Abstract
Objective
To evaluate the presence of pulmonary abnormalities in subjects with rheumatoid arthritis (RA)-related autoantibody (Ab) positivity without inflammatory arthritis (IA).
Methods
42 subjects without IA but with elevations of anti-cyclic citrullinated peptide antibodies and/or 2 or more rheumatoid factor isotypes (a profile that is 96% specific for RA), 15 Ab(−) controls and 12 patients with early established seropositive RA (<1 year duration) underwent spirometry and high-resolution computed tomographic (HRCT) lung imaging.
Results
The median age of Ab(+) subjects was 54 years-old, 52% were female and 38% were smokers (not significantly different than Ab(−) controls). No Ab(+) subject had IA on joint examination. On HRCT, 76% of Ab(+) subjects had airways abnormalities including bronchial wall thickening, bronchiectasis, centrilobular opacities and air trapping, compared to 33% of Ab(−) controls (p=0.005). The Ab(+) subjects had similar prevalence and type of lung abnormalities compared to patients with early RA. Two Ab(+) subjects with airways disease developed IA classifiable as articular RA ~13 months after lung evaluation.
Conclusion
Airways abnormalities that are consistent with inflammation are common in Ab(+) subjects without IA, and similar to airways abnormalities seen in early RA. These findings suggest that the lung may be an early site of autoimmune-related injury, and potentially a site of generation of RA-related autoimmunity. Further studies are needed to define the mechanistic role of lung inflammation in the development of RA.
Keywords: Rheumatoid arthritis, etiology, autoantibodies, preclinical, lung disease
Introduction
Multiple studies have identified a ‘preclinical’ phase of seropositive rheumatoid arthritis (RA) during which there are elevations of circulating RA-related autoantibodies (Abs) prior to the onset of symptomatic inflammatory arthritis (IA).(1) Lung disease has also been identified in early symptomatic RA as well as prior to the onset of articular symptoms of RA (2–4), perhaps because the lung is an early target of systemic RA-related autoimmunity, or because the lung is potentially a site of generation of RA-related autoimmunity due to initial autoimmune responses in the lung. This latter possibility is a consideration because Ab elevations prior to the onset of clinically-apparent RA suggest that RA may be generated at an extra-articular site.(5) To explore further the relationship between RA-related autoimmunity and inflammatory lung abnormalities in absence of clinically-apparent IA, we evaluated the lungs of RA-related Ab(+) subjects without IA, and compared these findings to Ab(−) subjects, and subjects with early, seropositive RA.
Methods
Study subjects
This study utilized the Studies of the Etiology of Rheumatoid Arthritis (SERA) project, a prospective cohort established to investigate the natural history of RA development. The SERA project is described elsewhere (6); briefly, however, probands with RA are identified, and their first-degree relatives without RA are recruited for prospective study; also, RA-related Ab(+) subjects without RA are recruited into SERA through community health-fair screening. Once enrolled, SERA subjects undergo standardized joint assessment (symptoms and a 68-joint examination) and the following Ab testing: RF isotypes IgM, IgA and IgG (ELISA (QUANTA Lite™ kits, INOVA Diagnostics, Inc.), anti-CCP2 (Diastat, Axis-Shield Diagnostics, Ltd.), and anti-CCP3.1 (IgA/IgG INOVA Diagnostics, Inc.). Positivity for each RF isotype was established based on levels positive in <5% of 491 blood donor controls; kit cut-offs were used for anti-CCP positivity (anti-CCP2 >5 units; anti-CCP3.1 ≥20 units).
For this lung study, Ab(+) subjects were recruited from 2 SERA study sites: Denver and Los Angeles (LA). These subjects did not have IA and were positive for anti-CCP2 or anti-CCP3.1, and/or 2 or more RF isotypes; this profile was 96% specific for established RA when tested in 200 SERA probands with RA and 200 blood donor controls and is therefore likely to reflect true RA-related autoimmunity. Ab(−) subjects were recruited as controls from the Denver SERA site, frequency matched to the Ab(+) subjects on age, gender, race and smoking history. To limit those exposed to radiation, the number of controls studied with HRCT was based on attaining 80% power to detect a ≥35% difference in the prevalence of lung abnormalities between Ab(+) and Ab(−) subjects (alpha 0.05). We recruited from Univ of Colorado clinics Early RA subjects who fulfilled 1987 American College of Rheumatology (ACR) RA criteria, were positive for RF and anti-CCP2, were <1 year since their RA diagnosis, and were without a clinical history of RA-related lung disease.
Lung study protocol
All subjects completed a standardized questionnaire regarding possible prior lung disease, underwent spirometry and high-resolution computed tomographic (HRCT) lung imaging; SERA subjects also underwent joint symptom assessment and a 68-joint examination by a trained examiner. HRCT was performed using multi-detector scanners, with helical supine inspiratory acquisition reconstructed contiguous (5 mm) images as well as 1 mm images reconstructed every 20 mm with high resolution reconstruction algorithms. Supine expiratory 1 mm axial images were obtained every 40 mm. Finally, prone inspiratory images were obtained with 1 mm collimation every 40 mm. HRCTs were reviewed by 2 chest radiologists blinded to subjects’ Ab or disease status; abnormalities were defined and scored using standard criteria (7), and classified as follows: 1) airways disease (bronchial wall thickening, bronchiectasis, centrilobular opacities [representing bronchiolar disease] and abnormal air trapping), and 2) parenchymal disease (alveolar infiltrates, nodules and interstitial lung disease/fibrosis). After initial independent interpretations, the radiologists reached a consensus on discrepant findings while still blinded to Ab/disease status; these final interpretations were used for analyses.
Statistical analysis
Non-parametric testing was used to compare findings between groups, and kappa statistics were used to determine inter-observer agreement for HRCT interpretations.
Ethical considerations
Institutional review boards at the participating institutions approved all study protocols.
Results
Subjects
56 SERA subjects were eligible by Ab(+) status; of these, 42 (75%) agreed to participate; 18 Ab(−) subjects were contacted to obtain the 15 enrolled in the study. Twelve of 15 (80%) Early RA subjects eligible for inclusion agreed to participate. The primary reasons that subjects declined to participate were concerns regarding imaging-associated radiation and possible discovery of non-RA related findings (e.g. lung cancer); only one Ab(+) case participated in the study to learn more about their existing lung problems.
Subject characteristics are reported in Table 1. There were no significant differences between Ab(+) and Ab(−) subjects in age, gender, race, smoking status, or self-reported history of health-care provider diagnosed chronic lung disease, bronchitis and pneumonia. When compared to the Ab(+) subjects, the Early RA subjects had non-statistically significant higher rates of chronic lung disease diagnosed prior to the onset of their RA.
Table 1.
Subject characteristics at the time of lung evaluation
| Variable | Autoantibody Positive Cases (N=42) |
Autoantibody Negative Controls (N=15) |
P-value+ | Early RA* (N=12) |
P-value++ |
|---|---|---|---|---|---|
| Age, median (quartiles 25, 75) | 54 (43, 62) | 53 (41, 67) | 0.890 | 53 (40, 59) | 1.000 |
| Female | 22 (52%) | 9 (60%) | 0.765 | 8 (67%) | 0.515 |
| Non-Hispanic White | 37 (88%) | 13 (87%) | 1.000 | 10 (83%) | 0.645 |
| ≥1 ‘shared epitope’ allele# | 22 (52%) | 8 (53%) | 1.000 | 6/7 (86%) | 0.215 |
| Ever smoked | 16 (38%) | 3 (20%) | 0.339 | 5 (42%) | 1.000 |
| Pack-years, median (range) | 7 (3,19) | 1 (1, 25) | 0.387 | 20 (16, 41) | 0.823 |
| Chronic lung disease** | 8 (19%) | 1 (7%) | 0.420 | 6 (50%) | 0.057 |
| Health-care provider diagnosed: | |||||
| Pneumonia | 18 (43%) | 2 (13%) | 0.109 | 5 (42%) | 1.000 |
| Acute bronchitis | 18 (43%) | 6 (40%) | 1.000 | 1 (8%) | 0.039 |
| Joint symptoms*** | 21 (50%) | 8 (53%) | 1.000 | Not assessed | Not assessed |
| 68-Joint count | |||||
| % with ≥1 tender joint | 8 (19%) | 2 (13%) | 1.000 | Not assessed | Not assessed |
| % with inflammatory arthritis | 0 (0%) | 0 (0%) | 1.000 | ||
| Autoantibody prevalence | |||||
| Anti-CCP2orCCP3.1 | 30 (71%) | N/A | N/A | 12 (100%) | N/A |
| Any CCP and any RF isotype | 16 (38%) | 11 (92%) | |||
| 2 or more RF isotypes | 20 (48%) | 12 (100%) | |||
| RF-IgM | 18 (43%) | 11 (92%) | |||
| RF-IgG | 24 (57%) | 9 (75%) | |||
| RF-IgA | 10(24%) | 9 (75%) |
The Early RA patients all fulfilled the 1987 ACR RA Classification Criteria (Arnett FC et al, Arthritis Rheum 1988) and were rheumatoid factor and anti-cyclic citrullinated peptide-2 positive; their median onset of arthritis symptoms prior to the lung study was 9 months, and the median time since diagnosis was 5 months. Disease-modifying anti-rheumatic drug use: 10/12 prednisone; 8/12 methotrexate; 4/12 hydroxychloroquine; 2/12 non-steroidal anti-inflammatory agents only.
Methodology for shared epitope testing provided in Kolfenbach et al Arthritis Rheum 2009.
Includes self-reported health-care provider diagnosed asthma, chronic bronchitis, emphysema, diagnosed prior to lung study (or diagnosis of RA) and ascertained after study enrollment. For Early RA subjects, 3 had a prior diagnosis of asthma, and 3 had a prior diagnosis of emphysema.
Results of standardized questionnaire performed at the time of lung study ascertaining subject-reported current or prior pain, stiffness or swelling in any of 68-joints.
P-value comparing autoantibody positive cases to autoantibody negative controls.
P-value comparing autoantibody positive cases to EarlyRA.
Abbreviations: RA = rheumatoid arthritis; N/A=not applicable.
Joint findings
At the time of lung evaluation, 50% of Ab(+) subjects and 53% of Ab(−) controls self-reported current or prior pain, stiffness or swelling in at least one joint; in all subjects, these were attributed to one of the following: prior injury, health-care provider diagnosed osteoarthritis, fibromyalgia, or hemochromatosis. All but 2 of the SERA subjects underwent simultaneous joint and lung evaluation, and none had evidence of synovitis. Of the two subjects (both Ab(+)) that were not examined, at the time of lung evaluation neither reported joint symptoms during telephone interview using the standardized SERA joint questionnaire (6), and one had a normal joint examination at a research visit 6 months prior.
HRCT and Spirometry
HRCT and spirometry results are reported in Table 2. HRCT demonstrated airways abnormalities in significantly more Ab(+) cases than Ab(−) controls (76% versus 33%, p=0.005) (representative images for airway thickening are provided in Figure 1). Significant differences in the prevalence of airways abnormalities between Ab(+) and Ab(−) subjects persisted even after subgrouping by smoking status, site of study, history of chronic lung disease and presence of joint tenderness. Airways abnormalities were present in 11/12 (92%) of Early RA subjects, a prevalence which was not significantly different than that seen in Ab(+) cases (p=0.421). Pulmonary parenchymal abnormalities were most prevalent in the Early RA subjects. There were no significant associations between subject-related factors within Ab(+) subjects (including specific Abs) and HRCT findings (data not shown), although the majority of subjects with abnormal spirometry also had HRCT evidence of airways disease (Table 2). After initial individual reviews, the two chest radiologists agreed on 59/69 (85.5%) interpretations, with a kappa of 0.68.
Table 2.
Pulmonary evaluation results
| Variable | Autoantibody Positive Cases (N=42) |
Autoantibody Negative Controls (N=15) |
P- value+ |
Early RA (N=12) |
P- value++ |
|---|---|---|---|---|---|
| Spirometry* | |||||
| FEV1/FVC ratio <70% predicted | 5 (12%) | 0 (0%) | 0.311 | 4 (33%) | 0.098 |
| Forced expiratory flow (25–75) <70% predicted | 13 (31%) | 2 (13%) | 0.187 | 6 (50%) | 0.307 |
| High-resolution computed tomography (HRCT) | |||||
| Any Airways Disease (all subjects)** | 32 (76%) | 5 (33%) | 0.005 | 11 (92%) | 0.421 |
| Bronchial wall thickening | 21 (50%) | 2 (13%) | 0.015 | 10 (83%) | 0.041 |
| Bronchiectasis | 6 (14%) | 1 (7%) | 0.662 | 2 (17%) | 1.000 |
| Centrilobular opacities | 10 (24%) | 1 (7%) | 0.256 | 6 (50%) | 0.148 |
| Air trapping | 29 (69%) | 1 (7%) | 0.000 | 10 (83%) | 0.474 |
| Airways disease, never smokers | 19/26 (73%) | 4/12 (33%) | 0.033 | 6/7 (86%) | 0.652 |
| Airways disease, no history of lung disease*** | 22/31 (71 %) | 5/14 (36%) | 0.047 | 5/6 (83%) | 1.000 |
| Airways disease, Denver participants only | 22/31 (71 %) | 5/15 (33%) | 0.025 | 11/12 (92%) | 0.237 |
| Airways disease, no joint tenderness | 26/34 (76%) | 5/13 (38%) | 0.020 | - | - |
| Parenchymal disease**** | 4 (10%) | 1 (7%) | 1.000 | 5 (42%) | 0.019 |
| Nodules | 4 (10%) | 0 (0%) | 0.564 | 3 (25%) | 0.175 |
| Alveolar infiltrates (ground glass opacities) | 0 (0%) | 1 (7%) | 0.263 | 2 (17%) | 0.046 |
| Lung fibrosis | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 1.000 |
|
Spirometry in subjects with airways disease identified by HRCT* |
|||||
| FEV1/FVC ratio <70% predicted | 4/32(13%) | 0/5 (0%) | - | 4/11 (36%) | - |
| Forced expiratory flow (25–75) <70% predicted | 12/32 (38%) | 0/5 (0%) | - | 6/11 (55%) | - |
Predictive values calculated according to the 3rd National Health and Nutritional Examination Survey (NHANES III)(Hankinson JL et al, Am J Resp Crit Care Med 1999); Obstructive disease = FEV1/FVC <70% of predicted; Reduced Forced Expiratory Flow (25–75) may be more sensitive for airways disease and obstruction than FEV1/FVC measurements (Ciprandi G et al, Am J Rhin 2006).
Airways disease included bronchial thickening, bronchiectasis, air trapping or centrilobular nodularity (these latter findings indicating small airways disease/inflammation); parenchymal disease = alveolar infiltrates (ground glass appearance on HRCT) and parenchymal nodules. Of note, air trapping results when small airways disease results in obstruction of airway outflow and subsequent overdistension of the alveoli on expiration which is seen on HRCT imaging as increased air density.
Chronic lung disease as assessed at the time of the lung study visit by questionnaire and including emphysema, asthma, chronic bronchitis as diagnosed by a health-care provider.
Parenchymal disease included ground-glass opacities, parenchymal nodules and lung fibrosis.
P-value comparing autoantibody positive cases to autoantibody negative controls.
P-value comparing autoantibody positive cases to Early RA.
Figure 1. High-resolution computed tomographic images demonstrating bronchial wall thickening.
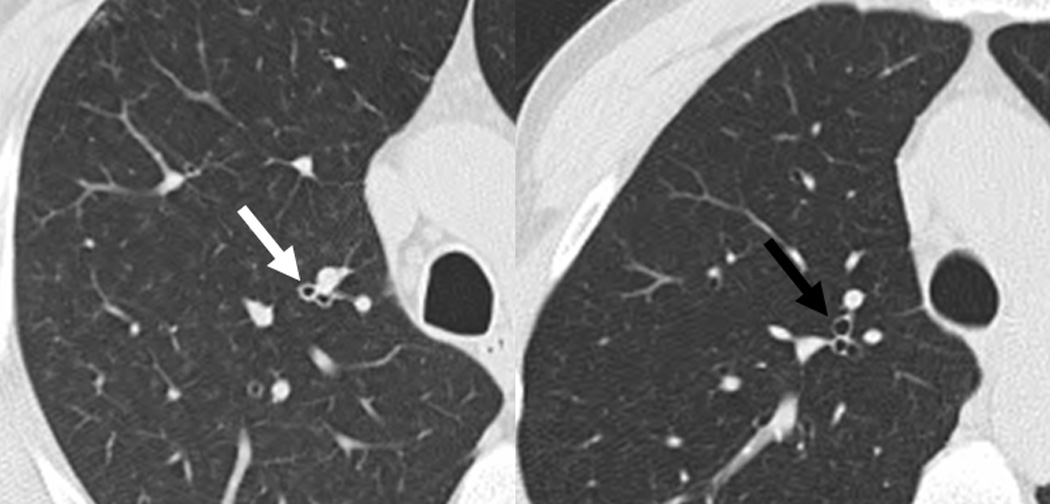
Images are from similar lung anatomic levels from a rheumatoid arthritis (RA)-related autoantibody positive case (left image; anti-cyclic citrullinated peptide antibody and rheumatoid factor isotypes IgM and IgA positive), and a RA-related autoantibody negative control (right image) that was matched to the case on age, sex and smoking status. The left image demonstrates bronchial wall thickening (white arrow). In contrast, the right image demonstrates a normal-appearing thin-walled bronchus (black arrow).
Post-lung study follow-up
Two subjects with airways abnormalities developed joint symptoms consistent with IA approximately 13 months after lung study. Both were subsequently diagnosed with RF and anti-CCP positive RA per 1987 ACR criteria.
Discussion
Herein we have identified airways abnormalities in a high proportion of RA-related Ab(+) subjects without IA. We have not obtained lung tissue from these Ab(+) subjects although, historically, biopsies from patients with established RA and similar HRCT findings have shown significant airways inflammation (2); therefore, we believe that the airways abnormalities seen in this study are due to inflammatory changes. Of note, spirometry was not significantly different between Ab(+) and Ab(−) subjects, although this is not unexpected as HRCT is a more sensitive measure for airways disease.(8)
However, while we believe that the airways abnormalities seen in these subjects are due to inflammation, the relationship between this inflammation and circulating RA-related Abs is unknown. It may be that these abnormalities are unrelated to Ab status, or that circulating RA-related autoimmunity generated outside the lung has targeted the airways. Alternatively, in the context of the hypothesis that RA-related autoimmunity is initiated at an extra-articular site (5), these findings may indicate that RA-related autoimmunity is initially generated in the lungs. This possibility is supported by the associations of inhaled factors (including smoking and dust) with increased risk for RA (9), as well as by the known immunobiology of the lung where inflammation and adaptive immune responses can develop in response to inhaled factors. In particular, Rangel-Moreno and colleagues have identified collections of organized lymphatic tissue termed ‘inducible bronchus associated lymphatic tissue’ (iBALT) in the lungs of patients with established RA and lung disease.(10) Importantly, they also demonstrated that plasma cells within iBALT from patients with RA and lung disease were reactive to the Fc portions of IgG and to citrullinated fibrinogen, suggesting that RF and antibodies to citrullinated protein antibodies (ACPAs) were being generated in the lungs although the initial trigger for the generation of iBALT and these Abs was not identified.(10) Furthermore, RF production in the lungs of patients with cystic fibrosis, likely due to chronic infection-related inflammation, supports the lung as a site of generation of RA-related autoimmunity.(11)
With these issues in mind, while speculative, in a model where RA-related autoimmunity is initiated in the lung, an environmental factor such as smoking or infection may trigger airways inflammation and local generation of autoantigens, similar to what has been demonstrated by Makrygiannakis et al who found elevated levels of citrullinated proteins in lung samples from smokers.(12) Subsequently, autoreactive cells and autoantibodies such as ACPAs may develop within the lung (9); local inflammation in the lung may also result in RF production (4), serving as a possible explanation for the high concordance of ACPA and RF elevations in patients with established RA. Once autoimmune factors develop, they may transmit from the lung to the circulation via regional lymphatics or translocation, and circulating autoimmunity may later trigger the characteristic IA of RA.
Based on this hypothetical model of RA development, the airways abnormalities seen herein suggest that the lungs of these Ab(+) subjects may harbor biologic factors that initially generate RA-related Abs. However, there are important caveats to these findings and resultant speculations regarding the initiation of RA in the lungs. First, the concept that RA is generated outside the joints is speculative, although data identifying RA-related Ab elevations prior to the onset of clinically-apparent IA support that RA may indeed be generated outside the joints.(1, 5) Second, it is uncertain that the Ab(+) subjects in this study are truly in a preclinical RA state since only 2 have developed classifiable RA; however, the presence of RA-related autoimmunity regardless of progression to future symptomatic IA is still relevant to understanding the pathogenesis of RA. Third, if RA does develop at an extra-articular site, the lung may not be the only site where this might occur, as data suggest other sites such as the periodontal region may be involved in RA pathogenesis.(13) Fourth, it may be that Ab elevations identified herein are non-specific factors associated with chronic lung inflammation or infection.(4, 14) Fifth, it is possible that the Ab(+) and Early RA subjects were inadvertently enriched with factors that may affect their airways including smoking and/or pre-existing lung disease, or that Ab(−) controls had healthier lungs than is typical, although subjects were not selected based on lung disease, and the prevalence of lung disease in these Early RA and Ab(−) subjects is not atypical given published findings.(2, 15) Finally, the Ab(+) subjects may have subtle joint inflammation that is undetected by physical examination suggesting their lung findings are not present in absence of synovitis. However, supporting that subjects’ clinical examinations are effective to rule-out synovitis, in preliminary study we found no evidence of synovitis on contrasted magnetic resonance imaging of the dominant-side metacarpal-phalangeal joints, wrists and metatarsal-phalangeal joints of a subset of 15 Ab(+) subjects with lung abnormalities (data not shown).
Conclusion
Airways abnormalities in RA-related Ab(+) individuals without apparent IA suggest that the lungs are an early target, or potentially a site of initial generation, of RA-related autoimmunity. These findings provide support for additional studies to evaluate the mechanisms by-which the lungs may be involved in RA development. These studies may include serial assessments examining the progression of lung abnormalities and circulating autoimmunity in relationship to the development of IA, tissue sampling to define the biology of lung injury and inflammation in relation to potential generation of RA-related Abs, and examination of genetic and environmental factors that may be associated with potential pulmonary generation of RA-related autoimmunity.
Acknowledgments
Grant Funding: NIH AR051461, AI50864, and AR051394; American College of Rheumatology Research and Education Foundation’s Within Our Reach Program; The Walter S. and Lucienne Driskill Foundation.
References
- 1.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36(2):213–241. doi: 10.1016/j.rdc.2010.02.001. Epub 2010/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443–448. doi: 10.1513/pats.200703-045MS. Epub 2007/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scand J Rheumatol. 2007;36(5):338–344. doi: 10.1080/03009740701393957. [DOI] [PubMed] [Google Scholar]
- 4.Gizinski AM, Mascolo M, Loucks JL, Kervitsky A, Meehan RT, Brown KK, et al. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin Rheumatol. 2009;28(5):611–613. doi: 10.1007/s10067-009-1128-9. Epub 2009/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Sande MG, de Hair MJ, van der Leij C, Klarenbeek PL, Bos WH, Smith MD, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis. 2011;70(5):772–777. doi: 10.1136/ard.2010.139527. Epub 2010/12/24. [DOI] [PubMed] [Google Scholar]
- 6.Kolfenbach JR, Deane KD, Derber LA, O'Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–1742. doi: 10.1002/art.24833. Epub 2009/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. Epub 2008/01/16. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009;136(6):1521–1528. doi: 10.1378/chest.09-0174. Epub 2009/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 10.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–3194. doi: 10.1172/JCI28756. Epub 2006/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiotz PO, Egeskjold EM, Hoiby N, Permin H. Autoantibodies in serum and sputum from patients with cystic fibrosis. Acta Pathol Microbiol Scand C. 1979;87(5):319–324. Epub 1979/10/01. [PubMed] [Google Scholar]
- 12.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 13.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233(1):34–54. doi: 10.1111/j.0105-2896.2009.00850.x. Epub 2010/03/03. [DOI] [PubMed] [Google Scholar]
- 14.Elkayam O, Segal R, Bendayan D, van Uitert R, Onnekink C, Pruijn GJ. The anti-cyclic citrullinated peptide response in tuberculosis patients is not citrulline-dependent and sensitive to treatment. Arthritis Res Ther. 2010;12(1):R12. doi: 10.1186/ar2913. Epub 2010/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copley SJ, Wells AU, Hawtin KE, Gibson DJ, Hodson JM, Jacques AE, et al. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251(2):566–573. doi: 10.1148/radiol.2512081242. Epub 2009/04/30. [DOI] [PubMed] [Google Scholar]


