. Children’s CD had long-term genetic and environmental implications for adult antisocial behavior, and hyperactivity was also a significant index of genetic risk. Although the outward expression may be somewhat different, the genetic link between externalizing problems in childhood and antisocial behavior in adulthood may reflect the intergenerational transmission of difficulties in behavioral or emotional regulation—traits with a strong genetic component (Calkins & Keane, 2009). Such behavioral and emotional dysregulation might also account for the bidirectional nature of the coercive process between parents and children (Patterson, 1982). Parents with ASP may be particularly susceptible to responding negatively to children’s externalizing behavior, giving rise to a reciprocal interaction that exacerbates both the parent and child behaviors. The analysis of specific parenting variables within an evocative genotype-environment framework is an important goal for our future research.
The pattern of transmissible effects for parental ASP on all three childhood behaviors is identical to that found for parental depression (Silberg et al., 2010), suggesting a nonspecific effect of parental psychopathology on children. Researchers widely recognize that both parental depression and antisociality create environmental adversity for their children, and these are likely mediated through similar parenting impairments that include a lack of parental warmth, inconsistent parenting, and hostility. Although specific parenting behaviors were not included in the present analysis, our recent analysis (Eaves et al., 2010) of longitudinal data on twins and their parents from the VTSABD and Young Adult Follow-Up (Silberg, Meyers, Pickles, Simonoff, & Eaves, 1996) showed an important mediating role of parental neglect on child conduct disorder over and above any indirect genetic correlation between parents and their children.
In addition to the mediating role of impaired parenting, moderating mechanisms such as genotype × environment interaction (GxE) or differences in genetic sensitivity to the environment might account for the differences in outcome in children exposed to the same family-wide environmental risk factors. Given the relatively small sample size, the current data set is not a model system for developing and testing new methods for detecting GxE interaction within a Children of Twins design, but this is clearly an important mechanism to consider in the future.
Despite the significant effect of both genetic and environmental factors on the transmission of risk from the parental ASP, effects specific to early childhood accounted for the majority of the variation in the three childhood behaviors. Significant juvenile specific effects underscore the need for including other environmental risk factors in our models such as other forms of parental psychopathology, family adversity, marital dysfunction, and peer influences.
That parenting can moderate a child’s liability to behavioral and emotional disturbance is well known. Because the Extended Children of Twins design can partition the environmental impact of parental ASP from any genetic risk the parents transmit to their children, we may be able to identify and target genuine family environmental factors for intervention. The different patterns of parental transmission highlight the need for designing behaviorally specific treatments. The importance of the family environment in CD and depression suggests a parent-based intervention that encourages parental warmth and attentiveness, clear limit setting, and the provision of a supportive family environment. The challenge to clinicians is that these parental behaviors are precisely those that ASP parents lack (Woodward, Taylor, & Dowdney, 1998).
When genetic factors are directly influential, as they clearly are with hyperactivity, a targeted intervention is indicated with the goal of involving both the parents and the child in formulating behavioral treatments for reducing genetic risk. If the genes and the environment are correlated, as they are in child CD (i.e., if genetic effects operate through increasing the likelihood that environmental risks would be experienced), genetic risks should be modifiable by interrupting the expression of the environmental factors. Valuable interventions are those that are focused early in the causal chain, with the goal of preventing the risk factor from occurring.
Limitations
The Children of Twins design is a powerful method for identifying effects of the family environment that are often undetected in traditional twin studies (Fonagy, 2003). However, the need for large sample sizes can prohibit the analysis of transmissible risks that are developmentally and gender specific (i.e., genotype × age and genotype × gender interaction).
Additional limitations include reliance on self-reported ratings of parental ASP and mother’s ratings of their children’s behavior via questionnaire. However, our twin correlations for parental ASP and juvenile behavior are consistent with those obtained using more rigorous diagnostic assessments (Eaves et al., 1997). To ensure comparability across studies we were constrained to analyze measures of child behavior used in the Virginia Twin Study of Adolescent Behavioral Development. Although the validity and reliability of these questionnaires are established, we recognize that these are not diagnostically based assessments.
The high degree of comorbidity among psychiatric disorders warrants a study of the pattern of transmissible family effects arising from multiple disorders (e.g., antisociality and depression). It has been shown that depression and antisocial behavior in mothers coveys a greater and different risk to the child compared with depression or antisocial behavior alone (Kim-Cohen, Caspi, Rutter, Tomas, & Moffit, 2006).
The most important limitation of the present analysis is the lack of inclusion of specific parenting behaviors that mediate the association between parental psychopathology and children’s behavioral and emotional disturbance. For more focused and effective family interventions, the elucidation of specific indices of the parenting environment that are involved in the causative process between parents and children are needed.
Key Points.
It is well established that antisocial behavior (ASB) in parents conveys a significant risk for behavioral and emotional problems in children. The nature of these associations is however, unclear.
Using an Extended Children of Twins design (E-COT), we sought to determine whether the association between antisocial parents and children’s psychopathology could be explained by a shared genetic liability, the direct causal impact of the family environment, or an indirect effect of the genes on the rearing environment that ASP parents provide.
The relationship between parental ASP and juvenile depression was solely environmental. Genetic and family environmental factors accounted for the resemblance between parents’ ASP and children’s conduct disturbance. The association between parental ASP and children’s hyperactivity was entirely genetic. All three childhood behaviors were risk factors for antisocial behavior in adulthood.
This study underscores the significant impact of parental ASB as both a genetic and family environmental risk factor for children’s psychopathology. Because we can disentangle the environmental effect of ASP from the transmission of genetic risk for these different child behaviors, we can better design behaviorally-specific environmental interventions for ASP parents and their children.
Table 6.
Proportions of variance (%) in juvenile outcome explained by sources in final “best’ model.
| Genes | Shared Environment |
Passive rGE |
Unique Environment |
|||
|---|---|---|---|---|---|---|
| Parental | Juvenile | Parental | Juvenile | |||
| Conduct disorder (Model 4) | 7.84 | 50.4 | 1.44 | 22.09 | 3.36 | 18.23 |
| Juvenile Depression (Model 5) | 0.00 | 42.3 | 1.96 | 46.24 | 0.00 | 9.5 |
| Child Hyperactivity (Model 3) | 5.33 | 36.00 | 0.00 | 0.00 | 0.00 | 58.67 |
Note: “Parental” genetic and environmental effects refer to contribution of parental ASP to the juvenile outcomes.
Acknowledgements
Supported by grants MH-55557, MH-62368 (JLS) and MH-068521 (L.J.E.) from the National Institute of Mental Health, U01 DA024413 (Eaves, Costello) from the National Institute of Drug Abuse, and UL1RR031990 from the National Center for Research Resources.
We want to thank Michael Rutter for his helpful comments to this manuscript, and the Mid-Atlantic Twin Registry (MATR) for the recruitment of twins and their families.
Footnotes
The authors have disclosed that they have no competing or potential conflicts of interest, financial or otherwise, relevant to the publication of this work.
References
- Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8–17 year olds: effects of age and gender. 2002;43(8):1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- Barber BK, Buehler C. Family cohesion and enmeshment. Journal of Marriage and the Family. 1996;58:443–441. [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Family transmission and heritability of childhood disruptive disorders. American Journal of Psychiatry. 2010;167:1066–1074. doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Developmental origins of early antisocial behavior. Development and Psychopathology. 2009;21:1095–1099. doi: 10.1017/S095457940999006X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronbach LJ. Essentials of psychological testing. 2nd ed. New York: Harper; 1960. [Google Scholar]
- D'Onofrio B, Slutske W, Turkheimer E, Emery RE, Harden KP, Heath A, et al. Intergenerational transmission of conduct problems: A children of twins study. Archives of General Psychiatry. 2007a;64(7):820–829. doi: 10.1001/archpsyc.64.7.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio B, Turkheimer E, Emery R, Maes H, Silberg J, Eaves L. A Children of Twins Study of parental divorce and offspring psychopathology. Journal of Child Psychology and Psychiatry. 2007b;48:667–675. doi: 10.1111/j.1469-7610.2007.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff ES, Pickles A, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Eaves L, Prom E, Silberg J. The mediating effect of parental neglect on adolescent and young adult antisociality:A longitudinal study of twins and their parents. Behavior Genetics. 2010;40(4):425–437. doi: 10.1007/s10519-010-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington DP. The development of offending and antisocial behaviour from childhood: key findings from the Cambridge study in delinquent development. Journal of Child Psychology and Psychiatry. 1995;36:929–964. doi: 10.1111/j.1469-7610.1995.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Farrington DP, Gundry G, West DJ. The familial transmission of criminality. Medicine, Science and the Law. 1975;15:177–186. doi: 10.1177/002580247501500306. [DOI] [PubMed] [Google Scholar]
- Feinberg M, Button T, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and adolescent antisocial behavior and depression: Evidence of genotype X parenting interaction. Archives of General Psychiatry. 2007;64:457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves L, Wormley B, Silberg J, Maes H, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61(7):738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fonagy P. The development of psychopathology from infancy to adulthood: The mysterious unfolding of disturbance in time. Infant Mental Health Journal. 2003;24:212–239. [Google Scholar]
- Goldstein R, Weissman M, Adams P, Horwath E, Lish J, Charnety D, et al. Psychiatric disorders in relatives of probands with panic disorder and/or major depression. Archives of General Psychiatry. 1994;5(15):383–394. doi: 10.1001/archpsyc.1994.03950050043005. [DOI] [PubMed] [Google Scholar]
- Harold G, Rice DF, Hay DA, Boivin M, van den Bree M, Thapar A. Familial transmission of depression and antisocial behavior symptoms: disentangling the contribution of inherited and environmental factors and testing the mediating role of parenting. Psychological Medicine. 2011;41:1175–1185. doi: 10.1017/S0033291710001753. [DOI] [PubMed] [Google Scholar]
- Heath AC, Eaves LJ. Resolving the effects of phenotype and social background on mate selection. Behavior Genetics. 1985;15:15–30. doi: 10.1007/BF01071929. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: Informativeness of different relationships. Behavior Genetics. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Henry B, Moffit TE, Robins L, Earls F, Silva P. Early family predictors of child and adolescent antisocial behavior. Who are the mothers of delinquents? Criminal Behaviour and Mental Health. 1993;3:97–118. [Google Scholar]
- Hewitt JK, Silberg JL, Rutter M, Simonoff E, Meyer JM, Maes H, et al. Genetics and developmental psychopathology: I Phenotypic assessment in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38:943–963. doi: 10.1111/j.1469-7610.1997.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Hoeve M, Dubasd J, Eichelsheim V, van der Laan P, Smeenk W, Gerris J. The relationship between parenting and delinquency: A meta-analysis. Journal of Abnormal Child Psycholgy. 2009;37:749–775. doi: 10.1007/s10802-009-9310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Smailes E, Cohen P, Kasen S, Brook J. Antisocial behaviour parental behaviour, problematic parenting, and aggressive offspring behaviour during adulthood: a 25 year longitudinal investigation. British Journal of Psychiatry. 2004;44:915–930. [Google Scholar]
- Kim-Cohen J, Caspi A, Rutter M, Tomas M, Moffit TE. The caregiving environments provided to children by depressed mothers with or without an antisocial history. American Journal of Psychiatry. 2006;163:1009–1018. doi: 10.1176/ajp.2006.163.6.1009. [DOI] [PubMed] [Google Scholar]
- Knapp M. Heath Economics. In: Rutter M, Bishop D, Pine D, Scott S, Stevenson J, Taylor E, Thapar A, editors. Rutter's Child and Adolescent Psychiatry. 5th ed. Oxford, UK: Wiley-Blackwell; 2008. pp. 123–133. [Google Scholar]
- Loeber R, Stouthamer-Loeber M. Family factors as correlates and predictors of juvenile conduct problems and delinquency. In: Tonry M, Norris N, editors. Crime and Justice. Chicago: University of Chicago Press; 1986. pp. 29–149. [Google Scholar]
- Macoby E, Martin J. Socialization in the context of the family: Parent-child interactions. In: Hetherington E, editor. Handbook of child psychology: Volume IV. Socialization, personality, and social development. New York: Wiley; 1983. [Google Scholar]
- Manuzza S, Klein RG. Long term prognosis in attention deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2000;9(3):711–726. [PubMed] [Google Scholar]
- Medland S, Keller MC. Modeling Extended Twin Family Data II: Power Associated With Different Family Structures. Twin Research and Human Genetics. 2009;12(1):19–25. doi: 10.1375/twin.12.1.19. [DOI] [PubMed] [Google Scholar]
- Nance WE, Corey LA. Genetic models for the analysis of data from the families of identical twins. Genetics. 1976;83:811–826. doi: 10.1093/genetics/83.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusyte J, Neiderhiser J, D'Onofrio B. Testing different types of genotype-environment correlation: An extended Children of Twins model. Developmental Psychology. 2008;44(6):1591–1603. doi: 10.1037/a0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Edition 2003. [Google Scholar]
- Patterson GR. Coercive family process. Eugene, OR: Castalia; 1982. [Google Scholar]
- Pilowsky D, Wickramaratne P, Talati A. Children of Depressed Mothers 1 Year After the Initiation of Maternal Treatment: Findings From the STAR*D-Child Study. American Journal of Psychiatry. 2008;165(9):1136–1147. doi: 10.1176/appi.ajp.2008.07081286. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley E, Eaves L, Foley D, Gardner C, Wormley B, Maes H, et al. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychological Medicine. 2009;39(4):579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial behavior by young people. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Rutter M, Tizard J, Whitmore K. Education, Heath, and Behaviour. London: Longman; 1970. [Google Scholar]
- SAS Institute. User's Guide: Statistics, Version 9.1.3. Cary N.C.: SAS Institute; 2002. SAS. [Google Scholar]
- Schachar RJ, Rutter M, Smith A. The characteristics of situationally and pervasively hyperactive children: Implications for syndrome definition. Journal of Child and Adolescent Psychiatric Nursing. 1981;22:375–392. doi: 10.1111/j.1469-7610.1981.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Scott S. Do parenting programmes for severe child antisocial behavior work over the longer term, and for whom? One year follow-up of a multi-centre controlled trial. Behavioural and Cognitive Psychotherapy. 2005;33(403):421. [Google Scholar]
- Silberg J, Maes HH, Eaves L. Genetic and environmental influences on the transmission of parental depression to children's depression and conduct disturbance. Journal of Child Psychology and Psychiatry. 2010;51(6):734–744. doi: 10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Meyers JM, Pickles A, Simonoff E, Eaves L. Heterogeneity among juvenile antisocial behaviors: Findings from the Virginia Twin Study of Adolescent Behavioral Development. In: Bock GR, Goode JA, editors. Genetics of criminal and antisocial behavior (Ciba Foundation Symposium no. 194. Chichester: Wiley; 1996. pp. 76–85. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer JM, Silberg JL, Maes H, Loeber R, et al. The Virginia Twin Study of Adolescent Behavioral Development: Influences of age, sex, and impairment on rates of disorder. Archives of General Psychiatry. 1997;54:801–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Singh AL, Donofrio BM, Slutske W, Turkheimer E, Emery RE, Harden KP, et al. Parental depression and offspring psychopathology: A children of twins study. Psychological Medicine. 2010;8:1–11. doi: 10.1017/S0033291710002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske W, D'Onofrio B, Turkheimer E, Emery RE, Harden KP, Heath A. Searching for an environmental effect of parental alcoholism on offspring alcohol use disorder: A genetically informed study of children of alcoholics. Journal of Abnormal Psychology. 2008;117(3):534–551. doi: 10.1037/a0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully E, Iacono W, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorder. American Journal of Psychiatry. 2008;165:1148–1154. doi: 10.1176/appi.ajp.2008.07091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L, Taylor E, Dowdney L. The parenting and family functioning of children with hyperactivity. Journal of Child Psychology and Psychiatry. 1998;39:161–169. [PubMed] [Google Scholar]