Abstract
ATP-binding cassette (ABC) transporters confer drug resistance against a wide range of chemotherapeutic agents, including nucleoside and nucleotide based drugs. While nucleoside based drugs have been used for many years in the treatment of solid and hematological malignancies as well as viral and autoimmune diseases, the potential contribution of ABC transporters has only recently been recognized. This neglect is likely because activation of nucleoside derivatives require an initial carrier-mediated uptake step followed by phosphorylation by nucleoside kinases, and defects in uptake or kinase activation were considered the primary mechanisms of nucleoside drug resistance. However, recent studies demonstrate that members of the ABCC transporter subfamily reduce the intracellular concentration of monophosphorylated nucleoside drugs. In addition to the ABCC subfamily members, ABCG2 has been shown to transport nucleoside drugs and nucleoside-monophosphate derivatives of clinically relevant nucleoside drugs such as cytarabine, cladribine, and clofarabine to name a few. This review will discuss ABC transporters and how they interact with other processes affecting the efficacy of nucleoside based drugs.
Keywords: ABC transporter, nucleoside drug, drug resistance, nucleoside kinase, cancer
1. ABC Transporters- historical background and transport mechanisms
Successful chemotherapy depends on achieving an adequate amount of a drug at an intracellular target. However, intracellular drug accumulation is the product of import and export processes that we now recognize occurs by families of membrane transporters. However, the importance of drug export was not recognized as a process affecting drug accumulation at first, and in cancer cells, reduced drug accumulation was mostly attributed to decreased uptake rather than export as a mechanism of drug resistance [1–3]. However, direct evidence for active export as a means of drug resistance was first demonstrated by Dano in 1973. Dano observed that “in resistant cells there is an outward flow of daunomycin against an electrochemical gradient that is carrier mediated, and dependent on the energy metabolism” [4]. This early theory accurately presaged the properties of the yet to be identified ATP-binding cassette (ABC) transporters. ABC transporters mediate the ATP- dependent, and unidirectional transmembrane extrusion of various compounds, both endogenous and exogenous (Table 1). Among the 48 ABC transporters identified in humans, those localized to the plasma membrane reduce intracellular drug concentrations by export [5, 6]. While ABC transporters interact with an array of structurally diverse chemotherapeutic agents and their metabolites to produce a multidrug resistance (MDR) phenotype, this review will primarily focus on nucleoside-based drugs and their relationship with ABC transporters.
Table 1.
Function and diseases/phenotypes caused by variation or deficiency of ABC genes.
| Gene | Alias | Location | Mendelian disorder/phenotype | Function/Drugs |
|---|---|---|---|---|
| ABCA1 | ABC1 | 9q31.1 | Tangier disease, FHDLD | Cholesterol efflux |
| ABCA2 | ABC2 | 9q34 | Early onset of Alzheimer disease? | Drug resistance |
| ABCA3 | ABC3 | 16p13.3 | Lung surfactant deficiency-NRD, ILD | Homeostasis of pulmonary surfactant |
| ABCA4 | ABCR | 1p22.1-p21 | Stargards/FFM, RP, CRD | Processing or transport of all trans-retinal in retinoid cycle |
| ABCA5 | 17q24 | |||
| ABCA6 | 17q24 | Putative role in lipid homeostasis | ||
| ABCA7 | 19p13.3 | |||
| ABCA8 | 17q24 | |||
| ABCA9 | 17q24 | Putative role in lipid homeostasis | ||
| ABCA10 | 17q24 | Lipid transport | ||
| ABCA12 | 2q34 | Lamellar and harlequin ichtyosis | Lipid trafficking in keratinocytes | |
| ABCA13 | 7p11-q11 | |||
| ABCB1 | P-GP, MDR | 7p21 | Multidrug resistance | |
| ABCB2 | TAP1 | 6p21 | Immune deficiency | Peptide transport |
| ABCB3 | TAP2 | 6p21 | Immune deficiency | Peptide transport |
| ABCB4 | MDR3 | 7q21.1 | PFIC-3 | PC transport |
| ABCB5 | 7p14 | Putative role in melanocytes and melanoma | ||
| ABCB6 | MTABC3, UMAT, PRP | 2q36 | Porphyrin transport | |
| ABCB7 | ABC7 | Xq12-q13 | XLSA/A | Fe-S cluster |
| ABCB8 | MABC1 | 7q36 | Protection against oxidative stress | |
| ABCB9 | 12q24 | |||
| ABCB10 | MTABC2, ABC-me | 1q42 | Putative heme transport | |
| ABCB11 | SPGP, BSEP | 2q24 | PFIC-2 | Bile salt transport |
| ABCC1 | MRP1 | 16p13.1 | Multidrug resistance | |
| ABCC2 | MRP2 | 10q24 | Dubin-Johnson syndrome | Organic anion transport |
| ABCC3 | MRP3 | 17q21.3 | Drug resistance | |
| ABCC4 | MRP4 | 13q32 | Nucleoside/nucleotide transport | |
| ABCC5 | MRP5 | 3q27 | Nucleoside/nucleotide transport | |
| ABCC6 | MRP6 | 16p13.1 | Pseudoxanthoma elasticum | |
| ABCC7 | CFTR | 7q31.2 | Cystic fibrosis | Chloride ion channel |
| ABCC8 | SUR1 | 11p15.1 | FPHHI | Sulfonylurea receptor, ion channel regulator |
| ABCC9 | SUR2 | 12p12.1 | DCVT | Sulfonylurea receptor ion channel regulator |
| ABCC10 | MRP7 | 6p21 | ||
| ABCC11 | MRP8 | 16q11-q12 | Dry ear wax | Nucleoside/nucleotide transport |
| ABCC12 | MRP9 | 16q11-q12 | ||
| ABCD1 | ALD | Xq28 | ALD | VLCFA |
| ABCD2 | ALDL1, ALDR | 12q11-q12 | ||
| ABCD3 | PXMP1, PMP70 | 1p22-p21 | Pristanic acid, DHCA/THCA | |
| ABCD4 | PMP69, P70R | 14q24.3 | ||
| ABCE1 | OABP | 4q31 | ||
| ABCF1 | ABC50 | 6p21.33 | ||
| ABCF2 | 7q36 | |||
| ABCF3 | 3q25 | |||
| ABCG1 | ABC8, White | 21q22.3 | Cholesterol transport | |
| ABCG2 | BCRP, MXR, ABCP | 4q22 | Porphyria (in mouse) | Multidrug resistance, protective role (hypoxia, etc) |
| ABCG4 | White2 | 11q23 | Cholesterol transport | |
| ABCG5 | White3 | 2p21 | Sitosterolemia | Sterol transport |
| ABCG8 | 2p21 | Sitosterolemia | Sterol transport |
FHDLD, familial hypoapoprtoteinemia; NRD, neonatal respiratory distress; ILD, pediatric interstitial lung disease; FFM, fundus flavimaculatis; RP, retinitis pigmentosum 19; CRD, cone-rod dystrophy; PFIC, progressive familial intrahepatic cholestasis; XLSA/A X-linked sideroblastic anemia and ataxia; FPHHI, familial persistent hyperinsulinemic hypoglycemia of infancy; DCVT, dilated cardiomyopathy with ventricular tachycardia; ALD, adrenoleukodystrophy; VLCFA, very long-chain fatty acids; DHCA, dihydroxycholestanoic acid; THCA; trihydroxycholestanoic acid. [5, 40, 129–137]
The first member of the ABC transporter superfamily was identified in 1976 by Ling and colleagues as a 170-kDa membrane glycoprotein overexpressed in colchicine resistant cell lines and referred to as a glycoprotein that reduces drug permeability (P-gp) [7]. Subsequently multiple laboratories cloned and identified the gene that encoded P-gp (P-gp/multidrug resistance (Mdr) aka ABCB1) which was often amplified in drug resistant cells [8–13]. For more details the reader is referred to recent reviews on P-gp [14–18]. The 48 human ABC proteins are separated into seven subfamilies (ABCA- to- ABCG) based on their gene structure, amino acid sequence, domain organization, and phylogenetic analysis [19]. All members except for ABCE and ABCF are localized to various membranes of cellular organelles (referred to ABC transporters hereafter). Insights into the biological function and endogenous substrates of many ABC transporters has been revealed by genetic deficiencies (Table 1). While some ABC transporters have trivial names that reflect, in some cases, their tissue of origin a new nomenclature was established by the Human Genome Organization guidelines in 1999 and P-gp for example is now referred to as ABCB1 (http://www.genenames.org/genefamily/abc.php).
Many years after the initial characterization of P-gp, two additional ABC transporters were identified by screening anthracycline resistant cell lines for drug resistance that was independent of functional P-gp. The first was identified by the laboratories of Cole and Deeley in 1991 and is referred to as multidrug resistance protein 1 (MRP1/ABCC1) [20]. The second breast cancer resistance protein (BCRP/ABCG2) was independently and almost concurrently identified by three laboratories each employing different strategies and assigning their own unique names (e.g., MXR, ABCP) [21–23]. Currently, P-gp (ABCB1), MRP1 (ABCC1) and ABCG2 are considered to be the “major” drug transporters for their roles in MDR phenotypes seen in many tumors and cell lines [24].
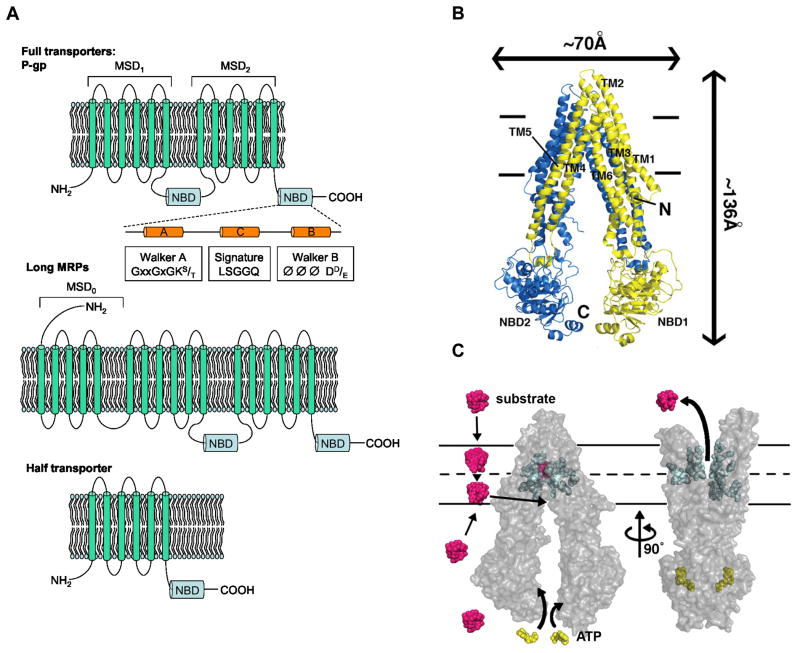
ABC proteins are characterized by Walker A and B motifs as well as a signature motif, which is unique to this superfamily, within the nucleotide-binding domain (NBD) (see Figure 1A)[11, 25–29]. A core unit of ABC transporters contains six or more α-helices in a membrane-spanning domain (MSD)[30, 31]. A functional ABC transporter requires two core units comprised of two NBDs and two MSDs [32–35]. A simple classification of ABC transporters is based upon the number of core units encoded in a single polypeptide (Figure 1A). ABC transporters are classified as “full” transporters if they contain two core units in tandem (MSD-NBD-MSD-NBD) that are active alone, whereas “half” transporters have only one core unit (an NBD and MSD), and therefore require to form either obligate homo- or hetero-dimers [36–40]. ABCD and ABCG subfamilies consist of half transporters, whereas both full and half transporters can be found in the ABCB subfamily. A subset of the ABCC subfamily members contains an amino terminal extension comprised of five additional transmembrane α-helices (termed MSD0). Recent electron cryomicroscopy studies of MRP1 suggested that MSD0 interacts with transmembrane helices from MSD1 and MSD2. These interactions might play a role in substrate recognition [41].
Figure 1.
Predicted two-dimensional topology of ABC transporters and Model of ABC transporter mechanism. A) The two membrane spanning domains (MSDs) and nucleotide binding domains (NBDs) are represented by P-gp and some MRP (ABCC) family members with some MRPs containing an additional N-terminal extension (MSD0), while some ABC transporters have only one MSD and NBD; B) A structure of P-gp in the inward facing conformation showing the transmembrane domains (TMs) and the NBDs; C) Transporter mechanism showing a large conformational change as a result of ATP binding. Substrates can enter by either the cytoplasmic face or extracellular face of an ABC transporter. Adopted from [43].
Recent structural studies of ABC transporters based on a bacterial protein, Sav1866 and a murine P-gp provided insights into the topology of the ABC transporter drug-binding site [42, 43]. The MSDs appear to form an internal cavity that binds substrates through multiple interactions (Figure 1B). For instance, studies with Sav1866 suggest that aromatic residues as well as charged amino acid residues provide an internal binding cavity that neutralizes charged compounds. This “drug-binding” pocket is thought to be highly flexible containing several “mini-pockets” that can accommodate not just structurally diverse classes of drugs, but more than one drug [44]. However, in the absence of additional ABC transporter structures, predicting whether a drug is a substrate for an ABC transporter is daunting and requires rigorous concurrent use of homology models, in silico prediction and biochemical studies.
The structures of the ABC transporters coupled with our knowledge of their transport biochemistry provide the following insights into the ABC transport mechanism [42, 43, 45–47]. The MSDs create an inverted “V” shape to accept substrates from either the cytoplasmic side or extracellular space with substrate binding increasing the affinity of the NBDs for ATP (see Figure 1B) [48, 49]. The binding and hydrolysis of ATP, by the NBDs, induces a conformational change in the MSDs yielding an outward opening permitting substrate/drug release into the extracellular milieu [50] (Figure 1C). Upon ATP hydrolysis, the NBD dimer disengages and the conformation of the transporter is restored to its original inward facing orientation possessing a high affinity for substrate. Further structural studies reveal interactions between the NBDs as well as the cytoplasmic loops. These interactions may be a key to understanding the transmission of conformational changes from MSDs to NBDs and vice versa.
In addition to their roles in the MDR phenotype, ABC transporters have “natural” roles in restricting drug and toxic compounds penetration by creating barriers between systemic blood circulation and organs such as: brain, gastrointestinal tract, and placenta [51–55]. This “protective role” is not their only function. ABC transporters have diverse physiological roles in regulating endogenous molecules affecting multiple pathways such as lipid and bile acid synthesis, antigen presentation, heme and iron homeostasis and signaling molecules such as cyclic nucleotides and ions, etc. (reviewed in an upcoming book). The crucial role that ABC transporters play in maintaining cellular homeostasis is in part illustrated by the fact that almost a third of the defective human ABC genes are associated with a disease phenotype [5]. Therefore, an understanding of their normal biological roles and structures will minimize unexpected adverse effects when designing inhibitors to overcome the MDR caused by transporters such as P-gp, MRP1 or ABCG2.
2. Nucleoside-based drugs- transporters and therapeutics
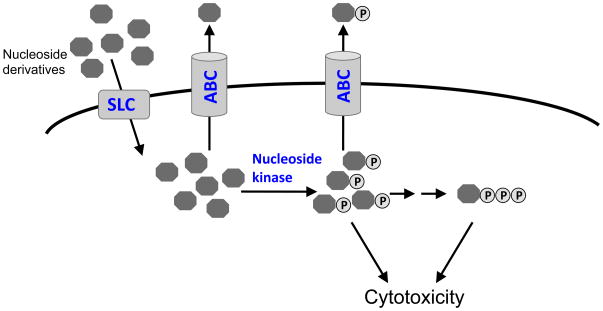
Structural analogs of natural nucleosides effectively insinuate themselves into cells by using the normal transport routes of endogenous nucleosides [56]. Subsequently, these nucleosides are activated by phosphorylation through endogenous nucleoside and nucleotide kinases. Mechanistically these nucleoside analogs are considered “antimetabolites” because they disrupt biosynthetic steps in DNA or RNA synthesis. Nucleoside-derived drugs are not only used as cytotoxic agents in the treatment of solid and hematological malignancies, but are also widely used as antivirals and immunosuppressants [57–59]. For example, nucleoside-derivatives used in the antiviral therapy typically inhibit reverse transcriptase by incorporation into the DNA chain, and due to their unnatural structure, terminate further chain elongation.
The biochemical properties of uptake carriers for nucleosides were well established prior to their identification and cloning [60–62]. While the solute carrier (SLC) superfamily consists of more than 300 members assigned to 51 subfamilies (SLC1-51), only two structurally unrelated but well defined nucleoside subfamily members exist, SLC28 and SLC29 [63]. These carriers mediate the uptake of natural nucleosides with their alternative names indicating their function: the cation ion symporter known as a concentrative nucleoside transporter (CNT, aka SLC28)[64, 65] and the facilitated diffusion carrier know as the equilibrative nucleoside transporter (ENT, aka SLC29)[66, 67], respectively. The three CNT members have different substrate specificity where CNT1 prefers pyrimidines, CNT2 accepts purine, and CNT3 transports both pyrimidine and purine molecules [65, 68–70]. The transport of nucleosides by CNTs is coupled to a sodium -gradient and is thereby electrogenic. Members of the ENT subfamily exhibit broad substrate specificity for nucleoside-derived molecules with ENT2 also transporting the precursors of nucleosides, the nucleobases [67, 71–74]. The ENT mediated direction of transport is dictated by nucleoside concentration. Therefore a high extracellular nucleoside concentration favors movement into the cell, conversely a large concentration of intracellular nucleoside switches transport to an outward direction, thus demonstrating ENTs are bidirectional.
The mRNA levels of ENT1 are highly variable among leukemic patients suggesting transcriptional regulation. The few polymorphic genetic variants reported for ENT1 do not affect transport [75] or require further investigation [76]. Both CNTs and ENTs exhibit a wide tissue distribution with an expression at the apical surface of epithelia, suggesting that they play an important role in uptake of nucleoside-derivatives administered orally [65, 67].
Although SLC22 transporters do not typically transport natural nucleosides, some family members especially organic anion and organic cation transporters (OAT, OCT, respectively) appear to transport nucleoside analogs such as thiopurines, 5-FU and many nucleoside-based antivirals [77–79].
Nucleoside drugs require metabolic activation by phosphorylation before interacting with their intracellular targets [80]. Some purine and pyrimidine-based drugs are phosphorylated by deoxycytidine kinase (dCK), whereby nucleobase drugs such as the thiopurines and 5-fluorouracil (5-FU) can be converted to nucleoside monophosphates by specific phosphoribosyltransferases that add a ribosyl phosphate group [81, 82]. Subsequently, additional phophorylation by kinases yields the nucleoside triphosphates. The nucleoside triphosphate form can be incorporated into growing DNA or RNA chains leading to inhibition of DNA polymerases, ligases, and/or endonucleases [83–86]. The nucleotide-derivatives can also interfere with de novo nucleotide biosynthesis (e.g., the 5-FU nucleotide, 5-FdUMP inhibits thymidylate synthase) or through other modes of action such as inhibiting ribonucleotide reductase or depleting endogenous nucleotide pools by feedback mechanisms [87, 88]. Analogs of natural purines and pyrimidines are used as templates to design chemotherapeutic drugs (Table 2). Many of these drugs have been very successful in treating or managing a variety of hematological malignancies as well as solid tumors and viral diseases. Specifically, 6-MP is effective in pediatric acute lymphoblastic leukemia (ALL) [89], and cytarabine and clofarabine are used as a frontline therapy for acute megaloblastic leukemia (AML) (Table 2) [90, 91].
Table 2.
Relationship between ABC transporters and nucleoside cancer drugs and their metabolties.
| Drugs | Transporters | Other factors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRP4 | MRP5 | MRP7 | MRP8 | ABCG2 | Uptake | kinase | ||||||
| resistance | transport | resistance | transport | resistance | transport | resistance | transport | resistance | transport | |||
| purine | ||||||||||||
| mercaptopurine | Xa | Xa | X | X | NCb | X | CNT3, ENT2, OAT1,3 | phosphoribosyl-transferase | ||||
| thioguanine | X | X | NC | NC | X | |||||||
| thioopurine metabolites | X | X | X | |||||||||
|
| ||||||||||||
| cladribine | X | NC | X | X | CNT2,3 ENT1,2 |
dCK | ||||||
| cladribine-MP | X | |||||||||||
| fludarabine | NC | NC | NC | CNT2, 3 ENT1,2 |
dCK | |||||||
| clofarabine | X | X | CNT2,3 ENT1,2 |
dCK | ||||||||
| clofarabine-MP | NTc | |||||||||||
|
| ||||||||||||
| PMEA (antiviral) | X | X | X | X | X | X | X | X | X | X | ||
|
| ||||||||||||
| pyrimidine | ||||||||||||
| cytarabine | NC | NC | X | X | NC, X | CNT1,3 ENT1,2 |
dCK | |||||
| cytarabine-MP | X | |||||||||||
| gemcitabine | NC | NC, X | X | NC, X | NC | CNT1,3 ENT1,2,3 |
dCK | |||||
|
| ||||||||||||
| Fluorouridine | ||||||||||||
| 5-fluorouracil | NC | NT | NC | X | NT, X | OAT2,3 | phosphoribosyl-transferase | |||||
| 5-FdUrd | NT | X | NT | |||||||||
| 5-dFUrd | NC | X | ||||||||||
| 5-FdUMP | X | X | ||||||||||
|
| ||||||||||||
| Reference | [100,106,107,110] | [106,109–112] | [116] | [113–115] | [118–120] | |||||||
resistance determined by cytotoxicity assay or transport determined by cellular uptake or membrane vesicles,
no resistance observed,
no transport detected. AZT; azathiopurine, MP; monophosphate, 5-FdUrd; 5′-fluoro-2′-deoxyuridine, 5-dFUrd; 5′-deoxy-5′-fluorouridine, 5-FdUMP; 5′-fluoro-2′-deoxyuridine monophosphate, PMEA; 9-(2-Phosphonylmethoxyethyl)-adenine.
3. Classical nucleoside resistance mechanisms
Tumors acquire resistance to nucleoside-derivatives by multiple mechanisms with many studies showing reduction in the intracellular concentration of phosphorylated metabolites. To achieve this, two classical mechanisms have been reported: defective nucleoside kinase activity that occurs secondary to mutation or impaired uptake. While other potential mechanisms of chemotherapeutic resistance such as alterations in amounts of a drug’s target protein or induction of drug metabolism pathways have been indentified, these will not be discussed in detail here.
Several enzymes are responsible for the intracellular phosphorylation of nucleoside drugs (nucleoside kinases). One example is deoxycytidine kinase (dCK), which phosphorylates clinically important nucleosides such as cytarabine, gemcitabine, cladribine, fludarabine, and clofarabine (see Table 2). Phosphorylation of nucleosides to their monophosphates is often a rate-limiting step in the activation of these drugs. Due to its important role in activation of the aforementioned drugs, the genetics of dCK has been extensively studied. The expression of dCK shows tissue specific variation, which could impact host toxicity. Further, dCK expression in tumor cells also varies [92, 93]. While transcription factors regulating dCK can impact dCK expression, genomic variation in dCK has revealed more than 60 single nucleotide polymorphisms (SNP). However, among these only three SNP’s produce amino acid changes (nonsynonymous SNP’s)[94]. Notably, these nonsynonymous SNP’s in dCK produce proteins with reduced kinase activity compared to the wild type protein. These findings suggest that ethnic differences in variant dCK might produce different responses to chemotherapy relying on dCK [94]. In addition, acquired resistance to cytarabine is associated with loss of dCK expression or activity thus providing evidence that lack of dCK activity is a bona fide mechanism for drug resistance [95–97].
Nucleoside resistance can arise by concurrent changes in more than one cellular factor [98]. For instance, HL-60 cell lines selected for resistance to the nucleosides cladribine or fludarabine exhibited differential changes in dCK activity depending upon which drug they were exposed to. In cells selected for cladribine resistance dCK activity was substantially reduced to ~10% of non-resistant cells whereas fludarabine resistant cells dCK activity was ~60% of non-resistant cells [96]. A logical explanation for the differential effect of these drugs on dCK was that cladribine has a 10-fold greater affinity for dCK than fludarabine. Therefore, the selective pressure to reduce dCK activity was greater for cladribine exposed cells than those exposed to fludarabine. Beside altered dCK, the uptake of radiolabeled nucleosides was also reduced in the cladribine-resistant cells, suggesting an alteration in drug transport as a concurrent mechanism of resistance. Further studies explored the relationship between uptake carriers and dCK using cell lines that acquired either high or low cytarabine resistance [97]. In cells with low resistance, a mutation in the uptake carrier, ENT1 was identified. These cells with mutant ENT1 showed reduced transport activity, but no alteration in dCK activity; therefore, it is likely that the reduced uptake mediated the resistance. In contrast, a cell line that was highly resistant to cytarabine showed no defect in uptake, but a strong reduction in dCK activity, which was due to a mutation in dCK gene. One explanation for the resistance by defective uptake being less profound is that at high concentrations of cytarabine the rate of passive diffusion of cytarabine exceeds that of the carrier-mediated uptake [99]. Therefore, resistance mediated by defects in nucleoside uptake carriers might be less effective because an alternate route of drug uptake (passive diffusion) is available.
4. Role of ABC transporters in nucleotide/nucleoside efflux
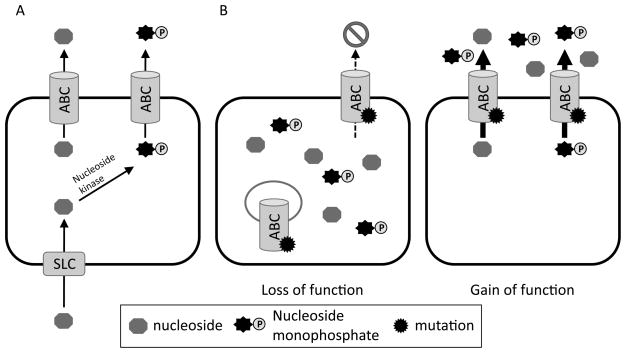
Initial studies of nucleoside resistance mechanisms might have overlooked the contribution of ABC transporters (Figure 2). One explanation is that export of nucleosides or nucleotide derivatives cannot be experimentally revealed by simply evaluating uptake or profiling the intracellular nucleotide drug metabolites. Another is that conventional wisdom believed phosphorylated metabolites were not exported, but were retained in the cell. Furthermore, export of nucelosides or nucleotides would resemble a defect in either nucleoside uptake or phosphorylation; therefore, specific strategies are required to discern their contribution (Figure 2). Because ABC transporters require cellular energy to export drugs, one approach to uncover the contribution of an ABC transporter to nucleotide or nucleoside export is to evaluate their accumulation after reducing a cell’s ATP by treatment with sodium azide and/or deoxyglucose [100], In contrast, a defect in a nucleoside kinase will result in reduced levels of phosphorylated nucleosides, whereas ATP reduction will increase drug accumulation if export occurs by an ABC transporter.
Figure 2.
ABC transporters can confer resistance to nucleosides and single-nucleotide polymorphisms (SNP’s) can modify ABC transporter function. A) The pathway of nucleoside uptake and activation by a nucleoside kinase can be disrupted by an ABC transporter. B) Single-nucleotide polymorphisms (SNP’s) can result in loss of function through mechanisms affecting protein targeting, stability etc. resulting in “loss of function” but SNP’s can also result in enhanced transport activity: “gain of function.” The arrow size indicates transport activity.
Members of the MRP subfamily (ABCC) are typically described as transporters of organic anionic conjugates such as glucuronide and glutathione. The first evidence that nucleotides could also be substrates for an ABCC family member was provided in 1999. MRP4 (ABCC4) was identified as the first ABC transporter capable of exporting nucleoside-monophosphates from mammalian cells [100]. The genesis of this discovery was using cell lines with acquired resistance to the cytotoxic effects of a purine nucleoside-monophosphate derivative (PMEA; 9-(2-phosphonylmethoxyethyl) adenine) that were developed by Fridland and colleagues [101]. These cell lines had an impaired ability to accumulate mono-phosphorylated nucelosides, and the accumulation defect was reversed by reduction in intracellular ATP. Furthermore, the stability of drug resistance during culture for many generations in the absence of drug suggested an amplification of a gene conferring PMEA resistance. The mechanistic basis of heritable and stable drug resistance due to amplification of a single gene that was a drug target was elucidated by Schimke and colleagues [102–104]. Typically, in gene amplification the number of copies of the target gene increases at its normal chromosomal address. While Southern blot screening of genomic DNA from PMEA sensitive and resistant cells revealed selective MRP4 gene amplification (compared to other ABC transporters), fluorescent in situ hybridization demonstrated that the MRP4 gene was focally amplified at its chromosomal address of 13q32 [100]. Further studies showing somatic cell-fusions confirmed that resistance to PMEA was dominant. These results implicated MRP4 as a bona fide drug resistance protein conferring nucleoside resistance, and accordingly, it was demonstrated that MRP4 exported not only PMEA, but also azidothymidine monophosphate (AZTMP).
Subsequent studies confirmed MRP4 role in resistance to nucleoside chemotherapeutic drugs such as the thiopurines, 6-mercaptopurine (6-MP) and 6-thioguanine (TG) by transfecting cells with expression constructs overexpressing MRP4 [105–107]. Mechanistically, thiopurine resistance was attributed to MRP4 mediated export of the thiopurine monophosphate derivatives (thioinosine-monophosphate and thioguanosine-monophosphate). This export was specific for mono-phosphates because di- and tri- phosphates were not exported and indicated that thiopurine monophosphates are major substrates of MRP4 [106]. While it has not been established that MRP4 overexpression contributes to impaired patient response in leukemia therapy, it is worth noting that MRP4 expression is highly variable among pediatric leukemia’s at diagnosis [108]. Noteworthy, a T-cell leukemia cell line (CEM-MPS) cultured in increasing concentrations of 6-mercaptopurine overexpressed MRP4 that was associated with acquired resistance to 6-MP and 6 TG. While this cell line overexpressed MRP4, the resistance appeared attributable to additional factors such as reduced expression of the equilibrative transporter (SLC28 family) and the concentrative transporters (SLC29 family) [107]. Notably expression of other candidate nucleoside/nucleotide exporters MRP5 and MRP8 were unchanged.
Other ABCC family members such as MRP5, MRP7, and MRP8 may also impact nucleoside-mediated resistance. Overexpression of MRP5 by expression vectors revealed that this closely related transporter conferred resistance to various nucleotide derivatives of thiopurines and PMEA [106, 109, 110]. To date, however it is unknown if cells that acquire resistance to thiopurines PMEA or other related nucleosides overexpress MRP5. Nonetheless, drug accumulation studies in cells engineered to overexpress MRP5 demonstrated lower levels of PMEA and thiopurine nucleotides, and MRP5 conferred resistance to 6-MP, 6-TG, and PMEA but not to other nucleoside derivatives such as 5-Fluorouracil (5-FU) and azathiopurine [109]. Accordingly, HPLC analysis of the intracellular concentrations of thiopurine metabolites revealed reduced concentrates of monophosphate forms. Furthermore, an analysis of the extracellular media for cells exposed to 6-MP showed that MRP5 exported 6-thio-IMP into the medium. Like MRP4, cells overexpressing MRP5 effluxed thiopurine metabolites such as thioinosine monophosphate. It is notable that thioxanthosine monophosphate was only exported by MRP5 and not MRP4. Although MRP4 and MRP5 appear to have differential affinity for these thiopurine metabolites, combination of these transporters would be very effective in exporting thiopurine monophosphates out of the cells.
It is notable that in addition to purine analogs, MRP4 and MRP5 also export pyrimidine nucleotides (MRP4=azidothymidine-monophosphate; MRP5= 5-fluordeoxyuride monophosphate). Although overexpression of MRP5 did not confer resistance to 5-FU in initial reports [109], recent studies demonstrated that cell lines engineered to overexpress MRP5 exports 5-FU monophosphorylated metabolites [111]. Intriguingly, a natural metabolite in pyrimidine biosynthesis, 5-dUMP accumulated in an ATP-dependent manner into inside-out membrane vesicles prepared from the cells expressing MRP5. This suggests that high levels of MRP5 might regulate normal pyrimidine biosynthesis by exporting 5-dUMP. In addition, MRP5 has been implicated in resistance to the deoxycytidine analog, gemcitabine, because MRP5 overexpression confers resistance to gemcitabine. For instance, the relationship between expression of MRP5 and gemcitabine cytotoxicity was inverse in non-small cell lung cancer cells, because siRNA against MRP5 increased sensitivity to the drug [112].
Other MRP family members that have been shown to extrude nucleotides are MRP7 and MRP8. Cell lines transfected with an MRP8 expression vector have been shown to export pyrimidine-based nucleotides such as 5-Fluorodeoxyurdine monophosphate (5-FdUMP) and this is associated with resistance to 5-FU derivatives [113]. These results suggested that MRP8 confers resistance to 5-FU by transporting its active metabolite, 5-FdUMP out of the cells rather than 5-FU itself. Furthermore, in a 5-FU resistant human small-cell lung cancer line, MRP8 mRNA but not MRP1-5 or MRP9 was upregulated [114]. This cell line showed cross-resistance with methotrexate, and reduction of MRP8 by siRNA sensitized the cells to 5-FU. Recent studies have shown that high expression of MRP8 but not MRP4 or MRP5 was associated with a poor treatment outcome for AML patients treated with cytarabine [115], a finding that is associated with reduced cytarabine intracellular metabolites.
Interestingly, MRP family members with three membrane-spanning domains (MSD): MRP1, as well as MRP2 and 3 have yet to be shown to transport nucleoside cancer drugs. However, a recent study suggested that cells transfected with MRP7 expression vectors have modest reductions in cytarabine accumulation and increased cytarabine resistance [116]. While these studies demonstrate that engineered overexpression of some MRP’s confers drug resistance, it is unknown if cells acquire MRP7 or MRP8 overexpression as drug resistance develops in therapy.
Another ABC transporter implicated in nucleoside transport is the “half transporter,” ABCG2. ABCG2 had been demonstrated to export the natural nucleotide, cyclic GMP [117], however recent studies indicate it transports a wide variety of therapeutically important nucleoside-derivatives used in the treatment of both cancer and viral infections [118–120]. The antiviral PMEA and the chemotherapeutic agent cladribine were recently shown to be ABCG2 substrates. These studies illustrated the substrate overlap between MRP4 and ABCG2, but more importantly showed how endogenous differences in tissue distribution of these transporters affected drug accumulation by using different combinations of ABCG2 and MRP4 knockout mice [118]. For example, lack of MRP4 affected PMEA accumulation in spleen suggesting the low contribution of ABCG2 in spleen but efficient efflux at other tissues such as kidney and liver. Intriguingly, ABCG2 expression in polarized cells cultured in a Transwell culture system (polarized cells are cultured on permeable membrane bathed in medium on their basal and apical surface [119]) export 6-MP from the basal compartment to the apical compartment. These findings appear relevant to chemotherapeutic response because a number of studies have found an association between ABCG2 overexpression and poor overall survival and relapse in AML patients [121–124]. Because nucleoside derivatives are frontline therapy for AML, knowledge of ABCG2 role in transporting nucleosides analogs and their derivatives will facilitate not just drug development, but increase therapeutic efficacy.
5. Transporter interaction with intracellular metabolic pathways
As mentioned above, intracellular concentrations of chemotherapeutic nucleosides- and their nucleotide-derivatives can be modulated by processes including uptake carriers, nucleoside, kinases and efflux transporters. The intracellular accumulation of phosphorylated nucleosides is essential for cytotoxicity and a reduction in nucleoside-monophosphate might impair therapy (Figure 2A). For example, if drug uptake is markedly reduced, the intracellular concentration of nucleoside may be below the affinity of the nucleoside kinase thereby leading to inadequate levels of active nucleotides and outcome associated with concomitant treatment failure. In addition, natural variation in nucleoside kinase levels or functionality (due to mutation) may affect therapeutic efficacy.
The possibility that nucleoside resistance mediated by ABCG2 was affected by intracellular metabolism was evaluated when two –independent cell lines, engineered for comparable ABCG2 expression and resistance to a prototypical ABCG2 substrate (mitoxantrone), had marked difference in resistance to clofarabine [120]. The ABCG2 resistance to clofarabine was strongly modulated by either up or down-regulation of dCK, the enzyme that activates clofarabine. Reducing dCK levels with a dCK siRNA enhanced ABCG2 mediated clofarabine resistance. In contrast, dCK overexpression reduced clofarabine resistance because the abundance of dCK resulted in a faster rate of clofarabine conversion to clofarabine-monophosphate. It was reasoned that if clofarabine –monophosphate was an ABCG2 substrate then greater resistance would have been expected. These studies suggested clofarabine-monophosphate was not an ABCG2 substrate, but clofarabine was. Based on this, membrane vesicle transport studies determined that clofarabine was an ABCG2 substrate. These studies showed that an ABC transporter could effectively derail the nucleoside phosphorylation pathway by directly exporting the nucleoside. Because ABCG2 has been shown to extrude nucleoside-monophosphates too, it suggests for some nucleosides, ABCG2 can have a powerful effect on response to nucleoside drugs. However, given that several ABC transporters export nucleotide-monophosphates, this finding suggests their role in affecting nucleoside activation and intracellular phosphorylation should also be considered.
6. SNPs in ABC transporters and the double-edge sword in therapeutic response
The function of ABC transporters can be modified by variation in the DNA sequence encoding the polypeptide. These SNP’s can produce changes in the amino-acid composition of the polypeptide. One such non-synonymous change is well known for ABCG2, the Arg482 to Gly substitution [125]. While this change alters substrate specificity, its therapeutic relevance is dubious considering it has yet to be identified in patient samples. In contrast, ethnic variation in non-synonymous SNP’s can have a profound effect if the allele is frequent and alters function. For instance, the ABCG2 Gln141K reduces the protein level and activity but is important because its frequency varies by ethnic group (35% in Asians an <1% in African Americans (Figure 2B)[126].
While recent studies have suggested that variation in MRP4 can affect therapeutic response by affecting host toxicity, it is well established that the lack of a thiopurine inactivating enzyme TPMT produces greater 6-MP toxicity. However, there are patients who are highly sensitive to 6-MP toxicity without the typical reason for sensitivity: a non-functional TPMT. MRP4 is highly expressed in myeloid progenitors, and a murine model mice lacking MRP4 exhibits high hematopoietic toxicity [127]. These studies identified a human MRP4 SNP that was associated with accumulation of thiopurine nucleotides at a high frequency among the Japanese population (>18%) and Asians (~4%) that resulted in impaired targeting to the plasma membrane producing a loss of MRP4 function. The contribution of this SNP to hematopoietic toxicity was confirmed in Japanese inflammatory bowel disease patients receiving the 6-MP prodrug, azathiopurine [128]. These results illustrate the delicate balance where too little of the ABC transporter function results in the in drug toxicity, whereas too much of it causes MDR.
7. Conclusion and perspective
The efficacy of the nucleoside derivatives relies on the formation and accumulation of phosphorylated metabolites. Nucleoside analogs are widely used in the treatment of both solid and hematologic malignancies with new nucleoside derivatives being developed to increase treatment efficacy. Although uptake carriers facilitate the movement of nucleosides into the cells, at a high plasma concentration, passive diffusion moves drugs into cells down their concentration gradient. However, the conversion of nucleosides to monophosphates is a rate-limiting step in drug activation. Thus a reduced or a loss in activity of the kinase reduces therapeutic efficacy. A newer concept reveals that impaired response to nucleoside drugs occurs by active efflux due to ABC transporters. Recent studies demonstrate that both ABCC family members and ABCG2 play an important role in intracellular accumulation of nucleotide metabolites. For drugs where both the nucleoside and its monophosphate are substrates of ABC transporters, drug efficacy will be strongly reduced and will appear independent of nucleoside kinase activity. However, if the ABC transporter has only affinity for the nucleoside and not the monophosphate form, a high rate of conversion to the monophosphate will result in accumulation of active metabolites with a very little contribution from ABC transporters. As presented here, an intricate cellular balance between uptake carriers, kinases, and ABC transporters determine the resistance to the nucleoside derivatives, and therefore, an understanding of substrate specificity for ABC transporters will be important in predicting drug efficacy.
Acknowledgments
We thank the Schuetz Lab members for critical review of the manuscript. This work was supported by NIH grants 2R01 GM60904, P30 CA21745, and CA21865 and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yu Fukuda, Email: Yu.Fukuda@stjude.org.
John D. Schuetz, Email: John.Schuetz@stjude.org.
References
- 1.Kessel D, Hall TC, Roberts D, Wodinsky I. Uptake as a determinant of methotrexate response in mouse leukemias. Science. 1965;150:752–4. doi: 10.1126/science.150.3697.752. [DOI] [PubMed] [Google Scholar]
- 2.Yesair DW, Kohner FA, Rogers WI, Baronowsky PE, Kensler CJ. Relationship of phthalanilide-lipid complexes to uptake and retention of 2-chloro-4′,4″-di(2-imidazolin-2-yl)terephthalanilide (NSC 60339) by sensitive and resistant P388 leukemia cells. Cancer Res. 1966;26:202–7. [PubMed] [Google Scholar]
- 3.Kessel D, Botterill V, Wodinsky I. Uptake and retention of daunomycin by mouse leukemic cells as factors in drug response. Cancer Res. 1968;28:938–41. [PubMed] [Google Scholar]
- 4.Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973;323:466–83. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- 5.Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–29. doi: 10.1016/S0076-6879(05)00024-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu CP, Hsieh CH, Wu YS. The Emergence of Drug Transporter-Mediated Multidrug Resistance to Cancer Chemotherapy. Mol Pharm. 2011 doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 7.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 8.Roninson IB, Abelson HT, Housman DE, Howell N, Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984;309:626–8. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- 9.Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–9. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- 10.Gros P, Croop J, Roninson I, Varshavsky A, Housman DE. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986;83:337–41. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gros P, Croop J, Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–80. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 12.Gros P, Ben Neriah YB, Croop JM, Housman DE. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986;323:728–31. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- 13.Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, et al. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–62. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 14.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–85. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 15.Callaghan R, Crowley E, Potter S, Kerr ID. P-glycoprotein: so many ways to turn it on. J Clin Pharmacol. 2008;48:365–78. doi: 10.1177/0091270007311568. [DOI] [PubMed] [Google Scholar]
- 16.Goda K, Bacso Z, Szabo G. Multidrug resistance through the spectacle of P-glycoprotein. Curr Cancer Drug Targets. 2009;9:281–97. doi: 10.2174/156800909788166493. [DOI] [PubMed] [Google Scholar]
- 17.Padowski JM, Pollack GM. Pharmacokinetic and pharmacodynamic implications of P-glycoprotein modulation. Methods Mol Biol. 2010;596:359–84. doi: 10.1007/978-1-60761-416-6_16. [DOI] [PubMed] [Google Scholar]
- 18.Wolf SJ, Bachtiar M, Wang J, Sim TS, Chong SS, Lee CG. An update on ABCB1 pharmacogenetics: insights from a 3D model into the location and evolutionary conservation of residues corresponding to SNPs associated with drug pharmacokinetics. Pharmacogenomics J. 2011;11:315–25. doi: 10.1038/tpj.2011.16. [DOI] [PubMed] [Google Scholar]
- 19.Allikmets R, Gerrard B, Hutchinson A, Dean M. Characterization of the human ABC superfamily: isolation and mapping of 21 new genes using the expressed sequence tags database. Hum Mol Genet. 1996;5:1649–55. doi: 10.1093/hmg/5.10.1649. [DOI] [PubMed] [Google Scholar]
- 20.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 21.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–9. [PubMed] [Google Scholar]
- 23.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 24.Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discov Today. 2008;13:379–93. doi: 10.1016/j.drudis.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–51. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–9. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 27.Gerlach JH, Endicott JA, Juranka PF, Henderson G, Sarangi F, Deuchars KL, et al. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986;324:485–9. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- 28.Higgins CF, Hiles ID, Salmond GP, Gill DR, Downie JA, Evans IJ, et al. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323:448–50. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- 29.Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, et al. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–5. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 30.Schrodt S, Koch J, Tampe R. Membrane topology of the transporter associated with antigen processing (TAP1) within an assembled functional peptide-loading complex. J Biol Chem. 2006;281:6455–62. doi: 10.1074/jbc.M509784200. [DOI] [PubMed] [Google Scholar]
- 31.Tusnady GE, Sarkadi B, Simon I, Varadi A. Membrane topology of human ABC proteins. FEBS Lett. 2006;580:1017–22. doi: 10.1016/j.febslet.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Berkower C, Michaelis S. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 1991;10:3777–85. doi: 10.1002/j.1460-2075.1991.tb04947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loo TW, Clarke DM. Reconstitution of drug-stimulated ATPase activity following co-expression of each half of human P-glycoprotein as separate polypeptides. J Biol Chem. 1994;269:7750–5. [PubMed] [Google Scholar]
- 34.Hrycyna CA, Ramachandra M, Germann UA, Cheng PW, Pastan I, Gottesman MM. Both ATP sites of human P-glycoprotein are essential but not symmetric. Biochemistry. 1999;38:13887–99. doi: 10.1021/bi991115m. [DOI] [PubMed] [Google Scholar]
- 35.Koch J, Guntrum R, Tampe R. Exploring the minimal functional unit of the transporter associated with antigen processing. FEBS Lett. 2005;579:4413–6. doi: 10.1016/j.febslet.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Kelly A, Powis SH, Kerr LA, Mockridge I, Elliott T, Bastin J, et al. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature. 1992;355:641–4. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- 37.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 38.Chloupkova M, Reaves SK, LeBard LM, Koeller DM. The mitochondrial ABC transporter Atm1p functions as a homodimer. FEBS Lett. 2004;569:65–9. doi: 10.1016/j.febslet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–9. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, et al. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–9. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg MF, Oleschuk CJ, Wu P, Mao Q, Deeley RG, Cole SP, et al. Structure of a human multidrug transporter in an inward-facing conformation. J Struct Biol. 2010;170:540–7. doi: 10.1016/j.jsb.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–5. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 43.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumacher MA, Brennan RG. Deciphering the molecular basis of multidrug recognition: crystal structures of the Staphylococcus aureus multidrug binding transcription regulator QacR. Res Microbiol. 2003;154:69–77. doi: 10.1016/S0923-2508(02)00013-X. [DOI] [PubMed] [Google Scholar]
- 45.Gaudet R, Wiley DC. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 2001;20:4964–72. doi: 10.1093/emboj/20.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–49. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–26. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–9. doi: 10.1021/bi973045u. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Pincheira R, Zhang JT. Dissection of drug-binding-induced conformational changes in P-glycoprotein. Eur J Biochem. 1998;255:383–90. doi: 10.1046/j.1432-1327.1998.2550383.x. [DOI] [PubMed] [Google Scholar]
- 50.Martin C, Higgins CF, Callaghan R. The vinblastine binding site adopts high-and low-affinity conformations during a transport cycle of P-glycoprotein. Biochemistry. 2001;40:15733–42. doi: 10.1021/bi011211z. [DOI] [PubMed] [Google Scholar]
- 51.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 52.Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, et al. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–8. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- 53.Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999;96:3900–5. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 55.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paterson AR, Kolassa N, Cass CE. Transport of nucleoside drugs in animal cells. Pharmacol Ther. 1981;12:515–36. doi: 10.1016/0163-7258(81)90096-6. [DOI] [PubMed] [Google Scholar]
- 57.Suhadolnik RJ. Naturally occurring nucleoside and nucleotide antibiotics. Prog Nucleic Acid Res Mol Biol. 1979;22:193–291. doi: 10.1016/s0079-6603(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 58.Hirsch MS, Schooley RT. Drug therapy. Treatment of herpesvirus infections. N Engl J Med. 1983;309:963–70. doi: 10.1056/NEJM198310203091607. [DOI] [PubMed] [Google Scholar]
- 59.Hirsch MS, Schooley RT. Drug therapy. Treatment of herpesvirus infections. N Engl J Med. 1983;309:1034–9. doi: 10.1056/NEJM198310273091706. [DOI] [PubMed] [Google Scholar]
- 60.Kessel D, Shurin SB. Transport of two non-metabolized nucleosides, deoxycytidine and cytosine arabinoside, in a sub-line of the L1210 murine leukemia. Biochim Biophys Acta. 1968;163:179–87. doi: 10.1016/0005-2736(68)90096-5. [DOI] [PubMed] [Google Scholar]
- 61.Cass CE, Paterson AR. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972;247:3314–20. [PubMed] [Google Scholar]
- 62.Paterson AR, Kolassa N, Cass CE. Transport of nucleoside drugs in animal cells. Pharmacology & therapeutics. 1981;12:515–36. doi: 10.1016/0163-7258(81)90096-6. [DOI] [PubMed] [Google Scholar]
- 63.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins Introduction. Pflugers Arch. 2004;447:465–8. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 64.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) Mol Membr Biol. 2001;18:65–72. doi: 10.1080/09687680010026313. [DOI] [PubMed] [Google Scholar]
- 65.Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–34. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- 66.Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001;18:53–63. [PubMed] [Google Scholar]
- 67.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 68.Ritzel MW, Yao SY, Huang MY, Elliott JF, Cass CE, Young JD. Molecular cloning and functional expression of cDNAs encoding a human Na+-nucleoside cotransporter (hCNT1) The American journal of physiology. 1997;272:C707–14. doi: 10.1152/ajpcell.1997.272.2.C707. [DOI] [PubMed] [Google Scholar]
- 69.Ritzel MW, Yao SY, Ng AM, Mackey JR, Cass CE, Young JD. Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol Membr Biol. 1998;15:203–11. doi: 10.3109/09687689709044322. [DOI] [PubMed] [Google Scholar]
- 70.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) The Journal of biological chemistry. 2001;276:2914–27. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- 71.Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, et al. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nature medicine. 1997;3:89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 72.Griffiths M, Yao SY, Abidi F, Phillips SE, Cass CE, Young JD, et al. Molecular cloning and characterization of a nitrobenzylthioinosine-insensitive (ei) equilibrative nucleoside transporter from human placenta. The Biochemical journal. 1997;328 (Pt 3):739–43. doi: 10.1042/bj3280739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Su SF, Dresser MJ, Schaner ME, Washington CB, Giacomini KM. Na(+)-dependent purine nucleoside transporter from human kidney: cloning and functional characterization. The American journal of physiology. 1997;273:F1058–65. doi: 10.1152/ajprenal.1997.273.6.F1058. [DOI] [PubMed] [Google Scholar]
- 74.Crawford CR, Patel DH, Naeve C, Belt JA. Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. The Journal of biological chemistry. 1998;273:5288–93. doi: 10.1074/jbc.273.9.5288. [DOI] [PubMed] [Google Scholar]
- 75.Osato DH, Huang CC, Kawamoto M, Johns SJ, Stryke D, Wang J, et al. Functional characterization in yeast of genetic variants in the human equilibrative nucleoside transporter, ENT1. Pharmacogenetics. 2003;13:297–301. doi: 10.1097/00008571-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Kim SR, Saito Y, Maekawa K, Sugiyama E, Kaniwa N, Ueno H, et al. Thirty novel genetic variations in the SLC29A1 gene encoding human equilibrative nucleoside transporter 1 (hENT1) Drug Metab Pharmacokinet. 2006;21:248–56. doi: 10.2133/dmpk.21.248. [DOI] [PubMed] [Google Scholar]
- 77.Ciarimboli G. Organic cation transporters. Xenobiotica. 2008;38:936–71. doi: 10.1080/00498250701882482. [DOI] [PubMed] [Google Scholar]
- 78.Minuesa G, Volk C, Molina-Arcas M, Gorboulev V, Erkizia I, Arndt P, et al. Transport of lamivudine [(-)-beta-L-2′,3′-dideoxy-3′-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J Pharmacol Exp Ther. 2009;329:252–61. doi: 10.1124/jpet.108.146225. [DOI] [PubMed] [Google Scholar]
- 79.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 80.Plunkett W, Saunders PP. Metabolism and action of purine nucleoside analogs. Pharmacol Ther. 1991;49:239–68. doi: 10.1016/0163-7258(91)90057-s. [DOI] [PubMed] [Google Scholar]
- 81.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3:415–24. doi: 10.1016/s1470-2045(02)00788-x. [DOI] [PubMed] [Google Scholar]
- 82.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 83.Kufe DW, Major PP, Egan EM, Beardsley GP. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980;255:8997–900. [PubMed] [Google Scholar]
- 84.Hentosh P, Koob R, Blakley RL. Incorporation of 2-halogeno-2′-deoxyadenosine 5-triphosphates into DNA during replication by human polymerases alpha and beta. J Biol Chem. 1990;265:4033–40. [PubMed] [Google Scholar]
- 85.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–7. [PubMed] [Google Scholar]
- 86.Ruiz van Haperen VW, Veerman G, Vermorken JB, Peters GJ. 2′,2′-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol. 1993;46:762–6. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- 87.Parker WB, Bapat AR, Shen JX, Townsend AJ, Cheng YC. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human DNA polymerases, DNA primase, and ribonucleotide reductase. Mol Pharmacol. 1988;34:485–91. [PubMed] [Google Scholar]
- 88.Plunkett W, Huang P, Gandhi V. Metabolism and action of fludarabine phosphate. Semin Oncol. 1990;17:3–17. [PubMed] [Google Scholar]
- 89.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–9. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 91.Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer. 2006;107:116–24. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- 92.Kakihara T, Fukuda T, Tanaka A, Emura I, Kishi K, Asami K, et al. Expression of deoxycytidine kinase (dCK) gene in leukemic cells in childhood: decreased expression of dCK gene in relapsed leukemia. Leuk Lymphoma. 1998;31:405–9. doi: 10.3109/10428199809059234. [DOI] [PubMed] [Google Scholar]
- 93.van der Wilt CL, Kroep JR, Loves WJ, Rots MG, Van Groeningen CJ, Kaspers GJ, et al. Expression of deoxycytidine kinase in leukaemic cells compared with solid tumour cell lines, liver metastases and normal liver. Eur J Cancer. 2003;39:691–7. doi: 10.1016/s0959-8049(02)00813-4. [DOI] [PubMed] [Google Scholar]
- 94.Lamba JK, Crews K, Pounds S, Schuetz EG, Gresham J, Gandhi V, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323:935–45. doi: 10.1124/jpet.107.128595. [DOI] [PubMed] [Google Scholar]
- 95.Verhoef V, Sarup J, Fridland A. Identification of the mechanism of activation of 9-beta-D-arabinofuranosyladenine in human lymphoid cells using mutants deficient in nucleoside kinases. Cancer Res. 1981;41:4478–83. [PubMed] [Google Scholar]
- 96.Mansson E, Spasokoukotskaja T, Sallstrom J, Eriksson S, Albertioni F. Molecular and biochemical mechanisms of fludarabine and cladribine resistance in a human promyelocytic cell line. Cancer Res. 1999;59:5956–63. [PubMed] [Google Scholar]
- 97.Cai J, Damaraju VL, Groulx N, Mowles D, Peng Y, Robins MJ, et al. Two distinct molecular mechanisms underlying cytarabine resistance in human leukemic cells. Cancer Res. 2008;68:2349–57. doi: 10.1158/0008-5472.CAN-07-5528. [DOI] [PubMed] [Google Scholar]
- 98.Bowen D, Diasio RB, Goldman ID. Distinguishing between membrane transport and intracellular metabolism of fluorodeoxyuridine in Ehrlich ascites tumor cells by application of kinetic and high performance liquid chromatographic techniques. J Biol Chem. 1979;254:5333–9. [PubMed] [Google Scholar]
- 99.Capizzi RL, Yang JL, Cheng E, Bjornsson T, Sahasrabudhe D, Tan RS, et al. Alteration of the pharmacokinetics of high-dose ara-C by its metabolite, high ara-U in patients with acute leukemia. J Clin Oncol. 1983;1:763–71. doi: 10.1200/JCO.1983.1.12.763. [DOI] [PubMed] [Google Scholar]
- 100.Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 101.Robbins BL, Connelly MC, Marshall DR, Srinivas RV, Fridland A. A human T lymphoid cell variant resistant to the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine shows a unique combination of a phosphorylation defect and increased efflux of the agent. Mol Pharmacol. 1995;47:391–7. [PubMed] [Google Scholar]
- 102.Alt FW, Kellems RE, Bertino JR, Schimke RT. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978;253:1357–70. [PubMed] [Google Scholar]
- 103.Schimke RT, Kaufman RJ, Alt FW, Kellems RF. Gene amplification and drug resistance in cultured murine cells. Science. 1978;202:1051–5. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- 104.Schimke RT. Gene amplification, drug resistance, and cancer. Cancer Res. 1984;44:1735–42. [PubMed] [Google Scholar]
- 105.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–54. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 106.Wielinga PR, Reid G, Challa EE, van der Heijden I, van Deemter L, de Haas M, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–31. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 107.Peng XX, Shi Z, Damaraju VL, Huang XC, Kruh GD, Wu HC, et al. Up-regulation of MRP4 and down-regulation of influx transporters in human leukemic cells with acquired resistance to 6-mercaptopurine. Leuk Res. 2008;32:799–809. doi: 10.1016/j.leukres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Sampath J, Adachi M, Hatse S, Naesens L, Balzarini J, Flatley RM, et al. Role of MRP4 and MRP5 in biology and chemotherapy. AAPS PharmSci. 2002;4:E14. doi: 10.1208/ps040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 111.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, 3rd, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–63. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 112.Oguri T, Achiwa H, Sato S, Bessho Y, Takano Y, Miyazaki M, et al. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther. 2006;5:1800–6. doi: 10.1158/1535-7163.MCT-06-0025. [DOI] [PubMed] [Google Scholar]
- 113.Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–14. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 114.Oguri T, Bessho Y, Achiwa H, Ozasa H, Maeno K, Maeda H, et al. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther. 2007;6:122–7. doi: 10.1158/1535-7163.MCT-06-0529. [DOI] [PubMed] [Google Scholar]
- 115.Guo Y, Kock K, Ritter CA, Chen ZS, Grube M, Jedlitschky G, et al. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: relation to long-term survival. Clin Cancer Res. 2009;15:1762–9. doi: 10.1158/1078-0432.CCR-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hopper-Borge E, Xu X, Shen T, Shi Z, Chen ZS, Kruh GD. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–84. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Wolf CJ, Yamaguchi H, van der Heijden I, Wielinga PR, Hundscheid SL, Ono N, et al. cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 2007;274:439–50. doi: 10.1111/j.1742-4658.2006.05591.x. [DOI] [PubMed] [Google Scholar]
- 118.Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, et al. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67:6965–72. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- 119.de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, et al. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7:3092–102. doi: 10.1158/1535-7163.MCT-08-0427. [DOI] [PubMed] [Google Scholar]
- 120.Nagai S, Takenaka K, Nachagari D, Rose C, Domoney K, Sun D, et al. Deoxycytidine kinase modulates the impact of the ABC transporter ABCG2 on clofarabine cytotoxicity. Cancer Res. 2011;71:1781–91. doi: 10.1158/0008-5472.CAN-10-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–7. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 122.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, et al. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 123.Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, et al. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108:685–96. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Damiani D, Tiribelli M, Michelutti A, Geromin A, Cavallin M, Fabbro D, et al. Fludarabine-based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk Res. 2010;34:942–5. doi: 10.1016/j.leukres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 125.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–9. [PubMed] [Google Scholar]
- 126.Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 127.Krishnamurthy P, Schwab M, Takenaka K, Nachagari D, Morgan J, Leslie M, et al. Transporter-mediated protection against thiopurine-induced hematopoietic toxicity. Cancer Res. 2008;68:4983–9. doi: 10.1158/0008-5472.CAN-07-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ban H, Andoh A, Imaeda H, Kobori A, Bamba S, Tsujikawa T, et al. The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2010;45:1014–21. doi: 10.1007/s00535-010-0248-y. [DOI] [PubMed] [Google Scholar]