Abstract
In spite of the recognition by the flow cytometry community of potential aerosol hazards associated with cell sorting, there has been no previous study that has thoroughly characterized the aerosols that can be produced by cell sorters. In this study an Aerodynamic Particle Sizer was used to determine the concentration and aerodynamic diameter of aerosols produced by a FACS Aria II cell sorter under various conditions. Aerosol containment and evacuation was also evaluated using this novel methodology. The results showed that high concentrations of aerosols in the range of 1–3 μm can be produced in fail mode and that with decreased sheath pressure, aerosol concentration decreased and aerodynamic diameter increased. Although the engineering controls of the FACS Aria II for containment were effective, sort chamber evacuation of aerosols following a simulated nozzle obstruction was ineffective. However, simple modifications to the FACS Aria II are described that greatly improved sort chamber aerosol evacuation. The results of this study will facilitate the risk assessment of cell sorting potentially biohazardous samples by providing much needed data regarding aerosol production and containment.
Introduction
Modern jet-in-air cell sorters are an invaluable tool for the isolation and study of a variety of cells and organisms. Due to the design requirements of these instruments, droplets are produced during normal operation that can be estimated to be between 80 to 300 μm in diameter dependent upon currently available nozzle diameters; and smaller satellite droplets are also formed as a result of droplet break-off (1). However, it is possible that during a partial nozzle obstruction this defined pattern of droplet formation may be disrupted and the stream may deviate and impact on hard surfaces within the sort chamber. As a result, aerosols are produced that cannot be described by simple fluid dynamic principles. In this regard, although the potential hazards associated with sorting of biohazardous samples have been recognized by the flow cytometry community and the International Society for the Advancement of Cytometry (ISAC) has published Biosafety guidelines in 1997 and updated standards in 2007 (2, 3), there has been no definitive study characterizing the size, concentration or distribution of aerosols capable of being produced by a cell sorter. The present study uses an Aerodynamic Particle Sizer, to evaluate aerosol production and containment on the FACS Aria II model of cell sorter.
Materials and Methods
Aerosol concentrations and aerodynamic diameter (AD) measurements were conducted on BD FACS Aria II model cell sorters (BD Biosciences, San Jose, CA) using an Aerodynamic Particle Sizer (UV-APS Model 3314, TSI, Inc, Shoreview, MN) equipped with a UV laser. High concentration aerosols (expected to be ≥ 600 particles/cm3) were measured by coupling the UV-APS with a Model 3302A Diluter (TSI, Inc.). Results were adjusted using the appropriate dilutor efficiency files as supplied by the manufacturer. Measurements were performed either on a cell sorter located within a Class II biological safety cabinet (BSC; BioProtect II, Baker Co, Sanford, ME). or on a non-enclosed cell sorter. Silicone conductive tubing (0.687-in. I.D.) was used to sample air at indicated locations. At least three 20-second consecutive samples were taken for each measurement. Since measured aerosols were expected to be liquid, results were adjusted according to efficiency measurements for liquid aerosols of Volckens and Peters (4) by creating efficiency files for the TSI Aerosol Instrument Manager (AIM) software. Efficiency values for AD greater than 10 μm were predicted by non-linear regression analysis (Sigma Plot, Systat Software Inc., San Jose, CA) of original data by Volckens and Peters.
For some experiments, aerosols originating from the cell sorter were distinguished from ambient particles with a UV-excitable dye (Clear Blue Fluorescent Water Tracer Dye (CBD); Risk Reactor, Santa Ana, CA) that was added to the sample tube. The optimal dye concentration in the sample tube was determined by first evaluating the concentration at which fluorescent aerosols (CBD at known concentration in phosphate buffered saline generated by a spray bottle) could be identified by the UV-APS operating at constant UV laser power and PMT voltage settings. This was found to be a 1:5000 dilution of the stock CBD. Second, the amount of sample volume dilution by the sheath fluid was calculated to be approximately 1:100, so an initial 1:50 dilution of the CBD would yield aerosols generated by the cell sorter containing CBD at the desired 1:5000 final dilution. This concentration was increased to the final concentration of 1:20–1:25 to ensure detection of smaller fluorescent aerosols. Most measurements were conducted with the UV PMT voltage set at 550. Data was exported from the TSI AIM software for plotting in SigmaPlot directly or after first analyzing in JMP 8.0 software (SAS Institute, Inc., Cary, NC) to ‘gate’ data on UV+ events; the cutoff used for UV+ events was established using ambient air measurements at the same settings used for CBD measurements. Statistical analysis of data collected during containment testing was performed using SigmaPlot statistical analysis features.
Aerosols were measured during normal operation and in ‘fail mode’. Fail mode, representing stream deviation due to a partial nozzle obstruction, was simulated by deviation of the center stream so that it impacted the side of the aspirator trough. This was done by loosening the sort block adjustment screws and rotating the sort block so that the center stream impacted the aspirator trough. Measurement variables included sheath pressure (20, 35 and 70 psi), aerosol management system (AMS) status, sort chamber door open vs. closed and measurement distance ranging from 0.5 cm to 33 cm from the sort chamber.
Results
Aerosol Characterization of FACS Aria II Cell Sorter in Fail Mode
Aerosol production by cell sorters is thought to be highest in the event of a partial obstruction of the nozzle and subsequent deviation of the sheath stream resulting in stream impact onto a solid surface such as the edge of the waste collection trough. This event was simulated (as described in the Materials and Methods) and aerosol measurements taken at distances indicated from the open sort chamber door using a 70 μm nozzle and with operating pressures at 20, 35 or 70 psi (Figure 1). Measurements were performed under two conditions: Aria cell sorter located within the Class II BSC (Figure 2 and Table 1) or non-enclosed Aria cell sorter (Figure 3 and Table 2).
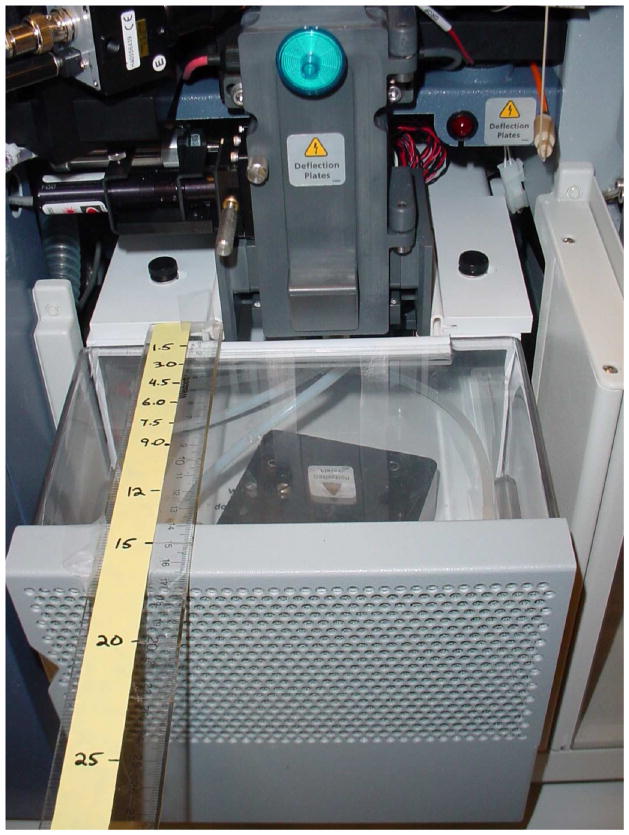
Figure 1.
photograph of aerosol measurement locations on top of FACS Aria collection chamber. Sample were taken on top of the collection chamber at the indicated locations. For measurements taken at 20cm and 25 cm, measurements were taken at the same relative height as closer measurements.
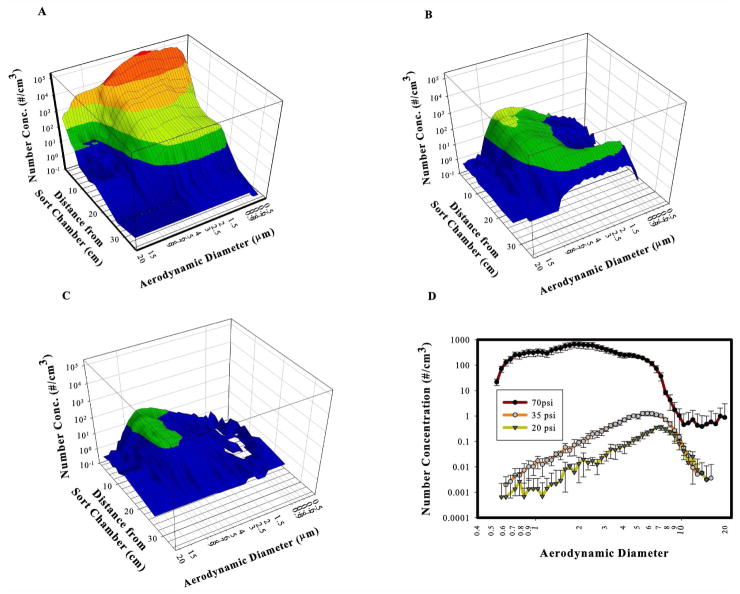
Figure 2.
UV-APS measurements of Aria cell sorter in fail mode in operational BSC at 70 psi (2a), 35 psi (2b) and 20 psi (2c). Figure 2d shows comparison at 3.0 cm for all pressures tested.
Table 1.
BSC
| Distance from stream (cm) | Pressure | Total Concentration (Avg #/cm3) | Mean Aerodynamic diameter (μm) | Median Aerodynamic diameter (μm) |
|---|---|---|---|---|
| 1.5 | 70 | 2.53 × 104 | 2.15 | 1.79 |
| 35 | 29.61 | 5.08 | 5.03 | |
| 20 | 5.92 | 5.79 | 5.70 | |
| 3.0 | 70 | 1.21 × 104 | 2.3 | 1.99 |
| 35 | 14.27 | 5.39 | 5.38 | |
| 20 | 2.96 | 6.51 | 6.64 | |
| 4.5 | 70 | 4.27 × 103 | 2.41 | 1.98 |
| 35 | 15.10 | 5.32 | 5.30 | |
| 20 | 3.84 | 6.66 | 6.68 | |
| 6.0 | 70 | 1.25 × 103 | 2.2 | 1.95 |
| 35 | 12.93 | 5.21 | 5.24 | |
| 20 | 2.53 | 6.25 | 6.27 | |
| 7.5 | 70 | 789 | 2.2 | 2.05 |
| 35 | 11.55 | 5.15 | 5.12 | |
| 20 | 2.28 | 6.17 | 6.19 | |
| 9.0 | 70 | 612 | 2.2 | 2.06 |
| 35 | 8.50 | 4.93 | 4.87 | |
| 20 | 2.22 | 5.85 | 5.85 | |
| 12 | 70 | 160 | 2.25 | 2.1 |
| 35 | 8.84 | 4.79 | 4.70 | |
| 20 | 2.04 | 5.59 | 5.60 | |
| 15 | 70 | 82 | 2.17 | 2.03 |
| 35 | 8.84 | 4.79 | 4.70 | |
| 20 | 0.94 | 5.57 | 5.52 | |
| 20 | 70 | 56 | 1.75 | 1.66 |
| 35 | 5.77 | 2.38 | 2.14 | |
| 20 | 0.08 | 3.20 | 3.42 | |
| 25 | 70 | 0.23 | 1.51 | 0.98 |
| 35 | 0.85 | 1.45 | 1.39 | |
| 20 | 0.01 | 0.75 | 0.69 |
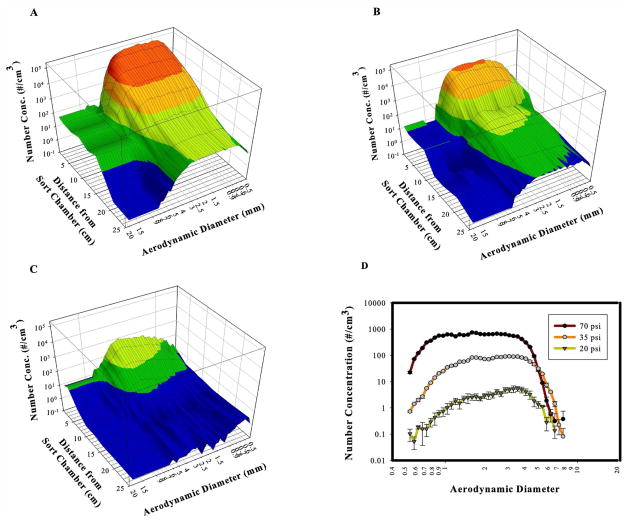
Figure 3.
UV-APS measurements of a non-enclosed Aria cell sorter in fail mode at 70 psi (3a), 35 psi (3b) and 20 psi (3c). Figure 3d shows comparison at 3.0 cm for all pressures tested.
Table 2.
non-enclosed
| Distance from stream (cm) | Pressure | Total Concentration (Avg #/cm3) | Mean Aerodynamic diameter (μm) | Median Aerodynamic diameter (μm) |
|---|---|---|---|---|
| 1.5 | 70 | 1.8 × 104 | 1.78 | 1.6 |
| 35 | 2.8 × 103 | 2.55 | 2.40 | |
| 20 | 150 | 2.74 | 2.7 | |
| 3.0 | 70 | 1.5 × 104 | 1.96 | 1.7 |
| 35 | 1.7 × 103 | 2.56 | 2.40 | |
| 20 | 75 | 2.8 | 2.76 | |
| 6.0 | 70 | 7.2 × 103 | 2.11 | 1.84 |
| 35 | 656 | 2.57 | 2.40 | |
| 20 | 25 | 2.82 | 2.77 | |
| 9.0 | 70 | 3.2 × 103 | 2.1 | 1.85 |
| 35 | 40 | 2.57 | 2.52 | |
| 20 | 5 | 2.71 | 2.64 | |
| 12 | 70 | 888 | 1.99 | 1.71 |
| 35 | 40 | 2.34 | 2.13 | |
| 20 | 1.2 | 2.06 | 1.66 | |
| 15 | 70 | 131 | 1.98 | 1.71 |
| 35 | 24.35 | 2.33 | 2.13 | |
| 20 | 0.45 | 1.41 | 0.82 | |
| 20 | 70 | 34 | 1.85 | 1.6 |
| 35 | 4.72 | 2.23 | 1.98 | |
| 20 | 0.5 | 1.66 | 0.89 | |
| 25 | 70 | 27 | 1.89 | 1.63 |
| 35 | 2.58 | 1.99 | 1.72 | |
| 20 | 0.43 | 1.29 | 0.78 |
Aerosol Measurement of FACS Aria within a BSC
All aerosols measured within the BSC were known to be derived from the cell sorter, since the HEPA filtered air is devoid of ambient particles in the range measured. The results are summarized in Figure 2a and Table 1 for measurements taken at 70 psi from an Aria II enclosed within an operating BSC. As expected, aerosol concentrations decreased with distance from the sort chamber (Figure 2a). An average of 25,303 particles/cm3 was measured at the closest location to the stream (1.5cm) and the median AD ranged between 1.5 to 2.0 μm (Table 1). Aerosol concentration decreased progressively with distance, from 1.5 to 20cm from the sort chamber, but rapidly decreased at 25 cm. The results also show that even at 25cm from the sort chamber, which was 8cm from the inner edge of the BSC front grille, aerosols were detected (0.23 particles/cm3).
At 35 psi, the highest aerosol concentration measured was 29 particles/cm3, an 850-fold reduction compared with 70 psi measurements. The median AD of aerosols at this pressure was more than twofold higher than at 70 psi, ranging from 4.5 to 5.4 μm for measurements taken between 1.5 to 15 cm from the sort chamber (Table 1). Similar to observations at higher pressure, aerosol concentration decreased with distance (Figure 2b) and median AD and concentration were decreased at distances of 15 cm or greater.
Aerosol concentrations were reduced by approximately 5-fold at 20 psi compared with measurements taken at 35 psi and were greater than 4000-fold reduced compared with 70 psi measurements (Figure 2c and Table 1). Median AD averaged 6 μm for measurements between 1.5 and 15 cm, compared with an average of 5 μm for 35 psi.
Figure 2d most clearly shows that of the pressures tested, the highest concentration of aerosols was at 70 psi, and because of the shift to higher AD at lower pressures, the greatest differences were measured within the range of 1 to 3 μm AD.
Aerosol Measurement of Non-enclosed FACS Aria
Although aerosol measurements taken with the Aria located in a BSC had the advantage that no ambient air background particles were present, it was possible that the downward air flow of the BSC may have affected the size distribution or concentration measurements. Measurements were repeated on a non-enclosed FACSAria. CBD was used for these measurements and data shown are for UV+ aerosols only. At 70 psi, aerosol concentration and median AD were comparable to measurements taken in the BSC; at 1.5 cm, there were 1.8 × 104/cm3 aerosols with an average median AD of 1.73 μm (Figure 3a and Table 2). Similar to measurements taken in the BSC, the concentration of aerosols was decreased at lower pressures (Figure 3b and 3c and Table 2) and the AD showed the same negative correlation with pressure as seen with the BSC measurements (Figure 3d). However, measurements of the non-enclosed cell sorter showed that aerosol concentration was much higher at both 35 psi and 20 psi compared with BSC measurements: 2.8 ×103/cm3 vs. 29/cm3 at 35 psi and 150/cm3 vs. 6/cm3 at 20 psi at 1.5 cm from sort chamber for UV+ and BSC measurements, respectively (Table 1 & 2). This is most clearly shown in Figure 4a. In addition, at these lower pressures, the median AD was much lower than when measured in the BSC; at 6.0 cm from the sort chamber, the median AD at 20 psi was 2.8 μm for UV+ vs. 6.27 μm for BSC measurements and at 35 psi was 2.4 μm for UV+ vs. 5.24 μm for BSC.
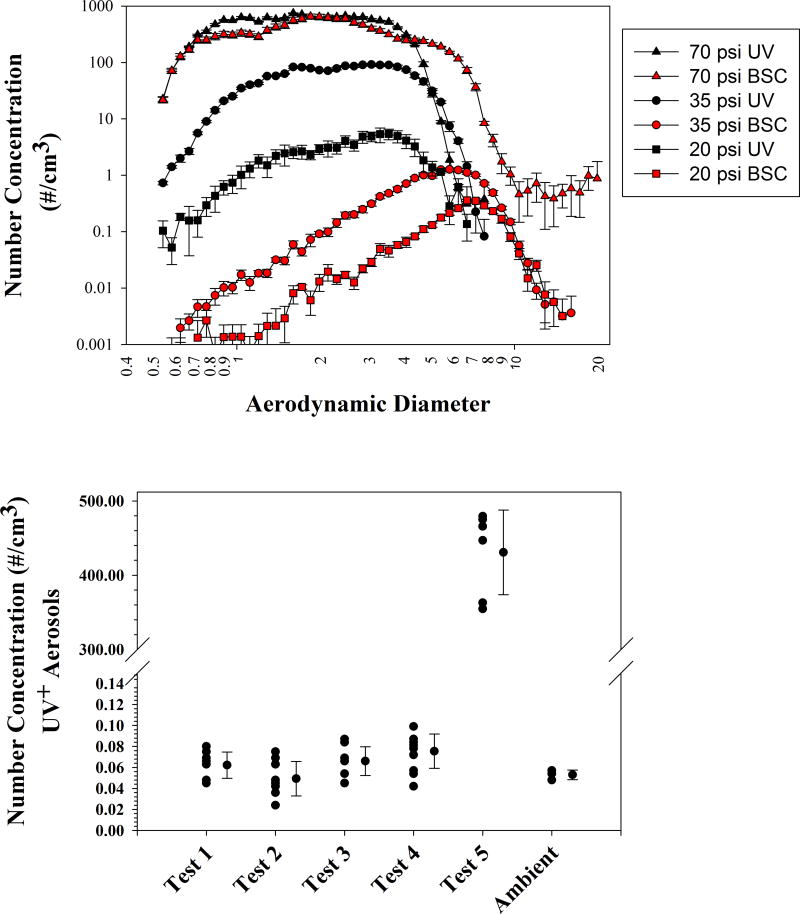
Figure 4.
Figure 4a. Comparison of BSC and non-enclosed aerosol measurements at 3.0 cm for 20, 25 and 70psi.
Figure 4b. Tests of Containment as outlined in Table 3. Tests 1–4 were not statistically significantly different from ambient (as determined by One-way ANOVA test with Holm-Sidak post hoc analysis). Ambient measurements were taken under conditions of Test 2, but with Aspirator door closed and no CBD used.
Tests of Containment and Aerosol Management System
The ability to detect and measure cell sorter-generated aerosols using the UV-APS presented the opportunity to evaluate the containment system design of the cell sorter. The FACS Aria cell sorter model has several features designed to contain aerosols: the sort chamber door is sealed with a rubber O-ring, the tube holders are also sealed with a rubber O-ring at the point of attachment just below the sort chamber, and the aspirator drawer (open during sorting) closes upon the detection of a stream perturbation to seal the sort chamber. The AMS provides aerosol evacuation through five hoses attached to the lower collection chamber. Containment and the efficacy of the AMS were tested under five conditions, under normal and fail mode with sort chamber door closed, and using CBD as a sample on a non-enclosed FACS Aria as outlined in Table 3.
Table 3.
Containment and AMS Tests
| Test | Conditions | Test purpose |
|---|---|---|
| 1 | Aspirator closed, AMS not active, no fail mode or sorting | Tests seals of sort chamber and aspirator drawer with undeflected stream but no sorting |
| 2 | Aspirator open, AMS not active, tube holder in place, test sort active, no fail mode | Tests sort chamber and tube holder seals during normal sorting without AMS |
| 3 | Aspirator open, AMS not active, tube holder in place, fail mode | Tests sort chamber and tube holder seals during fail without AMS |
| 4 | Aspirator drawer open, no tube holder, AMS active | Mimics fail mode during Automated Cell Deposition Unit (ACDU) sorting with AMS |
| 5 | Aspirator drawer open, no tube holder, AMS not active | Mimics fail mode during ACDU sorting without AMS |
| Ambient | No CBD: Aspirator closed, AMS not active, tube holder in place, test sort active, no fail mode | Ambient background |
As indicated, Tests # 1, 2 and 3 evaluated the containment design features under different conditions with sorting collection tube holder in place. Tests 4 & 5 evaluated the efficacy of the AMS during conditions similar to sorting into multiwell plates (no tube holder in place). All measurements were taken at 6 cm from the sort chamber door.
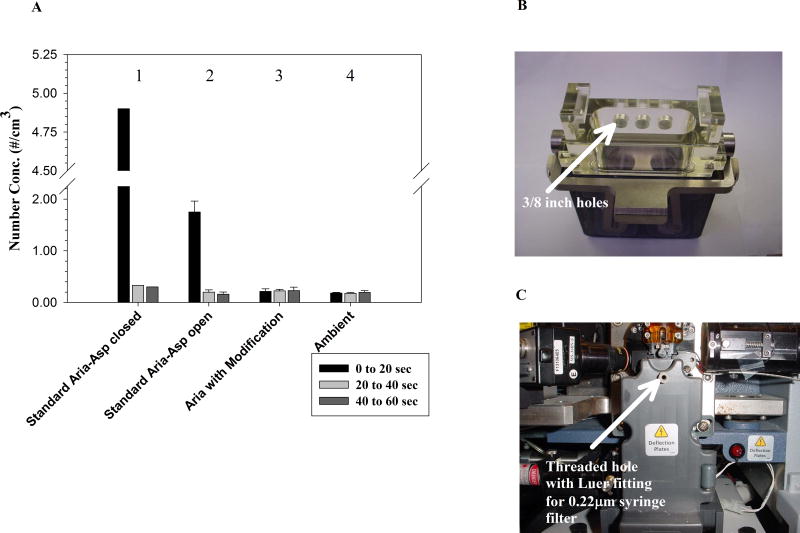
The results of tests # 1, 2 & 3 showed that the measured aerosol concentration of UV+ particles was not significantly different from ambient levels indicating that no aerosols escaped from the closed sort chamber during normal stream operation, test sort mode and fail mode (Figure 4b). Tests # 4 & 5 evaluated the efficacy of the AMS when set up for multiwell plate sorting and showed that under these conditions when the AMS is not operational (Test # 5), aerosols averaging 431/cm3 can escape. In contrast, with the AMS active at 20% (as recommended by the manufacturer) aerosols detected were at ambient levels (Test # 4; Figure 4b).
Test of Sort Chamber Evacuation Following Fail Mode
Although it was determined that the containment design of the sort chamber and the use of the AMS prevent aerosol escape as tested above, it was important to evaluate evacuation and containment in the context of procedures performed following a partial nozzle obstruction with stream deviation. Specifically, when procedures are followed by the operator to rectify a nozzle obstruction, is the instrument designed so that there is minimal risk of aerosol escape? The FACS Aria is equipped with a CCD camera that monitors droplet breakoff of the nozzle stream and in the event of a partial obstruction will signal the instrument to unload the sample, turn off the sheath stream, and close the aspirator drawer, which covers the sort collection tubes. It is then necessary for the operator to open the sort chamber to remove the nozzle and/or clean and dry droplet deflection plates. Consequently, tests were performed to determine whether aerosols generated within the sort chamber by the partial nozzle obstruction are evacuated prior to the task of opening the sort chamber. This was accomplished by mimicking normal sort conditions as closely as possible as follows: The tube holder was in normal sorting position, the sort chamber door was closed, and the aspirator drawer was in the open (sorting mode) position. Fail mode was induced, and then the stream was turned off, sample unloaded, aspirator drawer closed and after 15 sec had elapsed, the sort chamber door was opened and measurements taken. Three, 20- second samples were taken from a non-enclosed sorter operating at 70 psi (as detailed above) and using CBD as a sample.
The results showed that aerosols were detected (4.9/cm3) when the sort chamber door is opened 15 sec after the stream is shut off (Figure 5a; Exp. #1); aerosols then decreased to near ambient levels in the subsequent samples. Aerosols measured were reduced, but can still be detected (1.9/cm3) if, prior to opening the sort chamber door, the aspirator drawer is reopened and the AMS evacuation rate is increased to 100% (Figure 5a; Exp. #2). The above tests indicated that the design of the sort chamber, tube holders and AMS of the FACS Aria do not efficiently evacuate aerosols from the sort chamber following a nozzle obstruction with subsequent stream deviation. Therefore, it is possible that operators may be exposed to aerosols under these conditions.
Figure 5.
Figure 5a. Tests of Sort Chamber evacuation: Following fail mode, stream was shut off, aspirator drawer closed, AMS increased to 100% and after 15 sec had elapsed, sort chamber door was opened and measurements taken 6cm from sort chamber. Experiment #1 (1 measurement): aspirator drawer was left closed. Experiment #2 and #3: aspirator drawer was opened after stream was turned off, with no modification (#2; two replicates) and with modification (#3; three replicates). Experiment #4 is ambient measurements (two replicates). Three consecutive 20sec collections per replicate are shown. Results of Experiment #3 vs. #4 were not statistically significant at each 20sec time point (One-Way ANOVA).
Figure 5b. Modification to Aria Universal Top component of tube holder showing 3/8″ holes (arrows).
Figure 5c. Modification to Aria Sort Chamber door, showing threaded ¼″ hole to accommodate female Luer thread style fitting (1/4-28UNF) and 0.22mm Luer syringe filter installed.
Modification to Aria Tube Holder and Sort Chamber Door
It was reasoned that the inefficient evacuation of aerosols from the sort chamber was due to a lack of a communication between the sort chamber and the lower collection chamber; the O-ring of the tube holder and the closed design of the holder prevent the AMS negative airflow of the collection chamber from efficiently evacuating the sort chamber. Two simple modifications were made to increase the airflow between the sort and collection chambers. First, three holes were drilled into the Universal top component of the Aria II tube holder (Figure 5b). Second, a hole was drilled into the upper portion of the sort chamber door and was drilled so that it could be tapped to accommodate a ¼-28 Unified Fine (UNF) thread (Figure 5c). A female Luer thread style fitting was then installed in the door and a 0.22 μm syringe filter with Luer fitting was installed on the fitting. The holes in the tube holder top component permit airflow between the sort and collection chambers (when the aspirator door is open), while the sort chamber door hole provides filtered intake airflow.
Test of Modification
To verify that these modifications improve aerosol evacuation, the second test as outlined above was repeated with these modifications. Specifically, fail mode was induced, stream was turned off, sample unloaded and the aspirator door was closed. The aspirator door was then opened (using software control), the AMS evacuation rate increased to 100% and after 15 sec had elapsed the sort chamber door was opened and measurements taken. The results showed that with this simple modification, aerosols detected were not significantly different than ambient measurements (Figure 5a; Exp. # 3 vs. 4), indicating efficient evacuation from the sort chamber within 15 sec when the aspirator drawer is reopened and the AMS is increased to 100%. The tests were repeated on an Aria II in a BSC with identical results (data not shown).
Discussion
Establishment of biosafety policies for biomedical research involves a careful risk assessment of agents and procedures. Of particular concern are procedures that generate aerosols. Aerosols are the probable cause of many laboratory-associated infections (5) and infections in the laboratory may occur via the aerosol route even if not normally transmitted via aerosol in nature (6),(7). Risk assessment is facilitated by the availability of objective data to establish subsequent biosafety procedures. Although the production of aerosols by cell sorters has been previously recognized as a potential hazard (3), the present study was conducted to better define the risks associated with sorting of unfixed samples by characterizing the concentration and size distribution of aerosols capable of being produced.
Aerosol production was measured under the worst case scenario of a partial nozzle obstruction and the sort chamber door open. Aerosol characterization was performed on FACS Aria II cell sorters either a non-enclosed cell sorter or located within an operational BSC, operating at 20, 35 and at 70 psi. At 70 psi aerosol concentration and size distribution were generally comparable under both location conditions. However, at 20 and 35 psi, aerosols measured within the BSC were of lower concentration and higher AD. It would be difficult to determine all of the factors associated with this observation given the number of variables in this test system, but the lower aerosol concentration and higher AD found in the BSC measurements is most likely due to the increased susceptibility of the lower velocity, smaller AD particles to the effects of the 0.5 m/sec airflow of a BSC. That is, smaller, lower velocity particles were more rapidly dispersed by the airflow currents of the BSC. Therefore, results of tests taken on the non-enclosed sorter more accurately reflect aerosol distributions, especially at lower sheath pressures. The effect of the BSC airflow was also evidenced by the observed distribution of aerosols, specifically the rapid decrease in concentration observed at distances greater than 20 cm (Figure 2a). As shown in Figure 2 this rapid decrease in concentration between 20 cm and 25 cm from the sort chamber corresponds to measurements taken 5 cm to 10 cm from the edge of the Aria collection chamber. This is consistent with the predicted pattern of BSC air currents which would force aerosols along the top of the collection chamber and then downward toward the front grill of the BSC (see Figure 1).
There was a positive correlation between aerosol concentration and sheath pressure; at the highest sheath pressure tested of 70 psi, aerosol concentration was 1.8 × 104 particles/cm3 (non-enclosed) and 2.53 × 104/cm3 (BSC) when measured 1.5 cm from the sort chamber (Table 1 and 2). At this pressure, the median AD of aerosols measured ranged from 1.6 to 2.1 μm. The generation of a high concentration of aerosols of this size range by cell sorters is significant when considered with the following. It has been estimated that particles of diameters between 1 to 3 μm can remain suspended in a room almost indefinitely (8) and are associated with increased deposition and retention in the alveoli of the lung (reviewed in (9)). Most importantly, many studies have shown that aerosols in this size range are associated with infectivity (10–12) and for some pathogens a decreased median lethal dose, when compared with larger aerosols (13). The current study also clearly documents that sorters operating at high pressures (70 psi) are capable of producing a much higher concentration of aerosols than when operated at lower (35 psi and 20 psi) pressures (Figure 2d, and 3d) as previously suggested (3).
From the above discussion and as previously recommended (3), aerosol containment for cell sorters is essential for operator safety. The efficacy of aerosol containment on cell sorters has been previously tested with several different methods. The two most widely used methods are the bacteriophage T4 test and the GloGerm procedure. First described by Merrill (14) and later modified by Schmid, et al. (15), the bacteriophage T4 test is an adaptation of the method used to test for aerosol escape from zonal centrifugation (16). Disadvantages of this method, including a readout that is not available until the following day, led to the development of the alternative method using fluorescent melamine copolymer resin beads, trademarked GloGerm as first described by Oberyszyn and Robertson (17) and modified by Perfetto et al. (18). Most recently, containment on a FACS Aria was tested using a solution of radioactive Technetium-99m run as a sample and contamination assessed by measuring radioactivity using wipe tests, passive filter disks, collection of liquids and air sampling (19). Although this method is sensitive and quantitative, disadvantages such as a 60-hr down-time after testing, exposure to radioactivity and the requirement of a radioactive materials license make it impractical. Interestingly, in 1995, Ferbas et al. (20) tested the containment on an EPICS ELITE cell sorter using particle counter technology similar in principle to the UV-APS used in the present study, but lacking a laser to distinguish ambient from sorter-generated aerosols.
The aerosol containment engineering controls for the FACS Aria were evaluated in this study using the UV-APS. Similar to previous reports on this instrument (18, 19) the AMS, in concert with the engineering controls, including seals on the sort chamber door, tube holder seals and the aspirator drawer, were effective in containing aerosols during normal sorting modes and fail modes. However, data showed that an operational AMS was critical for containment of fail mode-induced aerosols when sorting into multiwell plates (Figure 4b), but even with an operational AMS, aerosols created by a fail mode were not efficiently evacuated from the sort chamber (Figure 5a). It is therefore possible that following a nozzle obstruction, upon opening the sort chamber door, the operator may be exposed to aerosols that are contained within the sort chamber. Modifications made to the tube holders and sort chamber door were demonstrated to be effective in rapidly evacuating fail mode-induced aerosols (15 sec) when the aspirator drawer was opened using software controls. By opening a communication to the sort chamber through the collection tube holder, this modification takes advantage of the high exhaust air flow rate of the AMS within the collection chamber (average of 7.2 m/sec per port (19)(21)).
In conclusion, this study details the potential aerosol hazard associated with droplet based cell sorters. Although other risk factors, such as the concentration of pathogen-containing aerosols during infectious cell sorting require further study, the documentation here of high concentration, small AD aerosols produced by cell sorters (particularly at high pressures) is invaluable in performing a comprehensive risk assessment for viable cell sorting. Further, this study demonstrates the utility of containment evaluation using a real-time, time-of-flight, fluorescence-based particle sizer (UV-APS). When contrasted with cascade impactor technology currently used with the GloGerm or T4 tests, this technology offers higher accuracy, is more quantitative and provides real-time, vs. time-consuming measurements. In spite of these advantages, widespread use of this instrument for this purpose is unlikely due to its high cost. However, it is feasible that a lower cost instrument designed to count particles (vs. size determination) having an AD in the range reported here (1–5 μm) and equipped with a lower cost violet (405nm) laser could potentially augment or supplant existing methods of aerosol containment testing.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH.
References
- 1.Pinkel D, Stovel R. Flow Chambers and Sample Handling. In: Van Dilla MA, Dean PN, Laerum OD, Melamed MR, editors. Flow Cytometry: Instrumentation and Data Analysis. London: Academic Press; 1985. pp. 77–128. [Google Scholar]
- 2.Schmid I. Biosafety guidelines for sorting of unfixed cells. Cytometry. 1997;28:99–117. doi: 10.1002/(sici)1097-0320(19970601)28:2<99::aid-cyto2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Schmid I, Lambert C, Ambrozak D, Marti GE, Moss DM, Perfetto SP. International Society for Analytical Cytology biosafety standard for sorting of unfixed cells. Cytometry A. 2007;71A:414–437. doi: 10.1002/cyto.a.20390. [DOI] [PubMed] [Google Scholar]
- 4.Volckens J, Peters TM. Counting and particle transmission efficiency of the aerodynamic particle sizer. Journal of Aerosol Science. 2005;36:1400–1408. [Google Scholar]
- 5.US Department of Health and Human Services Centers for Disease Control and Prevention and National Insitutues of Health. Biosafety in Microbiological and Biomedical Laboritories. 2009 http://www.cdc.gov/biosafety/publications/bmbl5.
- 6.Oh M, Kim N, Huh M, et al. Scrub typhus pneumonitis acquired through the respiratory tract in a laboratory worker. Infection. 2001;29:54–56. doi: 10.1007/s15010-001-0139-5. [DOI] [PubMed] [Google Scholar]
- 7.Pentella MA, Kostle PA, Desjardin L, Gilchrist MJR. Biosafety for Airborne Pathogens. In: Fleming DO, Hunt DL, editors. Biological Safety:Principles and Practices. 4. Washington, D.C: ASM Press; 2006. pp. 209–220. [Google Scholar]
- 8.Knight V. Viruses as agents of airborne contagion. Ann N Y Acad Sci. 1980;353:147–156. doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JH. Health-related aerosol measurement: a review of existing sampling criteria and proposals for new ones. J Environ Monit. 2005;7:1037–1053. doi: 10.1039/b509617k. [DOI] [PubMed] [Google Scholar]
- 10.Couch RB, Douglas RG, Jr, Lindgren KM, Gerone PJ, Knight V. Airborne transmission of respiratory infection with coxsackievirus A type 21. Am J Epidemiol. 1970;91:78–86. doi: 10.1093/oxfordjournals.aje.a121115. [DOI] [PubMed] [Google Scholar]
- 11.Cate TR, Couch RB, Fleet WF, Griffith WR, Gerone EPJ, Knight V. Production of tracheobronchitis in volunteers with rhinovirus in a small-particle aerosol. Am J Epidemiol. 1965;81:95–105. doi: 10.1093/oxfordjournals.aje.a120501. [DOI] [PubMed] [Google Scholar]
- 12.Alford RH, Kasel JA, Gerone PJ, KnightT V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 13.Day WC, Berendt RF. Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infect Immun. 1972;5:77–82. doi: 10.1128/iai.5.1.77-82.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill JT. Evaluation of selected aerosol-control measures on flow sorters. Cytometry. 1981;1:342–345. doi: 10.1002/cyto.990010507. [DOI] [PubMed] [Google Scholar]
- 15.Schmid I, Hultin LE, Ferbas J. Testing the efficiency of aerosol containment during cell sorting. Curr Protoc Cytom. 2001;Chapter 3(Unit) doi: 10.1002/0471142956.cy0303s01. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin CL, Lemp JF, Barbeito MS. Biohazards assessment in large-scale zonal centrifugation. Appl Microbiol. 1975;29:484–490. doi: 10.1128/am.29.4.484-490.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberyszyn AS, Robertson FM. Novel rapid method for visualization of extent and location of aerosol contamination during high-speed sorting of potentially biohazardous samples. Cytometry. 2001;43:217–222. [PubMed] [Google Scholar]
- 18.Perfetto SP, Ambrozak DR, Koup RA, Roederer M. Measuring containment of viable infectious cell sorting in high-velocity cell sorters. Cytometry A. 2003;52A:122–130. doi: 10.1002/cyto.a.10033. [DOI] [PubMed] [Google Scholar]
- 19.Wallace RG, Aguila HL, Fomenko J, Price KW. A method to assess leakage from aerosol containment systems: testing a fluorescence-activated cell sorter (FACS) containment system using the radionuclide technetium-99m. Applied Biosafety Journal. 2010;15:77–85. [Google Scholar]
- 20.Ferbas J, Chadwick KR, Logar A, Patterson AE, Gilpin RW, Margolick JB. Assessment of aerosol containment on the ELITE flow cytometer. Cytometry. 1995;22:45–47. doi: 10.1002/cyto.990220109. [DOI] [PubMed] [Google Scholar]
- 21.The modification described herein has been incorporated into FACS Aria cell sorters now being sold by BD Biosciences.