Abstract
Modification of GABAergic inhibition is an intensely investigated hypothesis guiding research into mechanisms underlying temporal lobe epilepsy (TLE). Seizures can be initiated by blocking γ amino butyric acid type A (GABAA receptors, GABARs), which mediate fast synaptic inhibition in the brain, and controlled by drugs that enhance their function. Derivatives of steroid hormones called neurosteroids are natural substances that physiologically enhance GABAR function and suppress seizures. GABAR structure, function, expression, assembly, and pharmacological properties are changed in the hippocampus of epileptic animals. These alterations render GABARs less sensitive to neurosteroid modulation, which may contribute to seizure susceptibility. Plasticity of GABARs could play a role in periodic exacerbation of seizures experienced by women with epilepsy, commonly referred to as catamenial epilepsy.
Introduction
Temporal lobe epilepsy (TLE) is characterized by recurrent unprovoked seizures arising from the mesial temporal lobe structures (Engel, Jr., 1996; Engel J Jr., 1997; Williamson et al., 1993). Many patients with TLE remain refractory to medical management and may require resective surgery. γ amino butyric acid type A (GABAA) receptors (GABARs) mediate fast inhibitory neurotransmission in the brain. In vivo studies demonstrated that many drugs known to cause seizures in the experimental animals and humans, such as penicillin (Gloor et al., 1979; Schwartzkroin and Prince, 1980), pentylenetetrazol, picrotoxin and bicuculline are GABAR antagonists (Gale, 1992; Gloor et al., 1967; Klitgaard et al., 1998). On the other hand, drugs that potentiate GABAR-mediated inhibition, including diazepam (Choi et al., 1977; Choi et al., 1981; Macdonald and Barker, 1978), Phenobarbital (MacDonald and Barker, 1979; MacDonald et al., 1989a; MacDonald et al., 1989b; Nicoll, 1972; Schmidt, 1963; Twyman et al., 1989) have an anticonvulsant action. These early observations suggested the hypothesis that compromised GABAergic inhibition could lead to the development of epilepsy.
Much of our current understanding of the role of GABARs in the pathogenesis of TLE comes from studies performed on the hippocampal formation. Patients with TLE have characteristic hippocampal pathology, which includes loss of CA1 and CA3 pyramidal neurons and hilar interneurons, with gliosis and sprouting of mossy fiber axons. There is similar hippocampal pathology in animal models of TLE (Buckmaster and Dudek, 1997a; Buckmaster and Jongen-Rêlo, 1999; Houser and Esclapez, 1996; Obenaus et al., 1993; Sloviter, 1987; Sun et al., 2007a). The hippocampal formation has a low threshold for seizures (Traub et al., 1989), and seizures originate in or propagate through the hippocampus in different epilepsy models (Bragin et al., 1999; Bragin et al., 2009). In this review we will discuss TLE-associated changes in GABARs in the hippocampus, with particular focus on the dentate gyrus granule cells (DGCs).
GABAR structure and plasticity in the hippocampus
The heteropentameric GABARs are formed from a family of 19 subunits, namely α (1–6), β (1–3), γ (1–3), δ, ε, π, and ρ (1–3) (Whiting et al., 1999). The subunit composition of GABARs determines their kinetic and pharmacological properties, as well as membrane localization (Olsen and Sieghart, 2009). The α1, α2, α4, α5, β1, β3, γ2 and δ subunit mRNA and peptide are highly expressed in the hippocampus (Sperk et al., 1997). The dentate granule cells (DGCs) and CA1 pyramidal neurons express α1βxγ2 and α2βxγ2 subunit-containing receptors; which mainly mediate fast synaptic inhibition (Prenosil et al., 2006; Sun et al., 2007b; Zhang et al, 2007). In addition, α4βxδ subunit-containing receptors are highly expressed on the DGCs (Wei et al., 2003; Zhang et al, 2007), whereas α5βxγ2 subunit-containing receptors are expressed on the CA1 neurons. These receptor types mediate a steady state background inhibition called tonic inhibition, in response to GABA spilled over from synaptic cleft, or released from neurons or glia via reverse transport (Caraiscos et al., 2004;Farrant and Nusser, 2005; Glykys et al., 2008; Mtchedlishvili and Kapur, 2006; Prenosil et al., 2006).
Reduced neurosteroid sensitivity of GABARs in epileptic animals
Reduced neurosteroid sensitivity of GABARs is a physiologically important alteration associated with epilepsy. Inhibitory neurosteroids have an anticonvulsant action (Belelli et al., 1989; Frye, 1995; Frye and Scalise, 2000; Herzog, 1999;Herzog and Frye, 2003; Kokate et al., 1996; Kokate et al., 1999). Neurosteroids interact with GABARs via two distinct sites (Hosie et al., 2006), such that at lower concentrations they allosterically activate the receptors, and at higher concentrations act like an agonist (Bianchi and MacDonald, 2003). However, neurosteroid sensitivity of GABARs expressed on the DGCs of epileptic animals is diminished. Allopregnanolone modulation of whole cell GABAR currents recorded from DGCs of epileptic animals was much lower than of those recorded from controls (Mtchedlishvili et al., 2001). This diminution was because of reduced neurosteroid sensitivity of both synaptic and tonic GABAR-mediated inhibition. Physiological concentrations of allopregnanolone failed to prolong the decay of mIPSCs in DGCs of epileptic animals (Sun et al., 2007b). Similarly, neurosteroids did not enhance tonic inhibition of DGCs from epileptic animals (Rajasekaran et al., 2010; Zhang et al., 2007). In addition, neurosteroid modulation of mIPSCs recorded from pyramidal and non-pyramidal neurons of piriform cortex of kindled animals was also diminished (Gavrilovici et al., 2006; Kia et al., 2011). Whether neurosteroid modulation of GABARs expressed in other regions such as CA1 pyramidal neurons, thalamic neurons, and cortical neurons is also decreased in epileptic animals is not known. However, an overall reduction in neurosteroid sensitivity of GABARs in epileptic animals may underlie increased susceptibility to seizures.
Factors underlying reduced neurosteroid sensitivity of synaptic and tonic inhibition appear to be complex. Expression, trafficking, and assembly of subunits composing synaptic GABARs is altered in epileptic animals. Immunohistochemical studies revealed higher expression of the α4 and γ2 subunits, mainly in the molecular layer of DGCs, in epileptic animals (Peng et al, 2004). Single-cell PCR amplification from DGCs of epileptic animals demonstrated increased α4 subunit mRNA expression and diminished α1 subunit expressin compared to controls (Brooks-Kayal et al, 1998). Furthermore, in epileptic animals the α4 subunit surface expression was increased and also its association with γ2 subunit (Rajasekaran et al., 2010). Increased co-localization of the α4 and γ2 subunits (Zhang et al., 2007) could explain synaptic expression of the α4γ2 subunit-containing receptors (Sun et al., 2007b). The intrusion of novel α4γ2 subunit-containing GABAR into synapses could contribute to diminished neurosteroid sensitivity of synaptic inhibition of DGCs. In addition, a recent study also demonstrated that increased β3 subunit phosphorylation was associated with reduced neurosteroid modulation of mIPSCs in the pyramidal neurons of piriform cortex from kindled animals (Kia et al., 2011).
The δ subunit-containing receptors, which mediate tonic inhibition of DGCs, have higher neurosteroid sensitivity (Mihalek et al., 1999; Wohlfarth et al., 2002). However, in the epileptic animals, total and surface expression of the δ subunit was reduced (Peng et al., 2004; Rajasekaran et al., 2010; Zhang et al., 2007). A larger fraction of δ subunit was retained in the endoplasmic reticulum in the hippocampus of epileptic animals (Rajasekaran et al., 2010), suggesting that impaired membrane insertion could underlie observed reduction in surface expression of the δ subunit-containing receptors. Proteins regulating trafficking of the δ subunit-containing receptors are not well understood. Brain derived neurotrophic factor (BDNF) and activation of calcium dependent protein kinase C (PKC) regulate surface expression of the δ subunit by reducing receptor internalization (Joshi and Kapur, 2009), and whether they play a role in epileptic animals is not known. Proteins chaperoning insertion of the δ subunit-containing receptors at the surface membrane once identified may reveal mechanisms of its reduced surface expression.
Reduced neurosteroid sensitivity contributes to seizure susceptibility
Reduced neurosteroid sensitivity of GABARs in the epileptic hippocampus has important physiological implications. Neurosteroids can be synthesized from circulating steroid hormones or de novo from cholesterol by neurons and glia [see review (Papadopoulos et al., 2006)]. Steroidogenic acute regulatory protein (StAR) transports cholesterol from cytoplasm to the outer mitochondrial membrane, where translocator proteins, also called as peripheral benzodiazepine receptors, carries it to the inner mitochondrial membrane (Rone et al., 2009). The enzyme cytochrome P450 side chain cleavage (P450ssc) then converts cholesterol to pregnanolone (Kimoto et al., 2001). Subsequent biosynthetic steps occur in the endoplasmic reticulum, and involve conversion of pregnanolone to progesterone by the enzyme 3β hydroxysteroid dehydrogenase (Ibanez et al., 2003). Next, progesterone is converted to allopregnanolone by a two step conversion process involving intermediate 5α dihydro progesterone. These reactions are catalyzed by sequential action of enzymes 5α reductase and 3α hydroxysteroid oxido-reductase (Georges, 2010). 5α reductase is the rate limiting enzyme in this pathway, whereas enzyme 3α hydroxysteroid oxido-reductase has bidirectional activity, and depending on availability of the substrate can convert 5α dihydro progesterone to allopregnanolone or vice a versa.
These biosynthetic enzymes are expressed in the hippocampus (Kimoto et al, 2001; Ibanez et al, 2003). Their immunoreactivity is predominately found in DGCs, pyramidal neurons, glia, and glutamatergic interneurons (Agis-Balboa et al, 2006). In contrast, neurosteroidogenic enzymes are not expressed in GABAergic interneurons. Endogenous neurosteroids appear to regulate kinetic properties of synaptic GABAR currents. Inhibition of enzyme 5α reductase by finasteride accelerates the decay of mIPSCs in lamina II neurons, presumably by reduced neurosteroid synthesis (Keller et al., 2004). In addition, progesterone and its neurosteroid derivatives also influence mRNA, protein, and surface expression of different GABAR subunits (Concas et al., 1998; Maguire et al., 2005; Maguire and Mody, 2007; Michael Foley et al., 2003; Sanna et al., 2009).
Women with epilepsy often experience menstrual cycle-associated seizure exacerbation commonly referred to as catamenial epilepsy (Reddy and Rogawski, 2000; Reddy and Rogawski, 2009). Cyclic fluctuations in progesterone and neurosteroid levels form high (during diestrus) to low (during estrus) associated with female reproductive cycle, are thought to create a seizure susceptibility window. We recently demonstrated regulation of seizure activity by endogenous neurosteroids (Lawrence et al., 2010). In the female epileptic animals, finasteride blockade of enzyme 5α reductase increased seizure frequency hundred folds when progesterone levels were higher. This was in accordance with the hypothesis that a withdrawal from higher progesterone and neurosteroid levels lowers the seizure threshold. Interestingly, finasteride also caused seizure exacerbation at physiological levels of progesterone, or in the animals with fixed progesterone levels. These studies revealed that a sudden decline in neurosteroid levels increased seizure frequency in epileptic animals, and the extent of seizure exacerbation was proportional to pre-finasteride neurosteroid levels. Increased seizure frequency under basal progesterone levels was most likely due to removal of control by endogenous neurosteroids, which could be synthesized locally from cholesterol.
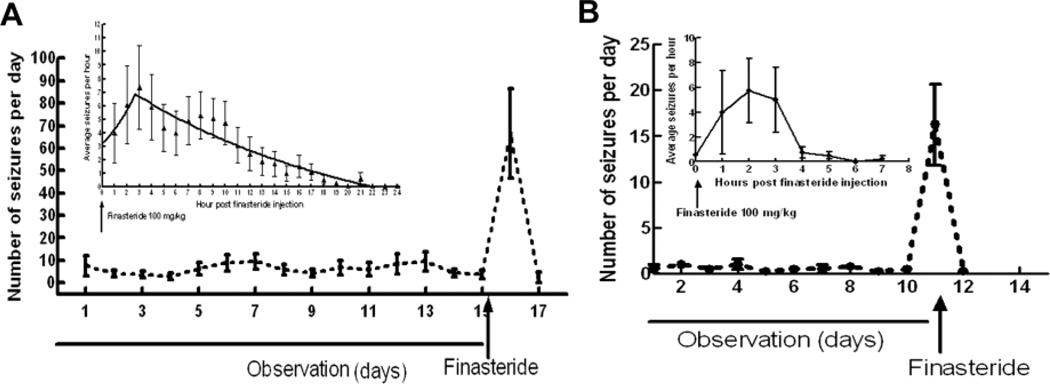
However, all these studies have mainly focused on role of neurosteroids in regulating seizure activity in female animals (Lawrence et al, 2010; Reddy and Rogawsky 2000; Reddy and Rogawski, 2001; Reddy et al, 2001). Since neurons and glia can synthesize neurosteroids de novo from cholesterol, endogenous neurosteroids are likely to regulate seizure frequency in male animals. We tested whether endogenous neurosteroids also regulate seizure activity in male epileptic animals. Following lithium pilocarpine induced status epilepticus (SE); male animals which developed spontaneous seizures were used 8–12 after SE. Hippocampal and cortical electrodes were implanted and following a week of recovery, basal seizure frequency was determined for 10 days. On the morning of 11th day, finasteride (100 mg/kg) was administered. Seizure frequency increased within an hour of finasteride administration and lasted for approximately 3 hours (Figure 1). In each of the animals (n=4), seizure frequency on the day of finasteride administration was higher than the average daily seizure frequency recorded for previous 10 days. Mean seizure frequency during the basal recording period was 0.5 ± 0.11 and increased to 16 ± 4 on the day of finasteride administration (p<0.05, chi square test). This pattern of seizure exacerbation was slightly different from that in the female epileptic animals. For example extent of increase in seizure frequency observed in male animals was not as much as in female animals, and seizure activity returned to the baseline comparatively faster. Some of these differences could be due to endogenous neurosteroid levels as well as time required to replenish them following finasteride treatment. However, despite these differences, the results demonstrate that endogenous neurosteroids regulate seizure activity in both male and female epileptic animals. We propose that finasteride administration precipitates seizures by reducing the neurosteroid levels below a critical threshold needed to activate the relatively neurosteroids insensitive GABARs expressed in the epileptic animals (Figure 2).
Figure 1.
Finasteride increased frequency of spontaneous seizures in epileptic animals. A: Administration of 100 mg/kg finasteride, a 5α reductase enzyme blocker, increased the frequency of spontaneous seizures in female epileptic animals (n=11). Increased seizure frequency returned to baseline 16–18 hrs after finasteride administration (inset) (modified from Lawrence et al, 2010). B: Treatment with finasteride (100 mg/kg) also increased frequency of spontaneous seizures in male epileptic animals (n=4). The seizures returned to baseline frequency 4–5 hrs after finasteride treatment (inset).
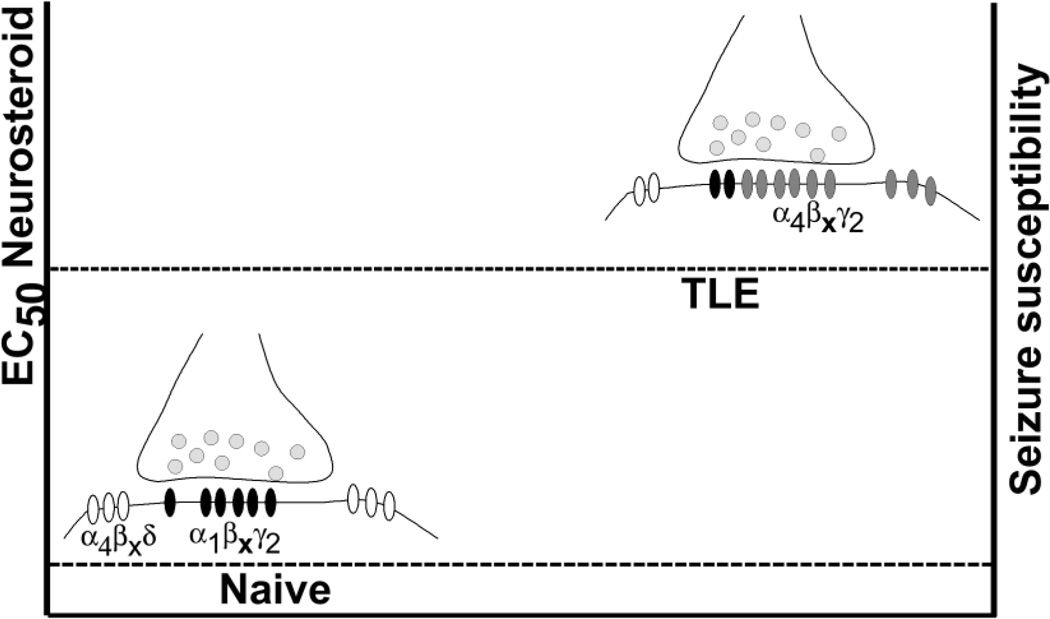
Figure 2.
A schematic showing increased synaptic expression of α4βxγ2 subunit-containing receptors, and reduced expression of α4βxδ subunit-containing receptors in the DGCs of epileptic animals. Expression of receptors with lower neurosteroid sensitivity is proposed to lower seizure threshold in epileptic animals, such that finasteride administration leads to seizure exacerbation.
Reduced neurosteroid sensitivity of GABARs in the DGCs of epileptic animals may also contribute to the breakdown of dentate gating function, hypothesized to underlie seizure spread in the hippocampus (Lothman et al., 1992). However, the gating function of DGCs is only transiently impaired during the course of seizure development (Pathak et al., 2007). Whether neurosteroid sensitivity of GABARs is also diminished during epileptogenesis is currently unknown. Two recent studies suggested an association between reduced neurosteroid sensitivity and epileptogenesis (Biagini et al., 2006; Biagini et al., 2009), which needs to be probed further.
Increased expression of synaptic GABAR in TLE
Another epilepsy associated alteration is augmentation of synaptic GABAR mediated inhibition. Early studies using electrophysiological, biochemical, and electron microscopic techniques revealed increased paired pulse inhibition (de Jonge and Racine, 1987; Tuff et al., 1983), higher ligand binding (Shin et al., 1985; Titulaer et al., 1995; Valdes et al., 1982; Fanelli and McNamara, 1983; McNamara et al., 1980), and greater synaptic expression of the β2/3 subunits (Nusser et al., 1998). Whole cell GABAR currents were larger in DCGs from epileptic animals compared to controls (Gibbs, III et al., 1997; Mtchedlishvilli et al, 2001). Subsequent studies revealed increased synaptic transmission; amplitude of sIPSCs (spontaneous inhibitory post synaptic currents) and mIPSCs (miniature inhibitory post synaptic currents) recorded from DGCs of epileptic animals was larger than that from controls (Cohen et al., 2003; Kobayashi and Buckmaster, 2003; Leroy et al., 2004; Otis et al., 1994; Sun et al., 2007b). Increased expression of GABARs in synapses may be a homeostatic response (Swanwick et al., 2006) to increased activity of recurrent seizures. On the other hand tonic inhibition of DGCs remained unaltered (Rajasekaran et al., 2010; Scimemi et al., 2005; Zhang et al., 2007). Reduced expression of the δ subunit-containing receptors appears to be compensated by increased expression of α4γ2 or α5γ2 subunit-containing GABARs, which maintains tonic inhibition (Rajasekaran et al, 2010; Zhan and Nadler, 2009). In addition to these changes in the DGCs, GABAR function in CA1 pyramidal neurons, thalamic neurons, piriform cortex and subiculum is also modified in epileptic animals (Gavrilovici et al., 2006; Knopp et al., 2008; Rajasekaran et al., 2007).
Frequency of synaptic currents recorded from DGCs of epileptic animals is lower than that recorded from controls. Loss of somatostatin-containing interneurons is thought to underlie this reduction (Buckmaster and Dudek, 1997b; Buckmaster and Jongen-Rêlo, 1999; Kobayashi and Buckmaster, 2003; Sun et al., 2007a). However changes in pre-synaptic release from GABAergic interneurons may contribute to this reduction in IPSC frequency (Zhang et al, 2009).
In addition, status epilepticus (SE) used to induce TLE also triggers neurogenesis of DGCs, which brings about network reorganization and hippocampal dis-inhibition (Parent et al., 1997; Scharfman et al., 2000). A fraction of new born DGCs migrate to ectopic locations such as dentate hilum and GABARs expressed on these neurons have distinct properties compared to those expressed on new born DGCs that stay in the granule cell layer (normotropic) (Zhan and Nadler, 2009). Although the amplitude of eIPSCs recorded from normotropic DGCs were larger than controls, those recorded from ectopic DGCs which migrated to the hilum were significantly attenuated, and suggested that homeostatic alterations occurring in the DGCs were absent in these neurons.
GABARs expressed in the epileptic animals have lower benzodiazepine sensitivity
Although expression of synaptic GABARs is increased in epileptic animals, their benzodiazepine sensitivity is reduced, which could have clinical implications. Benzodiazepines are important anti-convulsants used in the treatment of epilepsy, and increase GABAR function by allosteric modulation. In the DGCs of epileptic animals, bath application of zolpidem did not enhance GABA-evoked whole cell currents (Brooks-Kayal et al., 1998), and similarly diazepam also failed to increase the amplitude or prolong the decay of synaptic currents (Leroy et al., 2004; Sun et al., 2007b). Increased expression of α4γ2 subunit-containing receptors could explain these observations. The pharmacological properties of α4γ2 subunit-containing receptors are distinct from those containing α1γ2 subunits (Wafford et al., 1996). For example, flumazenil acts as a benzodiazepine antagonist at α1γ2 subunit-containing receptors; but has a low efficacy partial agonist-like action on α4γ2 subunit-containing receptors. Furthermore, α4γ2 subunit-containing receptors are also insensitive to modulation by zolpidem or diazepam whereas α1γ2 subunit-containing receptors get potentiated (Knoflach et al., 1996). In a recent study, neurosteroid withdrawal-induced seizures in kindled animals were associated with increased expression of α4γ2 subunit-containing receptors. Diazepam did not suppress withdrawal seizures however flumazenil was effective in preventing these seizures (Gangisetty and Reddy, 2010). This suggests that drugs that activate α4γ2 subunit-containing receptors may be better targets for treating withdrawal seizure.
Zinc sensitivity of GABARs is also increased in the epileptic animals (Brooks-Kayal et al., 1998; Buhl et al., 1996; Gibbs, III et al., 1997), and was proposed to play a role in epileptogenesis [see review (Dudek, 2001)]. It was proposed that zinc released from sprouted mossy fibers would inhibit GABARs expressed on the DGCs of epileptic animals and cause collapse of inhibition. However, in slice experiments activation mossy fibers could not release sufficient zinc to inhibit IPSCs recorded from DGCs of epileptic animals (Molnar and Nadler, 2001).
Potential signaling mechanisms associated with altered expression and subunit assembly of GABARs
Activation of different second messengers underlies observed changes in GABAR expression and subunit assembly. Mitogen activated protein kinases (MAP kinases), specifically extracellular receptor kinase 1/2 (ERK1/2), as well as signal transducer and activator of transcription (STAT), which are activated during SE and spontaneous seizures (Houser et al., 2008; Choi et al., 2003), are thought to play important role in regulation of GABARs. Increased BDNF expression following SE appears to regulate reduced expression of the α1 subunit, increased expression of the α4 subunit and increased assembly between the α4γ2 subunits observed in epileptic animals [see reviews (Brooks-Kayal et al., 2009; González and Brooks-Kayal, 2011)]. BDNF is proposed to increase phosphorylation CREB (cAMP-responsive element binding protein) through activation of RAS/MAP kinases, which in turn results in activation of ICER (Inducible cAMP Early Repressor) leading to decreased expression of α1 subunit. Similarly BDNF induced activation of PKC and MAP kinases is shown to activate transcription factor Egr3 (Early growth response factor) inducing expression of α4 subunit (Roberts et al., 2006). In addition ICER activity can also be regulated through JAK-STAT (Janus kinase/signal transducer and activator of transcription) signaling by BDNF (Lund et al., 2008). Drugs targeting these signaling mechanisms may have therapeutic potentials.
GABAR function and changes in excitability in TLE
Altered function and pharmacology of GABARs in epileptic animals may contribute to synchronization and emergence of seizures. Seizures arise, both in vitro and in vivo, due to the synchronous activation of large populations of neurons (Bragin et al., 2005; Dzhala and Staley, 2003; Khosravani et al., 2005; Miles and Wong, 1983; Traub et al., 1987a; Traub et al., 1993; Traub et al., 1996). The mechanism(s) underlying the generation of neuronal synchrony during seizures is an area of active study. GABAR activation via interneuronal activation can modulate the synchrony and firing of pyramidal neurons in a state-dependent manner (Jefferys and Haas, 1982; Lytton and Sejnowski, 1991; Miles and Wong, 1986; Traub et al., 1987c; Traub et al., 1987b; Wong et al., 1986). The loss of GABAR conductance can produce epileptic synchrony in the hippocampus by making it easier for a single CA3 pyramidal neuron to be activated by a single presynaptic counterpart (Miles and Wong, 1987a; Miles and Wong, 1987b). Thus, recurrent GABAergic inhibition onto CA3 pyramidal neurons via interneuronal input acts as a check to prevent recurrent excitation-induced bursting of CA3 pyramidal neurons. Enhancement of GABAR mediated conductance can desynchronize network synchrony in the CA3 region by significantly reducing the number of recruited pyramidal cells firing in each cycle (Traub et al., 1987a). In the neocortex, the onset of seizure activity is quickly followed by a strong interneuron-mediated feed forward (‘surround’) inhibition (Prince and Wilder, 1967; Trevelyan et al., 2006); however a breakdown of this GABAR mediated inhibition results in the spread of seizures (Trevelyan et al., 2007; Trevelyan, 2009).
It is important to note that even within the hippocampus; the neurons in various subfields have diverse impact on seizure generation and spread. For example, CA3 pyramidal neurons by virtue of their intrinsic bursting properties and high degree of recurrent excitatory connections can rapidly synchronize and propagate bursts, resulting in seizures (MacVicar and Dudek, 1980; Miles and Wong, 1983). In contrast, in the CA1 pyramidal neurons, where recurrent excitatory connections are minimal, synchronization occurs when inhibition maintained by GABAergic interneurons is suppressed (Jefferys and Haas, 1982; Kohling et al., 2000; Traub et al., 1993). In contrast to the pyramidal CA neurons, the DGCs typically generate single action potentials and do not generate bursts (Dzhala and Staley, 2003; Miles et al., 1984). DGCs also lack the recurrent connections necessary to synchronize and propagate bursts; therefore, unlike the pyramidal CA neurons, DGCs generally resist the spread of seizures. However, in TLE, sprouting of the mossy fibers provides a positive excitatory feedback onto the DGCs that leads to recurrent excitability of these neurons (Buckmaster et al., 2002; Dyhrfjeld-Johnsen et al., 2007; Nadler, 2003; Santhakumar et al., 2005; Sutula et al., 1992). We hypothesize that loss of neurosteroid sensitivity on DGCs increase their excitability. It is proposed that tonic inhibition mediated by GABAA receptors, primarily the δ and α5 subunit containing receptors, provides powerful shunting inhibition keeping neuronal excitability in check by potentially modulating the offset (threshold) of the I/O curve (Brickley et al., 1996; Semyanov et al., 2004; Bonin et al., 2007; Pavlov et al., 2009). Thus, tonic conductance mediated by these receptors increase the excitatory drive needed to induce action potential firing. The downregulation of these receptors in TLE can contribute to enhanced excitability (Mihalek et al., 2001; Glykys and Mody, 2006).
Conclusions
Studies over the past three decades have demonstrated a critical role of GABAR mediated neurotransmission in the generation of seizures, and the development of epilepsy. Subunit assembly, function, and pharmacological properties of GABAR expressed in TLE animals are distinct from those in naïve animals. Expression of synaptic receptors appears to be enhanced in the DGCs of epileptic animals; however, their neurosteroid and benzodiazepine sensitivity is diminished. Increased insertion of α4γ2 subunit-containing receptors at the synapses and reduced expression of α1 subunit appears to underlie these changes. Similarly, neurosteroid sensitivity of tonic inhibition is also reduced, along with reduced surface expression of δ subunit-containing receptors. Since endogenous neurosteroids regulate seizure activity, reduced neurosteroid sensitivity of GABARs has physiological significance in epileptic animals. Further studies are needed to determine whether reduction in neurosteroid sensitivity of GABARs is widespread in the epileptic brain. Also, uncovering mechanisms regulating trafficking of the δ subunit-containing GABARs, and novel α4γ2 subunit assembly in the epileptic hippocampus, will significantly advance our knowledge on GABAR mediated neurotransmission in the epileptic brain and its therapeutic relevance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 3.Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Experimental Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5 GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2006;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- 7.Bragin A, Azizyan A, Almajano J, Engel J., Jr The cause of the imbalance in the neuronal network leading to seizure activity can be predicted by the electrographic pattern of the seizure onset. J Neurosci. 2009;29:3660–3671. doi: 10.1523/JNEUROSCI.5309-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 9.Bragin A, Wilson CL, Fields T, Fried I, Engel J., Jr Analysis of seizure onset on the basis of wideband EEG recordings. Epilepsia. 2005;46(Suppl 5):59–63. doi: 10.1111/j.1528-1167.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 10.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Kayal AR, Raol YH, Russek SJ. Alteration of epileptogenesis genes. Neurotherapeutics. 2009;6:312–318. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol. 1997a;77:2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- 14.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997b;385:385–404. [PubMed] [Google Scholar]
- 15.Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckmaster PS, Zhang GF, Yamawaki R. Axon Sprouting in a Model of Temporal Lobe Epilepsy Creates a Predominantly Excitatory Feedback Circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 18.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi DW, Farb DH, Fischbach GD. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977;269:342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- 20.Choi DW, Farb DH, Fischbach GD. Chlordiazepoxide selectively potentiates GABA conductance of spinal cord and sensory neurons in cell culture. J Neurophysiol. 1981;45:621–631. doi: 10.1152/jn.1981.45.4.621. [DOI] [PubMed] [Google Scholar]
- 21.Choi JS, Kim SY, Park HJ, Cha JH, Choi YS, Kang JE, Chung JW, Chun MH, Lee MY. Upregulation of gp130 and differential activation of STAT and p42/44 MAPK in the rat hippocampus following kainic acid-induced seizures. Mol Brain Res. 2003;119:10–18. doi: 10.1016/j.molbrainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of GABAA receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jonge M, Racine RJ. The development and decay of kindling-induced increases in paired-pulse depression in the dentate gyrus. Brain Res. 1987;412:318–328. doi: 10.1016/0006-8993(87)91139-5. [DOI] [PubMed] [Google Scholar]
- 25.Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, Soltesz I. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol. 2007;97:1566–1587. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- 26.Dudek FE. Zinc and Epileptogenesis. Epilepsy Curr. 2001;1:66–70. doi: 10.1046/j.1535-7597.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23:7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel J, Jr, Williamson PD, Weiser HG. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. Mesial temporal lobe epilepsy. [Google Scholar]
- 29.Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 30.Fanelli RJ, McNamara JO. Kindled seizures result in decreased responsiveness of benzodiazepine receptors to GABA. J Pharmacol Exp Ther. 1983;226:147–150. [PubMed] [Google Scholar]
- 31.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA. The neurosteroid 3α, 5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3α, 5α-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- 34.Gale K. Role of GABA in the genesis of chemoconvulsant seizures. Toxicol Lett. 1992;64–65:417–428. doi: 10.1016/0378-4274(92)90215-6. [DOI] [PubMed] [Google Scholar]
- 35.Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABAA receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavrilovici C, D'Alfonso S, Dann M, Poulter MO. Kindling-induced alterations in GABAA receptor-mediated inhibition and neurosteroid activity in the rat piriform cortex. Eur J Neurosci. 2006;24:1373–1384. doi: 10.1111/j.1460-9568.2006.05012.x. [DOI] [PubMed] [Google Scholar]
- 37.Georges P. Progress in Brain Research Neuroendocrinology: The Normal Neuroendocrine System edited by Luciano, Martini. Elsevier; 2010. Steroidogenic Enzymes in the Brain: Morphological Aspects. Chapter 11. [Google Scholar]
- 38.Luciano M, editor. Neuroendocrinology: The Normal Neuroendocrine System. Elsevier; pp. 193–207. [Google Scholar]
- 39.Gibbs JW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 40.Gloor P, Hall G, Coceani F. Differential epileptogenic action of penicillin on cortical and subcortical brain structures. Electroencephalogr Clin Neurophysiol. 1967;23:491. [PubMed] [Google Scholar]
- 41.Gloor P, Pellegrini A, Kostopoulos GK. Effects of changes in cortical excitability upon the epileptic bursts in generalized penicillin epilepsy of the cat. Electroencephalogr Clin Neurophysiol. 1979;46:274–289. doi: 10.1016/0013-4694(79)90202-5. [DOI] [PubMed] [Google Scholar]
- 42.Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- 43.Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González MI, Brooks-Kayal A. Altered GABAA receptor expression during epileptogenesis. Neurosci Lett. 2011;497:218–222. doi: 10.1016/j.neulet.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herzog AG. Progesterone therapy in women with epilepsy: A 3-year follow-up. Neurology. 1999;52 doi: 10.1212/wnl.52.9.1917-a. 1917-191a. [DOI] [PubMed] [Google Scholar]
- 46.Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- 47.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 48.Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 49.Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibanez C, Guennoun R, Liere P, Eychenne B, Pianos A, El Etr M, Baulieu EE, Schumacher M. Developmental Expression of Genes Involved in Neurosteroidogenesis: 3β-Hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase in the rat brain. Endocrinology. 2003;144:2902–2911. doi: 10.1210/en.2002-0073. [DOI] [PubMed] [Google Scholar]
- 51.Jefferys JG, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300:448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- 52.Joshi S, Kapur J. Slow intracellular accumulation of GABAA receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5α-reduced neurosteroids is developmentally regulated and shapes GABAA receptor miniature IPSCs in lamina II of the spinal cord. J Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khosravani H, Pinnegar CR, Mitchell JR, Bardakjian BL, Federico P, Carlen PL. Increased high-frequency oscillations precede in vitro low-Mg seizures. Epilepsia. 2005;46:1188–1197. doi: 10.1111/j.1528-1167.2005.65604.x. [DOI] [PubMed] [Google Scholar]
- 55.Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SSG, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem. 2011;116:1043–1056. doi: 10.1111/j.1471-4159.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- 56.Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: NMDA and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- 57.Klitgaard H, Matagne A, Gobert J, Wnlfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 58.Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant GABAA receptors α4β2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 59.Knopp A, Frahm C, Fidzinski P, Witte OW, Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain. 2008;131:1516–1527. doi: 10.1093/brain/awn095. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kohling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 63.Kokate TG, Banks MK, Magee T, Yamaguchi Si, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in Mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- 64.Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABAA receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- 67.Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT Pathway. Sci STKE. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lytton WW, Sejnowski TJ. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol. 1991;66:1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- 69.Macdonald R, Barker JL. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurons. Nature. 1978;271:563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- 70.Macdonald RL, Barker JL. Anticonvulsant and anesthetic barbiturates: different postsynaptic actions in cultured mammalian neurons. Neurology. 1979;29:432–447. doi: 10.1212/wnl.29.4.432. [DOI] [PubMed] [Google Scholar]
- 71.Macdonald RL, Rogers CJ, Twyman RE. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurons in culture. J Physiol. 1989a;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macdonald RL, Rogers CJ, Twyman RE. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurons in culture. J Physiol. 1989b;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980;184:220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- 74.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 75.Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J. Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNamara JO, Peper AM, Patrone V. Repeated seizures induce long-term increase in hippocampal benzodiazepine receptors. Proc Natl Acad Sci USA. 1980;77:3029–3032. doi: 10.1073/pnas.77.5.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michael Foley C, Stanton JJ, Price EM, Thomas Cunningham J, Hasser EM, Heesch CM. GABAA α1 and α2 receptor subunit expression in rostral ventrolateral medulla in nonpregnant and pregnant rats. Brain Research. 2003;975:196–206. doi: 10.1016/s0006-8993(03)02635-0. [DOI] [PubMed] [Google Scholar]
- 78.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in GABAA receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miles R, Wong RK. Single neurons can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- 80.Miles R, Wong RK. Excitatory synaptic interactions between CA3 neurons in the guinea-pig hippocampus. J Physiol. 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987a;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miles R, Wong RK. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987b;329:724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- 83.Miles R, Wong RK, Traub RD. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience. 1984;12:1179–1189. doi: 10.1016/0306-4522(84)90012-5. [DOI] [PubMed] [Google Scholar]
- 84.Molnar P, Nadler JV. Lack of effect of mossy fiber-released zinc on granule cell GABAA receptors in the pilocarpine model of epilepsy. J Neurophysiol. 2001;85:1932–1940. doi: 10.1152/jn.2001.85.5.1932. [DOI] [PubMed] [Google Scholar]
- 85.Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- 87.Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 88.Nicoll RA. The effects of anesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972;223:803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 90.Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olsen RW, Sieghart W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of GABAA receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 94.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prenosil GA, Schneider G, Edith M, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- 99.Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. "Surround" inhibition. Arch Neurol. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- 100.Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajasekaran K, Kapur J, Bertram EH. Alterations in GABAA receptor mediated inhibition in adjacent dorsal midline thalamic nuclei in a rat model of chronic limbic epilepsy. J Neurophysiol. 2007;98:2501–2508. doi: 10.1152/jn.00139.2007. [DOI] [PubMed] [Google Scholar]
- 102.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- 103.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- 104.Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- 105.Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor α4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 107.Rone MB, Liu J, Blonder J, Ye X, Veenstra TD, Young JC, Papadopoulos V. Targeting and insertion of the cholesterol-binding translocator protein into the outer mitochondrial membrane. Biochemistry. 2009;48:6909–6920. doi: 10.1021/bi900854z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol. 2005;93:437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- 110.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt RF. Pharmacological studies on the primary afferent depolarization of the toad spinal cord. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- 112.Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980;183:61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- 113.Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends in Neurosciences. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Shin C, Pedersen HB, McNamara JO. GABA and benzodiazepine receptors in the kindling model of epilepsy: a quantitative radiohistochemical study. J Neurosci. 1985;5:2696–2701. doi: 10.1523/JNEUROSCI.05-10-02696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 117.Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv. Exp. Med. Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- 118.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 119.Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007a;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci. 2007b;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sutula TP, Golarai G, Cavazos J. Assessing the functional significance of mossy fiber sprouting. Epilepsy Res Suppl. 1992;7:251–259. [PubMed] [Google Scholar]
- 122.Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABA-ergic synapse strength is regulated by brain-derived neurotrophic factor. Mol Cell Neurosci. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Titulaer MN, Kamphuis W, Lopes da Silva FH. Long-term and regional specific changes in [3H]flunitrazepam binding in kindled rat hippocampus. Neuroscience. 1995;68:399–406. doi: 10.1016/0306-4522(95)00158-f. [DOI] [PubMed] [Google Scholar]
- 124.Traub RD, Borck C, Colling SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 125.Traub RD, Knowles WD, Miles R, Wong RK. Models of the cellular mechanism underlying propagation of epileptiform activity in the CA2-CA3 region of the hippocampal slice. Neuroscience. 1987a;21:457–470. doi: 10.1016/0306-4522(87)90135-7. [DOI] [PubMed] [Google Scholar]
- 126.Traub RD, Miles R, Wong RK. Models of synchronized hippocampal bursts in the presence of inhibition. I. Single population events. J Neurophysiol. 1987b;58:739–751. doi: 10.1152/jn.1987.58.4.739. [DOI] [PubMed] [Google Scholar]
- 127.Traub RD, Miles R, Wong RK, Schulman LS, Schneiderman JH. Models of synchronized hippocampal bursts in the presence of inhibition. II. Ongoing spontaneous population events. J Neurophysiol. 1987c;58:752–764. doi: 10.1152/jn.1987.58.4.752. [DOI] [PubMed] [Google Scholar]
- 128.Traub RD, Miles R, Jefferys JG. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol. 1993;461:525–547. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Traub RD, Miles R, Wong RK. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science. 1989;243:1319–1325. doi: 10.1126/science.2646715. [DOI] [PubMed] [Google Scholar]
- 130.Trevelyan AJ. The direct relationship between inhibitory currents and local field potentials. J Neurosci. 2009;29:15299–15307. doi: 10.1523/JNEUROSCI.2019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tuff LP, Racine RJ, Adamec R. The effects of kindling on GABA-mediated inhibition in the dentate gyrus of the rat. I. Paired-pulse depression. Brain Res. 1983;277:79–90. doi: 10.1016/0006-8993(83)90909-5. [DOI] [PubMed] [Google Scholar]
- 134.Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of GABA receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- 135.Valdes F, Dasheiff RM, Birmingham F, Crutcher KA, McNamara JO. Benzodiazepine receptor increases after repeated seizures: evidence for localization to dentate granule cells. Proc Natl Acad Sci. 1982;79:193–197. doi: 10.1073/pnas.79.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABAA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- 137.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whiting PJ. The GABAA receptor gene family: new targets for therapeutic intervention. Neurochem Int. 1999;34:387–390. doi: 10.1016/s0197-0186(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 139.Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 140.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong RK, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]
- 142.Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]